Abstract
Cytotoxic T lymphocytes and natural killer cells destroy target cells via the directed exocytosis of lytic effector molecules such as perforin and granzymes. The mechanism by which these proteins enter targets is uncertain. There is ongoing debate over whether the most important endocytic mechanism is nonspecific or is dependent on the cation-independent mannose 6-phosphate receptor. This study tested whether granzyme B endocytosis is facilitated by dynamin, a key factor in many endocytic pathways. Uptake of and killing by the purified granzyme B molecule occurred by both dynamin-dependent and -independent mechanisms. However most importantly, serglycin-bound granzyme B in high-molecular-weight degranulate material from cytotoxic T lymphocytes predominantly followed a dynamin-dependent pathway to kill target cells. Similarly, killing by live cytotoxic T lymphocytes was attenuated by a defect in the dynamin endocytic pathway, and in particular, the pathways characteristically activated by granzyme B were affected. We therefore propose a model where degranulated serglycin-bound granzymes require dynamin for uptake.
Introduction
Natural killer cells and cytotoxic T lymphocytes (CTLs) protect a whole organism against dangerous cells, such as those that are infected with virus or are tumorigenic.1 When natural killers and CTLs recognize a target cell, the latter is killed by either of 2 major pathways: the Fas-Fas ligand (FasL) pathway, or directional exocytosis of membrane-bound cytotoxic granules present in the cytoplasm of the killer cell. The granules mediate the demise of the target cell via the enclosed cytolytic molecules, which include, among others, a family of serine proteases called granzymes, and the pore-forming molecule, perforin (pfn).2-4 The granzymes, via a pfn-dependent mechanism, induce cell death following translocation across the plasma membrane of target cells. Inside the cell, each granzyme fulfills a critical nonoverlapping role to induce apoptosis by cleaving specific subsets of substrates, such as caspases5,6 and Bid7-10 by granzyme B (grB), and the SET complex11 by granzyme A. But in order to achieve cleavage of substrates, clearly a critical first step is the uptake of granzymes into the target cell.
The original model of granzyme internalization proposes translocation via a pfn pore in the plasma membrane. However, recent evidence suggests that granzymes are first internalized via endocytosis and then are released into the cytoplasm with the help of pfn by an unknown mechanism. This model is predominantly based on studies performed with grB, which has largely served as the granzyme prototype to date. The first evidence that granzyme uptake occurred by endocytosis was the finding that grB enters cells autonomously.12-14 Furthermore, grB binds to the cell surface in a concentration-dependent and saturable manner,12 suggesting a receptor-mediated endocytic mechanism. The grB endocytosis model has further developed with the identification of the cation-independent mannose 6-phosphate receptor (CI-MPR) as a receptor for grB,15 however a subsequent study has challenged whether the CI-MPR is critical for grB-mediated killing.16 Both studies demonstrated that the CI-MPR could serve as a receptor for endocytosis of grB, since cells displaying low surface levels of the CI-MPR (CI-MPR–) exhibited reduced grB binding and uptake.15,16 However, the latter study showed that the reduced grB accumulation in CI-MPR– cells could be compensated by increased incubation time or grB concentration,16 arguing that an alternate nonspecific mechanism for grB uptake was sufficient for the induction of apoptosis, which would undermine the relative importance of the CI-MPR.
Further questions concerning grB uptake have been raised due to the finding that granule-secreted grB is bound in a complex to the proteoglycan serglycin.17-19 Since most in vitro studies of grB have used the purified molecule, the following issues must be weighed against such studies. The first concern is whether a bulky complex including serglycin would allow the interaction of grB and a receptor. Secondly, the grB molecule alone has a positive surface charge,20,21 but when grB binds serglycin, since binding is charge-dependent,22 the charge must be masked. Therefore, the free grB molecule differs from serglycin-bound grB in that the former might interact with anything negatively charged on the cell surface, including phospholipid headgroups or glycosaminoglycans. Although this charge-dependent interaction between grB and the cell surface would be nonphysiologic, especially at relatively high concentrations of purified grB, it could be a sufficient means of grB uptake leading to target cell apoptosis.
To consider another aspect of grB uptake, the model system tested here explores a potential role for dynamin. Dynamin is a focal point for endocytic studies since it is a major factor in many endocytic events, including those of clathrin-coated pits and caveolae.23 Further, a dominant-negative mutant (K44A) of dynamin can be overexpressed in cells to effectively block dynamin-dependent endocytic pathways.24,25 While uptake and apoptosis induced by the purified grB molecule have been assessed in the present study, more importantly, induction of apoptosis by serglycin-complexed grB has been considered. Results indicated that the form of grB dictated the relative dependence on dynamin for endocytosis and induction of apoptosis.
Materials and methods
Cell lines and reagents
HeLa-DynWT (HtTa, wild-type dynamin) and HeLa-DynDN (HtTa, K44A dynamin) cells, maintained and induced as previously described,25,26 were generated in the laboratory of S. L. Schmid (Scripps Research Institute, La Jolla, CA). CI-MPR+ (MS9II) and CI-MPR– (MS) cells have been described previously.27,28 Isolation of human CTLs (hCTLs) has been described.29 Human replication–deficient AD type 5 d170-3 (AdV) was purified in our lab as described.30 Tetracycline hydrochloride (tet), mannose 6-phosphate (M6P) and glucose 6-phosphate (G6P) disodium salt dihydrates, as well as staurosporine were obtained from Sigma Aldrich (St Louis, MO). Western Blots were performed with mouse antihuman dynamin-1 (Hudy-1) monoclonal antibody (mAb; Upstate Biotechnology, Lake Placid, NY), mouse anti-grB mAb, Clone 2C5 (Santa Cruz Biotechnology, Santa Cruz, CA), or mouse anti-pfn mAb, clone HuPerf-2d4,31 a kind gift from Dr G. Griffiths (University of Oxford, United Kingdom), and a horse radish peroxidase–conjugated goat anti–mouse immunoglobulin G (IgG) secondary antibody (Ab; Biorad Laboratories, Hercules, CA). Fluorescein-conjugated transferrin (Tfn-FITC) was purchased from Molecular Probes (Eugene, OR). Goat antisera with specificity for the human CI-MPR was a kind gift from Dr W. Sly (Saint Louis University School of Medicine, St Louis, MO)32 and was detected with an R-phycoerythrin–conjugated donkey anti–goat IgG F(ab′)2 fragment from Jackson ImmunoResearch Laboratories (West Grove, PA). The grB-specific inhibitor was generously supplied by Dr N. A. Thornberry (Merck Research Laboratories, Rahway, NJ).33 The pfn, prepared as described,34 was kindly given by Dr D. Hudig (University of Nevada, Reno).
Preparation of grB, grB-A488
Human grB was purified from the cytotoxic granules of YT-Indy cells as described.35 GrB was concentrated over a centricon YM-10 centrifugal filter (Millipore, Bedford, MA) and then was derivatized using an Alexa Fluor 488 (A488) protein-labeling kit (Molecular Probes). GrB-A488 was resolved from unbound dye by passing the material through a Sephadex G-25 HiTrap Desalting Column (Amersham Biosciences, Piscataway, NJ) with phosphate-buffered saline (PBS). GrB enzymatic activity was monitored with the colorimetric substrate IEPD-pNA (200 μM; Kamiya Biomedical, Seattle, WA).36
Tfn-FITC and grB-A488 uptake assay
Suspended cells were incubated in solutions of 50 μg/mL Tfn-FITC or 8 μg/mL grB-A488, for up to 60 minutes: for binding, 2 × 105 cells in 10 μL prepared in labeling buffer (PBS, 0.1% [wt/vol] bovine serum albumin [BSA]) at 4° C; for uptake, 8 × 105 cells in 40 μL prepared in Dulbecco modified Eagle medium (DMEM) containing 0.1% (wt/vol) BSA at 37° C. Uptake reactions were stopped by adding 10 μL of the reaction sample to 190 μL ice-cold labeling buffer. For some samples, cells were preincubated in labeling buffer with 10 mM G6P or M6P for 20 minutes at 37° C. During uptake, buffer concentrations were adjusted with water to ensure a final [Na+] of approximately 160 mM. Labeled cells were incubated in 10 μL 7-amino-actinomycin D (7-AAD) solution (BD PharMingen, San Diego, CA) for 10 minutes at 4° C and then fixed in 2% (wt/vol) paraformaldehyde in PBS. Flow cytometric analysis was performed as previously described.37
Killing assay
grB/AdV and staurosporine. Cell monolayers (2.5 × 105 cells) were covered with 300 ng/mL grB and 500 plaque-forming units (pfu) per cell AdV, or 2.5 μM staurosporine in RHFM (RPMI 1640 supplemented with 25 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 10% [vol/vol] FBS, and 100 μM 2-mercaptoethanol [ME]). The staurosporine samples were matched with dimethyl sulfoxide (DMSO) controls. After a 2-hour incubation at 37° C, cells were lifted with trypsin/EDTA (ethylenediaminotetraacetic acid) and labeled with M30 mAb, as described in “Measurements of apoptosis.”
grB and pfn. Suspended cells (2 × 105) were incubated with 1 μg/mL purified grB and pfn (stored in IMAC control buffer [20 mM HEPES {pH 7.5}, 1 M NaCl, 0.05% NaN3, 0.1 M imidazole]; 10-fold final dilution). The final concentration of pfn was sublytic, as determined by 7-AAD exclusion. The final buffer also included 32% (vol/vol) DMEM, 8% (vol/vol) PBS, 0.1% (wt/vol) BSA, 20 mM HEPES (pH 7.2), and 2 mM CaCl2, so that in the final sample [Na+] was approximately 160 mM. After 2 hours at 37° C, samples were labeled with M30 mAb.
CTL degranulate material. To obtain the degranulate material, 6-well plates were coated with 5 μg/mL antihuman CD3, clone HIT3a (BD PharMingen) in carbonate coating buffer (15 mM Na2CO3, 35 mM NaHCO3, pH 9.6) for 1 hour at 37° C, then washed extensively with PBS. To each well, 0.5 mL of 107 cells/mL hCTL suspension was added in degranulation buffer (Phenol Red–free RPMI [Invitrogen Canada, Burlington, ON, Canada], 20% [vol/vol] PBS) and incubated for 4 hours at 37° C. Supernatants were collected and concentrated over a microcon YM-100 centrifugal filter at 1000g until a control filter with degranulation buffer ran dry. grB activity was estimated against a standard curve with the colorimetric substrate IEPD-pNA.36 The degranulate materials were then stored at –80° C for fewer than 24 hours prior to use in assays. For characterization, degranulate material was resolved on a 1% agarose gel in TBE (Tris/borate/EDTA) buffer, pH 7.4, and then vacuum transferred to polyvinylidene fluoride and probed for grB and pfn.
To kill, degranulate material retained by the filter was added to cells (2 to 2.5 × 105) at an equivalent of 125 to 1000 ng/mL grB activity. For some assays, this degranulate material was preincubated for 20 minutes at 37° C with 45 μM grB-specific inhibitor or an equivalent volume of DMSO (0.45% [vol/vol]), so that upon direct addition to samples, the final concentration was 20 μM inhibitor (or 0.2% DMSO). The final sample (25 μL) was prepared in degranulation buffer supplemented with 0.1% (wt/vol) BSA, 10 mM HEPES (pH 7.4), and 2 mM CaCl2. After 3 hours at 37° C, samples were labeled with M30 mAb.
CTLs. Cells were incubated at 37° C with hCTLs at approximately 1:1 (effector-target [E/T] ratio) in RHFM supplemented with 2 μg/mL concanavalin A (Sigma Aldrich) and then labeled with their respective apoptotic markers. Some assays were performed in the presence of EGTA (ethyleneglycoltetraacetic acid; 10 mM MgCl2, 5 mM EGTA, pH 8.0) or 300 ng/mL neutralizing antihuman Fas mouse monoclonal IgG1, clone ZB4 (Upstate Biotechnology). Alternatively, hCTLs were preincubated with 10 nM concanamycin A (Sigma Aldrich) for 2 hours at 37° C.
Measurements of apoptosis
Caspase activation was monitored with the mAb M30 (Roche, Laval, QC, Canada) along with the secondary Ab fluorescein–conjugated AffiniPure Donkey anti–mouse IgG (Jackson ImmunoResearch Laboratories) to specifically detect a caspase-cleavage product of cytokeratin 18.38 DNA fragmentation was monitored by TUNEL labeling (terminal deoxynucleotidyl transferase-mediated d-UTP nick-end labeling; Roche) after fixation in ice-cold methanol with 5% (vol/vol) acetic acid (at least 30 minutes at –20° C) and washing twice with PBS/0.1% (vol/vol) Tween-20. Loss of ΔΨm (mitochondrial inner membrane potential) was determined using 100 nM TMRE (Tetramethyl-rhodamine, ethyl ester, perchlorate; Molecular Probes). M30, TUNEL, and TMRE labeling were quantified by flow cytometry.37 When samples included both HeLa-Dyn and hCTLs, gates were set to exclude more than 95% of hCTLs.
Results
Regulation of dynamin expression in the HeLa-Dyn system
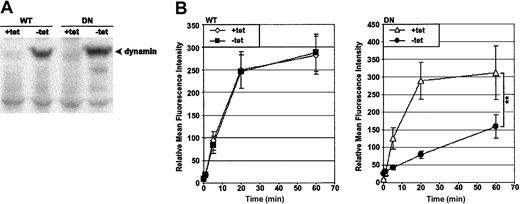
The model chosen to study dynamin-dependent endocytosis was HeLa cells transfected with the wild-type (WT) or the K44A dominant-negative (DN) human dynamin-1 cDNA;26 herein, the clones will be referred to as HeLa-DynWT or DN cells, respectively. The expression of the dynamin-1 cDNA was under the control of a tet-responsive promoter. As previous characterization has shown,25 preculturing of HeLa-DynWT or DN in the absence of tet for 48 hours induced overexpression of dynamin-1 protein, as detected by immunoblot for dynamin (Figure 1A).
Preculturing HeLa-DynDN clones in the absence of tet induces overexpression of DN dynamin and a block in Tfn-FITC endocytosis. HeLa-DynWT or DN cells were precultured in the presence of tet (+tet) to suppress expression from the transfected dynamin cDNA, or in the absence of tet (–tet) to induce it. (A) Immunoblot of cell lysates to detect dynamin. (B) Cells were incubated with Tfn-FITC at 37° C for up to 1 hour. Relative mean fluorescence intensity of viable cells was determined by flow cytometry. The mean ± SD of 4 independent experiments is shown (**.001 < P < .01).
Preculturing HeLa-DynDN clones in the absence of tet induces overexpression of DN dynamin and a block in Tfn-FITC endocytosis. HeLa-DynWT or DN cells were precultured in the presence of tet (+tet) to suppress expression from the transfected dynamin cDNA, or in the absence of tet (–tet) to induce it. (A) Immunoblot of cell lysates to detect dynamin. (B) Cells were incubated with Tfn-FITC at 37° C for up to 1 hour. Relative mean fluorescence intensity of viable cells was determined by flow cytometry. The mean ± SD of 4 independent experiments is shown (**.001 < P < .01).
To monitor the effect of DN dynamin expression on endocytosis, the probe of choice was fluorescently labeled transferrin (Tfn-FITC), a molecule shown to be dependent on dynamin for efficient endocytosis.24,25 Endocytic progress was monitored quantitatively by flow cytometry in cells incubated at 37° C for up to 60 minutes (Figure 1B). Tfn accumulated rapidly in cells not expressing dynamin (WT and DN +tet), and the rate of uptake was unaffected by overexpression of WT dynamin (WT –tet). In contrast, overexpression of DN dynamin (DN –tet) suppressed uptake of Tfn. These findings were supported by visual analysis of cells via confocal laser scanning microscopy (K.V., unpublished data, October 2002). This reflected previous findings that in HeLa-DynWT and DN cell lines, expression from the dynamin cDNA constructs was tightly regulated in a tet-dependent manner and, specifically, that DN dynamin induced a block in endocytosis.
Dominant-negative dynamin suppresses endocytosis of the grB molecule
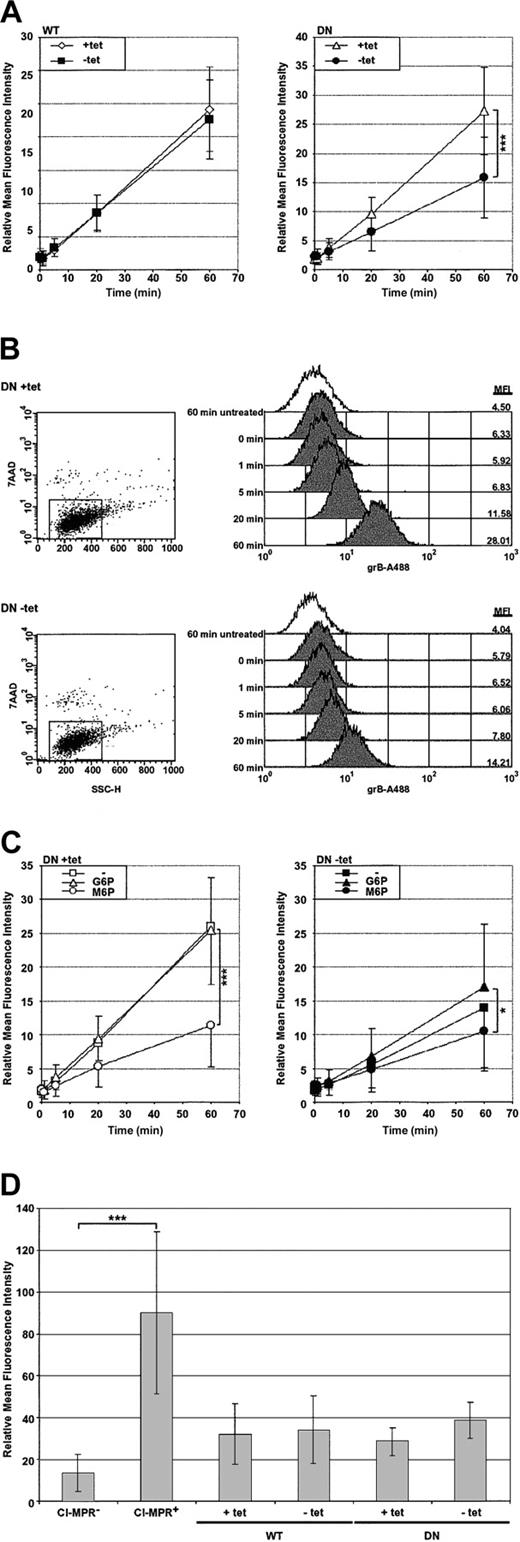
To begin, grB uptake was monitored in isolation to determine the effect of DN dynamin on uptake, which, for grB, is an event known to occur autonomously of pfn.12-14 In order to track grB, the purified grB molecule was covalently conjugated to the fluorescent tag Alexa 488 (grB-A488), and uptake was quantified by flow cytometry. Interestingly, a population of cells was observed that were nonviable and acquired high levels of fluorescence when cells were treated with the fluorescently labeled grB. To address this, cells were labeled with 7-AAD viability probe following the uptake assay, and in the population that did not stain with 7-AAD, uptake of the fluorescently labeled grB was tracked.
GrB uptake was monitored at 37° C over the course of an hour (Figure 2A-B). In nonexpressing HeLa-Dyn cells (WT and DN +tet) and in cells overexpressing WT dynamin (WT –tet), grB accumulated to significant levels over the period of an hour. In contrast, cells overexpressing DN dynamin (DN –tet) accumulated fluorescence at a significantly slower rate compared with the nonexpressing control cells (DN +tet). After 60 minutes, the DN dynamin–expressing cells showed an approximately 7-fold accumulation of fluorescence, compared with approximately 15-fold in the nonexpressing control cells, representing a 50% decrease in the rate of grB endocytosis. Thus, overexpression of dominant-negative dynamin significantly inhibited uptake of the grB molecule. It is apparent, however, that a significant level of grB endocytosis continued in the presence of dominant-negative dynamin, as has been shown previously.16
Uptake of the purified grB molecule is dependent on dynamin and the CI-MPR. HeLa-DynWT or DN cells were treated to suppress (+tet) or induce (–tet) overexpression from the transfected dynamin cDNA. The relative mean fluorescence intensity was determined by flow cytometry. (A) Cells were incubated with grB-A488 at 37° C for up to 1 hour, and viable cells were analyzed. (B) Representative flow cytometry data of grB-A488 uptake in HeLa-DynDN cells. The mean fluorescence intensity (MFI) is noted along the right-hand side of the histogram charts for cells—untreated at 60 minutes or exposed to grB-A488 from 0 to 60 minutes. (C) HeLa-DynDN cells were incubated with grB-A488 under normal conditions (–), or in the presence of 10 mM G6P or M6P, and viable cells were analyzed. (D) Cells displaying negligible or high surface levels of the CI-MPR (CI-MPR– or CI-MPR+, respectively) or HeLa-Dyn cells were surface labeled for the CI-MPR. The mean ± SD of 4 (A), 3 (C), or 5 (D) independent experiments is shown (*.01 < P < .05; ***P < .001).
Uptake of the purified grB molecule is dependent on dynamin and the CI-MPR. HeLa-DynWT or DN cells were treated to suppress (+tet) or induce (–tet) overexpression from the transfected dynamin cDNA. The relative mean fluorescence intensity was determined by flow cytometry. (A) Cells were incubated with grB-A488 at 37° C for up to 1 hour, and viable cells were analyzed. (B) Representative flow cytometry data of grB-A488 uptake in HeLa-DynDN cells. The mean fluorescence intensity (MFI) is noted along the right-hand side of the histogram charts for cells—untreated at 60 minutes or exposed to grB-A488 from 0 to 60 minutes. (C) HeLa-DynDN cells were incubated with grB-A488 under normal conditions (–), or in the presence of 10 mM G6P or M6P, and viable cells were analyzed. (D) Cells displaying negligible or high surface levels of the CI-MPR (CI-MPR– or CI-MPR+, respectively) or HeLa-Dyn cells were surface labeled for the CI-MPR. The mean ± SD of 4 (A), 3 (C), or 5 (D) independent experiments is shown (*.01 < P < .05; ***P < .001).
Dynamin-dependent uptake of the grB molecule occurs via the CI-MPR
Previous studies have indicated that receptor-mediated uptake of the purified grB molecule occurs via the CI-MPR.15,16 Uptake of the purified grB molecule was therefore monitored in response to blocks in endocytosis by the CI-MPR and/or dynamin. To interfere with CI-MPR–mediated uptake, receptors were saturated with 10 mM mannose 6-phosphate (M6P) or, as a control, with glucose 6-phosphate (G6P), and then uptake of grB-A488 was monitored (Figure 2C). Overall, treatment with G6P did not significantly affect the rate of fluorescence accumulation. In contrast, M6P significantly suppressed accumulation of fluorescence in normal cells (+tet). This indicated that uptake of grB was strongly dependent on the CI-MPR. In fact, comparable inhibition by M6P and DN dynamin was observed. Importantly, combining both defects did not have an additive effect with respect to attenuating grB uptake, but rather, the combined block inhibited uptake to a similar degree as using either block independently. This, therefore, suggested that when grB was taken up via the CI-MPR, it also relied on dynamin. This result is not surprising given that CI-MPR uptake is clathrin-mediated.39 Notably, differential expression of WT or DN dynamin did not alter CI-MPR surface expression (Figure 2D), therefore the decreased levels of grB uptake in the presence of DN dynamin could not be attributed to changes in CI-MPR surface levels.
Endocytosis-dependent apoptotic stimuli are sensitive to dominant-negative dynamin
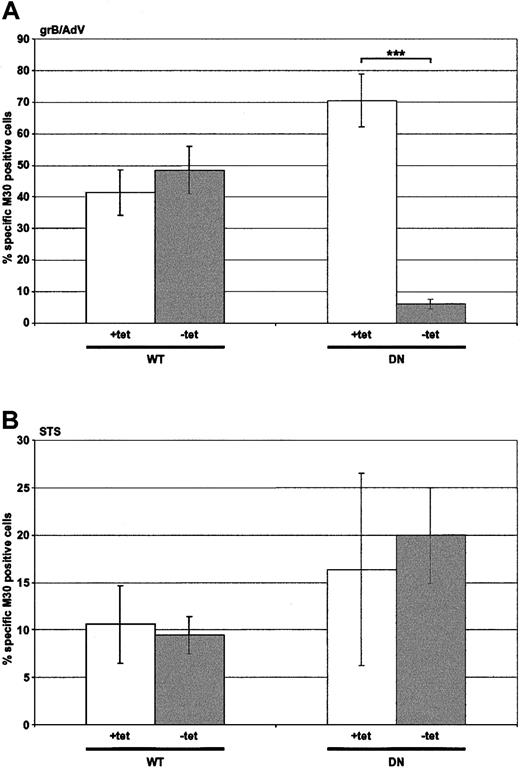
In order to test whether inhibition of endocytosis would result in attenuation of killing, adenovirus (AdV)–assisted grB entry was assessed (Figure 3A), since uptake of AdV itself is dynamin dependent.40 Importantly, though the read-out was apoptosis, which was dependent on the internalization of grB, this step was absolutely dependent on cointernalization of the AdV. Nonexpressing HeLa-Dyn cells (WT and DN +tet) or WT dynamin-overexpressing cells (WT –tet) displayed comparable caspase activation, as indicated by labeling with mAb M30. In contrast, in cells overexpressing DN dynamin (DN –tet) M30 labeling was effectively abolished, which indicated that AdV had not been effectively internalized. These data demonstrated that attenuation in cell death would be observed if the apoptotic stimulus was reliant on dynamin for endocytosis.
Death induced by grB and AdV, but not by staurosporine, is dependent on dynamin. HeLa-DynWT or DN cells were treated to suppress (+tet) or induce (–tet) overexpression from the transfected dynamin cDNA. Caspase activation was assessed by labeling with M30 mAb. The number of M30-positive cells was quantified by flow cytometry. (A) Cells were treated with grB and AdV for 2 hours at 37° C. (B) Cells were treated with staurosporine (STS) for 2 hours at 37° C. The mean ± SD of 4 independent experiments is shown (***P < .001).
Death induced by grB and AdV, but not by staurosporine, is dependent on dynamin. HeLa-DynWT or DN cells were treated to suppress (+tet) or induce (–tet) overexpression from the transfected dynamin cDNA. Caspase activation was assessed by labeling with M30 mAb. The number of M30-positive cells was quantified by flow cytometry. (A) Cells were treated with grB and AdV for 2 hours at 37° C. (B) Cells were treated with staurosporine (STS) for 2 hours at 37° C. The mean ± SD of 4 independent experiments is shown (***P < .001).
It was also important to consider whether the endogenous apoptotic machinery would be affected by the overexpression of dynamin or the conditions used to induce overexpression. Cell death induced by staurosporine was therefore monitored to address this question (Figure 3B). Similar levels of M30 labeling were observed whether cells did (–tet) or did not (+tet) overexpress either form of dynamin. These results suggested that the apoptotic machinery was unaffected, and any observed decreases in apoptosis in the following studies were due to a defect in the uptake of the stimulus, rather than a defect in the apoptotic machinery.
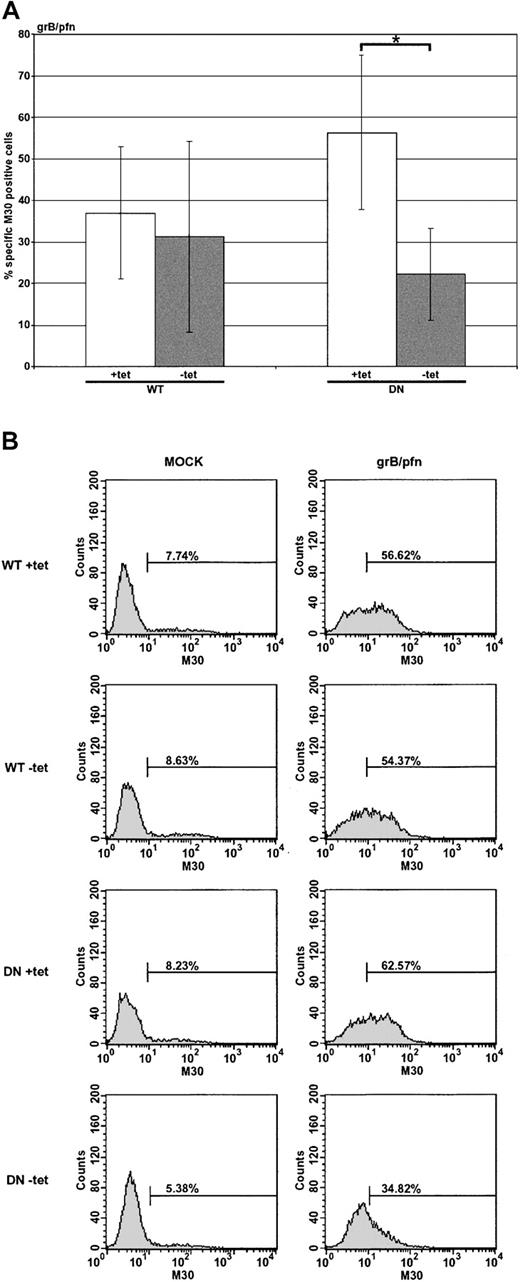
Dominant-negative dynamin suppresses apoptosis induced by the purified molecules grB and pfn
Although the evidence suggested that uptake of grB could occur in a dynamin-dependent manner, it was important to determine whether this occurred in the presence of pfn and, if so, whether such a pathway was important for induction of apoptosis (Figure 4A-B). Nonexpressing HeLa-Dyn cells (WT or DN +tet) were sensitive to treatment with purified grB and pfn, but apoptosis in cells overexpressing DN dynamin (DN –tet) was only half as much as the control. Notably, overexpression of WT dynamin (WT –tet) did not have the same inhibitory effect as DN dynamin. This suggested that overexpression of the DN dynamin limited the ability of grB and pfn to activate the caspase cascade. Presumably DN dynamin interfered with endocytosis of grB, though pfn may or may not have been affected. Interestingly, since killing was reduced only by half in the presence of DN dynamin, these data also implied the existence of dynamin-independent means of grB uptake, which is consistent with the grB-A488 uptake data (Figure 2A-B), as well as previous findings.16
Apoptosis induced by the purified molecules grB and pfn is sensitive to dominant-negative dynamin. HeLa-DynWT or DN cells were treated to suppress (+tet) or induce (–tet) overexpression from the transfected dynamin cDNA. Cells were treated with the purified molecules grB (1 μg/mL) and sublytic pfn for 2 hours at 37° C. Caspase activation was assessed by labeling with M30 mAb, followed by flow cytometry. (A) The mean ± SD of 3 (WT) or 4 (DN) independent experiments is shown (*.01 < P < .05). (B) Representative flow cytometry data of untreated (mock) or grB- and pfn-treated cells.
Apoptosis induced by the purified molecules grB and pfn is sensitive to dominant-negative dynamin. HeLa-DynWT or DN cells were treated to suppress (+tet) or induce (–tet) overexpression from the transfected dynamin cDNA. Cells were treated with the purified molecules grB (1 μg/mL) and sublytic pfn for 2 hours at 37° C. Caspase activation was assessed by labeling with M30 mAb, followed by flow cytometry. (A) The mean ± SD of 3 (WT) or 4 (DN) independent experiments is shown (*.01 < P < .05). (B) Representative flow cytometry data of untreated (mock) or grB- and pfn-treated cells.
Apoptosis induced by high-molecular-weight degranulate material is dynamin dependent
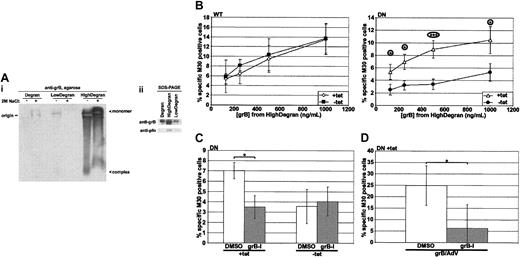
Mounting evidence has suggested that many molecules are involved in the granule-mediated killing pathway, and, in particular, serglycin is one such molecule and is proposed to usher grB17,19 and pfn18 from the granule to the target cell. To better mimic granule-mediated killing, degranulation of hCTLs was induced with an anti-CD3 mAb; then the degranulate material was fractionated over a 100-kDa cut-off centrifugal filter to separate the serglycin complex, an estimated 250 kDa, from smaller-molecular-weight material that might include free grB, 32 kDa. To compare and characterize the fractions, they were resolved on a neutral, nondenaturing 1% agarose gel and then blotted and probed for grB (Figure 5Ai). First, grB had primarily partitioned into the high-molecular-weight fraction of degranulate material (HighDegran). Second, grB in HighDegran migrated strongly toward the anode, which is consistent with migration of a grB-serglycin complex.19 To further test the nature of the complex, samples were pretreated with 2 M sodium chloride. This induced dissociation of the complex, though incompletely, and the monomeric, cationic grB migrated toward the cathode. Interestingly, the intensity of the monomeric band was greater than that of the complex, suggesting that the mAb detected the complexed protein less efficiently. To better compare the relative amounts of grB in the various degranulate materials, these were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), and an anti-grB immunoblot was performed (Figure 5Aii). The prominent band in the HighDegran fraction confirmed effective partitioning of grB into this fraction.
Serglycin-bound grB is taken up predominantly by a dynamin-dependent mechanism. hCTLs were induced to degranulate over immobilized anti-CD3 mAb, and the supernatant was collected and fractionated over a 100-kDa cut-off centrifugal filter. HeLa-DynWT or DN cells were treated to suppress (+tet) or induce (–tet) overexpression from the transfected dynamin cDNA and then incubated for 3 hours at 37° C with the high-molecular-weight fraction of degranulate material (HighDegran). Caspase activation was quantified by labeling with M30 mAb followed by flow cytometry. (A) Equivalent volumes of hCTL degranulate (Degran), low-molecular-weight degranulate fraction (LowDegran), and HighDegran were resolved on a gel prior to immunoblotting. (Ai) Samples, preincubated ± 2 M sodium chloride, were resolved on a 1% agarose gel in TBE, pH 7.4, and the blot was probed for grB. (Aii) Samples were resolved by SDS-PAGE, and the blot was probed for grB or pfn. (B) Cells were treated with various concentrations of HighDegran measured in nanograms per milliliter equivalents of grB activity. (C) HeLa-DynDN cells were treated with HighDegran (500 ng/mL grB activity) that had been pretreated with DMSO or grB-specific inhibitor (grB-I; 20 μM). (D) HeLa-DynDN +tet cells were treated with purified grB (500 ng/mL) pretreated with DMSO or grB-specific inhibitor (grB-I; 20 μM), along with AdV (500 pfu per cell). The mean ± SD of 5 (panel B, WT), 6 (panel B, DN), or 3 (C-D) independent experiments is shown (*.01 < P < .05; ***P < .001).
Serglycin-bound grB is taken up predominantly by a dynamin-dependent mechanism. hCTLs were induced to degranulate over immobilized anti-CD3 mAb, and the supernatant was collected and fractionated over a 100-kDa cut-off centrifugal filter. HeLa-DynWT or DN cells were treated to suppress (+tet) or induce (–tet) overexpression from the transfected dynamin cDNA and then incubated for 3 hours at 37° C with the high-molecular-weight fraction of degranulate material (HighDegran). Caspase activation was quantified by labeling with M30 mAb followed by flow cytometry. (A) Equivalent volumes of hCTL degranulate (Degran), low-molecular-weight degranulate fraction (LowDegran), and HighDegran were resolved on a gel prior to immunoblotting. (Ai) Samples, preincubated ± 2 M sodium chloride, were resolved on a 1% agarose gel in TBE, pH 7.4, and the blot was probed for grB. (Aii) Samples were resolved by SDS-PAGE, and the blot was probed for grB or pfn. (B) Cells were treated with various concentrations of HighDegran measured in nanograms per milliliter equivalents of grB activity. (C) HeLa-DynDN cells were treated with HighDegran (500 ng/mL grB activity) that had been pretreated with DMSO or grB-specific inhibitor (grB-I; 20 μM). (D) HeLa-DynDN +tet cells were treated with purified grB (500 ng/mL) pretreated with DMSO or grB-specific inhibitor (grB-I; 20 μM), along with AdV (500 pfu per cell). The mean ± SD of 5 (panel B, WT), 6 (panel B, DN), or 3 (C-D) independent experiments is shown (*.01 < P < .05; ***P < .001).
When cells were treated with the HighDegran alone, significant levels of M30 labeling were detected (Figure 5B), indicating that HighDegran could induce cell death. Remarkably, HighDegran pretreated with 20 μM of a grB-specific inhibitor (grB-I) poorly induced M30 labeling in cells, compared with the DMSO-pretreated HighDegran (Figure 5C; +tet). This degree of inhibition served as a measure of grB activity in HighDegran. To test if grB inhibition was quantitative, purified grB was pretreated with grB-specific inhibitor in parallel and then used to kill cells with AdV (Figure 5D).
Since grB-mediated killing is dependent on pfn,41,42 detection of grB-mediated killing in HighDegran implied that active pfn also was retained in the HighDegran. Indeed, anti-pfn immunoblot analysis of the degranulate fractions revealed that pfn had been effectively partitioned to the HighDegran (Figure 5Aii), likely via binding to serglycin, as has been previously found.18 Killing by HighDegran could be dramatically enhanced when exogenous pfn was added (K.V., unpublished data, May 2003). However, due to better representation of the in vivo granule-mediated killing model, treatment with HighDegran alone was chosen for further studies.
When the role of dynamin in killing by HighDegran was assessed (Figure 5B), DN dynamin overexpression (DN –tet) consistently suppressed induction of apoptosis compared with the control (DN +tet), though overexpressed WT dynamin (WT –tet) had no effect. It was therefore apparent that induction of cell death by HighDegran was reliant on dynamin-mediated endocytosis. Interestingly, killing by HighDegran was suppressed by overexpression of DN dynamin to a similar extent as by the grB-specific inhibitor. Importantly, treatment with both inhibitors in combination did not further attenuate cell death, but rather was similar to treatment with either inhibitor independently. This implied that the grB in HighDegran was dependent on dynamin for uptake. Since grB in the HighDegran is bound to serglycin (Figure 5Ai and previous studies17,18 ), it was concluded that serglycin-bound grB was critically dependent on dynamin for uptake into target cells.
CTL-mediated apoptosis is dependent on dynamin
A critical test of the model was to challenge the HeLa-Dyn targets with intact CTLs. Target cells were mixed with hCTLs (1:1 E/T ratio), and various phenotypes of apoptotic induction were assessed. In particular, apoptotic hallmarks known to be mediated by grB were evaluated: activation of caspases5,6 by M30 labeling; loss of mitochondrial inner membrane potential (ΔΨm)37,43 by loss of TMRE labeling; and oligomerization of DNA41,42 by TUNEL. Due to the distinct scatter patterns of HeLa-Dyn and hCTLs, it was possible to set the gate to exclude more than 95% of hCTLs from the analyzed cells and, therefore, predominantly monitor the HeLa-Dyn population.
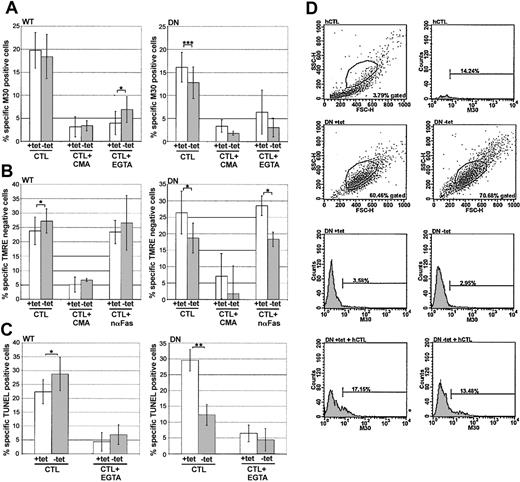
Compared with cells not expressing the transfected dynamin gene (WT +tet), the cells overexpressing WT dynamin (–tet) showed equivalent (M30; Figure 6A) or slightly enhanced (TMRE and TUNEL; Figure 6B and C, respectively) sensitivity to hCTL challenge. In contrast, cells overexpressing DN dynamin (–tet) demonstrated suppressed sensitivity to attack by hCTLs, as detected in all 3 apoptotic assays. These results suggested that CTL-mediated killing was dependent on a dynamin-mediated endocytic mechanism.
CTL-mediated killing is sensitive to dominant-negative dynamin. HeLa-DynWT or DN cells were treated to suppress (+tet) or induce (–tet) overexpression from the transfected dynamin cDNA. Cells were treated with CTLs (1:1 E/T ratio), with or without EGTA, concanamycin A (CMA), or neutralizing anti-Fas (nαFas). All assays were quantified by flow cytometry. (A) Caspase activation was assessed after 2 hours by labeling with M30 mAb. (B) Loss of ΔΨm was assessed after 2 hours by monitoring loss of TMRE labeling. (C) DNA oligomerization was assessed after 4 hours by TUNEL. (D) Representative flow cytometry data of hCTLs, or HeLa-DynDN ± tet ± hCTLs, as monitored by M30 mAb labeling. The mean ± SD of 4 (A,C) or 3 (B) independent experiments is shown (*.05 < P < .01; **.001 < P < .01; ***P < .001).
CTL-mediated killing is sensitive to dominant-negative dynamin. HeLa-DynWT or DN cells were treated to suppress (+tet) or induce (–tet) overexpression from the transfected dynamin cDNA. Cells were treated with CTLs (1:1 E/T ratio), with or without EGTA, concanamycin A (CMA), or neutralizing anti-Fas (nαFas). All assays were quantified by flow cytometry. (A) Caspase activation was assessed after 2 hours by labeling with M30 mAb. (B) Loss of ΔΨm was assessed after 2 hours by monitoring loss of TMRE labeling. (C) DNA oligomerization was assessed after 4 hours by TUNEL. (D) Representative flow cytometry data of hCTLs, or HeLa-DynDN ± tet ± hCTLs, as monitored by M30 mAb labeling. The mean ± SD of 4 (A,C) or 3 (B) independent experiments is shown (*.05 < P < .01; **.001 < P < .01; ***P < .001).
Since hCTLs kill not only via the granule pathway but are also able to use the FasL to induce apoptosis,44 the amount of FasL-mediated killing was measured by treating samples with EGTA to block degranulation, which is Ca2+ dependent, or by pretreating hCTLs with concanamycin A to block pfn-dependent killing.45 Alternatively, samples were treated with a neutralizing anti-Fas mAb (nαFas) to block FasL-mediated killing and measure granule-mediated killing alone. It is worth noting that none of these treatments had appreciable effects on hCTL scatter or labeling profiles. By all apoptotic measurements (Figure 6A-C), EGTA or concanamycin A treatment largely abrogated killing, or nαFas had minimal effect, suggesting that the hCTL killing observed was predominantly granule mediated. Together, these results suggested that during granule-mediated killing, some cytolytic factor was dependent on dynamin to mediate its proapoptotic effect. The fact that DN dynamin had a suppressive effect on grB-dependent apoptotic characteristics, including caspase activation, loss of ΔΨm, and DNA oligomerization suggested that at least killing through grB is dependent on dynamin-mediated endocytosis.
Discussion
The mechanism of cytolytic molecule delivery into target cells during granule-mediated killing has been a topic of debate in recent years. Despite the pervasiveness of a model where uptake of granzymes depends on passage through a perforin pore, mounting studies of grB have suggested that uptake of granzymes occurs primarily via an endocytic mechanism, and perforin is involved in the subsequent release of granzymes from the endosomes into the cytoplasm.2-4 The data presented in this study have addressed whether dynamin-mediated endocytosis is critical for granule-mediated killing. Importantly, the role of dynamin has been considered not only in the context of a purified component of the granule system, namely grB, but also of the more physiologically relevant degranulate material, as well as of intact CTLs. Although the purified grB molecule was only partially dependent on dynamin for endocytosis, grB in HighDegran, found to be serglycin bound, was taken up primarily in a dynamin-dependent manner. In support of this finding, granule-mediated killing by CTLs was significantly impaired by overexpression of DN dynamin. Thus, during granule-induced target-cell apoptosis, dynamin-mediated endocytosis of grB, and likely other granzymes, is a critical event.
The major caveat of grB endocytic studies to date has been that the primary focus has been the purified grB molecule, while in vivo degranulated grB is bound to the proteoglycan serglycin.17,18 To better address the in vivo scenario, CTL degranulate material was collected and fractionated to obtain the high-molecular-weight serglycin-bound grB. Killing by HighDegran was sensitive to overexpression of DN dynamin (Figure 5B). Further, quantification of grB activity in the HighDegran with a grB-specific inhibitor revealed that apoptosis induced by grB was blocked by DN dynamin (Figure 5C). Since native agarose analysis of HighDegran suggested that the grB was serglycin bound (Figure 5Ai), these findings suggest that serglycin-complexed grB is taken up predominantly by a dynamin-dependent mechanism. Since previous reports have indicated that degranulated grB is exclusively serglycin bound,17,18 these data imply that in vivo the dynamin-dependent endocytic mechanism is the primary means of grB uptake.
In support of a dynamin-dependent endocytosis model for degranulated granzymes, target cell death by granule-mediated CTL attack was reliant on dynamin (Figure 6A-C). In monitoring caspase activation, loss of ΔΨm, and DNA oligomerization, all 3 apoptotic markers during granule-mediated killing were suppressed by DN dynamin. These apoptotic markers are induced by grB,5,6,37,41-43 and in particular, caspase activation and DNA oligomerization cannot be effected by other granzymes.4 This indirectly supports the model that grB uptake during granule-mediated killing is dynamin dependent.
It is clear from the results that CTL-mediated killing is both dynamin dependent and independent. Although grB has been the particular focus of this study, due to the complexity of cytolytic granules, it is probable that factors other than grB are also reliant on dynamin. In particular, all granzymes are likely candidates. In contrast, some cytotoxic molecules must work independently of dynamin, as evidenced by CTL-mediated killing detected even in the presence of DN dynamin.
Although the presented results imply that serglycin-bound grB is taken up primarily in a dynamin-dependent manner, in contrast, uptake of free grB has been observed by both dynamin-dependent and -independent mechanisms. In Figure 2A, while uptake of the purified grB molecule was somewhat suppressed by DN dynamin, significant uptake still occurred. Similarly, apoptosis induced by the purified molecules grB and pfn was only partially sensitive to DN dynamin overexpression (Figure 4A). In contrast, in a comparable study, evidence suggested a lesser role for dynamin-dependent uptake.16 In this prior study, uptake of purified grB was minimally affected by DN dynamin, and an exclusively dynamin-independent mechanism was detected for cell death induced by purified grB and pfn, as measured by cell permeabilization and clonogenic survival. The contrasts between the previous and the present findings may be attributed in part to differences in assays. The previous study assessed uptake by quantitative confocal laser scanning microscopy, which is less sensitive than quantification by flow cytometry. The cell death assays also are distinct. Arguably, the M30 mAb assay used in the present study is more relevant to the study of grB uptake. To begin, the M30 assay specifically monitors activation of the cytoplasmic caspases, whereas the previous study assessed chromium release, which measures not only apoptosis, but also necrosis, which may be induced by numerous mechanisms. Further, the clonogenic survival assay may not be appropriate for studies where dynamin-dependent endocytosis has been blocked. Consider that even Tfn-FITC, a factor strongly dependent on dynamin for uptake, significantly accumulates in cells when added to cells for up to an hour (Figure 1B), indicating that the DN dynamin–overexpression system is relevant only for short-term assays. While the clonogenic survival assay is long term, the M30 mAb assay monitors immediate apoptotic induction, and so better assesses initial rates of uptake.
Methodology aside, both data sets are similar since they detect dynamin-independent uptake, suggesting that an alternate grB-uptake pathway exists. The question remains why the dynamindependent grB uptake is detected in some cases and not in others. Accumulating evidence suggests that the dynamin-independent uptake of the purified grB molecule may occur by binding to the cell surface via a charge-dependent mechanism. An important observation is that this alternate uptake pathway has been detected only when uptake of grB has been assessed in the absence of serglycin. The relevant distinction between free and serglycinbound grB is that the free molecule has a strong positive charge.20,21 Importantly, this charge on grB would be masked when complexed to serglycin, since binding is electrostatically mediated.22 The positive charge of free grB might allow interactions with negatively charged groups displayed on the cell surface, such as phospholipid headgroups and glycosaminoglycans. While the purified grB molecule could interact with the cell surface via electrostatic interaction, presumably this association would be of relatively low affinity compared with binding to a grB-specific receptor. Consequently, at low concentrations, free grB would first bind to and be internalized by the receptor in a dynamin-dependent manner. At high concentrations, free grB would bind to both the receptor and the cell surface, so that even if receptor-mediated endocytosis were blocked, adsorptive endocytosis would continue to take up the surface-bound grB. Consistent with this model, the accumulating data suggest that dynamin-independent uptake of purified grB is concentration dependent. The present study detected moderate dynamin-independent killing with 1 μg/mL purified grB (Figure 4), while previous findings demonstrated much stronger dynamin-independent killing when cells were treated, generally, with higher concentrations of purified grB.16
Previous reports have determined that the receptor for grB is the CI-MPR,15,16 an endocytic step that would be dynamin dependent.39 In support of this, uptake of the purified grB molecule was suppressed by M6P (Figure 2C). Furthermore, here and in another study,16 when grB uptake occurred in a dynamin-dependent manner, it was also CI-MPR mediated. The evidence presented here does not validate the direct assumption that, as for free grB, the CI-MPR is crucial for uptake of serglycin-bound grB. However, a study of CI-MPR involvement in grB uptake strongly implies that in vivo the CI-MPR is the primary receptor for uptake of cytolytic granule molecules.15 Strikingly, allograft transplant of CI-MPR– mouse tumor cells did not lead to rejection of the graft. Although a similar allograft-transplant experiment in a subsequent study led to rejection of the CI-MPR– graft,16 the relative importance of these data is a matter of further debate, since graft rejection was antibody mediated, though the interest was in cell-mediated killing. Nonetheless, the in vivo receptor(s) for the serglycin complexed cytolytic molecules will have important implications for therapies that might benefit from altered interactions between cytolytic molecules and target cells.
In summary, this study has presented evidence to suggest that uptake of the grB-serglycin complex is critically dependent on dynamin. Furthermore, granule-mediated killing by cytotoxic lymphocytes involves dynamin, arguably for assisting uptake of the serglycin-granzyme complex. Importantly, these data also make the distinction that though free grB uptake may occur by both dynamin-dependent and -independent mechanisms, the more physiologically representative serglycin-bound grB is primarily taken up in a dynamin-dependent manner. For future studies of granzyme uptake, these findings imply that appropriate selection of materials will be of the utmost importance for elucidating the physiologic model of granule-mediated killing.
Prepublished online as Blood First Edition Paper, January 22, 2004; DOI 10.1182/blood-2003-06-2156.
Supported by grants from the Canadian Institutes of Health Research (CIHR), and the National Cancer Institute of Canada. K.V. has received Studentships from the Alberta Heritage Foundation for Medical Research (AHFMR) and the Natural Sciences and Engineering Research Council of Canada. R.C.B. is a CIHR Distinguished Scientist, a Medical Scientist of the AHFMR, a Canada Research Chair, and a Howard Hughes International Scholar.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Ing Swie Goping, Dr Darren Roberts, and Catherine Ewen for helpful discussions. Thanks are also due to many for generously sharing their resources: Dr Brett Finlay for the HeLa-DynWT and DN clones, provided with permission of Dr Sandra L. Schmid; Dr Nancy Thornberry for the grB-specific inhibitor; Dr Dorothy Hudig for perforin; Dr William Sly for the goat antisera specific for the human CI-MPR; and Dr G. Griffiths for the perforin-specific mAb.