Abstract
Severe deficiency of the von Willebrand factor (VWF)-cleaving protease ADAMTS13 can lead to thrombotic thrombocytopenic purpura (TTP), a disease associated with the widespread formation of platelet-rich thrombi in many organs. Autoantibodies that inactivate ADAMTS13 are the most frequent cause of acquired TTP. Little is known about epitope specificity and reactivity of anti-ADAMTS13 antibodies. In this study, a series of ADAMTS13 domains were expressed in Escherichia coli, and the reactivity of purified recombinant fragments with anti-ADAMTS13 auto-antibodies from 25 patients with severe ADAMTS13 deficiency was evaluated in vitro. All TTP plasmas contained antibodies directed against the cysteine-rich spacer (cys-rich/spacer) domain of ADAMTS13. In the plasma of 3 patients, antibodies were detected that reacted exclusively with the cys-rich/spacer domain, underscoring the importance of this region for functional activity of ADAMTS13. In 64% of the plasmas, antibodies reacted with the 2 CUB domains, and in 56% they reacted with the isolated first thrombospondin type 1 (TSP-1) repeat and with the compound fragment consisting of the catalytic, the disintegrin-like, and the TSP1-1 domain. Less frequently, in 28% of the plasmas, antibodies reacted with the TSP1 repeats 2 to 8. Unexpectedly, antibodies reacted with the propeptide region in 20% of the plasmas. In conclusion, this study shows that even though anti-ADAMTS13 autoantibodies react with multiple domains of the protease, the cys-rich/spacer domain is consistently involved in antibody reactivity. (Blood. 2004;103:4514-4519)
Introduction
Abnormalities of von Willebrand factor (VWF) have a prominent role in the pathogenesis of thrombotic thrombocytopenic purpura (TTP), as indicated by the excessive accumulation of VWF in microvascular platelet thrombi found in the vascular lesions characteristic for TTP1 and by unusually large VWF (ULVWF) multimers detected in patients with the chronic, relapsing form of the disease.2 ULVWF was shown to interact efficiently with the platelet glycoprotein Ibα (GpIbα), a component of the GpIb/IX/V receptor for VWF expressed on platelets.3,4 Physiologically, efficient VWF-platelet interaction is of major importance for the recruitment of platelets to the site of vascular injury, but the prolonged, uncontrolled presence of ULVWF may cause platelet thrombus formation in the microcirculation, particularly under conditions of high shear stress.
The most favored hypothesis of the pathophysiology of TTP proposes that a still unknown precipitating event causes the excessive accumulation of ULVWF multimers on endothelial cell surfaces and in plasma that, in the absence of VWF-cleaving protease activity, leads to ULVWF-mediated platelet thrombus formation and ischemic symptoms in the circulation of multiple organs.
A VWF-cleaving protease, first isolated and characterized by Tsai5 and by Furlan et al,6 was shown to cleave VWF at position Tyr1605-Met1606, the peptide bond physiologically cleaved in vivo.7 Protein purification, N-terminal sequencing, and positional cloning identified the protease as a new member of the ADAMTS family of metalloproteases.8-10
This new protease was called ADAMTS13 (a disintegrin and metalloprotease with thrombospondin type 1 repeats).11 Complete lack of ADAMTS13 activity coupled with the presence of ULVWF multimers was first found in 4 patients with chronic relapsing TTP.12 Autoantibodies inactivating ADAMTS13 activity were subsequently described in a patient with acute, acquired TTP13 and were confirmed in a large proportion of patients diagnosed with this form of the disease.14,15
Autoantibodies inhibiting ADAMTS13 activity have since been detected in most patients with acquired TTP.14-19 However, acute TTP causing antibodies might not interfere with the activity assays20 or may be directed against molecules other thanADAMTS13, though physiologically interacting with ADAMTS13 activity.21 For instance, antibodies against CD36 (thrombospondin receptor) have been found in 23 out of 27 TTP plasmas analyzed22 and 8 out of 11 TTP patient plasmas reacted with an 85-kDa form of CD36.23
ADAMTS13 inhibitors have not been fully characterized at the molecular level. Recent publications pointed out the critical importance of the cysteine-rich spacer (cys-rich/spacer) region for substrate recognition and VWF cleavage.24,25 Competitive inhibition using a series of peptides derived from different ADAMTS13 regions further revealed that the C-terminus of ADAMTS13, including the thrombospondin type 1 (TSP1) repeats 2 to 8 and the CUB domains, are necessary for ADAMTS13 to bind to VWF under flow conditions.26,27 Against this background we were interested in a more detailed analysis of the autoimmune response leading to severely deficient ADAMTS13 activity in patients with acquired TTP. To map major epitopes of the human anti-ADAMTS13 antibody response in vivo, we expressed ADAMTS13 domains in Escherichia coli, either individually or as units of consecutive domains, and used the purified recombinant polypeptides as antigens in a series of Western blots.
Materials and methods
Expression and purification of ADAMTS13 fragments in E coli
DNA fragments coding for the various ADAMTS13 domains were generated by polymerase chain reaction (PCR) using Hot Star Taq DNA polymerase (Qiagen, Hilden, Germany) and the wild-type ADAMTS13 cDNA28 as a template. Primer sequences used for the amplification of the desired nucleotide regions are available on request. Resultant PCR fragments were cloned into the pBAD/Thio-TOPO expression vector using TOPO cloning technology according to the supplier's instructions (Invitrogen, Lofer, Austria). Fragments chosen to be expressed were the propeptide domain; the compound fragment containing the entire catalytic domain, the disintegrin-like domain, and the first thrombospondin type 1 repeat (TSP1-1) element (cat/dis/tsp1-1); the isolated TSP1-1 domain (tsp1-1); the cys-rich/spacer domain; a fragment containing TSP1 repeats 2 to 8 (tsp1/2-8); the 2 CUB domains (cub1 + 2) and, as a negative control, the his-tagged thioredoxin (thio/his) fragment. On transformation into E coli, TOP10 (Invitrogen) ADAMTS13 fragments were produced as thioredoxin fusion proteins with a C-terminal (his)6-tag attached to facilitate detection and purification. After inducting protein expression with 0.002% arabinose for 4 hours at 37°C, bacterial cells were collected and lysed in 300 mM NaCl, 50 mM sodium phosphate, pH 8.0, and 10 mM imidazole containing 1 mg/mL lysozyme. Recombinant fragments consisting of the propeptide, the tsp1-1, and the thio/his fusion portion (from the empty parental vector) were obtained from the soluble fraction, whereas the larger compound fragments cat/dis/tsp1-1, cys-rich/spacer, tsp1/2-8, and cub 1 + 2 were from the insoluble fraction. Soluble fragments were purified under native conditions using Ni2+-charged HiTrap chelating high performance (HP) columns (Amersham Biosciences, Freiburg, Germany) and increasing imidazole concentrations. Fragments obtained from inclusion bodies were treated with solubilization buffer (8 M urea, 20 mM sodium phosphate, pH 7.5, 0.5 M NaCl, 30 mM imidazole), applied to the HP columns, and eluted with increasing concentrations of imidazole. The cat/dis/tsp1-1 fragment was further purified by anion-exchange chromatography using Q Sepharose XL (Amersham Biosciences). Purity of the isolated recombinant fragments was judged by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and silver staining.
Plasma samples
Plasma samples 1 to 10 were collected at the Central Hematology Laboratory, University of Bern in Switzerland, and samples 11 to 25 were collected at the Angelo Bianchi Bonomi Hemophilia and Thrombosis Center in Milan, Italy. Measurement of ADAMTS13 activity was carried out according to Studt et al29 (plasmas 1-10) and to Gerritsen et al30 (plasmas 11-25). The inhibitory titer expressed in Bethesda units (BUs) was determined as described by Knöbl et al31 for plasmas 1 to 10 and by Lattuada et al32 for plasmas 11 to 25. A pool of normal human plasma (NHP; Baxter AG, Vienna, Austria) with no detectable ADAMTS13 inhibitor was used as a negative control.
Western blot analysis
Equivalent amounts of purified recombinant protein were reduced, denatured, subjected to 12% SDS-PAGE, and transferred to nitrocellulose membranes, as described.33 Purified recombinant ADAMTS13 (rADAMTS13), derived from mammalian cell culture,28 and the thioredoxin-his fusion portion, derived from E coli and expressed from the empty parental vector, were used as positive and negative controls, respectively.
Rabbit antihuman-rADAMTS13 antiserum was generated by repeated immunization of one New Zealand White rabbit (Charles River, Kisslegg, Germany) with purified, recombinant C-terminally his-tagged human ADAMTS13 (rADAMTS13-his). The first immunization was carried out using 20 μg rADAMTS13-his in complete Freund adjuvant, whereas the booster immunization was done with incomplete Freund adjuvant.
Citrated plasma samples, collected from patients with acute acquired TTP, were used as a source of anti-ADAMTS13 antibodies (primary antibody, diluted 1:500 in 10 mM Tris, pH 8.0, 150 mM NaCl, 0.05% Tween 20 containing 2% skim milk, and 5% TOP10 lysate). Bound human anti-ADAMTS13 antibodies were visualized using goat antihuman immunoglobulin specific for the α, γ, and μ chains (Sigma, Cambridge, United Kingdom) coupled to alkaline phosphatase (AP) and BCIP/NBT (Promega, Madison, WI) as substrate. His-tagged proteins were visualized using murine anti-his monoclonal antibody (Qiagen) and secondary AP-conjugated antimouse IgG (Sigma) antibody and BCIP/NBT. The rabbit anti-ADAMTS13-his antiserum was used in a 1:1000 dilution, and bound antibodies were detected by an AP-conjugated goat antirabbit IgG antibody (1:10 000, Promega) and BCIP/NBT.
Results
Expression of ADAMTS13 domains in E coli
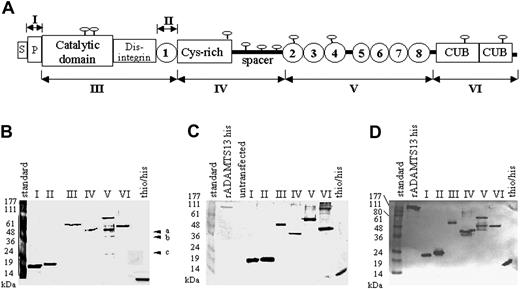
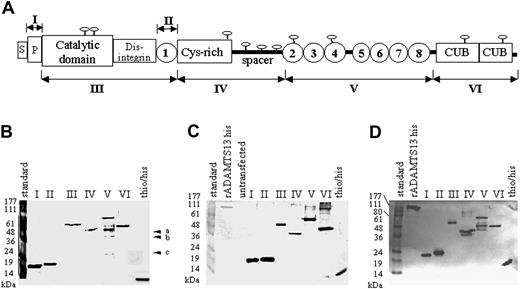
The ADAMTS13 fragments used for Western blot analysis spanned the propeptide region (amino acid residues 30-74; numbering of amino acids according to Zheng et al11 ), the cat/dis/tsp1-1 region (amino acid residues 75-439), the central tsp1-1 fragment (amino acid residues 386-439), the cys-rich/spacer domain (amino acid residues 440-685), the series of C-terminal TSP1 repeats 2 to 8 (amino acid residues 686-1131), and the cub1 + 2 domains corresponding to amino acid residues 1132-1427 (Figure 1A; Table 1). Predicted molecular weights of the various ADAMTS13 and thioredoxin-his fusion proteins are listed in Table 1.
ADAMTS13 fragments used for epitope mapping. (A) Domain organization of ADAMTS13 and a map of the ADAMTS13 fragments (I, II, III, IV, V, and VI) expressed in E coli. Indicated are the signal peptide (S), the propeptide (P), the catalytic, disintegrin, cys-rich, and spacer domains, the 2 CUB domains, and the 8 TSP type 1 repeats (1-8) (according to Zheng et al11 ). (B) Silver staining of purified recombinant ADAMTS13 fragments expressed in E coli (a-c). Contaminating bands derived from E coli that could not be removed during the purification process. (C) Western blot analysis of the purified recombinant ADAMTS13 fragments using an anti-histag antibody. (D) Western blot analysis of the purified recombinant ADAMTS13 fragments using a polyclonal rabbit antihuman ADAMTS13-his antiserum. Fragment I indicates propeptide; fragment II, tsp1-1; fragment III, cat/dis/tsp1-1; fragment IV, cys-rich/spacer; fragment V, tsp1/2-8; fragment VI, cub1 + 2.
ADAMTS13 fragments used for epitope mapping. (A) Domain organization of ADAMTS13 and a map of the ADAMTS13 fragments (I, II, III, IV, V, and VI) expressed in E coli. Indicated are the signal peptide (S), the propeptide (P), the catalytic, disintegrin, cys-rich, and spacer domains, the 2 CUB domains, and the 8 TSP type 1 repeats (1-8) (according to Zheng et al11 ). (B) Silver staining of purified recombinant ADAMTS13 fragments expressed in E coli (a-c). Contaminating bands derived from E coli that could not be removed during the purification process. (C) Western blot analysis of the purified recombinant ADAMTS13 fragments using an anti-histag antibody. (D) Western blot analysis of the purified recombinant ADAMTS13 fragments using a polyclonal rabbit antihuman ADAMTS13-his antiserum. Fragment I indicates propeptide; fragment II, tsp1-1; fragment III, cat/dis/tsp1-1; fragment IV, cys-rich/spacer; fragment V, tsp1/2-8; fragment VI, cub1 + 2.
Because many human plasma samples contain anti-E coli antibodies, the possibility that contaminating E coli proteins could give rise to false-positive results was taken into consideration. All ADAMTS13 fragments were purified to homogeneity and were shown by silver staining to be free from contaminating E coli cell debris (Figure 1B). All the fusion proteins, with one notable exception, were expressed and purified as single bands. The exception was the fragment tsp1/2-8, which was difficult to purify. Despite many efforts, we were unable to eliminate 3 contaminating protein bands of approximately 44 kDa, 36 kDa, and 25 kDa, apparently derived from E coli (Figure 1B). Western blot analysis using an anti-his-tag antibody confirmed that the purified ADAMTS13 fragments were isolated as single undegraded bands (Figure 1C) of the expected molecular weights.
In addition, all the purified fragments strongly reacted with a high-titer rabbit antihuman ADAMTS13 antiserum (Figure 1D) as single bands, and no cross-reaction with contaminating E coli material (ie, bands a, b, and c) was seen. The thio/his fragment most likely cross-reacts in this assay because it contains a his-tag and the rabbit anti-ADAMTS13 antiserum also contains anti-his-tag antibodies.
Western blot analysis of the patients' plasma samples
Twenty-five plasma samples from patients with idiopathic acquired TTP (Table 2) were selected for analysis. Patients had severe to moderately severe ADAMTS13 deficiency with residual ADAMTS13 activity varying from less than 3% to 10% of normal (Table 3). Titers of inhibitory antibodies ranged from 1 to 40 BU/mL.
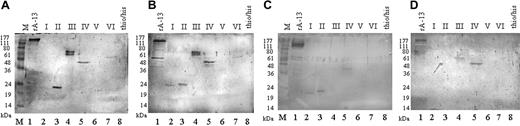
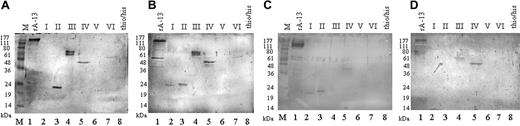
In this study we found no obvious correlations between the ADAMTS13 inhibitor titer of a patient's plasma, residual ADAMTS13 activity, and number or amino acid sequences of ADAMTS13 fragments recognized. A typical result of the Western blot analysis is shown in Figure 2A using plasma from patient 14 (Table 3) with a high ADAMTS13 inhibitory titer of 32 BU/mL. In this plasma we found anti-ADAMTS13 antibodies reactive with the tsp-1, the cat/dis/tsp1-1, the cys-rich/spacer, and the cub1 + 2 region.
Immunoblot analyses showing the domain-specific reactivity of TTP patient plasmas with different anti-ADAMTS13 inhibitor titers. Equivalent amounts of recombinant ADAMTS13 fragments were resolved on 12% SDS-PAGE and electroblotted. Plasma samples from patients 14 (32 BU/mL; panel A), 18 (6 BU/mL; panel B), 22 (10 BU/mL; panel C), and 3 (3 BU/mL; panel D) were used as sources of primary antibody and AP-conjugated antihuman immunoglobulin, and BCIP/NBT was used for visualization. Recombinant protein, lane 1: recombinant ADAMTS13 (rA-13) derived from HEK 293 cells. Purified ADAMTS13 fragments, lanes 2-7: propeptide (lane 2, I), tsp1-1 (lane 3, II), cat/dis/tsp1-1 (lane 4, III), cys-rich/spacer (lane 5, IV), tsp1/2-8 (lane 6, V), and cub1 + 2 (lane 7, VI). As a negative control, the thio/his fragment was applied (lane 8). M indicates molecular weight markers.
Immunoblot analyses showing the domain-specific reactivity of TTP patient plasmas with different anti-ADAMTS13 inhibitor titers. Equivalent amounts of recombinant ADAMTS13 fragments were resolved on 12% SDS-PAGE and electroblotted. Plasma samples from patients 14 (32 BU/mL; panel A), 18 (6 BU/mL; panel B), 22 (10 BU/mL; panel C), and 3 (3 BU/mL; panel D) were used as sources of primary antibody and AP-conjugated antihuman immunoglobulin, and BCIP/NBT was used for visualization. Recombinant protein, lane 1: recombinant ADAMTS13 (rA-13) derived from HEK 293 cells. Purified ADAMTS13 fragments, lanes 2-7: propeptide (lane 2, I), tsp1-1 (lane 3, II), cat/dis/tsp1-1 (lane 4, III), cys-rich/spacer (lane 5, IV), tsp1/2-8 (lane 6, V), and cub1 + 2 (lane 7, VI). As a negative control, the thio/his fragment was applied (lane 8). M indicates molecular weight markers.
All plasmas reacted with the full-length ADAMTS13 molecule (Figure 2; Table 3). More specifically, all plasmas tested contained antibodies directed against the cys-rich/spacer region. In 3 plasmas (from patients 1, 3, and 5; Table 3) antibodies reacted only with this region (Figure 2D), identifying it as essential for ADAMTS13 activity in vitro. Sixteen (64%) of the 25 plasmas contained antibodies directed against the polypeptide spanning the CUB1 and the CUB2 domains, making CUB the second most frequently recognized region. Fourteen plasmas (56%) contained antibodies against the composite fragment cat/dis/tsp1-1. Ten of the plasmas reactive with cat/dis/tsp1-1 also reacted with the isolated tsp1-1 fragment, indicating that they contained antibodies against this particular region. However, 4 plasmas (from patients 22, 23, 24, and 25; Table 3) contained antibodies strongly reactive with the isolated tsp1-1 fragment, but not with the tsp1-1 region, when the domain was presented in the context of the catalytic and disintegrin-like domain in the cat/dis/tsp1-1 fragment (Table 3; Figure 2C).
Only 7 plasmas (28%) contained antibodies reactive against ADAMTS13 fragment tsp1/2-8. Antibodies directed against the ADAMTS13 propeptide were detected in 5 (20%) of 25 plasmas (Figure 2B-C). Most plasmas contained antibodies reactive with 3 to 5 fragments, suggesting that the antibody response is directed against various epitopes located across the entire molecule.
To summarize, in plasma from 25 patients with acute TTP and severe ADAMTS13 deficiency, we found reactivity against the various ADAMTS13 fragments in the following order: cys-rich/spacer, 100%; cub1 + 2, 64%; cat/dis/tsp1-1, 56%; tsp1-1, 56%; tsp1-2/8, 28%; and propeptide, 20%.
Discussion
The multidomain structure of ADAMTS13 is highly conserved across mammals, birds, and fish,34 implying preserved and essential functions for each of the structural elements. Although the physiologic functions of most of the domains are unknown, they obviously act together in a concerted way to mediate ADAMTS13 function in vivo. The inhibition or depletion of ADAMTS13 activity, which is the cause of, or at least a major risk factor for, acquired TTP35 may, therefore, be attributable to various mechanisms, depending on the epitope bound by the autoimmune antibodies.
Autoantibodies inactivating ADAMTS13 activity are considered the most frequent cause of acute idiopathic TTP.14-16,18 However, the epitope specificity of such antibodies has not been analyzed in detail. We used Western blot to analyze plasma from patients with TTP attributed to acquired ADAMTS13 deficiency. The major limitations of Western blotting are that it does not address the ability of the detected antibodies to inhibit ADAMTS13 and that it does not reveal the extent to which the different antibodies contribute to the overall plasmatic inhibitor titer. Western blotting does, however, give a good overview of the regions and domains affected by an autoimmune response, and it allows major immunogenic regions to be identified.
Our results that all TTP plasma samples contained detectable antibodies to the cys-rich/spacer region are in accordance with recent data from Soejima et al,24 showing that a major epitope of anti-ADAMTS13 inhibitors resides within the cys-rich/spacer domain of ADAMTS13. Moreover, our results suggest that antibodies against the cys-rich/spacer region are necessary and sufficient to induce acquired ADAMTS13 deficiency because we found no patient without antibodies against the cys-rich/spacer region; in fact, 3 of our patients had antibodies that reacted with this particular region only. Conversely, given that Western blot analysis exclusively detects antibodies directed against linear epitopes, it must be taken into account that these 3 patients mounted antibody responses against 3-dimensional epitopes exhibited on other parts of ADAMTS13 or, apart from ADAMTS13, against binding partners of physiologic significance that had not been detected in our experiments. Hence, our results are in good agreement with the ADAMTS13 truncation experiments indicating that the cys-rich/spacer domains are necessary for VWF-cleaving activity.24,25
The importance of the cys-rich/spacer region for ADAMTS13 activity in vivo is also underscored by the relatively large number of ADAMTS13 missense mutations that map to this region and are associated with congenital TTP.10,36-39 An Arg-Gly-Asp motif potentially providing an integrin binding site is found within the cys-rich/spacer domains. Although mutation of the Arg-Gly-Asp sequence to Arg-Gly-Glu does not alter ADAMTS13 activity in vitro,24 anti-cys-rich/spacer antibodies might block specific Arg-Gly-Asp motif-mediated ADAMTS13 interactions and, therefore, contribute to TTP development.
Most (64%) patients' plasmas also reacted with the 2 CUB domains. CUB domains, uniquely found in ADAMTS13 among the known ADAMTS family members but present in other proteins, some of which are involved in developmental processes (embryogenesis, organogenesis, innervation), are thought to mediate protein-protein interactions.40 Competitive binding studies using short peptides derived from different domains of ADAMTS13 suggested that C-terminal regions downstream from the sixth TSP1 repeat mediate the binding of ADAMTS13 to VWF.26,27 In contrast, Banno et al41 showed recently that several in-bred and out-bred strains of mice contain an insertion in the ADAMTS13 gene that results in a C-terminal truncation of ADAMTS13 after TSP1 repeat 7, suggesting that the CUB domains, at least in the mouse, are dispensable for ADAMTS13 function in vivo. Anti-CUB domain antibodies might interfere with the substrate recognition process or, alternatively, might lead to an ADAMTS13 depletion by the formation of antibody-antigen complexes.
Other epitopes frequently recognized in this study reside in the isolated central tsp1-1 domain (56%) and in the fragment that includes the entire catalytic domain, the disintegrin-like domain, and central tsp1-1 domain. Both recombinant proteins were detected in 56% (14 of 25) of plasmas. Of the 14 plasmas reactive with cat/dis/tsp1-1, 10 cross-reacted with the isolated tsp1-1 fragment, indicating that they might have contained antibodies reactive with the full-length fragment or, alternatively, with the tsp1-1 portion only. Conversely, 4 of the plasmas containing antibodies binding to the isolated tsp1-1 fragment did not bind to the compound fragment cat/dis/tsp1-1. All fragments were used at the same concentration, approximately 200 ng per lane. This corresponds, on a molar basis, to a concentration of the isolated tsp1-1 fragment that is 3-fold higher than that of tsp1-1 in the compound fragment cat/dis/tsp1-1. If anti-tsp1-1 antibodies were present in low concentrations, the concentrated isolated fragment might have more easily detected them.
The tsp1-1 fragment is the major antigenic region on ADAMTS13, apart from the cys-rich/spacer and the CUB domains. TSP type 1 motifs have been identified in many different protein families and function as attachment sites for different cell types, proteins, and extracellular matrix components.42,43 The central TSP1 repeat contains a complete WXXW motif and a CSXS/TCG sequence, possibly facilitating interactions with the CD36 cell receptor expressed on a variety of cell types, including vascular endothelial cells.44,45 Soluble purified TSP1 has been shown to completely block ULVWF cleavage on the endothelial cell surface, but anti-CD36 antibodies did not, suggesting that ADAMTS13 might use different cell receptors for endothelial attachment.46,47 Antibodies directed against the tsp1-1 domain might interfere with the interaction of ADAMTS13 and binding partners important for its in vivo activity.
Only 28% of the TTP plasma samples recognized the fragment spanning TSP1 repeats 2 to 8, suggesting that this region is less immunogenic than the cys-rich/spacer domains. Alternatively, antibodies against tsp1/2-8 might have been directed primarily against conformational epitopes or might have been present in such low concentrations that we were unable to detect them. Interestingly, 20% of the tested plasma samples reacted with the propeptide fragment, suggesting that unprocessed ADAMTS13 containing the propeptide or soluble propeptide is sometimes present in plasma. Recently, the ADAMTS13 precursor, generated by mutation of the furin cleavage site or by expression in furin-deficient cells, was shown to be functionally active.48
In conclusion, this epitope mapping study provides detailed information about the specificity of ADAMTS13-inhibiting antibodies. We propose that the cysteine-rich and spacer domain, along with the CUB domains and the first TSP1 repeat, constitute major epitopes for inhibitory ADAMTS13 autoantibodies in patients with TTP.
Prepublished online as Blood First Edition Paper, March 4, 2004; DOI 10.1182/blood-2003-12-4165.
C.K. and B.P. contributed equally to this manuscript.
Four of the authors (C.K., B.P., F.D., and F.S.) are employed by Baxter BioScience, whose potential product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Flora Peyvandi for providing the patient data of plasmas 11 to 25 and Elise Langdon-Neuner for editing the manuscript.