Abstract
HMG-CoA reductase is the rate-limiting enzyme of the mevalonate pathway leading to the formation of cholesterol and isoprenoids such as farnesylpyrophosphate (FPP) and geranylgeranylpyrophosphate (GGPP). The inhibition of HMG-CoA reductase by lovastatin induced apoptosis in plasma cell lines and tumor cells from patients with multiple myeloma. Here we show that cotreatment with mevalonate or geranylgeranyl moieties, but not farnesyl groups, rescued myeloma cells from lovastatin-induced apoptosis. In addition, the inhibition of geranylgeranylation by specific inhibition of geranylgeranyl transferase I (GGTase I) induced the apoptosis of myeloma cells. Apoptosis triggered by the inhibition of geranylgeranylation was associated with reduction of Mcl-1 protein expression, collapse of the mitochondrial transmembrane potential, expression of the mitochondrial membrane protein 7A6, cytochrome c release from mitochondria into the cytosol, and stimulation of caspase-3 activity. These results imply that protein geranylgeranylation is critical for regulating myeloma tumor cell survival, possibly through regulating Mcl-1 expression. Our results show that pharmacologic agents such as lovastatin or GGTase inhibitors may be useful in the treatment of multiple myeloma.
Introduction
Prenylation is a class of lipid modification involving the covalent attachment of hydrophobic isoprenoid molecules to target proteins.1 The enzymes farnesyl transferase (FTase) and geranylgeranyl transferase (GGTase) catalyze the transfer and subsequent binding of farnesyl and geranylgeranyl isoprenoid moieties from farnesylpyrophosphate (FPP) and geranylgeranylpyrophosphate (GGPP), respectively, to a cysteine-containing motif located at or near the C-terminus of a target protein. Prenylation is essential for membrane attachment1 and the subsequent participation of prenylated proteins in diverse signaling pathways regulating cell growth and survival.2-5 Proteins that require geranylgeranylation or farnesylation for their function include guanosine triphosphate (GTP)-binding proteins such as the Rho family members Rac1, RhoA, and CDC42 (geranylgeranylation)6 and Ras (farnesylation).1,5 Oncogenic Ras mutations have been found in several human cancers including multiple myeloma.7,8 Based on in vitro studies indicating that the inhibition of farnesylation has antimyeloma activity,9-12 clinical studies have been initiated to evaluate the efficacy of FTase inhibitors in myeloma treatment.13
Multiple myeloma is an incurable neoplastic disease of the B-cell lineage, characterized by the presence of monoclonal plasma cells in the bone marrow. Although chemotherapy is initially effective in most patients with myeloma, multidrug-resistant disease eventually develops in all patients. Recently, we described that lovastatin effectively decreases the viability of myeloma cells from cell lines and patient samples, including those of patients with drug-resistant disease.14 In addition, low concentrations of lovastatin synergized with dexamethasone to induce plasma cell cytotoxicity.14 Lovastatin is a potent, competitive inhibitor of the enzyme 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase and is widely used for the treatment of hypercholesterolemia.15 HMG-CoA reductase is the rate-limiting enzyme of the mevalonate pathway, and it catalyzes the reduction of HMG-CoA to mevalonate, which is an intermediate in the synthesis of FPP and GGPP.16 The apoptotic effect of lovastatin on myeloma plasma cells is reversed by the addition of mevalonate.14 This suggests that downstream metabolites of mevalonate play a role in regulating lovastatin-induced apoptosis.
Recent reports imply that antiapoptotic and proapoptotic Bcl-2 family proteins are important regulators of cell survival and resistance to cytotoxic drugs. The antiapoptotic members of the Bcl-2 family, such as Bcl-2, Bcl-XL, and Mcl-1, protect against apoptosis by forming heterodimers with proapoptotic Bcl-2 family members, such as Bax and Bad.17,18 This prevents the collapse of the mitochondrial transmembrane potential and the release of cytochrome c from mitochondria, which may facilitate a change in Apaf-1 structure to allow procaspase-9 recruitment, processing, and activation. Activated caspase-9 then activates effector caspases such as caspase-3.17,18 Our work and that of others have shown that Bcl-2 protects myeloma cells against cytotoxic drugs and contributes to chemoresistance.19-22 Bcl-XL is up-regulated in MM cells at the time of relapse and correlates with a decreased response rate to subsequent chemotherapy.23 Recently it was reported that a threshold level of Mcl-1 expression is required to prevent apoptosis and to maintain viability of myeloma cells, marking Mcl-1 as a critical survival factor for myeloma cells.24-26
In this study, we examined the importance of protein farnesylation and geranylgeranylation for regulating the survival of myeloma tumor cells. We demonstrate that geranylgeranylation is essential for myeloma tumor cell survival through the regulation of Mcl-1 protein expression.
Patients, materials, and methods
Reagents
Lovastatin and simvastatin were obtained from Merck (Rahway, NJ) and were chemically activated by alkaline hydrolysis before use, as described previously.27 Pravastatin sodium was purchased from Bristol-Meyers Squibb (New Brunswick, NJ) and was dissolved in phosphate-buffered saline (PBS) (20 mM). Atorvastatin calcium was obtained from Pfizer GmbH (Freiburg, Germany) and dissolved in ethanol containing 3% dimethyl sulfoxide (DMSO; Riedel-de Haen, Seelze, Germany) (10 mM). Mevalonate and farnesol (FOH) were purchased from Sigma (St Louis, MO), and geranylgeraniol (GGOH) was obtained from ICN Biomedicals (Zoetermeer, The Netherlands). FOH and GGOH are metabolized to FPP and GGPP in the cells, respectively.28 FTI-277 and GGTI-298 were obtained from Calbiochem (Schwallbach, Germany).
Cell lines and patients
Plasma cell lines RPMI 8226 and U266 were obtained from the American Tissue Culture Collection (ATCC), and L363 was obtained from the German Collection of Microorganisms and Cell Cultures (GCMC). These cell lines were cultured in the absence of exogenous interleukin-6 (IL-6).29 The IL-6-dependent plasma cell line XG-1 was a kind gift of Dr B. Klein (Institute for Molecular Genetics, Montpellier, France).30 Cell lines were cultured in RPMI 1640 (Gibco, Breda, The Netherlands) supplemented with 10% fetal calf serum (FCS) (Integro, Zaandam, The Netherlands), 100 IU/mL penicillin, 100 μg/mL streptomycin, and 10 μM β-mercaptoethanol (growth medium). The IL-6-dependent cell line XG-1 was cultured in the continuous presence of exogenous IL-6 (1.25 ng/mL recombinant human IL-6 [rhIL-6]) (Roche, Almere, The Netherlands).
With informed consent, myeloma plasma cells were obtained from bone marrow aspirates taken from the posterior iliac crest in 4 patients and from peripheral blood in 1 patient with plasma cell leukemia. The plasma cell percentage in the patient samples varied from 12% to 96% of the mononuclear cells, as determined by the coexpression of CD38 (anti-CD38-fluorescein isothiocyanate [anti-CD38-FITC]; Immunotech, Marseille, France) and CD138 (anti-CD138-phycoerythrin [anti-CD138-PE]; Immunotech) by flow cytometric analysis (FACSCalibur, Becton Dickinson, Erembodegem, Belgium [BDIS]). Except for patient 2, whose bone marrow contained 96% myeloma cells, tumor cells were purified ex vivo from mononuclear cells obtained by Ficoll-Paque (Amersham; Pharmacia BiotechAB, Uppsala, Sweden) density centrifugation by magnetic cell sorting (MACS) based on CD138 expression. To this end, mononuclear cells were subsequently labeled with anti-CD138 (Immunotech) and rat antimouse immunoglobulin G1 (IgG1) microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) and then were separated using a high-gradient magnetic separation column placed in a strong magnetic field (Miltenyi), exactly following the instructions of the manufacturer. Samples obtained in this way contained more than 95% myeloma plasma cells as determined by analysis of CD38/CD138 coexpression. For experiments, myeloma cells were resuspended in RPMI 1640 (Gibco) supplemented with 10% FCS (Integro), 100 IU/mL penicillin, 100 μg/mL streptomycin, and 10 μM β2-mercaptoethanol. Approval was obtained from the University Medical Center Utrecht institutional review board for these studies (01/051-E). This study was performed according to the Helsinki agreement.
Cell viability
Viability of cells was examined by means of the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay as described previously.14 In short, cells were seeded in a concentration of 0.3 × 106/mL for the myeloma cell lines or 1 × 106/mL for the tumor cells of patients in a 96-well, flat-bottom plate (100 μL/well) (Nunc, Roskilde, Denmark) and were treated with lovastatin (for concentrations, see figure legends) alone or in the presence of mevalonate, FOH, or GGOH. Fixed concentrations of mevalonate (100 μM), FOH (10 μM), or GGOH (10 μM) were used. These concentrations proved to be optimal for rescuing myeloma cells from lovastatin-induced apoptosis (data not shown). Inhibition of FTase and GGTase I was accomplished by treating cells with FTI-277 and GGTI-298, respectively (for concentrations, see figure legends). After 2 or 4 days, 25 μL MTT (5 mg/mL) was added to each well. After 2-hour incubation at 37°C, the reaction was stopped by the addition of 100 μL 20% sodium dodecyl sulfate (SDS; Boehringer Mannheim, Mannheim, Germany)/0.025 M HCl/0.35 M HAc in a mixture of (1:1; vol/vol) N, N-dimethylformamide (Merck, Darmstadt, Germany) and distilled water. After overnight incubation at 37°C, the optical density of the samples was determined at 570 nm.
Apoptosis detection by annexin V staining
Myeloma cells (1.5 × 105 in 0.5 mL) were incubated with lovastatin (for concentrations, see figure legends) alone or in the presence of mevalonate (100 μM), FOH (10 μM), or GGOH (10 μM) in a 48-well plate (Nunc). Inhibition of FTase and GGTase I was accomplished by treating cells with FTI-277 and GGTI-298 (for concentrations, see figure legends), respectively. After 2 or 4 days, cells were harvested, washed in ice-cold PBS, and directly stained with annexin V-FITC (Nexins Research, Kattendijke, The Netherlands) and propidium iodide (PI). After 10 minutes of incubation at room temperature in the dark, cells were analyzed by flow cytometry (FACSCalibur; BDIS) as described previously.14 Apoptotic cells were defined as early apoptotic cells (annexin V-positive and PI-negative) and late apoptotic cells (annexin V-positive and PI-positive).
Apoptosis detection by APO2.7 staining
Myeloma cells (1.5 × 105 in 0.5 mL) were incubated with lovastatin (for concentrations, see figure legends) alone or in the presence of mevalonate (100 μM), FOH (10 μM), or GGOH (10 μM) in a 48-well plate (Nunc). Cells were washed in PBS with 2.5% FCS, permeabilized in 100 μg/mL digitonin (Sigma) in PBS, and incubated for 20 minutes on ice. Cells were washed and resuspended in 80 μL PBS with 2.5% FCS and 20 μL APO2.7-PE (Immunotech) and then incubated for 15 minutes at room temperature in the dark. After a washing step, cells were analyzed by flow cytometry (FACSCalibur; BDIS).
Caspase-3 activity
The caspase-3 activity assay (Roche) was used to determine caspase-3 activity. Briefly, cells were washed in ice-cold PBS and then resuspended in lysis buffer (1× dithiothreitol [DTT]) and incubated for 1 minute on ice. Supernatants were obtained after centrifugation at 14 000 rpm for 1 minute at room temperature. Supernatant was added to anti-caspase-3-coated wells and incubated at 37°C for 1 hour. After 3 washing steps, substrate solution (Ac-DEVD-AFC) was added, and the wells were incubated for 2 hours at 37°C. Fluorescence was measured with a 400-nm excitation filter and a 505-nm emission filter.
Cytochrome c ELISA
A cytochrome c enzyme-linked immunosorbent assay (ELISA) kit (MBL, Watertown, MA) was used to quantitate cytochrome c that was released from mitochondria into the cytosol. Briefly, cells were washed 3 times with ice-cold PBS and resuspended at a concentration of 5 × 106 cells/mL in 10 mM Tris-HCl (pH 7.5), 0.3 M sucrose, and a cocktail of protease inhibitors (Boehringer Mannheim). Cells were homogenized by douncing 10 times in a Dounce homogenizer with a sandpaper-polished pestle. After a centrifugation step at 4°C for 60 minutes at 14 000 rpm, the cytosolic fraction was present in the supernatant. Sixty microliters 1:5 diluted cytosolic fraction was mixed with 60 μL conjugate reagent. One hundred microliters mixture was added to anti-cytochrome c-coated wells and was incubated for 60 minutes at room temperature. After 4 washing steps, substrate solution was added, and the wells were incubated for 15 minutes at room temperature. After the stop solution was added, the absorbance of each well was read at 450 nm.
Measurement of mitochondrial transmembrane potential
Changes in mitochondrial transmembrane potential (ΔΨm) were evaluated by staining with 40 nM 3,3′-dihexyloxacarbocyanine iodide (DiOC6[3]; Molecular Probes, Leiden, The Netherlands). Cells were incubated with DiOC6[3] in PBS for 15 minutes at 37°C, washed, and resuspended in PBS. Cells were then analyzed on a flow cytometer (FACSCalibur; BDIS).
Western blotting
Cells (1 × 106 cells in 1.5 mL) were incubated for 2 or 4 days with lovastatin (for concentrations, see figure legends) in the presence or absence of mevalonate (100 μM), FOH (10 μM), or GGOH (10 μM). Inhibition of FTase and GGTase I was accomplished by treating cells with FTI-277 and GGTI-298 (for concentrations, see figure legends), respectively. After harvesting, whole-cell lysates were made by washing cells twice in ice-cold PBS and then resuspending them in lysis buffer (10 mM Tris-HCl [pH 7.6], 150 mM NaCl, 1% Triton X-100, and a cocktail of protease inhibitors [Boehringer Mannheim]) at 4°C for 6 minutes. Insoluble material was removed by centrifugation at 14 000 rpm for 6 minutes at 4°C. When membrane and cytosolic protein fractions were analyzed, cells were suspended in extraction solution (10% glycerol, 20 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 1 mM MgCl2, 1 mM EDTA [ethylenediaminetetraacetic acid], 1 mM DTT, and a cocktail of protease inhibitors [Boehringer Mannheim]). The lysate was sonicated twice for 5 seconds and centrifuged at 4°C for 30 minutes at 48 000g. The supernatant contained the proteins of the cytosolic fraction. The insoluble material was subsequently resuspended in solubilization buffer (1% NP-40, 20 mM HEPES, 20 mM MgAc, 5 mM NaF, 0.2 mM EDTA, 0.8 mM ethyleneglycotetraacetic acid [EGTA], 1 mM DTT, and a cocktail of protease inhibitors). After 2 rounds of sonification for 10 seconds, incubation for 30 minutes at 4°C, and centrifugation at 4°C for 30 minutes at 48 000g, proteins of the membrane fraction were present in the supernatant.
Protein concentrations were determined by the BCA assay (Pierce, Rockford, IL). Samples containing equal amounts of protein were mixed with 2× Laemmli sample buffer (0.125 M Tris [pH 6.9] with 4% SDS, 20% glycerol, and 10% β-mercaptoethanol) and were boiled for 5 minutes. Proteins were subsequently fractionated in 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) at room temperature and electrically transferred from the gel to a polyvinylidene fluoride (PVDF)-membrane (Bio-Rad). After blocking in 0.1% Tween-20, 5% skim powdered milk, 2% bovine serum albumin (BSA) in 10 mM Tris and 150 mM NaCl, the membranes were incubated with anti-Bcl-2 (DAKO, Glostrup, Denmark), anti-Mcl-1 (Santa Cruz Biotechnology, Santa Cruz, CA), anti-Bcl-XL (Santa Cruz), anti-Bax (Immunotech), anti-Rap1a (Santa Cruz), or anti-HDJ-2 (DnaJ) (Neomarkers; Lab Vision, Fremont, CA). Antibody binding was visualized with enhanced chemiluminescence (Amersham) detection with hyperfilm ECL (Amersham) after incubation with a horseradish peroxidase (HRP)-conjugated secondary antibody (DAKO). Finally, the membranes were extensively washed in PBS and reprobed with anti-α-actin (Sigma) as a control for equal loading of protein. Relative amounts of protein were determined by densitometry and expressed as a percentage of the solvent control.
Results
Inhibition of prenylation by lovastatin, FTI-277, and GGTI-298 in myeloma cell lines
The effect of lovastatin, FTase inhibitor FTI-277, and GGTase I inhibitor GGTI-298 on prenylation in myeloma cell lines was determined by analysis of the migratory behavior during electrophoresis of DnaJ, a protein prenylated exclusively by FTase,31,32 and of Rap1a, a protein prenylated exclusively by GGTase I.33 Inhibition of prenylation of these proteins can be monitored through immunoblotting, because their unprenylated forms display reduced mobility in SDS-PAGE compared with their prenylated versions. All experiments were performed with the myeloma cell lines RPMI 8226, L363, U266, and XG-1. For brevity, only representative results from the cell line RPMI 8226 are shown in Figure 1A. In solvent control-treated myeloma cell lines, DnaJ and Rap1a were in the processed, prenylated forms. Treatment with lovastatin inhibited the processing of DnaJ and of Rap1a in a dose-dependent way, resulting in unprenylated protein forms with reduced electrophoretic mobility (Figure 1A). The effect of lovastatin on prenylation was reversed by the addition of mevalonate (shown for 30 μM lovastatin and 100 μM mevalonate). Treatment with lovastatin (30 μM) in the presence of GGOH (10 μM), which is metabolized to GGPP in the cells,28 restored geranylgeranylation of Rap1a but had no effect on the inhibition of the farnesylation of DnaJ. In contrast, FOH (10 μM), which is metabolized to FPP,28 restored DnaJ farnesylation but not Rap1a geranylgeranylation. Inhibition of FTase by FTI-277 disrupted the processing of DnaJ in a dose-dependent way. Similarly, a dose-dependent mobility shift of Rap1a was observed on exposure to GGTI-298. The specificity of these prenylation inhibitors is illustrated by the lack of inhibition of Rap1a prenylation by FTI-277 and, similarly, the lack of inhibition of DnaJ prenylation by GGTI-298 (Figure 1B).
Effect of lovastatin, FTI-277, and GGTI-298 on farnesylation and geranylgeranylation. (A) RPMI 8226 cells were treated for 2 days with solvent control or lovastatin (30 μM) alone or in the presence of mevalonate (meva; 100 μM), GGOH (10 μM), or FOH (10 μM). For the dose response, RPMI 8226 cells were treated with solvent control or lovastatin (1, 2, 3, 4, 5, 10, 30, 50, or 100 μM). After protein isolation, prenylation status of DnaJ and Rap1a was determined by Western blot analysis. The faster-migrating band represents prenylated protein, and the slower band represents unprenylated protein. Data are representative of at least 3 independent experiments. (B) RPMI 8226 cells were treated for 2 days with solvent control, FTI-277 (2.5, 5, 10, 15, 20, or 30 μM), or GGTI-298 (2.5, 5, 10, 15, 20, or 30 μM). After protein isolation, prenylation status of DnaJ and Rap1a was determined by Western blot analysis. Data are representative of at least 3 independent experiments. (C) RPMI 8226 cells were exposed to solvent control or lovastatin (30 μM) alone or in the presence of mevalonate (100 μM), GGOH (10 μM), or FOH (10 μM). After a 2-day incubation, protein was separated in membrane and cytosolic fractions. RhoA was identified by Western blot analysis. Data are representative of 3 independent experiments.
Effect of lovastatin, FTI-277, and GGTI-298 on farnesylation and geranylgeranylation. (A) RPMI 8226 cells were treated for 2 days with solvent control or lovastatin (30 μM) alone or in the presence of mevalonate (meva; 100 μM), GGOH (10 μM), or FOH (10 μM). For the dose response, RPMI 8226 cells were treated with solvent control or lovastatin (1, 2, 3, 4, 5, 10, 30, 50, or 100 μM). After protein isolation, prenylation status of DnaJ and Rap1a was determined by Western blot analysis. The faster-migrating band represents prenylated protein, and the slower band represents unprenylated protein. Data are representative of at least 3 independent experiments. (B) RPMI 8226 cells were treated for 2 days with solvent control, FTI-277 (2.5, 5, 10, 15, 20, or 30 μM), or GGTI-298 (2.5, 5, 10, 15, 20, or 30 μM). After protein isolation, prenylation status of DnaJ and Rap1a was determined by Western blot analysis. Data are representative of at least 3 independent experiments. (C) RPMI 8226 cells were exposed to solvent control or lovastatin (30 μM) alone or in the presence of mevalonate (100 μM), GGOH (10 μM), or FOH (10 μM). After a 2-day incubation, protein was separated in membrane and cytosolic fractions. RhoA was identified by Western blot analysis. Data are representative of 3 independent experiments.
Prenylation is crucial for the membrane attachment of proteins. This is shown in Figure 1C for RhoA, which is geranylgeranylated by GGTase I. Lovastatin reduced the amount of RhoA present in the membrane protein fraction and increased the amount present in the cytosolic protein fraction when compared with the solvent control. Cotreatment of cells with mevalonate or GGOH, but not FOH, prevented the cytosolic accumulation and restored the membrane localization of RhoA.
Depletion of intracellular pools of geranylgeranylpyrophosphate induces apoptosis in myeloma cell lines
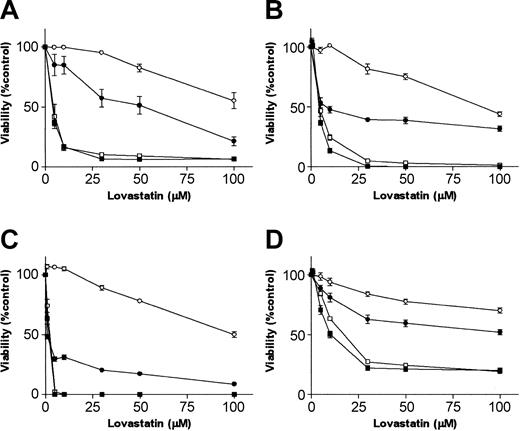
Previously we have shown that lovastatin reduces cell viability in myeloma cell lines and in ex vivo-purified myeloma tumor cells.14 Reduction of cell viability was abrogated by the addition of mevalonate. This suggests that the effect of lovastatin resulted from the inhibition of mevalonate formation and not from nonspecific cell toxicity. Here we examined the effect of the simultaneous exposure of cells to lovastatin and the isoprenoids GGOH or FOH, which are metabolites downstream of mevalonate. A dose-response analysis revealed reduced cell viability by lovastatin beginning at a concentration of 1 μM for XG-1 cells, 2 μM for RPMI 8226 and L363 cells, and 3 μM for U266 cells. Lovastatin sensitivity varied among the myeloma cell lines, with MTT50 (concentration that reduces cell viability 50%) ranging from 1.86 μM (XG-1) to 10.17 μM (U266) at day 4 (Figure 2). GGOH (10 μM), but not FOH (10 μM), prevented lovastatin-induced reduction of myeloma cell viability (Figure 2). This demonstrates that the inhibition of protein geranylgeranylation by lovastatin results in a reduction of cell viability.
Lovastatin reduces cell viability in plasma cell lines, which is restored by GGOH. Plasma cell lines (A) RPMI 8226, (B) L363, (C) XG-1, and (D) U266 were treated for 4 days with solvent control or different concentrations of lovastatin (5, 10, 30, 50, or 100 μM) alone (▪) or in the presence of mevalonate (100 μM; ○), GGOH (10 μM; •), or 10 μM FOH (10 μM; □). The percentage of viable cells, relative to the solvent control-treated cells, was measured by using MTT assay. Experiments were performed 3 times in triplicate. Data are presented as mean ± SEM.
Lovastatin reduces cell viability in plasma cell lines, which is restored by GGOH. Plasma cell lines (A) RPMI 8226, (B) L363, (C) XG-1, and (D) U266 were treated for 4 days with solvent control or different concentrations of lovastatin (5, 10, 30, 50, or 100 μM) alone (▪) or in the presence of mevalonate (100 μM; ○), GGOH (10 μM; •), or 10 μM FOH (10 μM; □). The percentage of viable cells, relative to the solvent control-treated cells, was measured by using MTT assay. Experiments were performed 3 times in triplicate. Data are presented as mean ± SEM.
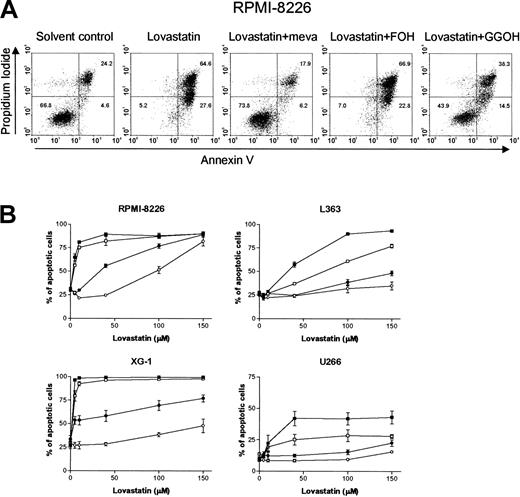
We investigated whether the reduction of cell viability was mediated by the induction of apoptosis by using the annexin V assay. Lovastatin induced apoptosis in a dose-dependent way in the U266, L363, RPMI 8226, and XG-1 cell lines. Mevalonate and GGOH prevented lovastatin-induced apoptosis. FOH was without effect (RPMI 8226 and XG-1) or had only partial protective effects (L363 and U266) (Figure 3A-B). These data were confirmed by the terminal deoxynucleotidyl transferase (TdT)-mediated deoxyuracil triphosphate (dUTP) nick-end labeling (TUNEL) assay (data not shown).
Lovastatin induces apoptosis in plasma cell lines by depletion of intracellular pools of GGPP. (A) RPMI 8226 cells were treated for 4 days with solvent control or lovastatin (30 μM) alone or in combination with mevalonate (meva; 100 μM), GGOH (10 μM), or FOH (10 μM). The percentage of apoptotic cells was examined by using the annexin V assay. The percentages of viable plasma cells (annexin V-/PI-), early apoptotic cells (annexin V+/PI-), and late apoptotic cells (annexin V+/PI+) in each dot plot are indicated in the corresponding quadrants. Results are representative of 3 experiments performed in triplicate. (B) RPMI 8226, L363, XG-1, and U266 cells were treated for 4 days with solvent control or different concentrations of lovastatin (5, 10, 40, 100, or 150 μM) alone (▪) or in combination with mevalonate (100 μM; ○), GGOH (10 μM; •), or FOH (10 μM; □), after which apoptosis was determined using the annexin V assay. Shown is the sum of the percentages of early and late apoptotic cells. Experiments were performed 3 times in triplicate. Data are presented as mean ± SEM.
Lovastatin induces apoptosis in plasma cell lines by depletion of intracellular pools of GGPP. (A) RPMI 8226 cells were treated for 4 days with solvent control or lovastatin (30 μM) alone or in combination with mevalonate (meva; 100 μM), GGOH (10 μM), or FOH (10 μM). The percentage of apoptotic cells was examined by using the annexin V assay. The percentages of viable plasma cells (annexin V-/PI-), early apoptotic cells (annexin V+/PI-), and late apoptotic cells (annexin V+/PI+) in each dot plot are indicated in the corresponding quadrants. Results are representative of 3 experiments performed in triplicate. (B) RPMI 8226, L363, XG-1, and U266 cells were treated for 4 days with solvent control or different concentrations of lovastatin (5, 10, 40, 100, or 150 μM) alone (▪) or in combination with mevalonate (100 μM; ○), GGOH (10 μM; •), or FOH (10 μM; □), after which apoptosis was determined using the annexin V assay. Shown is the sum of the percentages of early and late apoptotic cells. Experiments were performed 3 times in triplicate. Data are presented as mean ± SEM.
Identical results were obtained with other inhibitors of HMG-CoA reductase, including simvastatin and atorvastatin. Cells from the different myeloma cell lines were most sensitive to simvastatin, followed by lovastatin and atorvastatin. However, the hydrophilic HMG-CoA reductase inhibitor pravastatin had no effect (data not shown).
Inhibition of geranylgeranyl transferase I induces apoptosis in myeloma cell lines
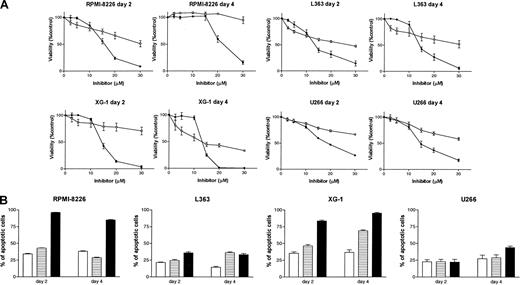
The importance of protein geranylgeranylation versus farnesylation for the regulation of survival of myeloma cells was further evaluated by treating cell lines with different concentrations of FTI-277 or GGTI-298 for 2 or 4 days. Cell viability was examined by MTT assay. GGTI-298 and FTI-277 reduced cell viability in a dose- and time-dependent way in the 4 plasma cell lines analyzed (Figure 4A). However, GGTI-298 had, by far, the most pronounced effect on cell viability when compared with FTI-277. The reduction of cell viability at days 2 and 4 by GGTI-298 (20 μM) in the cell lines varied from 52.9% to 86.2% and 40.4% to 98.7%, whereas the reduction by FTI-277 (20 μM) varied from 22.1% to 43.7% and 0% to 57.0%, respectively. The reduction of cell viability by GGTI-298 was mediated by the induction of apoptosis (Figure 4B). In all cell lines, treatment with GGTI-298 (20 μM) increased the percentage of apoptotic cells when compared with the solvent control. In contrast, FTI-277 (20 μM) induced low to moderate apoptosis in XG-1 and L363 cells and did not induce apoptosis in U266 and RPMI 8226 cells.
Inhibition of GGTase I by GGTI-298 reduces cell viability and induces apoptosis. (A) Plasma cell lines RPMI 8226, L363, XG-1, and U266 were treated for 2 or 4 days with solvent control, different concentrations of the farnesyl transferase inhibitor FTI-277 (2.5, 5, 10, 15, 20, or 30 μM; ○), or different concentrations of the geranylgeranyl transferase I inhibitor GGTI-298 (2.5, 5, 10, 15, 20, or 30 μM; •). The percentage of viable cells, relative to the solvent control-treated cells, was measured by MTT assay. Experiments were performed 3 times in triplicate. Data are presented as mean ± SEM. (B) Plasma cell lines RPMI 8226, L363, XG-1, and U266 were treated for 2 or 4 days with solvent control (□), FTI-277 (20 μM; ▤), or with GGTI-298 (20 μM; ▪). The percentage of apoptotic cells was determined by annexin V assay. Shown is the sum of the percentages of early and late apoptotic cells. Experiments were performed 3 times in triplicate. Data are presented as mean ± SEM.
Inhibition of GGTase I by GGTI-298 reduces cell viability and induces apoptosis. (A) Plasma cell lines RPMI 8226, L363, XG-1, and U266 were treated for 2 or 4 days with solvent control, different concentrations of the farnesyl transferase inhibitor FTI-277 (2.5, 5, 10, 15, 20, or 30 μM; ○), or different concentrations of the geranylgeranyl transferase I inhibitor GGTI-298 (2.5, 5, 10, 15, 20, or 30 μM; •). The percentage of viable cells, relative to the solvent control-treated cells, was measured by MTT assay. Experiments were performed 3 times in triplicate. Data are presented as mean ± SEM. (B) Plasma cell lines RPMI 8226, L363, XG-1, and U266 were treated for 2 or 4 days with solvent control (□), FTI-277 (20 μM; ▤), or with GGTI-298 (20 μM; ▪). The percentage of apoptotic cells was determined by annexin V assay. Shown is the sum of the percentages of early and late apoptotic cells. Experiments were performed 3 times in triplicate. Data are presented as mean ± SEM.
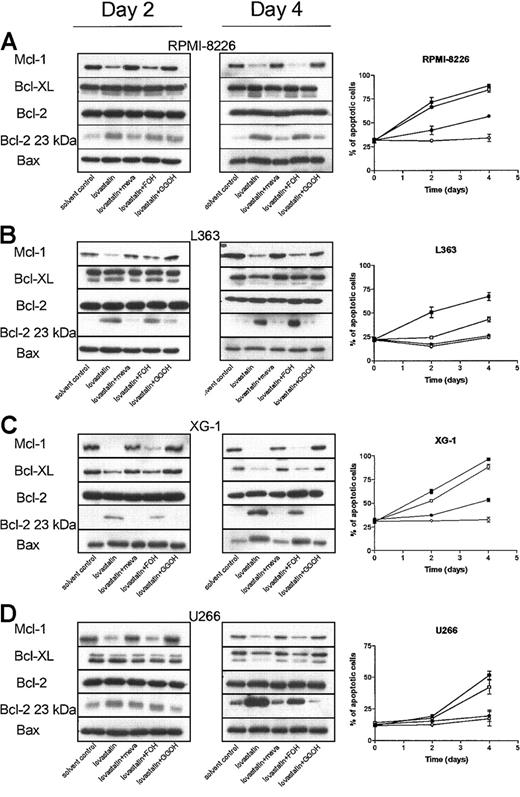
Inhibiting geranylgeranylation reduces Mcl-1 protein levels
Inhibiting geranylgeranylation, either by depleting intracellular pools of GGPP or inhibiting GGTase I, induces apoptosis in myeloma cell lines. To investigate how inhibiting geranylgeranylation results in apoptosis, the expression levels of several Bcl-2 family proteins were determined by Western blot analysis in cells treated with lovastatin in combination with mevalonate, FOH, or GGOH. Lovastatin treatment resulted in a down-regulation of Mcl-1 protein levels at days 2 and 4 in RPMI 8226, L363, U266, and XG-1 cells when compared with solvent control-treated cells (Figure 5). The Mcl-1 down-regulation by lovastatin preceded (U266 cells) or was concomitant (RPMI 8226, L363, and XG-1 cells) with apoptosis induction. Cotreatment of cells with mevalonate or GGOH restored Mcl-1 protein expression. Coincubation of myeloma cell lines with FOH was without effect. Lovastatin treatment also resulted in a reduction of Bcl-XL protein levels in U266, L363, and XG-1 cells and an increase in Bax protein levels in RPMI 8226 and XG-1cells. However, the altered expression levels of Bcl-XL and Bax were relatively late effects, observed only after 4 days of treatment. Bcl-XL was detected as a doublet of 29- to 31-kDa mass, representing 2 conformations of Bcl-XL that migrate differently in SDS-PAGE.23 Lovastatin did not alter the expression levels of Bcl-2. However, there was a time-dependent up-regulation of the proapoptotic 23-kDa Bcl-2 form, which could be visualized after long exposure of the film when compared with Bcl-2. Bcl-XS expression was not detected in the different plasma cell lines. Mevalonate and GGOH prevented the lovastatin-mediated reduction of Bcl-XL and the increase of Bax and the proapoptotic Bcl-2 form. In contrast, FOH was without effect. Similar results were obtained with simvastatin and atorvastatin, whereas pravastatin had no effect (data not shown).
Lovastatin reduces Mcl-1 protein levels by depletion of intracellular pools of GGPP. Plasma cell lines (A) RPMI 8226, (B) L363, (C) XG-1, and (D) U266 were treated for 2 or 4 days with solvent control, or lovastatin (5 μM for XG-1 and 30 μM for RPMI 8226, U266, and L363) alone (▪) or in combination with mevalonate (100 μM; ○), FOH (10 μM; □), or GGOH (10 μM; •). After protein isolation, Mcl-1, Bcl-XL, Bcl-2, and Bax were determined by Western blot analysis. Furthermore, the proapoptotic 23-kDa Bcl-2 form was detected after long exposure of the film. Data are representative of at least 3 independent experiments. The percentage of apoptotic cells was determined using the annexin V assay. Shown is the sum of the percentages of early and late apoptotic cells. Experiments were performed 3 times in triplicate. Data are presented as mean ± SEM.
Lovastatin reduces Mcl-1 protein levels by depletion of intracellular pools of GGPP. Plasma cell lines (A) RPMI 8226, (B) L363, (C) XG-1, and (D) U266 were treated for 2 or 4 days with solvent control, or lovastatin (5 μM for XG-1 and 30 μM for RPMI 8226, U266, and L363) alone (▪) or in combination with mevalonate (100 μM; ○), FOH (10 μM; □), or GGOH (10 μM; •). After protein isolation, Mcl-1, Bcl-XL, Bcl-2, and Bax were determined by Western blot analysis. Furthermore, the proapoptotic 23-kDa Bcl-2 form was detected after long exposure of the film. Data are representative of at least 3 independent experiments. The percentage of apoptotic cells was determined using the annexin V assay. Shown is the sum of the percentages of early and late apoptotic cells. Experiments were performed 3 times in triplicate. Data are presented as mean ± SEM.
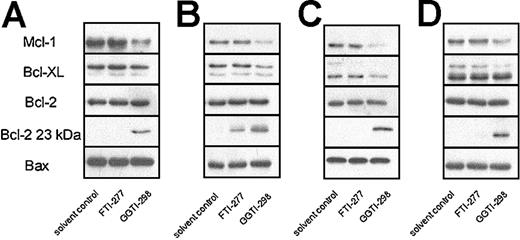
Similar to lovastatin, GGTI-298 also reduced Mcl-1 protein levels after 2 days in all 4 plasma cell lines tested (Figure 6). Furthermore, GGTI-298 treatment resulted in an increase of the proapoptotic 23 kDa Bcl-2 form and reduced Bcl-XL expression levels in L363 and XG-1 cell lines. FTI-277 did not alter Mcl-1, Bcl-XL, Bax, or Bcl-2 protein expression and did not consistently change expression of the proapoptotic 23-kDa Bcl-2 form.
GGTI-298 reduces Mcl-1 protein levels. Plasma cell lines RPMI 8226 (A), L363 (B), XG-1 (C), and U266 (D) were treated for 2 days with solvent control, FTI-277 (20 μM), or GGTI-298 (20 μM). After protein isolation, Mcl-1, Bcl-XL, Bcl-2, and Bax expression levels were determined by Western blot analysis. Furthermore, the proapoptotic 23-kDa Bcl-2 form was detected after long exposure of the film. Data are representative of at least 3 independent experiments.
GGTI-298 reduces Mcl-1 protein levels. Plasma cell lines RPMI 8226 (A), L363 (B), XG-1 (C), and U266 (D) were treated for 2 days with solvent control, FTI-277 (20 μM), or GGTI-298 (20 μM). After protein isolation, Mcl-1, Bcl-XL, Bcl-2, and Bax expression levels were determined by Western blot analysis. Furthermore, the proapoptotic 23-kDa Bcl-2 form was detected after long exposure of the film. Data are representative of at least 3 independent experiments.
Inhibition of geranylgeranylation results in reduced mitochondrial transmembrane potential, APO2.7 staining, release of cytochrome c, and caspase activation
Members of the Bcl-2 family are involved in the regulation of mitochondrial transmembrane potential, the release of cytochrome c, and the activation of caspase-3.17,18 Treating RPMI 8226, L363, U266, and XG-1 cells with lovastatin resulted in loss of the mitochondrial transmembrane potential, as shown in Figure 7A for RPMI 8226 cells. The collapse was time dependent (data not shown) and dose dependent (Figure 7B). APO2.7 reacts with a mitochondrial membrane protein (7A6 antigen) that is exposed in cells undergoing apoptosis. Lovastatin increased the percentage of APO2.7-positive cells in a time- (data not shown) and dose-dependent way (Figure 7A-B). This, together with the loss of the mitochondrial transmembrane potential, suggests that mitochondrial changes were induced by lovastatin.
Lovastatin treatment results in collapse of the mitochondrial transmembrane potential, APO2.7 staining, release of cytochromecinto the cytosol, and caspase-3 activation by depletion of GGPP. (A) RPMI 8226 cells were treated for 4 days with solvent control or lovastatin (30 μM) alone or in combination with mevalonate (meva; 100 μM), FOH (10 μM), or GGOH (10 μM). Collapse of the mitochondrial transmembrane potential was determined by staining the cells with DiOC6[3]. The fraction of cells with low (DiOC6[3]low) and the fraction of cells with intact (DiOC6[3]high) mitochondrial transmembrane potential are indicated in gates M1 and M2, respectively. APO2.7 staining was determined by flow cytometry in digitonin-permeabilized cells. The fraction of cells staining with APO2.7-PE (M2) and the fraction of cells not staining with APO2.7-PE (M1) are indicated. Results are representative of 3 experiments performed in triplicate (B) RPMI 8226 cells were treated for 4 days with solvent control or different concentrations of lovastatin (5, 10, 30, 50, 100, 150 μM) alone (▪) or in combination with mevalonate (100 μM; ○), FOH (10 μM; □), or GGOH (10 μM; •). Collapse of the mitochondrial transmembrane potential was determined by staining the cells with DiOC6[3]. Shown is the percentage of cells with low mitochondrial transmembrane potential (gate M1). APO2.7 staining was determined by flow cytometry in digitonin-permeabilized cells. Shown is the percentage of cells staining with APO2.7-PE (gate M2). Experiments were performed 3 times in triplicate. Data are presented as mean ± SEM. (C) RPMI 8226 cells were exposed to solvent control or lovastatin (30 μM) alone or in combination with mevalonate (meva; 100 μM), FOH (10 μM), or GGOH (10 μM) for 2 days, at which time the cells were harvested and cytochrome c in the cytosolic fraction was determined by ELISA. Experiments were performed 3 times in duplicate. Data are presented as mean ± SEM. (D) RPMI 8226 cells were exposed to solvent control or lovastatin (30 μM) alone or in combination with mevalonate (meva; 100 μM), FOH (10 μM), or GGOH (10 μM) for 2 days, at which time the cells were harvested and caspase-3 activation was assessed as described in “Materials and methods.” Experiments were performed 3 times in duplicate. Data are presented as mean ± SEM.
Lovastatin treatment results in collapse of the mitochondrial transmembrane potential, APO2.7 staining, release of cytochromecinto the cytosol, and caspase-3 activation by depletion of GGPP. (A) RPMI 8226 cells were treated for 4 days with solvent control or lovastatin (30 μM) alone or in combination with mevalonate (meva; 100 μM), FOH (10 μM), or GGOH (10 μM). Collapse of the mitochondrial transmembrane potential was determined by staining the cells with DiOC6[3]. The fraction of cells with low (DiOC6[3]low) and the fraction of cells with intact (DiOC6[3]high) mitochondrial transmembrane potential are indicated in gates M1 and M2, respectively. APO2.7 staining was determined by flow cytometry in digitonin-permeabilized cells. The fraction of cells staining with APO2.7-PE (M2) and the fraction of cells not staining with APO2.7-PE (M1) are indicated. Results are representative of 3 experiments performed in triplicate (B) RPMI 8226 cells were treated for 4 days with solvent control or different concentrations of lovastatin (5, 10, 30, 50, 100, 150 μM) alone (▪) or in combination with mevalonate (100 μM; ○), FOH (10 μM; □), or GGOH (10 μM; •). Collapse of the mitochondrial transmembrane potential was determined by staining the cells with DiOC6[3]. Shown is the percentage of cells with low mitochondrial transmembrane potential (gate M1). APO2.7 staining was determined by flow cytometry in digitonin-permeabilized cells. Shown is the percentage of cells staining with APO2.7-PE (gate M2). Experiments were performed 3 times in triplicate. Data are presented as mean ± SEM. (C) RPMI 8226 cells were exposed to solvent control or lovastatin (30 μM) alone or in combination with mevalonate (meva; 100 μM), FOH (10 μM), or GGOH (10 μM) for 2 days, at which time the cells were harvested and cytochrome c in the cytosolic fraction was determined by ELISA. Experiments were performed 3 times in duplicate. Data are presented as mean ± SEM. (D) RPMI 8226 cells were exposed to solvent control or lovastatin (30 μM) alone or in combination with mevalonate (meva; 100 μM), FOH (10 μM), or GGOH (10 μM) for 2 days, at which time the cells were harvested and caspase-3 activation was assessed as described in “Materials and methods.” Experiments were performed 3 times in duplicate. Data are presented as mean ± SEM.
Collapse of the mitochondrial transmembrane potential results in the release of several proapoptogenic factors such as cytochrome c from the mitochondria into the cytosol.17,18 Treating myeloma cell lines with lovastatin resulted in an increase of cytosolic cytochrome c, as shown for RPMI 8226 cells in Figure 7C.
Cytosolic cytochrome c promotes the activation of caspases through the cytochrome c/Apaf-1/caspase-9 pathway.17,18 To determine whether lovastatin-induced cytochrome c release resulted in the activation of caspase-3, cell lysates were analyzed for caspase-3 activity. Treatment of plasma cell lines with lovastatin activated caspase-3, as shown for RPMI 8226 cells in Figure 7D. Bcl-2 is a known substrate of caspase-3.34,35 Exposure of cell lines to lovastatin stimulates caspase-3 activity, which, in turn, results in the generation of the Bcl-2 cleavage product that promotes apoptosis. The time-dependent increase of the proapoptotic 23-kDa Bcl-2 form was demonstrated by Western blot analysis (Figure 5).
Importantly, incubating plasma cell lines with lovastatin in the presence of mevalonate or GGOH markedly prevented the collapse of the mitochondrial transmembrane potential, APO2.7 staining, the release of cytochrome c from mitochondria, and the activation of caspase-3 (as shown for myeloma cell line RPMI 8226 in Figure 7A-D). In contrast, the cotreatment of cells with FOH had no effect when compared with cells treated with lovastatin alone.
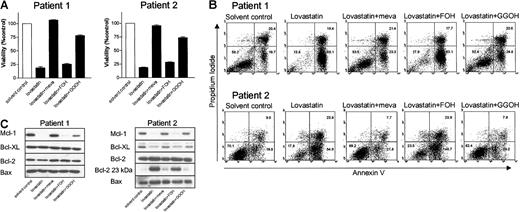
Inhibiting geranylgeranylation induces apoptosis in ex vivo purified tumor cells from myeloma patients
Studies were then conducted to determine the effect of lovastatin in purified tumor cells from patients with multiple myeloma (n = 5). Except for patient 2, who had 96% myeloma cells in her bone marrow, tumor cells were purified from mononuclear cells using MACS based on the expression of CD138. The percentage of tumor cells was greater than 95%. Treatment of ex vivo-purified myeloma cells with 30 μM lovastatin resulted in reduced myeloma cell viability in all patients (Table 1). Moreover, as in cell lines, treating ex vivo-purified myeloma tumor cells with lovastatin reduced Mcl-1 protein levels (Table 1). Similar to the effects in plasma cell lines, adding mevalonate or GGOH resulted in the complete inhibition of lovastatin-induced reduction of cell viability and lovastatin-induced apoptosis. However, the coincubation of cells with FOH had no effect. Cotreatment of tumor cells with mevalonate or GGOH restored Mcl-1 protein expression, whereas FOH was without effect. Figure 8 shows representative examples from 2 myeloma patients. In the tumor cells of patient 2, lovastatin treatment additionally reduced Bcl-XL protein expression (more than 50% reduction compared with the solvent control) and resulted in an increase of the proapoptotic Bcl-2 cleavage product.
Treatment of ex vivo-purified myeloma cells with lovastatin results in reduction of cell viability and induction of apoptosis by reducing Mcl-1 protein levels. Plasma cells from myeloma patient 1 were purified from bone marrow mononuclear cells by MACS based on CD138 expression. Plasma cell percentage was 97% after purification. Patient 2 had 96% myeloma cells in her bone marrow mononuclear cells; therefore, purification was not necessary. Tumor cells derived from patients 1 and 2 were treated for 4 days with solvent control or lovastatin (30 μM) alone or in the presence of mevalonate (meva; 100 μM), FOH (10 μM), or GGOH (10 μM). (A) The percentage of viable cells, relative to the solvent control-treated cells, was measured by using MTT assay. Experiments were performed once in triplicate. Data are presented as mean ± SEM. (B) The percentage of apoptotic cells was determined by the annexin V assay. The percentages of viable myeloma cells (annexin V-/PI-), early apoptotic cells (annexin V+/PI-), and late apoptotic cells (annexin V+/PI+) in each dot plot are indicated in the corresponding quadrants. (C) After protein isolation, Mcl-1, Bcl-XL, Bcl-2, and Bax expression levels were determined by Western blot analysis. Furthermore, the proapoptotic 23-kDa Bcl-2 form was detected after long exposure of the film.
Treatment of ex vivo-purified myeloma cells with lovastatin results in reduction of cell viability and induction of apoptosis by reducing Mcl-1 protein levels. Plasma cells from myeloma patient 1 were purified from bone marrow mononuclear cells by MACS based on CD138 expression. Plasma cell percentage was 97% after purification. Patient 2 had 96% myeloma cells in her bone marrow mononuclear cells; therefore, purification was not necessary. Tumor cells derived from patients 1 and 2 were treated for 4 days with solvent control or lovastatin (30 μM) alone or in the presence of mevalonate (meva; 100 μM), FOH (10 μM), or GGOH (10 μM). (A) The percentage of viable cells, relative to the solvent control-treated cells, was measured by using MTT assay. Experiments were performed once in triplicate. Data are presented as mean ± SEM. (B) The percentage of apoptotic cells was determined by the annexin V assay. The percentages of viable myeloma cells (annexin V-/PI-), early apoptotic cells (annexin V+/PI-), and late apoptotic cells (annexin V+/PI+) in each dot plot are indicated in the corresponding quadrants. (C) After protein isolation, Mcl-1, Bcl-XL, Bcl-2, and Bax expression levels were determined by Western blot analysis. Furthermore, the proapoptotic 23-kDa Bcl-2 form was detected after long exposure of the film.
Discussion
In this study we demonstrated that inhibiting protein geranylgeranylation induced apoptosis in myeloma plasma cells. Inhibition of geranylgeranylation was established in 2 independent ways—the depletion of intracellular pools of GGPP by lovastatin and the specific inhibition of GGTase I activity. Incubating myeloma cells with lovastatin effectively depleted pools of FPP and GGPP, resulting in a dose-dependent inhibition of protein farnesylation and geranylgeranylation. This was demonstrated by analysis of the migratory behavior of DnaJ and Rap1a during electrophoresis. Adding FOH, which is converted to FPP in cells,28 to lovastatin-treated myeloma cells restored farnesylation of DnaJ, and adding GGOH, which is converted to GGPP,28 restored geranylgeranylation of Rap1a. In addition, geranylgeranylation and farnesylation were specifically blocked dose dependently by GGTase I and FTase inhibitors GGTI-298 and FTI-277, respectively. In functional assays, adding GGOH, but not FOH, rescued myeloma cells from lovastatin-induced apoptosis. However, GGOH was less effective than mevalonate in the rescue of myeloma cell survival. This suggests that the depletion of other metabolites downstream of mevalonate may also contribute partly to the effects of lovastatin. The GGTase I inhibitor GGTI-298 also induced apoptosis in myeloma cells in a time- and dose-dependent way. Inhibition of geranylgeranylation by lovastatin and GGTI-298 preceded the reduction of cell viability. Rescuing farnesylation by adding FOH to lovastatin-treated cells was without effect. Furthermore, although the FTase inhibitor FTI-277 completely blocked farnesylation, it was less effective in inducing apoptosis than GGTI-298. These results point to an important role for geranylgeranylated proteins in regulating apoptosis in myeloma plasma cells.
Inducing apoptosis by inhibiting geranylgeranylation with lovastatin or GGTI-298 was preceded by or was concomitant with a consistent reduction of Mcl-1 protein expression in all myeloma cell lines and patient samples. Down-regulation of Mcl-1 protein expression at day 2 was accompanied by disruption of the mitochondrial transmembrane potential, increased APO2.7 staining, cytochrome c release, and activation of caspase-3, suggesting that apoptosis was carried out through the intrinsic cell-death pathway. In some cell lines and patient samples, a reduction of Bcl-XL expression levels or an induction of Bax or the proapoptotic 23-kDa Bcl-2 form was found. However, this was not a consistent finding or was only observed 4 days after the inhibition of geranylgeranylation. The observed temporal sequence in the regulation of expression of Bcl-2 family proteins suggests for the first time that down-regulation of Mcl-1 is the key event for inducing apoptosis through the inhibition of geranylgeranylation.
In multiple myeloma, Mcl-1 is one of the key regulators of apoptosis.24-26 Specific depletion of Mcl-1 by antisense oligodeoxynucleotides resulted in rapid cell death, whereas overexpression of Mcl-1 in myeloma cells delayed the activation of caspases in a manner consistent with the hypothesis that a threshold level of Mcl-1 is required for myeloma cell survival.24-26 In addition, Mcl-1 promotes cell viability under a variety of apoptosis-inducing conditions, including exposure to cytotoxic agents and withdrawal of required growth factors.36 Similarly, high levels of Mcl-1 have been correlated with failure to achieve complete remission to chemotherapy in chronic lymphocytic leukemia.37 It is unclear which pathway or pathways are involved in the down-regulation of Mcl-1 protein expression in myeloma cells after lovastatin treatment or GGTase I inhibition. Studies using various cell types imply that multiple pathways, including phosphatidylinositol 3 kinase (PI-3K), Janus kinase/signal transducer and activator of transcription 3 (JAK/STAT3), and MAPK/ERK kinase/extracellular signalrelated kinase (MEK/ERK), are involved in the regulation of Mcl-1 transcription.38 However, recent studies performed by Zhang et al39 suggest that Mcl-1 regulation in myeloma is more complex and may be independent of the signaling pathways mentioned.
Statins have been shown to induce apoptosis in other tumor cell lines and in ex vivo-purified tumor cells.14,40-42 As in myeloma, the effect of lovastatin could be attributed to inhibition of geranylgeranylation in acute myeloid leukemia (AML)40 and lung adenocarcinoma41 and in pulmonary smooth muscle cells.42 Identical results as presented here for myeloma were obtained with lymphoma cell lines and purified tumor cells from patients with B-cell non-Hodgkin lymphoma (N.v.d.D., manuscript in preparation). In addition to inducing apoptosis, recent studies have shown that statins also enhance new bone formation by stimulating osteoblast differentiation and activity43 and that they inhibit bone resorption by inhibiting osteoclast formation and function.44 This action may be beneficial in myeloma patients with bone disease. Furthermore, in various cell types, HMG-CoA reductase inhibitors inhibit the expression of cytokines, including the important myeloma growth factor IL-6.45 This indicates that in addition to inducing apoptosis by Mcl-1 down-regulation, these pleiotropic properties of statins may have important clinical implications in the treatment of multiple myeloma.
It is unclear which geranylgeranylated target protein(s) are involved in regulating myeloma tumor cell apoptosis. Possible geranylgeranylated target proteins include RhoA, CDC42, and Rac1. These small GTP-binding proteins are involved in important cellular functions, such as organization of the cytoskeleton.46 In addition, they are also involved in regulating apoptosis and proliferation6,47-49 by activating various signaling pathways including PI-3K,50,51 several serine/threonine kinases,52 NF-κB,53,54 and SAPK/JNK.55,56 Furthermore, Rac1, RhoA, and CDC42 have been demonstrated to have transforming and oncogenic potential in cell lines.57-60 Whether one of the GTP-binding proteins mentioned is involved in the regulation of myeloma cell survival or apoptosis is under investigation.
In summary, we demonstrated that inhibition of geranylgeranylation rather than inhibition of farnesylation reduces cell viability of myeloma plasma cells by inducing apoptosis. Apoptosis triggered by the inhibition of geranylgeranylation was associated with reduced Mcl-1 protein expression, which, in turn, resulted in collapse of the mitochondrial transmembrane potential, cytochrome c release from mitochondria into the cytosol, and stimulation of caspase-3 activity. These results show that geranylgeranylation of proteins is a key event in the regulation of myeloma tumor cell survival. Furthermore, our results suggest that pharmacologic agents such as lovastatin and GGTase inhibitors may be useful in the treatment of multiple myeloma.
Prepublished online asBlood First Edition Paper, July 10, 2003; DOI 10.1182/blood-2003-03-0970.
Supported by a grant from the Dutch Cancer Society (K.W.F.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


![Figure 7. Lovastatin treatment results in collapse of the mitochondrial transmembrane potential, APO2.7 staining, release of cytochrome c into the cytosol, and caspase-3 activation by depletion of GGPP. (A) RPMI 8226 cells were treated for 4 days with solvent control or lovastatin (30 μM) alone or in combination with mevalonate (meva; 100 μM), FOH (10 μM), or GGOH (10 μM). Collapse of the mitochondrial transmembrane potential was determined by staining the cells with DiOC6[3]. The fraction of cells with low (DiOC6[3]low) and the fraction of cells with intact (DiOC6[3]high) mitochondrial transmembrane potential are indicated in gates M1 and M2, respectively. APO2.7 staining was determined by flow cytometry in digitonin-permeabilized cells. The fraction of cells staining with APO2.7-PE (M2) and the fraction of cells not staining with APO2.7-PE (M1) are indicated. Results are representative of 3 experiments performed in triplicate (B) RPMI 8226 cells were treated for 4 days with solvent control or different concentrations of lovastatin (5, 10, 30, 50, 100, 150 μM) alone (▪) or in combination with mevalonate (100 μM; ○), FOH (10 μM; □), or GGOH (10 μM; •). Collapse of the mitochondrial transmembrane potential was determined by staining the cells with DiOC6[3]. Shown is the percentage of cells with low mitochondrial transmembrane potential (gate M1). APO2.7 staining was determined by flow cytometry in digitonin-permeabilized cells. Shown is the percentage of cells staining with APO2.7-PE (gate M2). Experiments were performed 3 times in triplicate. Data are presented as mean ± SEM. (C) RPMI 8226 cells were exposed to solvent control or lovastatin (30 μM) alone or in combination with mevalonate (meva; 100 μM), FOH (10 μM), or GGOH (10 μM) for 2 days, at which time the cells were harvested and cytochrome c in the cytosolic fraction was determined by ELISA. Experiments were performed 3 times in duplicate. Data are presented as mean ± SEM. (D) RPMI 8226 cells were exposed to solvent control or lovastatin (30 μM) alone or in combination with mevalonate (meva; 100 μM), FOH (10 μM), or GGOH (10 μM) for 2 days, at which time the cells were harvested and caspase-3 activation was assessed as described in “Materials and methods.” Experiments were performed 3 times in duplicate. Data are presented as mean ± SEM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/9/10.1182_blood-2003-03-0970/6/m_h82135137007.jpeg?Expires=1765885109&Signature=wh5JhecKh8QVHCZV9uHwyJx2ME~xQxJxOiz9WLxDqIDzq1ZR5Bcx6pGwzMJr12ziUSo0aiLIZbVOYSIGimffJbc~HatDlY6acds2QCiw0G3aM0kLrBZZxgplaV1kC7qWqwD~jBm34yG4N9GSuV7AJTeMMqP1l6hPgoFna19fE3neuZr0hbazI5FOJHkffDD~lUgPjVLS3IRADperWQDO3vdRLqdN2m-BBZ6ff6XAVKSwe1Lw83dUX3VIX96K0M1MwZ8o4Xl1alWHDQfKn4zsYdxiiqU2XFFga05fmFW-~AWWhT1bT5eHd8FrlD0Hbxya2rvdq04uLBgoWZS3~b8XGA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)