Abstract
Vascular endothelial cells are able to sense changes in the forces acting on them and respond, for instance, by modifying expression of a range of genes. However, there is little information on how such responses are integrated to modify homeostatic functions. We hypothesized that different shear stresses experienced in different regions of the circulation might influence endothelial sensitivity to inflammatory stimuli. We cultured human endothelial cells in tubes and exposed them for varying periods to shear stresses ranging from those typically found in postcapillary venules to those in arteries. When tumor necrosis factor-α was included in the flow cultures, we found startling differential effects of shear stress on the ability of endothelial cells to induce adhesion and migration of flowing neutrophils. Compared with static cultures, endothelial cells cultured at low shear stress (0.3 Pa) captured similar numbers of neutrophils but failed to induce their transendothelial migration. After exposure of endothelial cells to high shear stress (1.0 or 2.0 Pa), capture of neutrophils was largely ablated. The modification in response was detectable after 4 hours of exposure to flow but was much greater after 24 hours. From analysis of gene expression, loss of capture or migration was attributable to reduction in tumor necrosis factor–induced expression of selectins or CXC-chemokines, respectively. Thus, conditioning of endothelial cells by different flow environments may underlie variations in susceptibility to inflammation between different tissues or parts of the vascular tree.
Introduction
The vascular endothelium forms an interface between the blood and underlying tissue, regulating transport of soluble substances and immune cells between these compartments. The endothelial cells lining vessels are subjected continually to hemodynamic forces, including shear stress exerted by flowing blood, which influence the transport processes. The magnitude of the shear stress varies greatly according to the location in the vascular system, typically being more than 1 Pa in arteries, approximately 0.1 to 0.5 Pa in postcapillary venules, and even lower in sinusoidal organs such as the liver or spleen.1 It has become increasingly recognized that endothelial cells can sense changes in shear stress and modify their cytoskeletal structure and expression of a wide variety of genes accordingly.2,3 However, the influence of long-term differences in shear stress between sites or of shifts in shear stress on integrated, homeostatic functions such as inflammation remains unclear. It is known that expression of various adhesion molecules and of the chemokine monocyte chemotactic protein-1 (MCP-1) can be modified by exposure to shear stress in otherwise resting endothelial cells.4-7 Nevertheless, it is not known how continuous exposure of endothelial cells to different stresses affects their ability to respond to inflammatory cytokines and then support each stage of leukocyte adhesion and migration.
Modulation of inflammatory responses by the hemodynamic environment is potentially important. Local variations in flow patterns and shear stresses in the arterial tree have been associated with development of atheromatous plaques that contain immune cells recruited from the blood.8,9 Moreover, in a broader sense, the inflammatory response to cytokines may vary between organs and vascular beds because the endothelial cells are conditioned differently by the local level of shear stress. For instance, it is well known that recruitment of leucocytes in inflamed tissue is concentrated in postcapillary venules where shear stress is low.10,11 This localization is only partly explained by the fact that increasing shear stress makes the binding of leukocytes to endothelial receptors less efficient and may also reflect a difference in the responsiveness of endothelial cells between vessels.12 We, therefore, set out to examine how the integrated physiologic response of leukocyte recruitment was influenced by exposure of endothelial cells for different periods to different levels of shear stress, covering the range typically experienced in vivo. Our approach was rather different from most previous work, which has concentrated on the response of endothelial cells to fluid stress per se. Modulation of endothelial state was tested using a well-characterized model of human umbilical vein endothelial cells treated with tumor necrosis factor-α (TNF-α), which support capture of flowing neutrophils through expression of selectin adhesion molecules.13-15 The neutrophils then become activated by surface-presented CXC-chemokines, which induce their firm integrin-mediated attachment and onward migration through the endothelial monolayer.
Materials and methods
Culture of endothelial cells under static or flow conditions
Human umbilical vein endothelial cells (HUVECs) were isolated as previously described and maintained in medium 199 (M199; Invitrogen, Paisley, United Kingdom) containing 20% fetal calf serum, 28 μg/mL gentamycin, 2.5 μg/mL amphotericin B, and 1 ng/mL epidermal growth factor (all from Sigma Chemical, Poole, United Kingdom) until confluent.16 Primary cultures were dissociated with trypsin/EDTA (ethylenediaminetetraacetic acid) (Sigma) and passaged into rectangular glass capillaries (microslides; internal width 3 mm, depth 0.3 mm) which had been coated with collagen/gelatin as described.17 Seeding was at a density that yielded confluent monolayers within 24 hours. Each experiment used first passage HUVECs from a different donor.
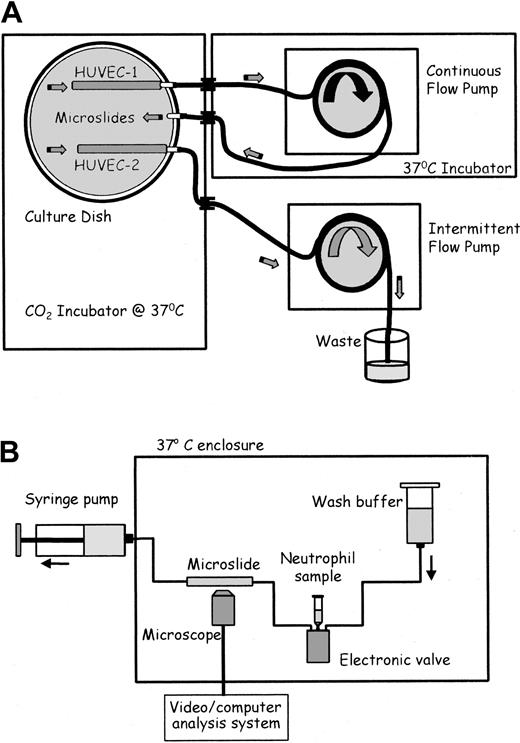
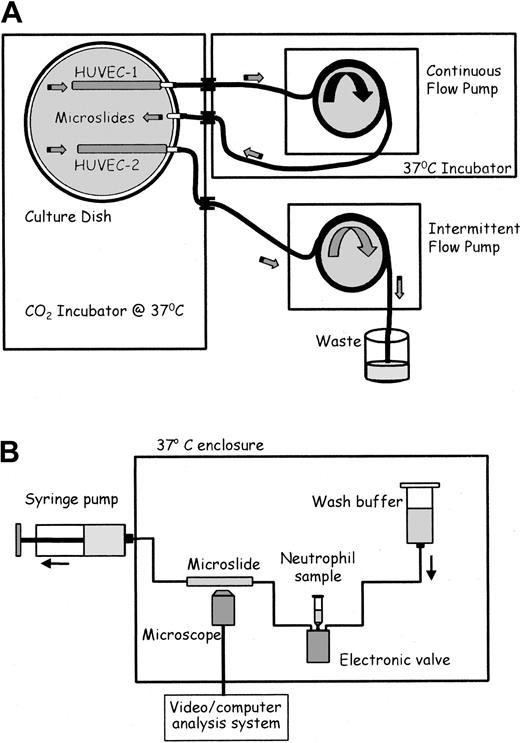
After seeding with HUVECs for 1 hour, microslides were placed into specially constructed glass dishes and attached to glass tubing that had been fused into the wall (adapted from Cooke et al16 ; Figure 1A). Silicon tubing (Tygon R1000; Fisher, Loughborough, United Kingdom) was connected to each external arm. The dish contained culture medium and was placed in a humidified CO2 incubator (Nuaire DH; Triple Red, Thame, Oxfordshire, United Kingdom). The tubing was passed through a port in the incubator wall. The tubing from 2 adjacent arms (one attached to a microslide and one empty; Figure 1A) was connected and placed into a multichannel, 8-roller pump (model 502S; Watson Marlow, Falmouth, United Kingdom) forming a continuous flow loop. The bore of the pump tubing was chosen to give the desired flow rate and hence wall shear stress (0.3, 1.0, or 2.0 Pa) in the microslide for each experiment, for a single pump speed. The pump and external tubing were enclosed in a Perspex box, thermostatically regulated at 37°C. The tubing from a separate microslide in each dish was connected to a separate pump. This pumped a small amount of medium through the microslide once an hour, to enable prolonged growth under our standard, static conditions.16,17 Three separate dishes could be cultured in parallel at any time.
Schematic diagrams of flow culture system and flow-based adhesion assay. (A) Flow culture system. Medium in the culture dish was constantly perfused at a chosen shear stress through the microslide containing HUVEC-1 and perfused for 30 seconds each hour at low shear stress through the microslide containing HUVEC-2. The 2 microslides received identical medium. (B) Flow-based adhesion assay. Either neutrophil suspension or wash buffer was perfused at a wall shear stress of 0.1 Pa through the microslide. HUVECs and neutrophils adhering to it were continually observed and recorded by phase-contrast videomicroscopy.
Schematic diagrams of flow culture system and flow-based adhesion assay. (A) Flow culture system. Medium in the culture dish was constantly perfused at a chosen shear stress through the microslide containing HUVEC-1 and perfused for 30 seconds each hour at low shear stress through the microslide containing HUVEC-2. The 2 microslides received identical medium. (B) Flow-based adhesion assay. Either neutrophil suspension or wash buffer was perfused at a wall shear stress of 0.1 Pa through the microslide. HUVECs and neutrophils adhering to it were continually observed and recorded by phase-contrast videomicroscopy.
Three protocols were used: (1) HUVECs were exposed to shear stress of 0.3 Pa for 24 hours immediately after seeding, and then 2, 5, or 100 U/mL human recombinant TNF-α (expressed in Escherichia coli; Sigma) was added to each dish and culture continued for a further 4 hours under flow; (2) HUVECs were cultured under static conditions for 24 hours and then exposed to shear stress of 0.3 Pa. TNF (100 U/mL) was added either simultaneously to the imposition of flow, after 4 hours or after 24 hours, and culture continued for a further 4 hours (total culture under flow = 4, 8, or 28 hours); (3) HUVECs were cultured under static conditions for 24 hours and then exposed to shear stress of 0.3, 1.0, or 2.0 Pa for 28 hours, with 100 U/mL TNF (Sigma) added for the last 4 hours. The control, static microslides attached to the third arms of each dish were exposed to identical recirculated medium for identical periods.
Isolation of neutrophils
Blood was collected from healthy volunteers into K2EDTA, (Sarstedt, Leicester, United Kingdom) and used within 2 hours of venipuncture. Neutrophils were isolated by centrifuging the whole blood at 800g for 30 minutes over a 2-step density gradient consisting of equal quantities of Histopaques 1119 and 1077 (Sigma) as described.17 The neutrophil layer was aspirated from the density interface and washed twice in phosphate-buffered saline (PBS) containing 1 mM Ca2+, 0.5 mM Mg2+, 0.15% culture-tested bovine serum albumin (Sigma), and 5 mM glucose (PBS/BSA). Neutrophils were counted using a Coulter counter (Coulter Electronics, Harpenden, Essex) and suspended at 106/mL in PBS/BSA.
Adhesion and migration of flowing neutrophils
Adhesion assays were performed as previously described (Figure 1B).13,14,17 Microslides containing confluent HUVECs were glued to a glass microscope slide, mounted on a microscope stage, and viewed by phase-contrast videomicroscopy. One end of the microslide was attached via silicon rubber tubing to a Harvard syringe pump (Harvard Apparatus, South Natic, MA), allowing the control of the flow rate through the microslide. The other end of the microslide was attached by silicon rubber tubing to an electronic valve (Lee Products, Gerards Cross, United Kingdom), permitting smooth switching between neutrophil suspension and cell-free buffer. Following insertion of the microslide into the flow system, HUVECs were washed with PBS/BSA to remove residual TNF-α. A 4-minute bolus of neutrophils was perfused through the microslide, followed by washout with PBS/BSA, all at a flow rate equivalent to a wall shear stress of 0.1 Pa. This wall shear stress is adequate to ensure that binding to HUVECs requires selectin expression and cannot occur directly through integrin-mediated adhesion.13,17 Videomicroscopic recordings were made throughout of neutrophils adherent to the HUVECs along the centerline of the microslide and were analyzed off-line using a computerized image analysis system (ImagePro; DataCell, Finchampstead, United Kingdom). Adherent neutrophils were counted after completion of the bolus and corrected per millimeter squared per 106 cells perfused. After 5 minutes of washout, adherent cells were classified as (1) rolling slowly over the surface (velocity about 5-10 μm/s), (2) activated on the surface (phase bright, and stationary or migrating slowly), (3) transmigrated (phase dark and migrating at about 10-15 μm/min under the HUVECs).14 Adherent cells were easily distinguished from nonadherent cells, which were visible only as faint streaks.
Evaluation of gene expression by reverse transcriptase polymerase chain reaction (RT-PCR)
RNA was extracted from HUVECs within microslides using Trizol extraction (Invitrogen). Reverse transcription of single stranded cDNA and PCR were conducted as described.18 Primers and PCR reaction conditions for β-actin and interleukin-8 (IL-8) were those previously published.19 Primers and PCR conditions for growth-related oncogene-α (GRO-α) were from BD Clontech United Kingdom (Basingstoke, United Kingdom). Primers and PCR conditions for intercellular adhesion molecule-1 (ICAM-1) were from R&D Systems Europe (Abingdon, United Kingdom). Primers for E-selectin and P-selectin were designed in house and synthesized by Alta Bioscience (Birmingham, United Kingdom); E-selectin forward, 5′-TTC GCC TGT CCT GAA GGA TG-3′; E-selectin reverse, 5′-TCA GTT GAA GGC CGT CCT TG-3′; P-selectin forward, 5′-AGC CTG GAT TGT TCT GAC AC-3′; P-selectin reverse 5′-TCT GCA TGC TGG AGT TAC TG-3′. Reaction conditions were 1 × (95°C, 5 minutes), 1 × (55°C, 5 minutes), 35 × (72°C, 1 minute; 94°C, 30 seconds; 55°C, 30 seconds), 1 × (72°C, 5 minutes). Amplified products were analyzed on 2% agarose gel containing ethidium bromide, and band density was measured using a scanning densitometer.
Immunofluorescence and flow cytometry
HUVECs in microslides were fixed with 0.5% formaldehyde at 4°C for 2.5 minutes and washed with PBS/BSA. Mouse monoclonal antibody (mAb) against E-selectin (clone 1.2B6), against ICAM-1 (clone 6.5B5), or mouse immunoglobulin G (IgG) control (all from DakoCytomation, Ely, United Kingdom) was diluted to 0.3 μg/mL in PBS containing 2% normal goat, injected into microslides and incubated for 1 hour at room temperature. Monolayers were washed with PBS/BSA and incubated for 1 hour with fluorescein isothiocyanate (FITC)–conjugated goat antimouse IgG (DakoCytomation). The monolayers were treated with trypsin/EDTA at room temperature for 4 minutes, and the cells were flushed from the microslide and washed with PBS/BSA. The ratio of median fluorescence intensities for E-selectin or ICAM-1 versus IgG control was measured using a FACScan flow cytometer (Becton Dickinson, Oxford, United Kingdom).
Assay of IL-8 in perfusates and Western blotting of proteins extracted from HUVECs
In one series of experiments, static and flow cultures were maintained in separate dishes. Medium that had been passed to “waste” through static cultures at intervals, and medium that had been recirculated in the flow cultures were collected, both after 24 hours of initial culture, and after an additional 4 hours of culture with 100 U/mL TNF. The concentration of IL-8 in the perfusate was measured using a Duoset kit sandwich enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (R&D Systems Europe). Concentrations were converted to IL-8 released in nanogram per microslide per hour to take into account the different volumes of perfusate and times over which IL-8 was collected.
In similar experiments, HUVECs were extracted from the microslides either after the initial 24 hours of culture or after the additional 4 hours of culture with TNF, using radioimmunoprecipitation assay (RIPA) buffer containing 1% NP40, 0.1% sodium dodecyl sulfate (SDS), 0.5% sodium deoxycholate, 150 mM NaC1, 50 mM Tris (tris(hydroxymethyl)aminomethane) pH 8.0, 1 mM EDTA, 50 mM NaF, 1 mM NaVO4,10 μg/mL leupeptin, 10 μg/mL aprotinin, 200 μg/mL benzamidine, 8 μg/mL calpain inhibitor I, 8 μg/mL calpain inhibitor II, 200 μg/mL phenylmethylsulfonyl fluoride, and 2 μg/mL pepstatin A. Protein (30 μg per lane, pooled from duplicate microslides) was separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes. Membranes were blocked in 20% low-fat milk (Marvel; Premier Brands UK, Spalding, UK) in Tris Tween-20 buffer, pH 7.5, and probed with mAb against TNF-receptor 1 or TNF-receptor 2 (0.2 μg/mL; sc7895 or sc1072; Santa Cruz Biotechnology, Santa Cruz, CA), or against P-selectin (0.5 μg/mL; rabbit polyclonal antibody; gift from Dr Michael Berndt, Melbourne, Australia) or IL-8 (1 μg/mL; mAb 208; R&D Systems Europe) or against β-actin (1 μg/mL; Sigma) as a loading control. Blots were developed with an appropriate horseradish peroxidase (HRP)–conjugated secondary antibody (DakoCytomation) and visualized using an enhanced chemiluminescence method (Pierce, Rockford, IL).
Results
Neutrophil behavior on HUVECs treated with different concentrations of TNF in static or low shear cultures
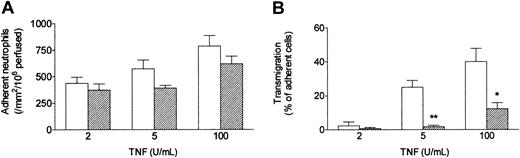
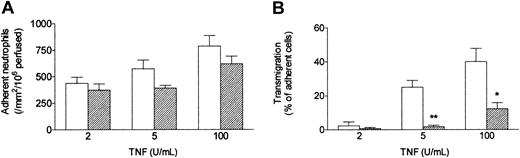
When a bolus of neutrophils was perfused over HUVECs that had been cultured with TNF under conventional static conditions, they adhered efficiently. As previously described,13 increasing the dose of TNF had a modest effect on the level of adhesion (Figure 2A) but markedly altered the behavior of the adherent cells (Figure 2B). At 2 U/mL only about 5% of the adherent neutrophils migrated through the endothelial monolayer within 5 minutes of completion of the bolus, whereas at 100 U/mL about 40% transmigrated. When HUVECs were exposed to a wall shear stress of 0.3 Pa (approximating that found in postcapillary venules) for 24 hours immediately after seeding, and TNF was added for a further 4 hours at this stress, neutrophil behavior was markedly altered. Although the absolute numbers of adherent neutrophils were only slightly (but significantly) lower than those detected using static cultures, the proportion transmigrating through shear-conditioned HUVECs was reduced by about 75% (Figure 2B). It was also notable that approximately 90% of neutrophils attached to shear-conditioned HUVECs were rolling rather than stationary (data not shown).
Effect of exposing HUVECs to flow on their response to different concentrations of TNF. Response was assessed by (A) number of adherent neutrophils, and (B) percentage of adherent neutrophils transmigrating through the monolayer. HUVECs were cultured static (□) or exposed to a shear stress of 0.3 Pa (▨) for 28 hours, with TNF added for the last 4 hours, followed by flow-based adhesion assay. Data are mean ± SEM from 3 or 4 experiments at each concentration of TNF. Analysis of variance (ANOVA) showed significant effect of culture conditions on adhesion (P < .05) and transmigration (P < .01). *P < .05, **P < .01 compared with results for static cultures by paired t test.
Effect of exposing HUVECs to flow on their response to different concentrations of TNF. Response was assessed by (A) number of adherent neutrophils, and (B) percentage of adherent neutrophils transmigrating through the monolayer. HUVECs were cultured static (□) or exposed to a shear stress of 0.3 Pa (▨) for 28 hours, with TNF added for the last 4 hours, followed by flow-based adhesion assay. Data are mean ± SEM from 3 or 4 experiments at each concentration of TNF. Analysis of variance (ANOVA) showed significant effect of culture conditions on adhesion (P < .05) and transmigration (P < .01). *P < .05, **P < .01 compared with results for static cultures by paired t test.
Time course of response of HUVECs to conditioning at low shear stress
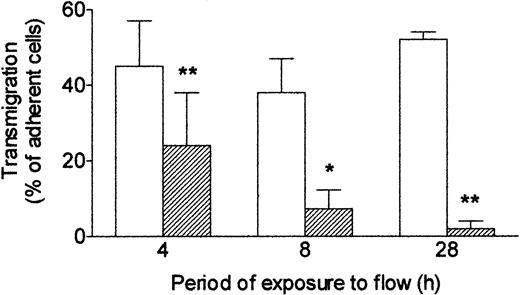
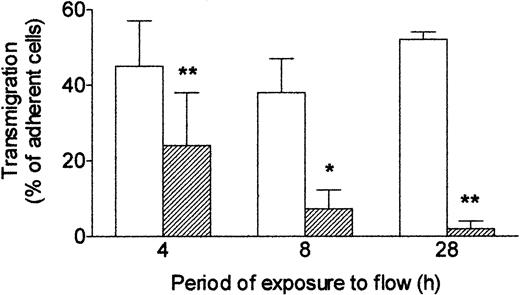
Next, we investigated how long it was necessary to expose endothelial cells to shear stress to modify their response to TNF. HUVECs were first cultured for 24 hours under static conditions to form a confluent monolayer, and then flow (wall shear stress = 0.3 Pa) was imposed. If flow was started and TNF added at the same time, then after 4 hours, a significant impairment of transmigration was noted compared with cells treated with TNF under static conditions throughout (Figure 3). If a 4-hour flow conditioning was used before addition of TNF for a further 4 hours, then migration was further reduced. A 24-hour period of flow conditioning was sufficient to reduce transmigration induced by TNF by more than 90% with this protocol (Figure 3). Thus, increasing the period of exposure to shear stress before exposure to TNF steadily reduced endothelial responsiveness, assessed by ability to induce neutrophil transmigration.
Effect of exposing HUVECs to flow for varying times on their response to TNF. Response was assessed by the percentage of adherent neutrophils transmigrating through the monolayer. HUVECs were cultured static (□) or exposed to shear stress of 0.3 Pa (▨) for 4, 8, or 28 hours. TNF (100 U/mL) was added for the last 4 hours, followed by flow-based adhesion assay. Data are mean ± SEM from 3 or 4 experiments of each duration. ANOVA showed significant effect of culture conditions on transmigration (P < .01). *P < .05, **P < .01 compared with results for static cultures by paired t test.
Effect of exposing HUVECs to flow for varying times on their response to TNF. Response was assessed by the percentage of adherent neutrophils transmigrating through the monolayer. HUVECs were cultured static (□) or exposed to shear stress of 0.3 Pa (▨) for 4, 8, or 28 hours. TNF (100 U/mL) was added for the last 4 hours, followed by flow-based adhesion assay. Data are mean ± SEM from 3 or 4 experiments of each duration. ANOVA showed significant effect of culture conditions on transmigration (P < .01). *P < .05, **P < .01 compared with results for static cultures by paired t test.
Neutrophil adhesion to HUVECs treated with TNF in cultures exposed to high shear stress
Because conditioning at low shear stress effectively abolished migration but had a modest effect on capture of flowing neutrophils, we extended studies to include exposure to higher shear stresses of 1.0 or 2.0 Pa (approximating values found in arteries). HUVECs required 24 hours of static culture to establish monolayers that could withstand such high shear stress, because some cells detached if flow was imposed immediately after seeding. When HUVECs were then conditioned at wall shear stress of 1.0 or 2.0 Pa for 24 hours before addition of 100 U/mL TNF for 4 hours, there was dramatic reduction in neutrophil capture compared with static cultures or those maintained at 0.3 Pa (Figure 4). Stable adhesion was absent after culture at the highest stress. We did observe some neutrophils making short-lived attachments to the endothelial cells (typical duration < 1 second), but these attachments did not survive the washout period. It may be noted also that the levels of adhesion to the control, static cultures tended to decrease the higher the shear stress to which their paired flow culture was exposed (Figure 4). However, this trend was not statistically significant.
Effect of exposing HUVECs to flow at different shear stresses on their response to TNF. Response was assessed by the number of adherent neutrophils. HUVECs were cultured static (□) or exposed to flow (▨) at a shear stress of 0.3, 1.0, or 2.0 Pa for 28 hours with 100 U/mL TNF added for the last 4 hours, followed by flow-based adhesion assay. Data are mean ± SEM from 3 to 5 experiments at each stress. ANOVA showed significant effect of the level of stress on adhesion (P < .01). *P < .02 compared with results for static cultures by paired t test.
Effect of exposing HUVECs to flow at different shear stresses on their response to TNF. Response was assessed by the number of adherent neutrophils. HUVECs were cultured static (□) or exposed to flow (▨) at a shear stress of 0.3, 1.0, or 2.0 Pa for 28 hours with 100 U/mL TNF added for the last 4 hours, followed by flow-based adhesion assay. Data are mean ± SEM from 3 to 5 experiments at each stress. ANOVA showed significant effect of the level of stress on adhesion (P < .01). *P < .02 compared with results for static cultures by paired t test.
Effects of shear stress on expression of chemokines by HUVECs
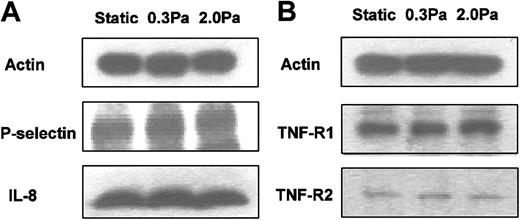
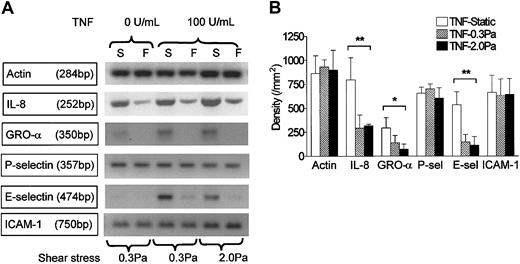
RT-PCR was carried out using mRNA from endothelial cells cultured in the microslides for 26 hours under static conditions, or at 0.3 or 2.0 Pa, with or without 100 U/mL TNF added for the last 2 hours. We found mRNA for the chemokines IL-8 and GRO-α in HUVECs tended to be up-regulated by treatment with TNF but markedly reduced by flow culture (Figure 5). We could not detect mRNA for ENA-78 in HUVECs, although positive control mRNA from a bile duct epithelial cell line was strongly positive (not shown). We have not been able to demonstrate chemokines directly on the surface of HUVECs by ELISA or immunofluorescence labeling. Instead, we assayed release of IL-8 by HUVECs. Although the rate of IL-8 release during 24 hours of culture without TNF (0.05 ng/microslide per hour) was greatly increased during 4 hours of culture with 100 U/mL TNF (1.7 ng/microslide per hour), culture at 0.3 or 2.0 Pa had little effect on secretion induced by TNF (1.5 ng/microslide per hour or 1.6 ng/microslide per hour, respectively; means from 2 experiments in each case, in which each sample was tested in triplicate). Because this result was somewhat unexpected, we also used Western blotting to assess total cellular IL-8 in protein extracted from microslides treated with TNF. Again, there was little difference between IL-8 protein levels in lysates from TNF-treated HUVECs that had been cultured static, or at 0.3 or 2.0 Pa (Figure 6a).
Effect of exposing HUVECs to flow on their expression of genes for chemokines and adhesion receptors in response to TNF. (A) Stained gels showing DNA amplified by RT-PCR from mRNA extracted from HUVECs that were cultured for 26 hours under static conditions (S) or exposed to shear stress of 0.3 Pa or 2 Pa (F). TNF (100 U/mL) was added for the last 2 hours. Actin is shown as a loading control unmodified by treatment. Gels are from individual experiments, which were carried out on 3 occasions. (B) Densitometry of DNA bands obtained by RT-PCR for HUVECs treated with 100 U/mL TNF and cultured under static conditions or exposed to shear stress of 0.3 Pa or 2.0 Pa. Data are mean ± SEM from 3 experiments under each condition. ANOVA showed significant effect of culture conditions on expression of IL-8, GRO-α, and E-selectin (*P < .05, **P < .01).
Effect of exposing HUVECs to flow on their expression of genes for chemokines and adhesion receptors in response to TNF. (A) Stained gels showing DNA amplified by RT-PCR from mRNA extracted from HUVECs that were cultured for 26 hours under static conditions (S) or exposed to shear stress of 0.3 Pa or 2 Pa (F). TNF (100 U/mL) was added for the last 2 hours. Actin is shown as a loading control unmodified by treatment. Gels are from individual experiments, which were carried out on 3 occasions. (B) Densitometry of DNA bands obtained by RT-PCR for HUVECs treated with 100 U/mL TNF and cultured under static conditions or exposed to shear stress of 0.3 Pa or 2.0 Pa. Data are mean ± SEM from 3 experiments under each condition. ANOVA showed significant effect of culture conditions on expression of IL-8, GRO-α, and E-selectin (*P < .05, **P < .01).
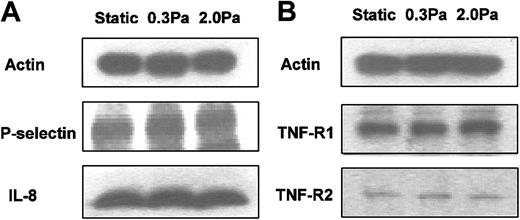
Western blots from HUVECs cultured static or at 0.3 or 2.0 Pa. (A) IL-8 and P-selectin for HUVECs that had been cultured for 24 hours static or under flow, and then a further 4 hours with 100 U/mL TNF. (B) TNF receptors 1 and 2 (p55TNFR and p75TNFR) for HUVECs that had been cultured for 24 hours static or under flow. Actin is shown as a loading control in each case. Positions of bands for P-selectin and IL-8 were determined by comparisons to purified proteins run in separate lanes and to molecular weight standards. Bands for TNF receptors were consistent with known molecular weights. Western blots are from individual experiments, which were repeated on 2 to 4 occasions with similar results.
Western blots from HUVECs cultured static or at 0.3 or 2.0 Pa. (A) IL-8 and P-selectin for HUVECs that had been cultured for 24 hours static or under flow, and then a further 4 hours with 100 U/mL TNF. (B) TNF receptors 1 and 2 (p55TNFR and p75TNFR) for HUVECs that had been cultured for 24 hours static or under flow. Actin is shown as a loading control in each case. Positions of bands for P-selectin and IL-8 were determined by comparisons to purified proteins run in separate lanes and to molecular weight standards. Bands for TNF receptors were consistent with known molecular weights. Western blots are from individual experiments, which were repeated on 2 to 4 occasions with similar results.
Effects of shear stress on expression of adhesion molecules and TNF receptors by HUVECs
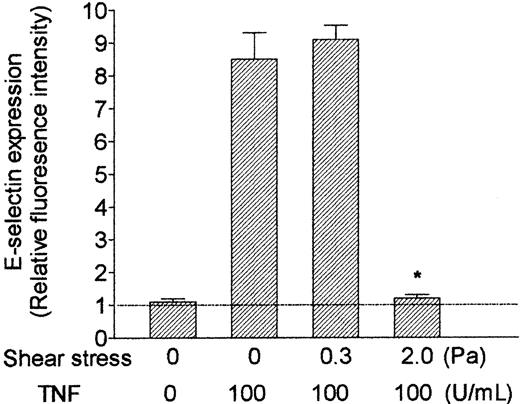
RT-PCR also showed that mRNA level for E-selectin was up-regulated by TNF and markedly reduced by shear stress (Figure 5). E-selectin protein was clearly demonstrable on the surface of TNF-treated HUVECs using immunofluorescence labeling and flow cytometry. Although the surface level was unaltered by culture at 0.3 Pa, it was reduced to baseline by culture at 2.0 Pa (Figure 7). In contrast, mRNA for P-selectin was not up-regulated by TNF or modified by flow culture (Figure 5). Neither ELISA nor immunofluorescence labeling reproducibly quantified P-selectin on the surface of endothelial cells grown under the conditions used here, although we have detected low levels in conventional cultures treated with TNF.13 However, we could detect bands when extracts from HUVECs were subject to Western blotting using a polyclonal antibody against P-selectin. The density of bands did not vary consistently when we compared TNF-treated HUVECs cultured under static conditions, at 0.3 Pa or at 2.0 Pa (Figure 6A). Examining expression of ICAM-1, we found that mRNA levels were not influenced by culture under flow in the presence or absence of TNF (Figure 5). Although surface expression of ICAM-1 was easily detectable by flow cytometry, we found no consistent differences between the levels expressed in TNF-treated cells cultured static, at 0.3 Pa, or at 2.0 Pa (ie, intensity of fluorescence labeling relative to nonspecific control 11.8 ± 2.5, 12.8 ± 2.3, 12.9 ± 3.8, respectively; mean ± SEM from 3 experiments).
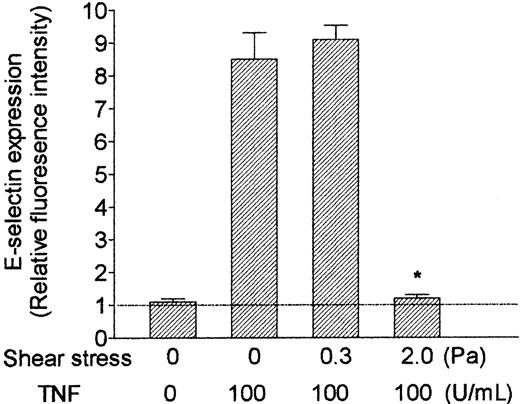
Effect of exposing HUVECs to flow on their up-regulation of surface expression of E-selectin in response to treatment with TNF. HUVECs were cultured under static conditions or exposed to shear stress of 0.3 Pa or 2.0 Pa for 28 hours, with 100 U/mL TNF added for the last 4 hours. Data are mean ± SEM from 3 experiments using immunofluorescence labeling and flow cytometry. Values are expressed relative to intensity of fluorescence for HUVECs labeled with nonspecific control antibody. *P < .05 compared with HUVECs treated with TNF under static conditions. Horizontal line represents relative fluorescence intensity (set at 1).
Effect of exposing HUVECs to flow on their up-regulation of surface expression of E-selectin in response to treatment with TNF. HUVECs were cultured under static conditions or exposed to shear stress of 0.3 Pa or 2.0 Pa for 28 hours, with 100 U/mL TNF added for the last 4 hours. Data are mean ± SEM from 3 experiments using immunofluorescence labeling and flow cytometry. Values are expressed relative to intensity of fluorescence for HUVECs labeled with nonspecific control antibody. *P < .05 compared with HUVECs treated with TNF under static conditions. Horizontal line represents relative fluorescence intensity (set at 1).
Finally, we investigated effects of 24-hour exposure to flow on expression of TNF receptors 1 and 2 (p55TNFR and p75TNFR, respectively), as this could affect subsequent response to added TNF. Fluorometric analysis of total binding of FITC-conjugated TNF to the surface of HUVECs was not sufficiently sensitive to demonstrate differences between specifically and nonspecifically labeled cells. Nevertheless, Western blotting showed distinct bands for the 2 receptors at the expected molecular weights. However, these did not vary consistently when static cultures were compared with those exposed to 0.3 or 2.0 Pa for 24 hours (Figure 6B).
Discussion
The tumor necrosis factor family of ligands and receptors are key regulators of inflammation.20 Endothelial cells treated with tumor necrosis factor-α, in particular, are able to induce each stage in the capture and transendothelial migration of flowing leukocytes in vitro and in vivo.13,21-23 Here, we have shown for the first time that the ability of endothelial cells to respond to TNF by inducing adhesion and migration of neutrophils is highly dependent on the physical environment to which the endothelial cells have been exposed. Exposure of cultures to increasing levels of shear stress was associated with progressive suppression of their response. In consequence, for fluid shear stresses spanning those typically found in the vascular tree, the higher the shear stress during culture, the less effective were the endothelial cells in supporting subsequent neutrophil recruitment (always tested at a wall shear stress of 0.1 Pa). An interesting differential response was observed, in that exposure to low (“venular”) shear stress inhibited ability to support neutrophil transmigration but had relatively little effect on numbers captured from flow. Exposure to high (“arterial”) shear stress modified the endothelial response to TNF to the extent that neutrophil capture was barely detectable.
Many studies have shown that endothelial cells are sensitive to the forces exerted on them in the circulation.2,3,24 Endothelial cells can respond rapidly to changes in shear forces by releasing vasoactive substances such as nitric oxide, or more slowly through modification of gene expression. Recent studies have used microarray techniques to catalog the extensive changes in gene expression that can be induced when endothelial cultures are exposed to different levels of shear stress for varying periods.25-27 The goals of the present study were different from most previous work, in that we did not concentrate on the effects of flow per se. Instead, we aimed to evaluate how variation in the shear environment would affect the ability of endothelial cells to support an integrated response such as leukocyte recruitment, following an inflammatory challenge. Endothelial cells are continually exposed to shear stress in vivo, and we hypothesized that they might adapt to local variations in stress by changing their phenotype. Indeed, we were able to show that endothelial cells were “conditioned” by the level of shear stress, so that their responses to TNF were modulated. This response was not a transient phenomenon. Although changes in response were detectable after 4 hours of exposure to shear stress, they were progressively more marked, the longer the exposure.
The adhesion model used in the present study has been carefully characterized.13-15 Capture of flowing neutrophils is supported by E-selectin and P-selectin at a high TNF level but dependent mainly on E-selectin at a lower concentration. There is no evident role for adhesion mediated by L-selectin. Immobilization and migration following capture require activation of β2-integrins and stimulation of cellular motility, arising from ligation of neutrophil CXC-chemokine receptor-2. The β2-integrins themselves interact with endothelial immunoglobulin superfamily member ICAM-1 and, possibly, other unidentified receptors during transmigration.28,29 To explain the changes in neutrophil behavior noted here, we examined endothelial expression of adhesion receptors and CXC-chemokines. The inability of HUVECs exposed to high shear stress to capture flowing neutrophils could be attributed to inhibition of TNF-induced up-regulation of E-selectin. This was demonstrable at the level of mRNA. Moreover, surface presentation of E-selectin, which was plentiful after TNF treatment of static cultures, was absent after treatment of cultures at high shear stress. Flow culture induced no change in mRNA for P-selectin, but neither was this gene up-regulated by TNF. Western blotting indicated that exposure to shear stress had little effect on total cellular content of P-selectin for HUVECs treated with TNF. P-selectin is stored in preformed granules, and it is possible that its translocation to the endothelial surface was impaired in flow-conditioned cells. However, P-selectin was barely detectable by ELISA or by immunofluorescence and flow cytometry, and no reduction could be quantified. Neutrophil capture was reduced to occasional short-lived attachments for endothelial cells cultured at high shear stress and treated with TNF. This suggests that a low residual density of P-selectin did exist.30
Impairment of neutrophil transmigration on HUVECs exposed to low shear stress, when capture remained quite efficient, may have arisen from changes in production of chemokines. The CXC-chemokines IL-8, ENA-78, and GRO-α have all been reported as expressed by HUVECs under some stimuli.31,32 In TNF-treated HUVECs, we detected mRNA for IL-8 and GRO-α but not for ENA-78. Levels of expression were markedly reduced for HUVECs conditioned by either low or high shear flow. However, we found that exposure to shear stress hardly affected the secretion of IL-8 or total cellular content of IL-8, for HUVECs treated with TNF. This is puzzling. Others showed that up-regulation of IL-8 mRNA in HUVECs treated with TNF was not associated with an increase in cell content of IL-8, the protein being rapidly secreted.33 Thus, although shear regulation of IL-8 mRNA would not necessarily influence cell content, it would be expected to reduce secretion. Functionally, it is the surface-bound IL-8 that is important, but we were not able to quantify this fraction. In any case, it is likely that GRO-α may have assisted IL-8 to induce migration on static HUVECs, because previously we could not inhibit transmigration using an IL-8–neutralizing antibody alone.13 Another possibility that we examined was that lack of ligand for β2-integrins impaired the transmigration phase. However, exposure of HUVECs to low or high shear stress did not reduce mRNA or surface levels of ICAM-1, so that failure to migrate is unlikely to have resulted from lack of a counter receptor for β2-integrins.
Others have shown that the levels of expression of various endothelial adhesion molecules are modified by culture under different conditions of flow in the absence of chemical agonists.4-6,34 Expression of ICAM-1, for instance, was up-regulated by shear stress, but in a biphasic manner, with a return to basal level by 24 hours.5 mRNA for the chemokine MCP-1 was up-regulated soon after initial exposure to shear stress but then down-regulated later.7 However, these studies did not quantify expression when shear stress and cytokine stimulation were combined.
There remains the question of the mechanism(s) by which the endothelial response to TNF was modified. There are 2 endothelial receptors for TNF-α, p55TNFR (which is present both on the surface and in an intracellular pool) and p75TNFR (which is largely surface presented) (reviewed in Ledgerwood et al35 and MacEwan36 ). Both are believed to contribute to the full proinflammatory response induced by TNF. We examined total protein levels of the 2 receptors by Western blotting and found that they were not markedly altered by exposure to shear stress for 24 hours. Although the impaired response to TNF does not seem to have arisen from lack of receptors, the functional significance of the separate surface and intracellular receptor pools and the balance between them remains uncertain.35 Thus, one should be conservative in drawing conclusions from total protein levels alone. Changes in TNF-driven signaling pathways or downstream transcription factors might, however, explain changes in responses. TNF-induced activation of mitogen-activated protein kinase that acts upstream of gene transcription factors was reduced by exposure of endothelial cells to shear stress for a few minutes.37 In addition, expression of TNF-receptor–associated factor-3 (TRAF-3) was up-regulated during 12 to 18 hours of exposure to shear stress of approximately 0.5 to 4.5 Pa.38 TRAF-3 was able to inhibit responses to ligation of endothelial CD40 (a member of the TNF-receptor family), including up-regulation of mRNA for MCP-1. Further studies will be necessary to define the mechanisms underlying the endothelial conditioning seen here.
It is also interesting to consider whether changes in soluble factors released by endothelial cells could have had feedback effects on responses. In our circulatory culture model, a small endothelial surface area (1.5 cm2) was perfused with a relatively large fluid volume (about 50 mL), and so such effects should be minimized. Endothelial cells in control microslides were exposed to the same recirculating medium, so that the differences noted between “static” and “flow” cultures must have been due to the flow itself. There was a slight downward trend in adhesion levels for endothelial cells in control microslides in dishes used for increasing shear stress regimes. However, there was no downward trend in the percentage of transmigration, and levels were similar to those observed in independent static microslides cultured in separate dishes (data not shown). Thus, any substances released (eg, at high shear) had quite small effects.
The results presented here have several implications. They make it clear that endothelial cells cultured under different physical conditions can show different responses during functional studies carried out in vitro. This result is consistent with the concept that the artificial state of endothelial cells in static culture returns toward the in vivo phenotype under flow.39 Moreover, they suggest that sensitivity of different vessels to inflammatory stimuli in vivo may depend on the local shear stress. Inflammation in vivo may involve a range of cytokines and activating agents, and combinations of cytokines can yield different endothelial responses compared with TNF alone.40,41 Thus, care should be taken in generalizing the present results obtained in studies of TNF alone. Nevertheless, our results may help explain the well-known observation that in the arterial tree, local regions of disturbed flow and low shear stress (eg, around bends and bifurcations) are associated with development of atheromatous plaques that contain inflammatory cells.8,9 Endothelial cells in regions with low levels of shear stress are likely to be more responsive to inflammatory mediators than cells in areas of high shear. It is also believed that local spatial gradients and temporal variations in shear stress may predispose to development of atheroma, for instance affecting endothelial proliferation and adhesion molecule expression directly.6,42 However, effects of such flow patterns on cytokine responses are not known.
Shear stress also varies between the arterial and venous sides of the microcirculation, and, in inflammation, leukocyte adhesion is concentrated in postcapillary venules in which shear stress is lowest. Low shear will assist adhesion directly, but endothelial cells in these venules should be in their most responsive state. In fact, comparison of levels of leukocyte adhesion and local shear stresses measured in arterioles and venules have suggested that endothelial cells in arterioles are inherently less able to support leukocyte attachment.12 It is noteworthy that in a murine model of ischemia and reperfusion, arterioles were observed to support increased leukocyte adhesion after a period of reduced flow.43 Finally, certain organs, such as the lungs and liver, have unusually low fluid shear stresses overall, which might influence regional responses to inflammatory stimuli. Thus, local hemodynamics, along with other factors, such as signals from underlying stromal cells,44 may constitute a complex system whereby sensitivity to inflammation is made appropriate to local requirements or becomes disturbed in vascular pathology.
Prepublished online as Blood First Edition Paper, June 26, 2003; DOI 10.1182/blood-2003-01-0080.
Supported by a Programme Grant from the British Heart Foundation (No. RG/2000011), a British Heart Foundation Non-Clinical Lectureship Award (BS/97001) (G.E.R.), and a Project Grant from the British Heart Foundation (No. PG/2001019).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.