Abstract
Mutations of the Wiskott-Aldrich syndrome protein (WASP) gene result either in the classic Wiskott-Aldrich syndrome (WAS) or in a less severe form, X-linked thrombocytopenia (XLT). A phenotype-genotype correlation has been reported by some but not by other investigators. In this study, we characterized WASP gene mutations in 50 Japanese patients and analyzed the clinical phenotype and course of each. All patients with missense mutations were WASP-positive. In contrast, patients with nonsense mutations, large deletions, small deletions, and small insertions were WASP-negative. Patients with splice anomalies were either WASP-positive or WASP-negative. The clinical phenotype of each patient was correlated with the presence or absence of WASP. Lack of WASP expression was associated with susceptibility to bacterial, viral, fungal, and Pneumocystis carinii infections and with severe eczema, intestinal hemorrhage, death from intracranial bleeding, and malignancies. Rates for overall survival and survival without intracranial hemorrhage or other serious complications were significantly lower in WASP-negative patients. This analysis provides evidence for a strong phenotype-genotype correlation and demonstrates that WAS protein expression is a useful tool for predicting long-term prognosis for patients with WAS/XLT. Based on data presented here, hematopoietic stem cell transplantation should be considered, especially for WASP-negative patients, while the patients are young to improve prognosis.
Introduction
The Wiskott-Aldrich syndrome (WAS) is a rare X-linked immunodeficiency disorder characterized by thrombocytopenia and small platelets, eczema, recurrent infections, and increased risk for autoimmunity and malignancy.1 The gene responsible for this syndrome, WAS protein (WASP), consists of 12 exons containing 1823 base pairs. WASP encodes a 502–amino acid (aa) protein that is expressed selectively in hematopoietic stem cell–derived lineages and is involved in cell signaling and cytoskeleton reorganization.2-5 Subsequent sequence analysis of WASP demonstrated that X-linked thrombocytopenia (XLT), a congenital disorder characterized by thrombocytopenia and small platelets but, in general, without the other complications of WAS, is a mild allelic variant caused by mutations of the WASP gene.6,7 A correlation between clinical phenotype and genotype was reported independently by several investigators8-10 but was not observed by others.11 One of the difficulties in establishing a phenotype-genotype correlation for WAS/XLT has been the lack of carefully collected clinical data over time and uncertainty about a common denominator. Here we report the WASP gene mutations identified in 50 WAS/XLT patients from 43 unrelated Japanese families and the effects these mutations have on the expression of the WAS protein. The clinical course of each patient has been recorded longitudinally, and a correlation has been established between the clinical phenotype, the extent of the mutation, and the presence or absence of WASP in patient-derived Epstein-Barr virus (EBV)–transformed B-lymphoblastoid cell lines (B-LCLs).
Patients, materials, and methods
Patients and clinical phenotypes
Fifty affected members of 43 unrelated families with WAS or XLT were included in this analysis. Twelve patients had been described previously.12-14 Using a simple scoring system,7,15 the severity of WAS/XLT-associated symptoms was estimated and expressed as a score of 1 to 5. A score of 1, assigned to patients with only thrombocytopenia and small platelets, and a score of 2, assigned to patients with additional findings of mild, transient eczema or minor infections, identified XLT patients. Those with treatment-resistant eczema and recurrent infections in spite of optimal treatment received a score of 3 (mild WAS) or 4 (severe WAS). Regardless of the original score, if any patient then had autoimmune disease or malignancy, the score was changed to 5.
Approval for these studies was obtained from the Tokyo Medical and Dental University institutional review board. Informed consent was provided according to the Declaration of Helsinki.
Cell lines
B-LCLs were established by inoculating peripheral blood mononuclear cells (PBMCs) from healthy controls and WAS patients with EBV-containing supernatant, as described elsewhere.12
DNA purification and sequencing of genomic DNA
Genomic DNA was extracted from granulocytes or B-LCLs using Sepa-Gene (Seikagaku kogyo, Tokyo, Japan). Purified genomic DNA samples were amplified with primer pairs designed to span each exon and exon/intron junction, and the specific causative mutation was identified by direct sequencing as described previously.12
RNA isolation, RT-PCR, and sequencing of cDNA
RNA isolation from B-LCLs, using the Wizard SV-total RNA isolation kit (Promega, Madison, WI), and reverse transcription–polymerase chain reaction (RT-PCR), using the RT-PCR kit (Stratagene, La Jolla, CA), were performed according to the manufacturers' instructions. WASP cDNA was amplified by PCR in 2 overlapping fragments using the previously reported primers.12 PCR products were purified by agarose gel electrophoresis. PCR products were subcloned into pGEM-T Easy Vector System II (Promega). Subcloned cDNA was sequenced using the Big Dye Terminator cycle sequencing kit (Perkin-Elmer, Foster City, CA) and an ABI 310 genetic analyzer (Perkin-Elmer).
Anti-WASP antisera and Western blot analysis
Rabbit anti-WASP antibody (Ab 503) was made against a synthetic peptide (aa's 209-226 of WASP), as described previously.9 B-LCLs from healthy control subjects and WAS patients were suspended at 1.0 × 107/mL in lysis buffer containing 1% Nonidet P-40, 1 mM phenylmethylsulfonyl fluoride (PMSF), 0.5% aprotinin, and 10 μg/mL leupeptin at pH 7.5 and were kept on ice for 30 minutes. The protein concentration was determined in each lysate by the Protein Assay Kit (Bio-Rad Laboratories, Hercules, CA). From each sample, 40 μg total protein was loaded onto a sodium dodecyl sulfate (SDS) polyacrylamide gel, electrophoresed, and transferred to a polyvinylidene difluoride (PVDF) membrane (Bio-Rad). After blocking with 5% nonfat milk, the membranes were incubated with anti-WASP Ab 503 at 1:5000 dilutions for 1 hour at room temperature. After washing with 0.1% Tween/phosphate-buffered saline 4 times, the membranes were incubated with alkaline phosphatase-conjugated goat antirabbit immunoglobulin (Promega) at a concentration of 1:7500 for 30 minutes at room temperature. Results were visualized by incubation with AP buffer (100 mM Tris-HCl, pH 9.5; 100 mM NaCl; and 5 mM MgCl2).
Statistics
Probabilities of outcomes were calculated using the Kaplan-Meier product limit estimate. Univariate comparisons of survival were made using the log-rank test. Comparisons of the data between WASP-positive and -negative groups were made using the Student t test and the Mann-Whitney U test.
Results
WASP mutations and protein expression by B-LCL from WAS/XLT patients
Thirty-three different mutations of the WASP gene were identified in 43 unrelated families studied (Tables 1 and 2). To investigate the relationship between clinical features and WASP protein expression, we performed Western blot analysis of B-LCL lysates using the polyclonal anti-WASP Ab 503. Blots for selected WAS/XLT patients are shown in Figure 1.
Western blot analysis of EBV-transformed cell lysates from WAS/XLT patients. Typical blots are shown. Patient numbers and descriptions of the mutations are shown in Table 1. (A) WASP-positive patients. (B) WASP-negative patients.
Western blot analysis of EBV-transformed cell lysates from WAS/XLT patients. Typical blots are shown. Patient numbers and descriptions of the mutations are shown in Table 1. (A) WASP-positive patients. (B) WASP-negative patients.
All 16 patients with missense mutations expressed normal-sized WASP (Figure 1A). Four patients from 3 unrelated families (patients 1, 2, 8, and 13) who had normal amounts of WASP had isolated thrombocytopenia and received a score of 1. The other 12 patients with missense mutations had a reduced amount of normalsized WASP; 7 had a clinical score of 1 or 2, 1 had a score of 3, and 4 had autoimmune renal disease (score 5A).
WASP was not detected in the 3 unrelated patients with nonsense mutations in exons 1 and 2 (patients 17, 18, 19), but it was present as a truncated protein in the 2 brothers with nonsense mutations in exon 10 (patients 20, 21).
WASP could not be detected in cell extracts from any of the 9 unrelated patients with deletions within the WASP gene or from the patient with deletion of the entire WASP gene (Figure 1B). All patients with deletions had a classical WAS phenotype (scores 3 to 5, except for a patient with a score of 2) (Table 2). The only patient with a nucleotide insertion did not express WASP.
B-LCL extracts from 11 of 18 patients with splice-site mutations showed reduced amounts of normal-sized WASP protein (Figure 1A). It is of interest that normally spliced products and abnormally spliced products were detected in cDNA from these patients (Table 1). B-LCL extracts from the other 7 patients with splice-site mutations lacked WASP protein expression (Figure 1B).
WASP expression correlates with clinical characteristics
To correlate clinical phenotype with genotype, we divided the patients into 2 groups according to the expression of full-length WASP protein. WASP-positive patients were defined as expressing normal or reduced amounts of full-length WASP protein, as detected by Western blot analysis. WASP-negative patients were defined as having either no detectable WASP protein or C-terminal–truncated WASP protein. The entire medical history of each patient was reviewed and retrospectively analyzed and scored at the time of mutation analysis.
Table 3 summarizes the clinical phenotypes and the WAS/XLT scores of the patients. Twenty-seven patients were defined as WASP-positive and 23 patients as WASP-negative. Of 27 WASP-positive patients, 21 patients (78%) had no episodes of recurrent or life-threatening infections. On the other hand, 21 (91%) of 23 WASP-negative patients had a history of recurrent or life-threatening infections. The age distribution of 6 WASP-positive patients who had score of 3 or more (mean age, 7.7 years; range, 1-24 years) was even younger than that of 21 WASP-positive patients who had score 1 or 2 (mean age, 13.2 years; range, 2-61 years).
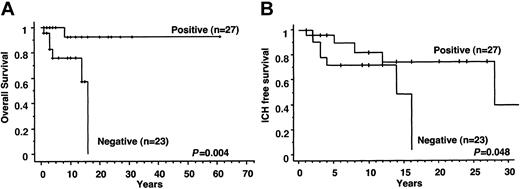
Twenty-six (96%) of 27 WASP-positive patients were alive at the time of this analysis compared with only 17 (74%) of 23 WASP-negative patients. Five patients (including an 8-year-old WASP-positive patient with a score of 4) died because of intracranial hemorrhage (ICH), 1 patient died of JC virus encephalitis after bone marrow transplantation (BMT), and 1 patient died of EBV-associated B-cell lymphoma. Of those alive, the WASP-positive patients were significantly older than the WASP-negative patients (mean ages, 12.1 years and 6.1 years, respectively; P < .0001). Mean age at death of the WASP-negative patients was 6.8 years (range, 1-16 years). Overall survival for WASP-positive and WASP-negative WAS/XLT patients is shown in Figure 2A. The 10-year probability of survival was 92.3% (range, 84.9%-100%) for WASP-positive patients and 76.0% (range, 65.3%-86.7%) for WASP-negative patients. More strikingly, the 20-year probability of survival was 92.3% (range, 84.9%-100%) for WASP-positive patients and 0% for WASP-negative patients (P = .004). Thus, WASP expression is closely linked to the overall outcome of the disease and has a strong prognostic value for patients with WASP gene mutations. ICH-free survival is shown in Figure 2B. The 10-year probability of ICH-free survival was 81.8% (range, 71.9%-91.7%) for WASP-positive and 70.6% (range, 59.2%-82.0%) for WASP-negative patients. The 30-year probability of ICH-free survival was 36.8% (range, 9.9%-63.5%) for WASP-positive and 0% for WASP-negative patients (P = .048).
WASP expression and probability of survival of patients. (A) Overall survival of the patients. Positive and negative indicate the survival curves of patients found positive or negative for WASP on Western blot analysis. (B) Intracranial hemorrhage-free survival of WAS/XLT patients. Positive and negative indicate the survival curves of patients found positive or negative for WASP on Western blot analysis.
WASP expression and probability of survival of patients. (A) Overall survival of the patients. Positive and negative indicate the survival curves of patients found positive or negative for WASP on Western blot analysis. (B) Intracranial hemorrhage-free survival of WAS/XLT patients. Positive and negative indicate the survival curves of patients found positive or negative for WASP on Western blot analysis.
WASP expression, platelet abnormalities, and hemorrhagic episodes
Patient age on first visit was similar between the 2 groups (Table 3). Most of the patients had symptoms related to thrombocytopenia. More WASP-positive patients had petechiae and more WASP-negative patients had hematorrhea as an initial symptom. By definition, WAS and XLT patients have thrombocytopenia and small platelets in common and, as a result, lifelong hemorrhagic diathesis. To determine whether the presence or absence of WASP influences bleeding tendency, we compared platelet data and hemorrhagic incidences in the 2 groups of patients (Table 4). ICH was seen in 5 of 27 WASP-positive and 6 of 23 WASP-negative patients. Of note, 4 WASP-negative patients died as a result of hemorrhage compared with only 1 WASP-positive patient. Episodes of intestinal bleeding were significantly more frequent in WASP-negative patients (P < .0001) (Table 4).
Platelet abnormalities (numbers, size) were the same between the 2 groups of patients. Numbers of megakaryocytes in the bone marrow were normal or increased in 11 (40%) of 28 patients tested (no difference between the 2 groups). We were unable to detect megakaryocytes in the bone marrow aspirates of 7 (46%) of 15 WASP-negative patients and 1 (8%) of 13 WASP-positive patients (P = .07). Most of the patients in each group had increased platelet-associated immunoglobulin G (IgG), and no significant differences were observed among them.
WASP expression, immunologic defects, and infections
To minimize the difference in age distribution between the 2 groups, 6 adult WASP-positive patients (patients 6, 10, 12, 15, 49, 50) were excluded from the analysis of frequency of infection. WASP-negative patients were found to have significantly more episodes of infection than WASP-positive patients (0.67 episodes/patient-year and 0.14 episodes/patient-year, respectively; P < .0001) (Table 5). Bacterial infections (otitis media, skin abscess, pneumonia, enterocolitis, meningitis, sepsis, urinary tract infection, and others) were observed 4 times more frequently in WASP-negative patients than in WASP-positive patients (0.40 events/patient-year and 0.10 events/patient-year, respectively; P = .003). Viral infections were more frequent in WASP-negative patients than in WASP-positive patients (0.17 events/patient-year and 0.05 events/patient-year, respectively). WASP-negative (but not WASP-positive) patients were highly susceptible to herpes simplex virus (HSV) infections (13 episodes). Cytomegalovirus (CMV) encephalitis occurred in 2 WASP-negative patients and CMV hepatitis in 3 WASP-positive patients. WASP-negative patients were also reported to have extensive candidiasis (9 patients), aspergillosis (3 patients), and Pneumocystis carinii pneumonia (PCP) (2 patients). On the other hand, recurrent herpes zoster was only observed in WASP-positive patients (2 patients).
The bias of antimicrobial prophylaxis did not contribute to the susceptibility of infections seen in WASP-negative patients (Table 5). All WASP-positive patients except one did not receive antimicrobial prophylaxis. On the other hand, 13 (56.5%) of 23 WASP-negative patients received antimicrobial prophylaxis after clinical or molecular diagnosis. Thirteen of 14 received trimethoprim-sulfamethoxazole and amphotericin B syrup. PCP was observed before the prophylaxis started in 2 patients affected, indicating the importance of prophylaxis by trimethoprim-sulfamethoxazole. Only 1 WASP-negative patient received acyclovir to prevent recurrent herpes infections. Candidiasis (4 patients) and aspergillosis (1 patient) developed even under prophylactic treatment with amphotericin B syrup, indicating the limitation of this prophylaxis. These observations strongly support aggressive prophylactic therapy with fungal and antiviral agents (such as acyclovir) and with PCP for WASP-negative patients.
Lymphocyte subsets and in vitro lymphocyte proliferation to mitogens (phytohemagglutinin [PHA] or concanavalin A [ConA]) were normal in both groups of patients, as has been reported previously.1 Serum IgG concentrations were normal or increased in both groups. Low serum IgM concentrations were observed in 23% of the WASP-positive and 20% of WASP-negative patients, and increased serum IgA concentrations were observed in WASP-positive (53%) and in WASP-negative (36%) patients. These immunologic data were not significantly different between the 2 groups.
WASP expression and atopic diseases
The severity of eczema was estimated by the scoring system, described in “Patients, materials, and methods.” As shown in Table 6, only 1 WASP-positive patient had persistent difficulty controlling eczema; 16 patients (59%) never had or had only transient and mild eczema. In contrast, severe eczema was reported in 11 WASP-negative patients; eczema was transient in 1 and absent in 1 WASP-negative patient. Asthma and food allergy, an often reported complication associated with atopic dermatitis,17 was observed in 8 patients (16%) only and did not correlate with the expression of WASP. Very high IgE concentrations (more than 1000 IU/mL) were found in 62% of WASP-negative patients compared with 25% of WASP-positive patients (P = .05). IgE concentrations did not correlate with the severity of eczema.
WASP expression and autoimmune disorders
Autoimmune diseases and malignancies are frequent complications observed in WAS patients.1 As shown in Table 7, autoimmune/inflammatory diseases were seen almost equally as much in WASP-positive and WASP-negative patients (26% and 22%, respectively). Five WASP-positive patients (19%) had IgA nephropathy. Age at onset was 10 to 20 years, and the disease progressed to chronic renal failure in 2 of the 5 patients at the ages of 22 and 24, respectively.
WASP expression and malignancies
During the observation period, malignancies developed in 5 WASP-negative patients (Table 7). Lymphoma developed in 2 patients and was related to EBV infection in one of them, as reported previously.18 EBV-related lymphoma was detected at age 1, and the patient died at age 2. Myelodysplasia developed in 3 patients. None of the WASP-positive patients had a malignancy. However, we obtained positive family histories from 2 unrelated kinships in which the surviving patients were WASP-positive. Each family reported the death of an affected male relative; 1 died of malignant lymphoma at age 32 and the other of a brain tumor at age 38.
WASP expression and response to therapy
Hematopoietic stem cell transplantations (HSCTs) were performed in 3 of 27 WASP-positive and in 12 of 23 WASP-negative patients. Eleven patients received bone marrow from HLA-matched donors (sibling, 1; unrelated donor, 10), and 1 patient received bone marrow from an HLA-haploidentical donor. Three patients underwent reconstitution with cord blood stem cells from partially matched, unrelated donors. Mean observation time was 2.2 years (range, 0.25-8.0 years), and all but 1 patient are alive. Three patients rejected grafts, and 2 patients achieved partial grafting after the first HSCT (Table 8). Two patients achieved engraftment after second HSCT, and 1 patient achieved it after receiving donor lymphocyte transfusions. A 14-year-old WASP-negative patient died after undergoing BMT from an unrelated HLA-matched donor. Initially he achieved partial engraftment, but EBV-related posttransplantation lymphoproliferative disease developed. Treatment with donor lymphocyte transfusions resolved the lymphoproliferation. However, HSV and fungal pneumonitis then developed, and he died 200 days after BMT of JC-virus–related progressive multifocal leukoencephalopathy.
Splenectomy was performed in 10 patients (5 WASP-positive, 5 WASP-negative). Good responses (platelet counts higher than 100 × 109/L) were achieved in 5 patients. All 5 WASP-positive patients survived without ICH after splenectomy for an average of 17.0 years (range, 0.5-59 years). In contrast, 2 of the 5 WASP-negative patients died of ICH after splenectomy. After splenectomy all patients were placed on prophylactic antibiotics. In spite of this, 2 WASP-positive and 3 WASP-negative patients acquired but survived pneumococcal sepsis/meningitis.
High-dose intravenous immunoglobulin therapy was given to 24 patients (13 WASP-positive, 11 WASP-negative). No patient had a good response; therefore, it is no longer recommended for treatment of WAS-associated thrombocytopenia. Prednisolone or methylprednisolone pulse therapy was attempted in 26 patients. Sixteen patients (62%) did not respond, and 10 patients (38%) achieved transient responses to this therapy.
Discussion
To compile this comprehensive phenotype-genotype study, we collected clinical and molecular data from a population of patients treated at major medical centers in Japan for classic WAS or XLT. Clinical data were collected over many years and addressed disease severity, complications, clinical outcomes, and extent of immunodeficiency. Each patient underwent an extensive molecular evaluation that included mutation analysis of WASP, both at the genomic DNA and at the transcriptional/translational levels. Thirty-three unique WASP mutations were observed in the 43 unrelated families enrolled in the study, demonstrating that mutations were highly diverse in Japanese patients, as had been observed in other populations.9,10,19 In agreement with previous reports, missense and nonsense mutations were found predominantly in exons 1 and 2, and splice-site mutations were found within the C-terminal half of the gene; insertions and deletions were distributed throughout the WASP gene. To test the hypothesis that clinical phenotype correlates with the presence or absence of WASP, we divided the patients into 2 groups. Patients with normal or reduced amounts of full-length mutated WASP were categorized as WASP-positive patients (group 1), and those with no detectable or truncated WASP were categorized as WASP-negative patients (group 2). Group 1 consisted of 27 WASP-positive and group 2 of 23 WASP-negative subjects. As expected, patients with missense mutations expressed normal-sized WASP, often at a decreased level. Patients with nonsense mutations either lacked WASP or had truncated protein if the mutation affected exons at the C-terminal end. Patients with deletions and the only patient with an insertion did not express WASP, presumably because of frameshift and early termination of transcription. Some patients with splice-site mutations had multiple splicing products and expressed WASP, but others failed to express detectable WASP.
Using a scoring system, most patients with missense mutations had a score of 1 or 2 (XLT phenotype). In contrast, patients who had nonsense mutations or deletions/insertions had scores of 3 to 5 (WAS phenotype). Two thirds of WASP-positive patients with splice-site mutations had mild disease with scores of 1 and 2, possibly because of the presence of small quantities of normally spliced WASP. All patients with splice-site mutations who were WASP-negative had severe disease with scores of 3 to 5.
A striking correlation between WASP expression and susceptibility to infection was observed. The number of bacterial and viral infections per patient-year was almost 4 times higher in WASP-negative patients than in WASP-positive patients. Fungal infections and P carinii pneumonia were only observed in WASP-negative patients. Interestingly, standard screening tests to assess immune responses were minimally affected, and no difference was observed between the 2 groups of patients. In-depth evaluation of the immune system in WAS patients has revealed reproducible defects; lymphocytes from patients with classic WAS fail to proliferate in vitro when exposed to anti-CD3 mAb.20 If they are immunized with bacteriophage ΦX174, WAS patients have severely defective in vivo antibody responses characterized by failure for isotype switching and for developing immunologic memory.21 In contrast, after bacteriophage immunization, patients with XLT are able to isotype switch and to develop immunologic memory though it is usually less than that of healthy controls.22 The increased incidence of infections and the severe defect in antibody production justifies prophylactic treatment of WAS patients with intravenous immunoglobulin infusions and, in those younger than 3 years of age, with trimethoprim-sulfamethoxazole and anti-herpes virus prophylaxis.
Platelet abnormalities are characteristic findings in patients with WASP mutations, but no differences in platelet number or size between the 2 groups were observed. Almost half the patients from each group had normal or increased numbers of megakaryocytes in the bone marrow. However, the proportion of patients with undetectable megakaryocytes was greater in WASP-negative than in WASP-positive patients (46% vs 8%, respectively). Nevertheless, as shown in Table 4, the number of serious episodes of bleeding (intracranial hemorrhage, intestinal bleeding) was higher in the WASP-negative group. One patient (3.7%) from the WASP-positive group and 4 patients (17.4%) from the WASP-negative group died as a result of hemorrhage.
As expected, the severity of eczema correlated with the expression of WASP. Only 1 of 27 WASP-positive patients had severe eczema compared with 11 of 23 WASP-negative patients. High IgE levels (more than 1000 IU/mL) were observed in most (62%) WASP-negative patients compared with only 25% in the WASP-positive group, strongly suggesting that the lack of WASP is directly or indirectly responsible for the atopic diathesis observed in WAS patients.
Whereas malignancies did not develop in any of the 27 WASP-positive patients during the observation period, malignancies were diagnosed in 5 of 23 WASP-negative patients (lymphoma, 2; myelodysplasia, 3). This observation correlates with the findings of others, who reported that the incidence of malignancies is low in XLT patients compared with classic WAS (H.D.O., unpublished observation; J.A. Winkelstein, personal communication).
In contrast, autoimmune/inflammatory diseases were equally frequent in WASP-positive and WASP-negative patients (26% and 22%, respectively). Characteristic nephropathy with IgA deposits was observed in 5 of 27 WASP-positive patients, but not in WASP-negative patients. IgA nephropathy progressed through the years and led to chronic renal failure in 2 patients. The incidence of nephropathy in patients with classic WAS was reported previously (5 of 32 patients),23 and there are some case reports of XLT with nephropathy.24-26 It is possible that IgA nephropathy required the generation of specific IgA antibody, which may be less likely to be produced in WASP-negative patients with defective isotype switching. In WAS patients there were some defects of glycosylation.27 The O-glycan side chains in the hinge of the glomerular IgA1 were highly underglycosylated in IgA nephropathy.28 Thus the impaired glycosylation of IgA1 molecules caused by mutated WASP might have contributed to the pathogenesis of IgA nephropathy seen in the patients.
Finally, we observed a striking difference in long-term outcomes. All but 1 of the 27 WASP-positive patients were alive at the completion of the study, compared with only 17 of 23 WASP-negative patients. Five patients (including an 8-year-old WASP-positive patient with a score of 4) died of intracranial hemorrhage, 1 patient died of JC virus encephalitis after BMT, and fatal EBV-associated B-cell lymphoma developed in 1 patient. Of those alive, the WASP-positive patients were significantly older than the WASP-negative patients (mean ages, 12.1 years and 6.1 years, respectively; P < .001). The 10-year probability rate of survival was higher for WASP-positive patients than for WASP-negative patients (92.3% vs 76.0%). Furthermore, when we calculated the 20-year probability rate of survival, none of the WASP-negative patients were expected to survive, whereas 92.3% of WASP-positive patients were expected to survive (P = .004). As shown in Figure 2B, intracranial hemorrhage-free survival was highly dependent on WASP expression. The 30-year probability of intracranial hemorrhage-free survival is 36.8% for WASP-positive and 0% for WASP-negative patients. Thus, the expression of WASP is closely linked to the overall disease outcome and has a strong prognostic value for patients with WASP gene mutations.
This retrospective analysis revealed that bone marrow transplantation was more frequently performed in WASP-negative patients (12 of 23) than in WASP-positive (3 of 27). Only 1 patient, a 14-year-old WASP-negative patient who underwent BMT from an unrelated HLA-matched donor, died of complications related to the procedure, which included EBV-related lymphoproliferative disease and JC-virus–associated progressive multifocal leukoencephalopathy. Both groups responded equally to splenectomy; however, 2 of the WASP-negative patients who underwent splenectomy died of intracranial hemorrhage, and sepsis/meningitis developed in 5 (2 from WASP-positive and 3 from WASP-negative group), developed in spite of antibiotic prophylaxis. Neither high-dose intravenous immunoglobulin infusions (IVIG) nor prednisone had a significant impact on thrombocytopenia in either group of patients, and neither is recommended in WAS/XLT.
This retrospective study demonstrates a strong phenotype-genotype correlation in patients with WASP mutation. Although this has been reported by others,9,10,29 more detailed observations, such as the data related to thrombocytopenia, types of infections, incidence and course of autoimmune and malignant diseases, response to therapies, and prognosis, especially in a large number of XLT patients, gives us more informations to understand and to manage this complex disease. There is some overlap between the 2 groups, however, as has been observed by others.11 Infants and young children may not yet have developed the final clinical phenotype; other genes may influence the severity of the clinical symptoms; some patients are exposed to more pathogenic microorganisms. Recently, reversal of the WASP gene mutations to normal has been observed in several WAS patients, resulting in improvement of the clinical phenotype.30,31 The guarded long-term prognosis of patients with the classic WAS phenotype associated with the complete absence of WASP is a strong indication for early stem cell transplantation using matched related or unrelated bone marrow or cord blood stem cells because these methods were shown to have high probability of hematopoietic and immunologic reconstitution, as seen in this and other studies.32 If there is a suitable donor, HSCT can also be a treatment of choice for WASP-positive patients with the XLT phenotype because of their equivalent or higher frequency of complications, among them ICH, autoimmune diseases, and IgA nephropathy, that lead to chronic renal failure. Prospective studies of HSCT for XLT are needed to establish the method and to assess the safety of HSCT for this patient group.
Splenectomy should be carefully indicated in WASP-negative patients if they have no suitable HSCT donor because 3 patients acquired pneumococcal sepsis/meningitis and 2 patients died because of ICH after splenectomy in our study in spite of the favorable outcomes of a previous report.33 Indicating splenectomy for WASP-positive patients remains controversial. Further study is needed to establish the standard treatment regimen for WAS and XLT patients.
Prepublished online as Blood First Edition Paper, September 11, 2003; DOI 10.1182/blood-2003-05-1480.
Supported in part by grants from the Ministry of Education, Science and Culture of Japan (S.N. and M.K.), the Kawano Masanori Memorial Foundation for the Promotion of Pediatrics (S.N.), the National Institutes of Health (HD 17427) (H.D.O.), the March of Dimes and Birth Defects Foundation (6-0116) (H.D.O.), the DeJoria Wiskott-Aldrich Research Fund (H.D.O.), the Jeffrey Modell Foundation (H.D.O.), and the Immunodeficiency Foundation (H.D.O.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The following doctors allowed us to study their patients and provided material that was invaluable for this investigation:Akihiko Nomura, Akihiro Yaginuma, Akitoshi Kinoshita, Atsushi Shibuya, Chihiro Kawakami, Hirokazu Inoue, Hiroshi Komatsu, Hirotake Takahashi, Hiroyuki Takimoto, Jinya Itoh, Kaname Araida, Kazuhiro Ohga, Kei Ohnuma, Kenji Katamura, Kiyoshi Kawakami, Koichi Matsumoto, Masaaki Kumagai, Masafumi Kaneko, Masahiro Sako, Miho Maeda, Naoko Hashimoto, Narumi Hibi, Noriko Obuchi, Shinichiro Nishimura, Shoichi Ohga, Sumi Yoshimoto, Takahiro Uehara, Yukino Itakura, Takashi Hamasaki, Takashi Onodera, Toshisada Morita, Yoshihisa Nagatoshi, Yoshihisa Ohishi, Yoshiko Matsuda, Yoshiyuki Ohashi, Yuichi Akiyama, Miharu Yabe, Yoshiteru Osone, Masataka Ichikawa, and Yuji Miyajima.