Abstract
Megakaryocytes skip late anaphase and cytokinesis during endomitosis. We found normal expression and localization of a fundamental regulator of mitosis, Aurora-B/AIM-1, during prophase in polyploidizing mouse bone marrow megakaryocytes. At late anaphase, however, Aurora-B/AIM-1 is absent or mislocalized. Megakaryocytes treated with a proteasome inhibitor display Aurora-B/AIM-1 properly expressed and localized to the midzone, suggesting that protein degradation contributes to this atypical appearance. In contrast, survivin, an Aurora-B/AIM-1 coregulator of mitosis, is not detected at any stage of the endomitotic cell cycle, and in most megakaryocytes proteasome inhibition does not rescue this phenotype. To further explore the importance of reduced Aurora-B/AIM-1 for polyploidization, it was overexpressed in megakaryocytes of transgenic mice. The phenotype includes increased transgenic mRNA, but not protein, in polyploidy megakaryocytes, further suggesting that Aurora-B/AIM-1 is regulated at the protein level. Aurora-B/AIM-1 protein is, however, elevated in diploid transgenic megakaryocytes. Transgenic mice also exhibit enhanced numbers of megakaryocytes with increased proliferative potential, and some mice exhibit mild decreases in ploidy level. Hence, the molecular programming involved in endomitosis is characterized by the mislocalization or absence of at least 2 critical mitotic regulators, Aurora-B/AIM-1 and survivin. Future studies will examine the impact of survivin restoration on mouse megakaryocyte polyploidization.
Introduction
Megakaryocytopoiesis consists of a series of processes that involve the initial expansion of committed precursor cells, endomitosis, which results in increased numbers of chromosomes in each cell (yielding polyploid cells), the final maturation of the cytosol, and the subsequent production of platelets.1 Although most polyploid megakaryocytes in the bone marrow are 16N, some can acquire a DNA content of up to 128N, where 2N is the DNA content of a diploid somatic cell. Other cell types under stress and transformed cells also become polyploid (for a review, see Zimmet and Ravid2 ). It is now generally accepted that the endomitotic cell cycle in megakaryocytes consists of a DNA replication S-phase, an M-phase with multiple pole spindles but an aborted anaphase B and cytokinesis, and a Gap-phase that would allow re-entry to the next round of S-phase (for a review, see Ravid et al3 ). It has been shown that the G1-phase cyclin, cyclin D3, is an important inducer of megakaryocyte polyploidization. Cyclin D3 overexpression in megakaryocytes increased the number of megakaryocytes and the ploidy level.4,5 The G2/M-phase cyclin, cyclin B1, on the other hand, has been shown to be reduced in polyploidizing megakaryocytic cell lines compared with proliferating cell lines.6,7 Similarly, cyclin B1-dependent cdc2 kinase activity was found to be reduced in other megakaryocytic cell lines.8-10 In polyploidizing primary megakaryocytes, cyclin B1 has been clearly detected at early mitosis (using immunohistochemistry),11,12 with no apparent reduction in protein level, compared with proliferating cells (using immuno-flow cytometry).13 In the primary cell system and in the cell lines, the anaphase-promoting complex that degrades cyclin B at early anaphase is intact.7,14 A recent study, also using primary megakaryocytes and immunohistochemical analysis, supported earlier findings that cyclin D3 is elevated in polyploidizing megakaryocytes and concluded that cyclin B1 is detectable at early mitosis during endomitosis, though at significantly lower levels in high-ploidy megakaryocytes, compared with lower-ploidy cells (both cell types were captured while cycling).15 This suggested that the profile of cell cycle regulators might be different between low-ploidy and high-ploidy bone marrow megakaryocytes and that regulation in the latter cells is reminiscent of that in megakaryocytic cell lines. A common finding in all these megakaryocytic systems is the detection of cyclin B1 during early mitosis, which would induce chromosome condensation, and its subsequent degradation at anaphase, which would allow cells to reenter the S-phase. The destruction of cyclin B1 permits reassembly of the replication-initiation complex, which is essential for controlling the re-entry of the next round of DNA synthesis even in the absence of mitotic events, as found in yeast cells.16 It is possible then that reduced levels of cyclin B during endomitosis in high-ploidy megakaryocytes, compared with those of lower ploidy, allows this mitotic checkpoint to be bypassed prematurely, leading to further polyploidization.
Although re-entry into the S-phase in polyploidizing megakaryocytes might be induced by increased levels of G1-phase cyclins and potentially by fine changes in cyclin B1 levels, the mechanism that leads these cells to skip late mitosis and cytokinesis remains to be determined. AIM-1 kinase, also known as Aurora-B (and, hence, referred to here as Aurora-B/AIM-1), is a member of the recently discovered Aurora/Ipl1-kinase superfamily, which regulates mitosis and cytokinesis. Aurora-B/AIM-1 belongs to the second Aurora kinase subfamily, consisting of human AIK2/STK12 and Aurora1, mouse STK1 and ARK2, rat Aurora-B/AIM-1, and Caenorhabditis elegans AIR-2. Aurora-B/AIM-1 plays multiple roles in mitotic events, including chromosome segregation and cytokinesis (for reviews, see Adams et al,17 Lens and Medema,18 and Terada19 ). Various target molecules of Aurora-B/AIM-1 have been identified to date. Myosin II regulatory light chain (MRLC) has been shown to be a target for Aurora-B/AIM-1 kinase,20 and histone H3 is proposed to be the target of the kinase complex that contains Aurora-B/AIM-1.21 Phosphorylation of the inner centromere protein, INCENP, by Aurora-B/AIM-1 stimulates this kinase activity.22 Aurora-B/AIM-1 regulates the spindle checkpoint by targeting BubR1 to the kinetochores.23 Aurora-B/AIM-1 is present in a complex with the kinase survivin, to which it directly binds, and with other proteins such as INCENP. Aurora-B/AIM-1 phosphorylates the cleavage furrow–specific vimentin and controls vimentin filament segregation in cytokinetic processes.18,19,24 MgcRacGAP, previously known as a GAP for Rac/Cdc42, is functionally converted to a RhoGAP through phosphorylation by Aurora-B/AIM-1 during cytokinesis.25 Hence, changes in levels or localization of Aurora-B/AIM-1 or survivin, also termed chromosome passenger proteins (for reviews, see Adams et al,17 Lens and Medema,18 and Terada19 ), are likely to affect normal progression through the mitotic cell cycle. In accordance, levels of these kinases are up-regulated in a variety of cancer cells and tissues (summarized under http://cgap.nci.nih.gov/Genes/GeneFinder).
It was shown by our group and by others that Aurora-B/AIM-1 mRNA is down-regulated during the polyploidization of megakaryocytes26,27 and that its overexpression prevents ploidy promotion in cell lines treated with phorbol ester.26 We proposed that Aurora-B/AIM-1 down-regulation might be an important factor for, but not necessarily an inducer of, megakaryocyte polyploidization. No full reports are available, however, regarding the expression and localization of Aurora-B/AIM-1 throughout the endomitotic cell cycle in bone marrow megakaryocytes or the importance of regulated Aurora-B/AIM-1 expression during megakaryocyte development. We describe here that though Aurora-B/AIM-1 is properly expressed and localized during early mitosis in polyploidizing bone marrow megakaryocytes, it is either absent or diffused throughout the nucleus at late anaphase of the endomitotic cycle. Some of the polyploidizing megakaryocytes treated with a proteasome inhibitor display Aurora-B/AIM-1 properly localized to the midzone at anaphase, suggesting that protein degradation is an important facet of this phenotype. In contrast, survivin, an Aurora-B/AIM-1 partner protein, is absent throughout the cell cycle in polyploidizing megakaryocytes, and proteasomal inhibition does not rescue this phenotype in most megakaryocytes, suggesting regulation at a transcriptional level as well. Our study further indicates that Aurora-B/AIM-1 mRNA overexpression in the megakaryocytes of transgenic mice does not result in Aurora-B/AIM-1 protein up-regulation in polyploid cells. In diploid transgenic megakaryocytes, this protein is abundant, leading to increased proliferation potential.
Materials and methods
Generation of transgenic mice
A vector containing the rat 1.1-kb platelet factor 4 (PF4) promoter linked to 1.7 kb of the 3′ end of the human β-globin gene (including the 3′ intron and a poly A signal; base pairs 71022-72711 from the translation start of the clone, described in GenBank accession number NT_000007) was linearized with SacI and NheI sites between these gene fragments. The rat Aurora-B/AIM-1 1.2-kb cDNA (GenBank accession number D89731) construct, starting with the N-terminal FLAG-tag sequence and ending with a stop codon, was released from a pcDNA3 vector with HindIII and XbaI digestion and then ligated, with the above PF4 vector, with SacI-HindIII linker sequence AGCTAGCT (the sites NheI and XbaI are cohesive). The ligation mixture was transformed into Escherichia coli DH5α, and positive colonies were identified. The resultant PF4-AIM-β-globin plasmid was purified using the CsCl method and freed of vector sequences with NdeI (which cut immediately upstream to the PF4 promoter and at the end of the β-globin sequence). Intron sequences, including the β-globin gene portion, have been used in a variety of transgenic constructs, resulting in increased levels of transgenic mRNA.28 Given that Aurora-B/AIM-1 1.2-kb cDNA contains a stop codon, the translated protein does not contain β-globin. It must be noted that the tag at the 5′ end was shown in our laboratory not to affect the stability of Aurora-B/AIM-1 protein because the half-lives of the endogenous and transfected tagged proteins were similar. (Half-life determination was pursued in Y10/L8057 and HeLa cells, as we describe in Zhang et al7 and H.G.N., unpublished data, January 2003). The 4-kb PF4-AIM globin DNA was purified by electroelution and was injected into the pronuclei of mouse fertilized eggs (FVB strain; Jackson Laboratories, Bar Harbor, ME). Positive transgenic founders were identified through Southern blot analysis on mousetail genomic DNA using a radiolabeled AIM-1 probe, all as previously described. In all analyses, age-matched (6- to 10-week-old) and sex-matched F2 and higher-generation mice were used.
Transgenic mRNA expression level
To detect the message of interest in rare megakaryocytes, a method has been developed to amplify the total cDNA pool. Only mRNA with a poly-A tail and an intact 5′ cap would be amplified with this method. Thus, a large pool of cDNA could be generated from full-length mRNA. This would allow high-efficiency detection of low-level messages from limited amounts of RNA. To achieve this, first-strand DNA was reverse-transcribed with 1 to 2 μg bone marrow total RNA using an oligonucleotide modified from the Clontech Smart III cDNA library construction kit (BD Bioscience, Franklin Lakes, NJ). The poly-A–anchoring coding sequence (CDS) oligonucleotide sequence was 5′ GCAGTGGTAACAACGCAGAGTACT(30)VN 3′, and the Smart oligonucleotide sequence was 5′ GCAGTGGTAACAACGCAGAGTACGCGGG 3′, which would allow the reverse transcriptase (RT) to switch templates and continue replicating to the end of the oligonucleotide after stopping at the 5′ end of mRNA. The full-length cDNA pool was amplified using the polymerase chain reaction (PCR) primer, 5′ GCAGTGGTAACAACGCAGAGT 3′. Manufacturer-suggested conditions were used for the first-strand synthesis and PCR reactions. The optimum number of PCR cycles in our system was 19. PCR products (5-8 μL) were used in Southern hybridization with radiolabeled DNA probes. Autoradiographic x-ray film was used to detect the message. Probes used were radiolabeled cDNAs of rat Aurora-B/AIM-1 (to detect transgene expression), human β-globin (to detect transgene expression), rat PF4 (a marker for megakaryocytes), and rat GAPDH (a marker for all cell types that typically yielded a similar level product in all samples). These probes (300-600 bp) were generated in our laboratory by RT-PCR and were confirmed by DNA sequencing.
Transgenic Aurora-B/AIM-1 protein detection
Bone marrow cells were isolated from the femurs of transgenic or control mice, as previously described.4 Bone marrow cells suspended in phosphate-buffered saline (PBS) were centrifuged onto (1 × 105 cells/slide) Colofrost Plus microscope slides (Fisher Scientific, Pittsburgh, PA) with a Cytospin3 (Shandon, Pittsburgh, PA) for 5 minutes at 120g. Cells were air dried and fixed in 1% formaldehyde with 0.1% Triton X-100 buffered in PBS for 10 minutes. Slides were washed 4 times with PBS and then blocked with 2% goat serum and 1% bovine serum albumin (BSA) in PBS for 30 minutes at room temperature. Mouse anti–Aurora-B/AIM-1 antibody (BD Transduction Laboratories, Lexington, KY) (1 μg/mL) or antirat Aurora-B/AIM-1 (1:200 dilution)26 was reacted with the cells for 1 hour at room temperature, and slides were then washed 4 times with PBS. As specified, a solution of rhodamine-conjugated goat antimouse immunoglobulin G (IgG; Molecular Probes, Eugene, OR) at 100-fold dilution in PBS or Alexa Fluor 488–conjugated goat antimouse IgG (Molecular Probes) at 200-fold dilution in PBS was subsequently added to the slides for 1 hour at room temperature. Slides stained with rhodamine were subsequently incubated with fluorescein-isothiocyanate (FITC)–conjugated rat antimouse CD41 (integrin αIIb) (10 μg/mL) for 1 hour. All slides were stained with 1 μM Hoechst (Sigma, St Louis, MO) for 10 minutes and subsequently were washed 4 times with PBS. Slides were mounted with ProLong Antifade Kit (Molecular Probes) and were observed under an Olympus (Melville, NY) IX70 inverted fluorescence microscope. Images were documented using a Hamamatsu charge-coupled device (CCD) camera (C4742-95; Hamamatsu City, Japan) and were analyzed with OpenLab (Improvision, Lexington, MA) software.
Determination of Aurora-B/AIM-1 and survivin localization throughout the megakaryocytic cell cycle
Bone marrow cells were freshly prepared from the femurs of control or transgenic mice by flushing femur cavities with Iscove modified Dulbecco medium (IMDM) through 26-gauge needles. Cells (1 × 106 cells/mL) were cultured with bone marrow stromal cells for 4 days in IMDM containing 10% fetal calf serum (FCS), mouse interleukin-3 (IL-3) (5 ng/mL), mouse stem cell factor (50 ng/mL), and recombinant mouse thrombopoietin (TPO) (50 U/mL). Recombinant mouse TPO was prepared from the supernatants of COS-7 cells transfected with mouse TPO cDNA expression vector.29 For proteasome inhibitor experiments, bone marrow cells were cultured for approximately 6 hours in the absence (vehicle used) or presence of 20 μM MG132 (Z-Leu-Leu-Leu-H-aldehyde) (Sigma). We chose 6 hours for experiments involving treatment with MG132 because of findings from preliminary experiments—that is, within 6 hours of treatment, Aurora-B was displayed at the highest levels compared with nontreated cells, indicating that at this time, the drug had a maximal effect on the steady state level of this mitotic protein (H.G.N., unpublished results, January 2003, with Y10/L8057 megakaryocytes and HeLa cells). A longer incubation period (more than 10 hours) initiates the accumulation of trypan-blue cells. Bone marrow cells were centrifuged for 10 minutes at 90g. Smear samples on slides were fixed with 100% methanol for 5 seconds at room temperature. Slides were washed with PBS for 10 minutes at room temperature and then blocked with 1% BSA in PBS for 30 minutes at 37° C. Mouse anti–Aurora-B/AIM-1 antibody (BD Transduction Laboratories) (1 μg/mL) was reacted with the cells for 1 hour at 37° C. After 5 rinses with PBS, slides were incubated with Cy 3-conjugated donkey antimouse IgG (Jackson ImmunoResearch Labs, West Grove, PA) at 400-fold dilution in 1% BSA/PBS and FITC-conjugated rat antimouse CD41 (integrin αIIb) (10 μg/mL) for 30 minutes at 37° C. Slides were washed with PBS 5 times and were mounted in 90% glycerol/PBS containing 0.1% P-phenylenediamine and 0.2 μg/mL 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) (Sigma). Cells incubated only with the secondary antibody were used as negative controls. When indicated, similar procedures were used to react with anti–α-tubulin monoclonal antibody (Biosys, Compiegne, France) at 100-fold dilution in 1% BSA/PBS, anti-survivin (Santa Cruz, CA) at 100-fold dilution in 1% BSA/PBS, or antihuman centromere30 at 300-fold dilution in 1% BSA/PBS. Secondary antibodies were as indicated, including Alexa Fluor 488–conjugated goat antirat IgG (Molecular Probes) at 200-fold dilution in 1% BSA/PBS, Alexa Fluor 546–conjugated goat antirabbit IgG (Molecular Probes) at 300-fold dilution in 1% BSA/PBS, or FITC-conjugated goat antihuman IgG (Jackson ImmunoResearch) at 200-fold dilution in 1% BSA/PBS. Cells were observed under an Olympus BX60-34-FLBD1 fluorescence microscope (Olympus Optical, Tokyo, Japan) at a final magnification of × 1500. All digital images were captured and produced with Mac SCOPE software (Mitani, Fukui, Japan). Ploidy level was determined by comparing DNA (stained with DAPI) content in a cell of interest with that of diploid bone marrow cells. Approximately 1 × 104 megakaryocytes were analyzed for the studies presented in Figures 1, 2, 3, 4, 5.
Aurora-B/AIM-1 expression in primary megakaryocytes at interphase. Mouse primary megakaryocytes were stained with DAPI (blue; i), anti–Aurora-B/AIM-1 antibody (red; ii), or FITC-conjugated anti-CD41 (green; iii). Triple staining (iv). Shown are representative images captured as described in “Materials and methods.” (A) Mature megakaryocyte (not cycling) with ploidy of 16N at interphase. (B) Polyploidizing megakaryocyte with ploidy of 8N at interphase/early prophase. (C) Polyploidizing megakaryocyte with ploidy of 32N at interphase/early prophase. Original magnification, × 1500.
Aurora-B/AIM-1 expression in primary megakaryocytes at interphase. Mouse primary megakaryocytes were stained with DAPI (blue; i), anti–Aurora-B/AIM-1 antibody (red; ii), or FITC-conjugated anti-CD41 (green; iii). Triple staining (iv). Shown are representative images captured as described in “Materials and methods.” (A) Mature megakaryocyte (not cycling) with ploidy of 16N at interphase. (B) Polyploidizing megakaryocyte with ploidy of 8N at interphase/early prophase. (C) Polyploidizing megakaryocyte with ploidy of 32N at interphase/early prophase. Original magnification, × 1500.
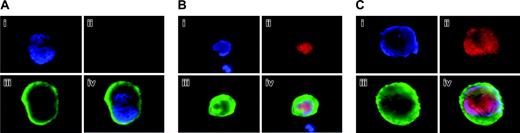
Localization and expression of Aurora-B/AIM-1 at mitosis. Mouse primary megakaryocytes were stained with DAPI (blue; i) or anti–Aurora-B/AIM-1 antibody (red; ii) and were subjected to double staining (iii) or triple staining (iv) with FITC-conjugated anti-CD41 (green). Shown are representative images captured as described in “Materials and methods.” (A) Megakaryocyte with ploidy of 16N at prophase. (B) Megakaryocyte with ploidy of 4N at anaphase. (C) Megakaryocyte with ploidy of 16N at anaphase. (D) Megakaryocyte with ploidy of 16N at anaphase. Original magnification, × 1500.
Localization and expression of Aurora-B/AIM-1 at mitosis. Mouse primary megakaryocytes were stained with DAPI (blue; i) or anti–Aurora-B/AIM-1 antibody (red; ii) and were subjected to double staining (iii) or triple staining (iv) with FITC-conjugated anti-CD41 (green). Shown are representative images captured as described in “Materials and methods.” (A) Megakaryocyte with ploidy of 16N at prophase. (B) Megakaryocyte with ploidy of 4N at anaphase. (C) Megakaryocyte with ploidy of 16N at anaphase. (D) Megakaryocyte with ploidy of 16N at anaphase. Original magnification, × 1500.
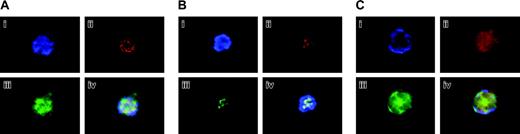
Confirmation of Aurora-B/AIM-1 localization at mitosis. (A) 16N mouse primary megakaryocyte at prophase. Cells were stained with DAPI (blue; i), anti–Aurora-B/AIM-1 antibody (red; ii), and anti–α-tubulin antibody (green; iii), or with triple staining (iv). (B) 8N mouse primary megakaryocyte at prophase. Cells were stained with DAPI (blue; i), anti–Aurora-B/AIM-1 antibody (red; ii), anti-centromere antibody (green; iii), or with triple staining (iv). (C) Mouse primary 16N megakaryocyte at anaphase. Cells were stained with DAPI (blue; i), anti–Aurora-B/AIM-1 antibody (red; ii), or anti–α-tubulin antibody (green; iii). Triple staining (iv). Shown are representative images captured as described in “Materials and methods.” Original magnification, × 1500.
Confirmation of Aurora-B/AIM-1 localization at mitosis. (A) 16N mouse primary megakaryocyte at prophase. Cells were stained with DAPI (blue; i), anti–Aurora-B/AIM-1 antibody (red; ii), and anti–α-tubulin antibody (green; iii), or with triple staining (iv). (B) 8N mouse primary megakaryocyte at prophase. Cells were stained with DAPI (blue; i), anti–Aurora-B/AIM-1 antibody (red; ii), anti-centromere antibody (green; iii), or with triple staining (iv). (C) Mouse primary 16N megakaryocyte at anaphase. Cells were stained with DAPI (blue; i), anti–Aurora-B/AIM-1 antibody (red; ii), or anti–α-tubulin antibody (green; iii). Triple staining (iv). Shown are representative images captured as described in “Materials and methods.” Original magnification, × 1500.
Aurora-B/AIM-1 is localized to the midzone in MG132-treated megakaryocytes at anaphase. Mouse primary megakaryocytes were treated with 20 μM proteasome inhibitor MG132 for 6 hours. Cells at anaphase were stained with DAPI (blue; i) or anti–Aurora-B/AIM-1 antibody (red; ii) and were subjected to double staining (iii) or triple staining (iv) with FITC-conjugated anti-CD41 (green). (A-B) Representative images from 2 different analyses captured as described in “Materials and methods.” Original magnification, × 1500.
Aurora-B/AIM-1 is localized to the midzone in MG132-treated megakaryocytes at anaphase. Mouse primary megakaryocytes were treated with 20 μM proteasome inhibitor MG132 for 6 hours. Cells at anaphase were stained with DAPI (blue; i) or anti–Aurora-B/AIM-1 antibody (red; ii) and were subjected to double staining (iii) or triple staining (iv) with FITC-conjugated anti-CD41 (green). (A-B) Representative images from 2 different analyses captured as described in “Materials and methods.” Original magnification, × 1500.
Survivin expression in primary megakaryocytes. Mouse primary megakaryocytes were treated with or without 20 μM proteasome inhibitor MG132 for 6 hours. Cells at prophase were stained with DAPI (blue; i), anti–Aurora-B/AIM-1 antibody (red; ii), or anti-survivin (green; iii). Triple staining (iv). Shown are representative images captured as described in “Materials and methods.” (A) Polyploidizing megakaryocyte with ploidy of 16N at prophase. (B) MG132-treated polyploidizing megakaryocyte with ploidy of 8N at prophase. In approximately 100 megakaryocytes examined (treated or not treated with MG132), survivin was not detected. Similar results were noted for cells at anaphase (not shown). (C) Only one 8N megakaryocyte at prophase displayed survivin after MG132 treatment. Original magnification, × 1500.
Survivin expression in primary megakaryocytes. Mouse primary megakaryocytes were treated with or without 20 μM proteasome inhibitor MG132 for 6 hours. Cells at prophase were stained with DAPI (blue; i), anti–Aurora-B/AIM-1 antibody (red; ii), or anti-survivin (green; iii). Triple staining (iv). Shown are representative images captured as described in “Materials and methods.” (A) Polyploidizing megakaryocyte with ploidy of 16N at prophase. (B) MG132-treated polyploidizing megakaryocyte with ploidy of 8N at prophase. In approximately 100 megakaryocytes examined (treated or not treated with MG132), survivin was not detected. Similar results were noted for cells at anaphase (not shown). (C) Only one 8N megakaryocyte at prophase displayed survivin after MG132 treatment. Original magnification, × 1500.
In situ hybridization
Rat Aurora-B/AIM-1 riboprobes were synthesized from Aurora-B/AIM-1 cDNA (GenBank accession number D89731) using the Riboprobe In Vitro Transcription System (Promega, Madison, WI) according to the manufacturer's instructions. Rat and mouse (GenBank accession number BC003261) Aurora-B/AIM-1 cDNAs share more than 90% identity in nucleotide sequences. In situ hybridization was performed as previously reported.5 Briefly, whole mice were perfused with 4% paraformaldehyde in PBS. The fixed spleen was embedded in paraffin, sectioned, and mounted onto slides. These slides were then treated with proteinase K, acetylated, prehybridized, and hybridized with antisense or sense riboprobe made from rat Aurora-B/AIM-1 cDNA (subcloned in pcDNA3 vector). Slides were washed in 5 × SSC (0.75 M NaCl, 75 mM Na3Ci, pH 7.0) containing 10 mM dithiothreitol (DTT) at 50° C for 30 minutes and subsequently at 65° C for 20 minutes in 2 × SSC containing 50% formamide and 10 mM DTT. Slides were then treated with RNase A before being exposed to Kodak NTB-2 emulsion (Eastman Kodak, Rochester, NY). After 6-week exposure, slides were developed and subsequently stained with hematoxylin and eosin. Slides were examined under an Olympus (Melville, NY) IX70 inverted microscope. Images were captured with a Hamamatsu CCD camera C4742-95 and were analyzed with OpenLab software (Improvision, Lexington, MA).
Acetylcholinestrase assay
Megakaryocyte number was scored based on a positive signal in acetylcholinesterase assay, as previously reported.4 Briefly, 1 to 4 × 105 bone marrow cells from transgenic or wild-type control mice were centrifuged onto Colofrost Plus microscope slides (Fisher Scientific) for 5 minutes at 120g using a Cytospin3 (Shandon). Slides were then air dried and fixed in 2% formaldehyde buffered in PBS for 45 minutes at room temperature. After fixation, the slides were washed twice with PBS and then treated at room temperature for 4 to 16 hours with the substrate staining solution (0.5 mg/mL acetylthiocholine iodide (Sigma), 0.1 M sodium phosphate, pH 6.0, 5 mM sodium citrate, 3 mM CuSO4, and 0.5 mM K3Fe(CN)6). After the reaction, slides were washed 3 times with PBS, air dried, coated with Crysalmount (Biomedia, Foster City, CA), covered with coverslips, and inspected under a light microscope, by which the orange/brown cells were identified as acetylcholinesterase-positive and thus megakaryocyte-positive.
Platelet number and blood cell counts
Mouse blood collected from the tail vein was diluted in IsoFlow Sheath Fluid (Beckman Coulter, Miami, FL) supplemented with 4 mM EDTA (ethylenediaminetetraacetic acid) (2 μL blood in 4 mL). Diluted blood was again diluted 10 times, and platelet number was scored on a Beckman Coulter Z2 counter.
CFU-Mk assay
The MegaCult-C serum-free system from StemCell Technologies (Vancouver, BC, Canada) was used to score the megakaryocyte colony-forming unit (CFU-Mk) assay. A combination of cytokines containing 50 ng/mL human TPO, 10 ng/mL murine IL-3, 20 ng/mL human IL-6, and 50 ng/mL human IL-11 was used for culturing 1 × 105 bone marrow (BM) cells per assay. Cells were cultured in collagen-based gel for 7 days before final staining with the acetylcholinesterase assay for 4 hours. Colonies with 3 or more megakaryocytes were counted as megakaryocytic colonies or as mixed megakaryocytic colonies if negatively stained cells were also present in the same colonies. The final CFU-Mk number was the combination of megakaryocytic colonies and mixed megakaryocytic colonies. Megakaryocyte number in each CFU was also counted.
Ploidy analysis
Ploidy profiles were analyzed as previously reported.6 Briefly, freshly derived bone marrow cells were washed in PBS and stained for 10 minutes with propidium iodide solution (PI; Sigma) (0.05 mg/mL in 0.1% Triton X-100, 4 mM sodium citrate, pH 7.4, and 100 μg/mL DNase-free RNase A). An equal volume of salt solution (0.05 mg/mL PI, 0.1% Triton X-100, and 400 mM NaCl) was added. Cells were filtered through a 250-μm mesh filter and were subjected to flow cytometry analysis on a FACScan system. Data were collected and analyzed using the CellQuest program (Becton Dickinson, San Jose, CA).
Results
Expression of Aurora-B/AIM-1 and survivin and their localization in resting and polyploidizing bone marrow megakaryocytes
We sought to examine Aurora-B/AIM-1 expression and subcellular localization during the endomitotic cell cycle because Aurora-B/AIM-1 is a regulator of mitosis and cytokinesis and because polyploidizing megakaryocytes skip late anaphase. This was of particular interest because it was reported that Aurora-B/AIM-1 protein is reduced in a nonsynchronized, polyploidizing megakaryocytic cell line (measured by Western blotting).26 It is unclear whether this represents a property of a cell line and, if not, whether Aurora-B/AIM-1 is reduced at a particular stage of the endomitotic cell cycle. In the current study, we focused on the in situ examination of primary bone marrow megakaryocytes (through immunohistochemistry) because of their scarcity in the marrow (consisting of less than 0.02% of total cells). Analyses presented in Figures 1, 2, and 3 are representative of more than 104 megakaryocytes examined, and the images are characteristic of patterns observed. Figure 1 illustrates mouse bone marrow megakaryocytes at interphase that were stained with anti–Aurora-B/AIM-1 antibody (red), anti-CD41 antibody (green), and DAPI (blue). CD41 is a megakaryocyte-specific cell surface marker, and it was confirmed that the cells shown were all CD41-positive megakaryocytes. Figure 1A shows a 16N megakaryocyte in which no Aurora-B/AIM-1 was expressed (Figure 1B), as would be typical of a cell in the G1/S- or G0-phase.31 Figures 1B and 1C show 8N and 32N megakaryocytes, respectively, and Aurora-B/AIM-1 was expressed and localized in the nuclei of these cells (Figure 1A-B), as would be typical of cells in the G2-phase.
Figure 2 exhibits polyploidizing megakaryocytes during mitosis, identified based on the morphology and localization of the chromosomes, as previously described.11 Figure 2A exhibits a representative 16N megakaryocyte at prophase, with punctuate staining of Aurora-B/AIM-1; this protein was clearly expressed and properly localized to the centromeres, as found during normal cell division.31,32 Figure 2B shows a 4N megakaryocyte at anaphase, which was going to divide normally into two 2N megakaryocytes. In this megakaryocyte, the chromosomes appeared bipolar, and Aurora-B/AIM-1 was specifically localized to the midzone (the open space between the mass of chromatids), as typically found during normal cell division. In contrast, Figure 2C-D demonstrates that Aurora-B/AIM-1 was not localized to the midzone in polyploidizing megakaryocytes at anaphase A. In a megakaryocyte shown in Figure 2C (16N cell), Aurora-B/AIM-1 was present but was not localized to the midzone; it appeared diffused (Figure 2B-C). In Figure 2Dc, 16 distinct round masses of 2N chromatids appeared oriented toward the spindle poles, but Aurora-B/AIM-1 was not detected at the midzone. The number of sister chromatid sets appeared as a ball (mass) in DAPI staining (blue), typical of anaphase. As noted in previous reports, chromosome segregation is asymmetrical.14
Figure 3 represents an additional examination set, focusing on similar ploidy cells at different cell cycle stages. Figures 3A and 3C focus on cells stained with anti–Aurora-B/AIM-1 (red), anti–α-tubulin (green), and DAPI (blue) at prophase or anaphase, respectively. The α-tubulin is a spindle marker, and it was confirmed that the spindles were detected inside the chromosomes (not close to the membrane and not pulled toward the poles) at prophase and that the spindles were positioned to pull the sister chromatid sets toward the poles (the spindles were localized outside or close to the membrane) at anaphase. Figure 3B shows cells stained with anti–Aurora-B/AIM-1 (red), anti-centromere (green), and DAPI (blue) at prophase. It was confirmed that Aurora-B/AIM-1 was localized in centromeres. In all our analyses, we detected polyploidy megakaryocytes at late anaphase that essentially lacked Aurora-B/AIM-1 (25% of the cells at this cell cycle stage, as represented in Figure 2D) or contained it in a diffused pattern (75% of the cells at this cell cycle stage, as represented in Figure 3A).
These atypical patterns of Aurora-B/AIM-1 at this specific cell cycle phase could be attributed to a primary effect on factors responsible for regulating Aurora-B/AIM-1 protein localization and on subsequent (or simultaneous) protein degradation. Aurora-B/AIM-1 belongs to a family of proteins that are degraded through the proteasome pathway.33 If protein degradation contributed to the above phenomenon, inhibiting proteasome-mediated protein degradation would have reversed the phenotype. Figure 4 shows polyploid megakaryocytes captured at anaphase. It demonstrates that treating mouse bone marrow megakaryocytes with the proteasome inhibitor MG132 resulted in polyploidizing cells in which Aurora-B/AIM-1 protein was properly localized to the midzone at anaphase. In Figure 4 chromatids were structured similarly to the megakaryocytes in Figures 3C and 2D, which were all at anaphase. Aurora-B/AIM-1 was, however, specifically localized to the midzone at anaphase only in MG132-treated cells. We did not observe this phenomenon in approximately 1 of 3 megakaryocytes at anaphase, possibly because of the proteasome treatment of cells at a cell cycle stage when Aurora-B/AIM-1 had been already degraded. These results suggest that protein degradation contributed to the atypical profile of Aurora-B/AIM-1 expression and localization during late anaphase in polyploidizing megakaryocytes.
As indicated by the background, Aurora-B/AIM-1 was typically found in a complex with survivin; hence, we examined whether the characteristic detection of survivin in the midzone in mitotic cells persisted in polyploidizing megakaryocytes. In contrast to Aurora-B/AIM-1, which was apparent at prophase but not at late anaphase in polyploidizing megakaryocytes, survivin was not detected at all throughout the endomitotic cell cycle in megakaryocytes. Cells at interphase, prophase, or prometaphase, identified based on the criteria described here, completely lacked survivin. Examples are shown in Figure 5 (in the same cells, Aurora proteins were properly localized at prophase; data not shown). It has been reported that survivin is degraded through the proteasome pathway.34 Treatment with the proteasome inhibitor MG132 did not rescue the phenotype (Figure 5B). We detected a megakaryocyte that expressed survivin at prophase after MG132 treatment (Figure 5C), but this was only 1 in approximately 100 megakaryocytes surveyed. This represents a rare case. Hence, we suggest that the lack of survivin during polyploidization is not caused by a primary effect on protein degradation. Taken together, our study identified 2 chromosome passenger proteins, critical regulators of anaphase and cytokinesis, as being atypically expressed or localized during the endomitotic cell cycle in megakaryocytes.
Generation of transgenic mice overexpressing Aurora-B/AIM-1 mRNA in megakaryocytes
In a previous study, we observed decreased Aurora-B/AIM-1 transcription during polyploidization of a megakaryocytic cell line.27 Others have also reported a significant decrease in Aurora-B/AIM-1 mRNA in polyploidizing megakaryocytic cell lines and decreased ploidy in megakaryocytic cell lines overexpressing this protein.26 To explore the importance of regulated Aurora-B/AIM-1 expression in promoting polyploidy in vivo, we specifically overexpressed this cDNA in megakaryocytes of transgenic mice using the platelet factor 4 (PF4) promoter.35 Transgenic DNA (Figure 6A)36 was injected into pronuclei of fertilized eggs from FVB mice. Eight transgenic founder lines of PF4–Aurora-B/AIM-1 have been identified with a copy number of genes integrated ranging from 5 to 25 (Figure 6B), of which TG58 and TG74 had the highest transgenic Aurora-B/AIM-1 messages (Figure 6C). As often observed in other transgenic lines,37 transgene copy number did not correlate with expression level. Transgene mRNA was examined with specific Aurora-B/AIM-1 primers and with primers to β-globin (unique to the transgenic construct), using a new method of quantitation that we developed in this study (“Materials and methods”). The relative abundance of megakaryocytes in each specific bone marrow preparation could vary because of sample processing (particularly because megakaryocytes are rare in the marrow). To account for this variable, a megakaryocyte marker, PF4 mRNA, was also examined. The ratio of Aurora-B/AIM-1 mRNA/PF4 mRNA was the highest for TG58 > TG74. Other transgenic lines expressed the transgene at low levels (Figure 6C) or not at all. Aurora-B/AIM-1 mRNA was detected in wild-type bone marrow samples only after prolonged exposure of the blots, likely because this gene transcription is cell cycle regulated,26 whereas the number of cycling cells in the marrow is small. In our subsequent studies, we focused on F1 and higher-generation mice (age- and sex-matched, as indicated in “Materials and methods”) derived from the 2 transgenic lines, TG58 and TG74. As previously reported,4,35 the PF4 promoter drove a tissue-specific expression of the transgene.
Transgenic mice overexpressing Aurora-B/AIM-1. (A) Scheme of the PF4-Aurora-B/AIM-1 transgenic DNA construct. Not shown here is a short FLAG sequence engineered at the 5′ end of the cDNA, as detailed in “Materials and methods.” Rat Aurora-B/AIM-1 cDNA (indicated here as AIM-1) is under the control of the megakaryocyte-specific 1.1-kb rat PF4 promoter. The 3′ end of the gene was replaced with human β-globin genomic sequences. Restriction enzyme digestion sites are indicated: NdeI,B(BamHI), K (KpnI), and ApaI. (B) Southern blot analysis of genomic DNA extracted from mouse tails using radiolabeled rat Aurora-B/AIM-1 cDNA as a probe. DNA was digested with BamHI, yielding 2 fragments of 0.6 and 0.8 kb. The position of endogenous Aurora-B/AIM-1 is also indicated (mAIM-1). Transgene copy number (fold over endogenous Aurora-B/AIM-1 gene) ranged from 5 to 25. (C) Message level in total mouse bone marrow was monitored as described in “Materials and methods.” The cDNA was separated on 1% agarose gel, which was stained with ethidium bromide (EtBr) as shown. An evenly distributed appearance of bands is in accordance with our nonspecific PCR amplification of total cDNA derived from cells. Specific message level was detected by Southern blot analysis using radiolabeled Aurora-B/AIM-1, β-globin, or PF4 cDNAs, as detailed in “Materials and methods.” Shown are results from 1 of 3 representative experiments. In this study and in previous studies with PF4-driven genes, we noted reductions in PF4 mRNA in some of the lines. We do not believe this has an impact on megakaryopoiesis because the phenotype of these various models is typically different and because nonchallenged PF4 null mice have a level of CFU-Meg similar to that of wild-type mice.44
Transgenic mice overexpressing Aurora-B/AIM-1. (A) Scheme of the PF4-Aurora-B/AIM-1 transgenic DNA construct. Not shown here is a short FLAG sequence engineered at the 5′ end of the cDNA, as detailed in “Materials and methods.” Rat Aurora-B/AIM-1 cDNA (indicated here as AIM-1) is under the control of the megakaryocyte-specific 1.1-kb rat PF4 promoter. The 3′ end of the gene was replaced with human β-globin genomic sequences. Restriction enzyme digestion sites are indicated: NdeI,B(BamHI), K (KpnI), and ApaI. (B) Southern blot analysis of genomic DNA extracted from mouse tails using radiolabeled rat Aurora-B/AIM-1 cDNA as a probe. DNA was digested with BamHI, yielding 2 fragments of 0.6 and 0.8 kb. The position of endogenous Aurora-B/AIM-1 is also indicated (mAIM-1). Transgene copy number (fold over endogenous Aurora-B/AIM-1 gene) ranged from 5 to 25. (C) Message level in total mouse bone marrow was monitored as described in “Materials and methods.” The cDNA was separated on 1% agarose gel, which was stained with ethidium bromide (EtBr) as shown. An evenly distributed appearance of bands is in accordance with our nonspecific PCR amplification of total cDNA derived from cells. Specific message level was detected by Southern blot analysis using radiolabeled Aurora-B/AIM-1, β-globin, or PF4 cDNAs, as detailed in “Materials and methods.” Shown are results from 1 of 3 representative experiments. In this study and in previous studies with PF4-driven genes, we noted reductions in PF4 mRNA in some of the lines. We do not believe this has an impact on megakaryopoiesis because the phenotype of these various models is typically different and because nonchallenged PF4 null mice have a level of CFU-Meg similar to that of wild-type mice.44
Increased Aurora-B/AIM-1 protein level is only detected in small megakaryocytes
To examine whether the Aurora-B/AIM-1 protein level is also elevated in transgenic megakaryocytes, we performed immunohistochemistry on bone marrow cells derived from transgenic mice using an anti–Aurora-B/AIM-1 antibody. As discussed in numerous studies involving analysis of transgenic megakaryocytes (eg, see Ravid et al38 ), this cell type is scarce in the marrow (less than 0.02%), prohibiting quantitative analyses such as Western blotting. Positive fluorescence staining of Aurora-B/AIM-1 throughout the cell (in a diffused pattern) was detected in transgenic megakaryocytes, which were counterstained for the megakaryocyte surface marker CD41 (Figure 7, Table 1). To our surprise, those strong positive signals were only detected in small megakaryocytes of TG58 and TG78, not in large megakaryocytes. As to the localization of transgenic Aurora-B/AIM-1 throughout the cell cycle in polyploidizing large transgenic megakaryocytes, it followed the pattern found for wild-type cells (as in Figures 1 and 2), indicating that the transgenic protein was regulated similarly to the endogenous one. In some large megakaryocytes of TG58 mice (approximately 20 of 100 examined), weak, diffused staining of Aurora-B/AIM-1 (over background) was detected throughout the cell.
High Aurora-B/AIM-1 protein level is only detected in transgenic, small, CD41+ megakaryocytes. Immunohistochemical staining of bone marrow cells isolated from wild-type or transgenic mice. Aurora-B/AIM-1 was stained with rhodamine-conjugated antibody (red). The megakaryocyte surface marker CD41 was stained with FITC (green). Cell nuclei were stained with Hoechst (blue). Large and small CD41+ cells (megakaryocytes) are indicated with arrows. Small CD41+ cells have diameters in the range observed for all other bone marrow cells (noted by phase-contrast microscopy). Small megakaryocytes in transgenic mice showed strong, diffuse Aurora-B/AIM-1 staining. Original magnification, × 200.
High Aurora-B/AIM-1 protein level is only detected in transgenic, small, CD41+ megakaryocytes. Immunohistochemical staining of bone marrow cells isolated from wild-type or transgenic mice. Aurora-B/AIM-1 was stained with rhodamine-conjugated antibody (red). The megakaryocyte surface marker CD41 was stained with FITC (green). Cell nuclei were stained with Hoechst (blue). Large and small CD41+ cells (megakaryocytes) are indicated with arrows. Small CD41+ cells have diameters in the range observed for all other bone marrow cells (noted by phase-contrast microscopy). Small megakaryocytes in transgenic mice showed strong, diffuse Aurora-B/AIM-1 staining. Original magnification, × 200.
Ectopic Aurora-B/AIM-1 message expression is under the control of the PF4 promoter, which is active in small and large megakaryocytes.38 Figure 6 indicates transgene expression at the mRNA level, but in this PCR assay we were unable to focus on large megakaryocytes. In situ hybridization of spleen sections, using an Aurora-B/AIM-1 riboprobe, indicated that the Aurora-B/AIM-1 message is present at low levels in wild-type, large megakaryocytes but abundant in all large megakaryocytes of transgenic mice (Figure 8). Therefore, even though we are able to elevate the Aurora-B/AIM-1 message level in transgenic mice, after a certain point of megakaryocytic differentiation, an Aurora-B/AIM-1 protein regulatory mechanism took over and down-regulated Aurora-B/AIM-1 protein, suggesting that mature megakaryocytes have an internal mechanism to reduce levels of excessive Aurora-B/AIM-1 protein.
In situ hybridization of Aurora-B/AIM-1 mRNA. In situ hybridization was performed to examine the message level of Aurora-B/AIM-1 in transgenic megakaryocytes. Microscopic images shown here demonstrate that morphologically recognizable large megakaryocytes4 (identified by arrows) in the spleen of transgenic mice (TG 58 or TG 74) have elevated levels of the Aurora-B/AIM-1 message compared with control wild-type (WT) counterparts of similar cell size. Some small-diameter cells showed a strong positive signal in the wild-type or transgenic samples (not shown). Approximately 30 polyploid megakaryocytes of similar diameter were surveyed in each preparation. Original magnification, × 200.
In situ hybridization of Aurora-B/AIM-1 mRNA. In situ hybridization was performed to examine the message level of Aurora-B/AIM-1 in transgenic megakaryocytes. Microscopic images shown here demonstrate that morphologically recognizable large megakaryocytes4 (identified by arrows) in the spleen of transgenic mice (TG 58 or TG 74) have elevated levels of the Aurora-B/AIM-1 message compared with control wild-type (WT) counterparts of similar cell size. Some small-diameter cells showed a strong positive signal in the wild-type or transgenic samples (not shown). Approximately 30 polyploid megakaryocytes of similar diameter were surveyed in each preparation. Original magnification, × 200.
Megakaryocyte number, ploidy level, and platelet number in Aurora-B/AIM-1 transgenic mice
In view of the reports that high levels of Aurora-B/AIM-1 are associated with abnormal cell growth,39 we sought to examine effects of this transgene on megakaryocyte number. Two independent assays, as shown in Table 2, indicated that megakaryocyte number was greater by approximately 30% in adult transgenic mice (TG58 and TG74) compared with control. This was not accompanied by a significant change in platelet level (Table 2). Additionally, only a few of the TG58 mice displayed reduced ploidy levels (Table 3). This might be related to fine variances in the regulation of the Aurora-B/AIM-1 level throughout the cell cycle in different offspring of TG58. Reduced ploidy levels were associated with an increase in the percentage of tetraploid cells. Increased tetraploidy was also observed in Chinese hamster embryo fibroblasts overexpressing Aurora-B/AIM-1.40
The number of CFU-Mk progenitors is not increased in transgenic mice, but those progenitors have a higher tendency to proliferate. One possible reason for the increased megakaryocyte number in transgenic mice could be Aurora-B/AIM-1–induced generation of megakaryocyte progenitors. Another reason could be that higher levels of Aurora-B/AIM-1 in small diploid megakaryocytes drive the cells to proliferate. To examine these possibilities, serum-free CFU-Mk assay was performed on bone marrow cells derived from transgenic or control mice. No significant change in CFU-Mk colony number was detected in transgenic mice compared with wild-type mice (Figure 9A). Further counting of megakaryocyte numbers per CFU-Mk colony indicated an increase in the average number of megakaryocytes per colony in the transgenic mice (Figure 9B). Increased levels of Aurora-B/AIM-1 protein in small megakaryocytes tended to promote proliferation.
CFU-Mk assay. Bone marrow cells (105) extracted from each mouse femur were cultured in collagen-based, serum-free medium for 7 days. Cultures were dried, fixed, and assayed for acetylcholinesterase activity. Colonies with 3 or more megakaryocytes were scored as CFU-Mk (Meg), and the rest were scored as nonmegakaryocyte colonies (non-Meg). (A) Average numbers of CFU-Mk from TG58 and TG74 compared with wild-type (WT) mice. (B) Average numbers of megakaryocytes in CFU-Mk colonies from Aurora-B/AIM-1 transgenic lines TG58 and TG74 compared with wild-type mice. Numbers in transgenic mice were approximately 40% greater than in wild-type mice (P < .01; Student t test). As shown, the average megakaryocyte number in a wild-type colony was 8.4. (C) Percentage of megakaryocyte colonies that had higher-than-average megakaryocyte number (8.4). Megakaryocyte colonies TG58 and TG74 had more cells than wild-type (P < .01; Student t test). Data presented are mean ± SD of 6 mice per experimental group, each assayed in duplicate.
CFU-Mk assay. Bone marrow cells (105) extracted from each mouse femur were cultured in collagen-based, serum-free medium for 7 days. Cultures were dried, fixed, and assayed for acetylcholinesterase activity. Colonies with 3 or more megakaryocytes were scored as CFU-Mk (Meg), and the rest were scored as nonmegakaryocyte colonies (non-Meg). (A) Average numbers of CFU-Mk from TG58 and TG74 compared with wild-type (WT) mice. (B) Average numbers of megakaryocytes in CFU-Mk colonies from Aurora-B/AIM-1 transgenic lines TG58 and TG74 compared with wild-type mice. Numbers in transgenic mice were approximately 40% greater than in wild-type mice (P < .01; Student t test). As shown, the average megakaryocyte number in a wild-type colony was 8.4. (C) Percentage of megakaryocyte colonies that had higher-than-average megakaryocyte number (8.4). Megakaryocyte colonies TG58 and TG74 had more cells than wild-type (P < .01; Student t test). Data presented are mean ± SD of 6 mice per experimental group, each assayed in duplicate.
Discussion
Aurora-B/AIM-1 and survivin are mitotic regulators that are localized to the centromeres at early mitosis. During anaphase, these proteins become localized at the midzone (for reviews, see Adams et al,17 Lens and Medema,18 and Terada19 ). Our study indicates that Aurora-B/AIM-1 is normally expressed and localized during early mitosis in polyploidizing mouse megakaryocytes. In contrast, Aurora-B/AIM-1 expression and localization are atypical during anaphase in this cell type. Similarly, it has been reported that the midzone at anaphase is void of Aurora-B/AIM-1.41 We report that Aurora-B/AIM-1 is not absent in all cells at anaphase, but in some cells at this cell cycle stage it appears diffused throughout the nucleus. Survivin is, however, absent throughout the cell cycle. Interestingly, a main kinesin, MKLP, has been reported as normally present in the midzone at anaphase in this cell type.14 Hence, it is unlikely that endomitosis involves a regulator, which prevents the formation of a typical midzone and, consequently, leads to aberrant localization of all midzone-associated proteins. Survivin disappearance precedes that of Aurora-B/AIM-1 during the endomitotic cell cycle. Our data favor a model by which regulators of survivin expression/localization and a consequent or independent regulation of Aurora-B/AIM-1 localization and degradation are targets of control during megakaryocyte polyploidization. Aurora-B/AIM-1 belongs to a family of proteins that are degraded through the proteasome pathway,33 and accordingly it is ubiquitinated (H.G.N., unpublished data, May 2003, using methods as in Zhang et al7 ). Survivin is also degraded through the proteasome pathway.34 Inhibition of proteasome-mediated protein degradation leads to proper localization of Aurora-B/AIM-1 at the midzone during anaphase in some polyploidizing megakaryocytes, suggesting that degradation processes contribute to Aurora-B/AIM-1 elimination from the midzone (directly or indirectly by a potential effect on an associated protein). Treatment with a proteasome inhibitor, however, does not lead to proper expression levels of survivin in most megakaryocytes, suggesting regulation at the transcriptional level. A study in proliferating human cells provides evidence that the depletion of survivin by survivin antisense oligonucleotide treatment resulted in a dim and diffused appearance Aurora-B.42 In polyploidizing megakaryocytes, prophase is marked by the proper localization of Aurora-B/AIM-1 despite a lack of survivin. It is possible that in polyploidizing cells a complex of survivin with Aurora-B/AIM-1 is important for the stabilization/localization of the latter protein only at anaphase, the absence of which might lead to polyploidization. Future studies involving the restoration of survivin in megakaryocytes will examine this contention.
Aurora-B/AIM-1 mRNA down-regulation is associated with megakaryocyte polyploidization,26,27 whereas the overexpression of wild-type Aurora-B/AIM-1 reduces the level of phorbol ester-induced polyploidization.26 Because Aurora-B/AIM-1 mRNA was reported to be reduced during megakaryocyte polyploidization,26,27 it is possible that combined regulation at the mRNA and protein levels ensures moderate abundance of Aurora-B/AIM-1 at late mitosis in polyploidizing megakaryocytes. In the current study, we examined whether deregulated expression of Aurora-B/AIM-1 impacted proliferation and polyploidization rates in megakaryocytes in vivo. Continuous expression might be achieved by the use of a promoter, such as the PF4 promoter, that is not cell cycle regulated. A major consideration with this overexpression strategy is the potential accumulation of Aurora-B/AIM-1 at levels much greater than those found in normally proliferating cells. This has been a concern in a vast number of studies involving transgenic overexpression to examine the in vivo functional relevance of a cell cycle regulator. We resorted toAurora-B/AIM-1 cDNAoverexpression by the tissue-specific PF4 promoter, assuming that if Aurora-B/AIM-1 is regulated at the protein level, it will be only mildly elevated in transgenic megakaryocytes.
When the Aurora-B/AIM-1 message was overexpressed in megakaryocytes in vivo, the corresponding protein level was not proportionally elevated in any of the large megakaryocytes we studied, indicating an internal mechanism that removed excess Aurora-B/AIM-1 protein efficiently. This was not reminiscent of any other PF4-driven transgenes; in previous cases, the transgenic protein was readily detectable in mature polyploid megakaryocytes.35,37,43 The level of Aurora-B/AIM-1 transgenic protein was high, however, at the early stages of megakaryocytopoiesis. The internal mechanism that reduced Aurora-B/AIM-1 protein in large transgenic megakaryocytes most likely did not involve reduced protein translation. The 5′ and 3′ nontranslated regions in our Aurora-B/AIM-1 transgene are not related to Aurora-B/AIM-1; thus, the likelihood is low that the transgene is regulated at the translational level or that it is regulated differently in small and large megakaryocytes. The fact that Aurora-B/AIM-1 is highly abundant in small megakaryocytes, but not in large transgenic megakaryocytes that express high levels of Aurora-B/AIM-1 mRNA, suggests that Aurora-B/AIM-1 degradation in these cells is highly effective. It has been noted that not every small transgenic megakaryocyte has an elevated Aurora-B/AIM-1 level, creating a distinction between small megakaryocytes that tolerate high levels of this protein and megakaryocytes that start to remove Aurora-B/AIM-1 efficiently. Analysis also shows that higher levels of Aurora-B/AIM-1 in early megakaryocytes induce them to proliferate, as indicated by colony-forming assays and by increases in megakaryocyte numbers in the marrow. Higher levels of Aurora-B/AIM-1 in transgenic mice could overload the degradation system and delay the timing of entry into endomitosis. Some of the Aurora-B/AIM-1 transgenic line TG58 mice displayed accumulated tetraploid cells and inhibited ploidy levels. Interestingly, a recent study showed that overexpression of Aurora-B/AIM-1 in Chinese hamster embryo cells induced tetraploidy.40 The finding that few, not all, TG58 mice have reduced ploidy levels might be related to fine differences in Aurora-B/AIM-1 protein levels among mice of the same founder line. It is possible that re-entering endomitosis (and reaching high ploidy) requires the amount of Aurora-B/AIM-1 protein to be below a certain level, and this might vary among megakaryocytes in the marrow, depending on their cell cycle synchrony. An additional variable phenomenon observed involves higher incidences of lethality among newly born pups in the F1 generation of line TG58 (approximately 25% of the pups). This lethality was associated with hemorrhages, but because of the size of the newborns and the detection of lethality after the fact, we could not estimate platelet levels in these mice. The phenomenon was essentially selected out by subsequent breeding of the viable mice. It is possible that conditional overexpression of this transgene will enable the examination of effects of higher dosages of protein expression on platelet levels and lethality in transgenic mice.
In summary, this is the first paper to describe a lack of survivin throughout polyploidization in mouse megakaryocytes and an atypical localization and abundance of Aurora-B/AIM-1 at late anaphase. Our study also indicates that proteasome-dependent degradation is a primary regulator of Aurora-B/AIM-1 abundance during endomitosis. Future studies will aim at exploring the mechanisms of down-regulation of these proteins and their potential interdependence. Given that these kinases are known regulators of anaphase and cytokinesis, it is possible that their atypical distributions during mitosis contribute to the promotion of polyploidization. Our investigation also indicates that in vivo overexpression of Aurora-B/AIM-1 increases the number of megakaryocytes with a higher proliferation potential.
Prepublished online as Blood First Edition Paper, January 29, 2004; DOI 10.1182/blood-2003-09-3365.
Supported by National Institutes of Health grant HL58547 (K.R.).
Y.Z. and Y.N. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Michael Long for critically reading this paper and Dr Hou Xiang Xie and Robin MacDonald at the transgene facility at Boston University School of Medicine.