Abstract
The growth of Lewis lung carcinoma (LLC) was sustained in plasminogen-deficient mice when transplanted into the dorsal skin but was dramatically suppressed in another anatomic location, the footpad. This unanticipated negative effect of plasminogen deficiency on footpad tumor growth was entirely relieved by superimposing a deficit in fibrinogen. This finding was not simply an unusual feature of LLC tumors—T241 fibrosarcoma growth in the footpad was also restricted by plasminogen deficiency in a fibrinogen-dependent manner. The probable mechanistic basis for suppression of tumor growth was revealed through transmission electron microscopy studies of tumor tissues. Occlusive microvascular thrombi were commonplace within footpad tumors from plasminogen-deficient mice, whereas no such lesions were observed within either dorsal skin tumors from plasminogen-deficient mice or footpad tumors from mice that also lacked fibrinogen. The data infer that tumor growth in the footpad of plasminogen-deficient mice is compromised as a function of the formation and persistence of vaso-occlusive thrombi that limit tumor blood supply. These studies indicate that plasminogen and fibrinogen can serve as critical determinants of tumor growth, but their relative importance is dependent on the tumor microenvironment. Furthermore, these studies suggest that one target of plasmin(ogen) relevant to tumor progression in vivo is intravascular fibrin.
Introduction
The conversion of plasminogen to the active serine protease, plasmin, is a frequently recognized feature of many physiologic and pathologic processes involving extracellular matrix dissolution and remodeling. In addition to serving a vital role in the clearance of intravascular fibrin-rich thrombi, plasmin-mediated extracellular proteolysis appears to be important in a diverse spectrum of biologic processes, including wound healing, mammary gland involution, liver repair/regeneration, neuronal degeneration in the central nervous system, peripheral nerve regeneration, vessel wall disease, the inflammatory response, and tumor progression.1-9 Consistent with the prevailing notion that productive matrix degradation and remodeling require the intricate regulation of extracellular proteolysis, plasmin formation is carefully controlled by the expression, localization, and regulation of tissue-type plasminogen activator (tPA), urokinase-type plasminogen activator (uPA), uPA receptor (uPAR), plasminogen activator inhibitor-1 (PAI-1), α2-plasmin inhibitor, and other cell-associated and secreted factors.
Studies of mice with selected deficits in plasminogen activation system components and coagulation factors have underscored that one fundamental role of plasminogen/plasmin in vivo is fibrinolysis. In this regard, many of the severe pathologic manifestations of plasminogen deficiency, including widespread ischemic organ damage, wasting, impaired wound healing, diminished peripheral nerve regeneration, and early mortality, were effectively remedied if fibrinogen deficiency was genetically superimposed.6,8 However, detailed studies of mutant mouse lines have also unambiguously shown that the plasminogen activation system has critical physiologic and pathologic roles in vivo that are independent of fibrinogen/fibrinolysis. One context in which a fibrin-independent pathologic role of the plasminogen activation system has been well documented is in the central nervous system. Here, plasmin contributes to excitotoxin-induced neurodegeneration within the hippocampus through a mechanism that is clearly independent of fibrinogen/fibrinolysis and appears to be coupled to local laminin degradation.3,10 The most dramatic illustration of a physiologic role of the plasminogen activation system apart of fibrinolysis was documented in the context of liver repair after hepatotoxin injury. In this experimental setting, the efficient clearance of necrotic debris and cellular reorganization of damaged liver tissue was shown to be dependent on uPA and plasminogen, but this dependence could not be fully corrected by the elimination of fibrin(ogen).2,11 Unfortunately, the identity of any biologically relevant nonfibrin target of plasmin important in hepatic repair remains unknown. Given the ability of plasmin to directly degrade several common extracellular matrix components (eg, fibronectin, laminin), activate protease zymogens (eg, matrix metalloproteases), and activate latent growth factors, it is conceivable that multiple nonfibrin targets of plasmin are biologically relevant in vivo, particularly in complex processes such as tumor growth and metastasis.
A specific link between the plasminogen activation system and cancer has been established through extensive studies of human and animal tumor biology (for reviews, see Andreasen et al,1 Duffy et al,12 Johnsen et al,13 and Dano et al14 ). The expression of plasminogen activator, plasminogen activator receptor, and plasminogen activator inhibitor by stromal or cancer cells has been frequently documented.15,16 It has been inferred from these findings that plasmin-mediated proteolysis contributes to the local extracellular matrix degradation associated with tumor stroma formation, angiogenesis, and tumor dissemination. Consistent with this view, expression of plasminogen activator, uPAR, and PAI-1 within tumor tissues has been correlated with poor prognoses for a variety of human cancers.12,14,17,18 A causal link between the plasminogen activation system and tumor progression has been implied from multiple reports indicating that inhibitors or antagonists of plasminogen activator or uPAR diminish the metastatic potential of transplantable tumor lines.19-30 A particularly provocative link between plasminogen and tumor angiogenesis was made in studies showing that angiostatin, a derivative of plasminogen, effectively inhibited tumor angiogenesis and growth.31-33 Finally, studies of mice with selected deficits in plasminogen activation system components have supported the hypothesis that these factors contribute significantly to tumor biology. However, the findings with genetically modified mice have sometimes been unexpected or dependent on the tumor model. Notably, several studies suggest that either PAI-1 deficiency or PAI-1 overexpression reduces tumor angiogenesis and tumor growth,34-38 whereas other compelling studies conclude that neither PAI-1 deficiency nor PAI-1 overexpression alters tumor growth.39 The influence of other plasminogen activation system components on tumor growth has been similarly inconsistent. No difference in tumor growth was observed in comparative studies of control and plasminogen-deficient mice using an oncogene-driven mouse mammary tumor model,40 but the loss of plasminogen activator or of plasminogen was shown to greatly attenuate T241 fibrosarcoma tumor growth.36,41 The importance of plasminogen as a determinant of metastatic potential also appears to vary with the experimental setting and with the route or site of metastatic lesion formation.40,42 Taken together, persuasive data support a role for the plasminogen activation system in tumor pathophysiology, at least within specific contexts, but the precise mechanisms by which this system supports (or hinders) tumor growth and dissemination remain to be defined.
Based on the wealth of data implying that plasmin(ogen) is likely to be important in tumor biology, the early observation that Lewis lung carcinoma (LLC) primary tumor growth in the dorsal subcutis was not strongly influenced by the loss of plasminogen was an enigma.42 This finding prompted us to transplant LLC cells into an alternative location, the mouse footpad. These studies revealed the unexpected finding that primary LLC tumor growth in plasminogen-deficient mice is highly dependent on anatomic location. We report here that, unlike LLC tumors grown in the dorsal subcutis, the loss of plasminogen dramatically diminished tumor growth in the footpad relative to control animals. This restriction in tumor growth in plasminogen-deficient mice was not associated with any apparent difference in vascular density. Rather, electron microscopy revealed a significant loss of tumor vascular patency. Furthermore, superimposing a genetic deficit in fibrinogen relieved the occlusion of tumor vasculature and the restriction of tumor growth in plasminogen-deficient mice. These studies suggest that though plasminogen and fibrinogen can serve as critical determinants of tumor growth, their importance is dependent on tumor location and that one target of plasmin(ogen) relevant to tumor progression in vivo appears to be fibrin.
Materials and methods
Transgenic mice
The generation of gene-targeted mice with single and combined deficits in plasminogen and fibrinogen (Aα chain) has been previously described.8,43,44 All mice enrolled in these studies were inbred into a C57BL/6J background (Jackson Laboratories, Bar Harbor, ME) and were previously shown to be histocompatible with transplanted LLC cells.42,45 Mice were genotyped by multiplex polymerase chain reaction analysis of DNA obtained from ear biopsies as previously described.8 All experimental mice were 7 to 14 weeks of age at the beginning of experiments. Age- and sex-matched cohorts of mice of the following 4 genotypes were enrolled: Plg+/–/FibAα+/–, Plg–/–/FibAα+/–, Plg+/–/FibAα–/–, and Plg–/–/FibAα–/–. It should be noted that the Bβ chain of fibrinogen appears to be rate limiting for fibrinogen assembly and secretion46 ; therefore, hemizygous (FibAα+/–) mice have circulating fibrinogen levels that are approximately 80% those of wild-type (FibAα+/+) mice.44 Study protocols were approved by the Children's Hospital Research Foundation Institutional Animal Care and Use Committee and were in accordance with the guidelines of the National Institutes of Health.
Tumor cell transplantation
These studies used 2 C57Bl/6-derived tumor cells lines, Lewis lung carcinoma and T241 fibrosarcoma, which were transplanted into immune-competent C57Bl/6-inbred mice. Green fluorescence protein (GFP) expressing Lewis lung carcinoma (LLCGFP) cells and T241 fibrosarcoma cells (kindly provided by Dr Y. Cao, Laboratory of Angiogenesis Research, Karolinska Institute, Stockholm, Sweden) were cultured in vitro as previously described.47 Tumor cell viability was determined by trypan blue exclusion and was always 95% or greater. Mice were anesthetized by inhalation of 2% isoflurane (Ohmeda PPD, Liberty Corner, NJ), and 50 μL LLCGFP cell suspension (2.5 × 105 cells) was injected subcutaneously into the right rear footpad or the dorsal subcutis overlying the lower part of the thoracic spine. Alternatively, a 50-μL suspension of T241 cells (7.5 × 105 cells) was injected into either the right rear footpad or the dorsal subcutis. Tumor volume in the dorsal subcutis was estimated as a function of calipered tumor length and width, whereby tumor volume = [(length)(width2)](π/6).48 Footpad tumor size was tracked over time as a function of dorsal-ventral tumor thickness calipered at the level of the metatarsals, subtracting the thickness of the foot before tumor cell inoculation. Tumor mass was directly determined at the time of killing. The tumor-bearing foot and the contralateral foot were removed by disarticulation of the ankle joint. Footpad tumor mass was estimated by subtracting the mass of the non–tumor-bearing foot from the mass of tumor-bearing foot.
Histologic analysis
Fixed tissue was embedded in paraffin, sectioned, and stained with hematoxylin and eosin. Fibrin(ogen) immunostaining and antiplatelet endothelial cell adhesion molecule (anti-PECAM) (PharMingen, San Diego, CA) immunostaining were performed using a rabbit antimouse polyclonal antiserum as described previously.45 Neutrophils in the tumor tissue sections were detected using Leder stain. Macrophages were stained with the macrophage-specific rat monoclonal antibody F4/80 (Abcam, Cambridge, United Kingdom), using the Vectastain ABC detection system with biotinylated rabbit antirat immunoglobulin G (IgG), avidin-phosphatase conjugate, and Vector Red as substrate (Vector Laboratories, Burlingame, CA).
Electron microscopic analysis
Tumor tissues for electron microscopy were collected from mice carrying LLCGFP tumors grown in the footpad or in the subcutaneous tissue of the back and were immediately fixed in 3% glutaraldehyde in 0.15 M cacodylate buffer, pH 7.4. After 1 to 2 hours of fixation, the samples were transferred to a solution of 1% osmic acid in cacodylate buffer and were postfixed for an additional hour. Samples were then dehydrated in graded alcohol solutions and processed in LX112 embedding media. Thin sections were stained with uranyl acetate before evaluation by transmission electron microscopy. The grid was surveyed to confirm the widespread presence of tumor cells and to evaluate the status of tumor-associated blood vessels for evidence of thrombosis. At least 5 randomly selected blood vessels were scored for patency in each tumor section evaluated by electron microscopy.
Results
Tumor location and plasminogen are interconnected determinants of tumor growth
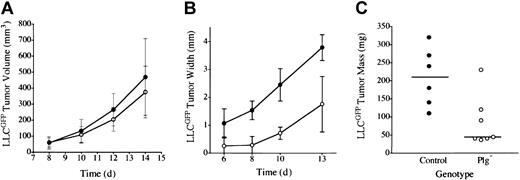
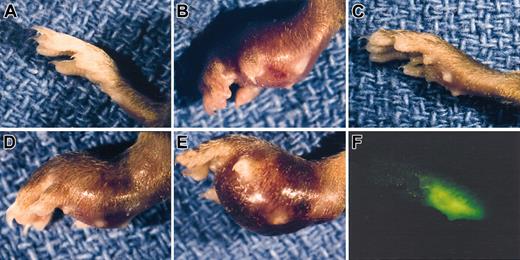
Earlier studies of LLC indicated that plasminogen was not a major determinant of primary tumor growth in the dorsal subcutis, spontaneous hematogenous metastasis to the lung, or secondary tumor growth in the lung.42 However, to further test the concept that plasmin(ogen) can serve as an important determinant of tumor growth in at least certain contexts, we transplanted LLC tumors to another anatomic location, the mouse footpad. In these studies, we used LLC cells expressing LLCGFP that could be more readily recognized in vivo. Consistent with previous results, LLCGFP tumor growth was similar in control and plasminogen-deficient mice when grown in the dorsal subcutis (Figure 1A). In striking contrast, serial calipation (Figure 1B), direct weight measurement (Figure 1C), and mere visual inspection (Figure 2) revealed a dramatic diminution in LLCGFP tumor growth within the footpads of plasminogen-deficient mice compared with control animals. This experiment was repeated 3 times with similar results. The impediment to tumor growth observed in Plg– mice was so substantial that a week or more after tumor cell transplantation, it was often difficult to distinguish the tumor-bearing feet in Plg– mice from the contralateral non–tumor-bearing feet (Figure 2C; see representative data). However, the presence of LLCGFP-derived tumor tissue within these otherwise normal-appearing feet could be readily established by gross examination using a fluorescence-equipped stereomicroscope (Figure 2C,F; bright-field and fluorescence images of tumor-bearing foot from plasminogen-deficient mouse 14 days after transplantation). Thus, gross evaluation and microscopic analysis of tissue sections (see “Microscopic analysis”) indicated that tumors were established in the footpads of plasminogen-deficient mice, but the tumors grew far more slowly than they did in control mice. This genotype-dependent difference in tumor growth was apparent within 6 to 10 days, when footpad tumors in control mice were still relatively small (approximately 10 mm3 in control mice) but were well above the size where tumor growth would be expected to depend on a supportive vasculature.49 This profound difference in tumor growth in the footpad could not be accounted for by a trivial, though genotype-related, immunologic response (perhaps against GFP) in that no such impediment to tumor growth was observed after the transplantation of LLCGFP cells into the dorsal subcutis of control or plasminogen-deficient mice. Furthermore, there was no indication of tumor rejection based on microscopic analyses of tumor tissues (see “Microscopic analysis”). Lymphocytic infiltrates that would have been expected to be a prominent feature in the context of any tissue rejection were distinctly inconspicuous in LLCGFP tumor tissue, regardless of mouse genotype and tumor location.
Plasminogen is a determinant of LLC tumor growth through a mechanism linked to anatomic location. (A) Tumor mass after LLCGFP transplantation into the dorsal subcutis of control (•, n = 4) and plasminogen-deficient (○, n = 5) mice was estimated by serial calipation. Tumor sizes were not significantly different between the cohorts at any time point. Direct tumor weight measurements made at the time of animal killing were also not significantly different (data not shown). Tumor size after LLCGFP transplantation into the footpad was estimated by serial calipation of tumor width (B) and direct tumor weight at day 14 (C) in the footpad of control (•, n = 6) and plasminogen-deficient (○, n = 7) mice. Footpad tumors in plasminogen-deficient mice were significantly smaller than in control animals at every time point, including the final weight measurement (P < .02 at each comparison; Mann-Whitney U test). Horizontal lines in panel C indicate median values. Error bars indicate standard deviation from the mean.
Plasminogen is a determinant of LLC tumor growth through a mechanism linked to anatomic location. (A) Tumor mass after LLCGFP transplantation into the dorsal subcutis of control (•, n = 4) and plasminogen-deficient (○, n = 5) mice was estimated by serial calipation. Tumor sizes were not significantly different between the cohorts at any time point. Direct tumor weight measurements made at the time of animal killing were also not significantly different (data not shown). Tumor size after LLCGFP transplantation into the footpad was estimated by serial calipation of tumor width (B) and direct tumor weight at day 14 (C) in the footpad of control (•, n = 6) and plasminogen-deficient (○, n = 7) mice. Footpad tumors in plasminogen-deficient mice were significantly smaller than in control animals at every time point, including the final weight measurement (P < .02 at each comparison; Mann-Whitney U test). Horizontal lines in panel C indicate median values. Error bars indicate standard deviation from the mean.
Gross appearance of LLC footpad tumors in mice with single and combined deficits in plasminogen and fibrinogen. A normal, non-tumor–bearing foot (A) is shown along with examples of LLCGFP footpad tumors 14 days after inoculation into control (B), Plg– (C,F), Fib– (D), and Plg–/Fib– (E) mice. Despite the minimal difference in size between the Plg– tumor-bearing foot (C) and a non-tumor–bearing foot (A), the presence of tumor tissue could be easily appreciated using a fluorescence-equipped stereomicroscope (F). Note that the foot in panel F is the same foot shown in panel C and is photographed at the same magnification.
Gross appearance of LLC footpad tumors in mice with single and combined deficits in plasminogen and fibrinogen. A normal, non-tumor–bearing foot (A) is shown along with examples of LLCGFP footpad tumors 14 days after inoculation into control (B), Plg– (C,F), Fib– (D), and Plg–/Fib– (E) mice. Despite the minimal difference in size between the Plg– tumor-bearing foot (C) and a non-tumor–bearing foot (A), the presence of tumor tissue could be easily appreciated using a fluorescence-equipped stereomicroscope (F). Note that the foot in panel F is the same foot shown in panel C and is photographed at the same magnification.
Footpad tumor growth is restored in plasminogen-deficient mice by genetically superimposing fibrinogen deficiency
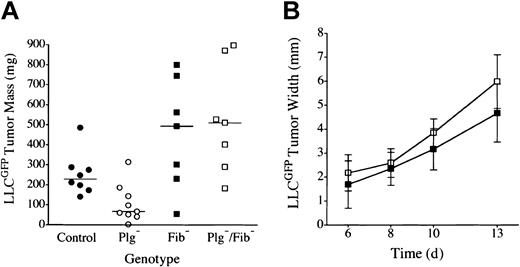
Plasminogen could influence tumor growth through plasmin-mediated proteolysis of multiple targets (eg, laminin, fibronectin, protease zymogens, latent growth factors, fibrin) or through nonproteolytic plasminogen derivatives (eg, angiostatin). Fibrin(ogen) seemed to be the least likely plasmin target relevant to tumor growth because fibrinogen was itself not a significant determinant of LLC tumor growth in the dorsal subcutis and because fibrin deposition within these tumors was relatively modest45,47 (see “Microscopic analysis”). Nevertheless, given that one universally recognized role of plasmin is fibrin clearance, we directly tested whether a genetically imposed loss of fibrin(ogen) would restore tumor growth in the footpads of plasminogen-deficient mice. Cohorts of C57Bl/6-inbred mice with single and combined deficits in plasminogen and fibrinogen were challenged by the transplantation of LLCGFP cells into either the footpad or the dorsal subcutis, and tumor growth was monitored as described above. Consistent with previously published findings42,47 and our findings, no significant difference was observed in dorsal skin tumor growth (serial volume estimates made by calipation) or final mass (established by direct weight measurements) in cohorts of control, Plg–, Fib–, and Plg–/Fib– mice (data not shown). In contrast, tumor growth was obviously impeded in the footpads of the plasminogen-deficient cohort. More significantly, the poor tumor growth observed in the footpads of plasminogen-deficient mice was effectively relieved, and robust tumor growth restored, in mice that also lacked fibrinogen (Figures 2, 3). Indeed, footpad tumors tended to be larger in fibrinogen-deficient mice than in control animals, though the difference did not reach statistical significance. Although superficial hemorrhage was occasionally encountered in large tumors regardless of genotype, the restoration of tumor mass in plasminogen-deficient mice lacking fibrinogen was clearly a reflection of tumor expansion and not hemorrhage based on microscopic analyses of tissue sections (see “Microscopic analysis”). This experiment was repeated 3 times with similar results.
LLC tumor growth is restored in the footpad of plasminogen-deficient mice by genetic elimination of fibrinogen. (A) Weights of footpad tumors in cohorts of control •, Plg– ○, Fib– ▪, and Plg–/Fib– □ mice analyzed in parallel are shown 14 days after transplantation of LLCGFP. The weight distribution of footpad tumors was significantly smaller in Plg– mice than in control animals (P < .02), consistent with 2 other independent experiments. The elimination of fibrinogen relieved the impediment in tumor growth in Plg– mice. Indeed, footpad tumors from Plg–/Fib– mice were larger than those derived from control mice, though this difference was not statistically significant (P = .1). Plasminogen was not a significant determinant of tumor mass in the absence of fibrinogen (P = .7). Horizontal lines indicate median values. (B) Serial tumor width measurements demonstrated similar tumor expansion in fibrinogen-deficient mice with (▪, n = 7) and without (□, n = 7) plasminogen (P > .1 at each time point). All P values were established using the Mann-Whitney U test. Error bars indicate standard deviation from the mean.
LLC tumor growth is restored in the footpad of plasminogen-deficient mice by genetic elimination of fibrinogen. (A) Weights of footpad tumors in cohorts of control •, Plg– ○, Fib– ▪, and Plg–/Fib– □ mice analyzed in parallel are shown 14 days after transplantation of LLCGFP. The weight distribution of footpad tumors was significantly smaller in Plg– mice than in control animals (P < .02), consistent with 2 other independent experiments. The elimination of fibrinogen relieved the impediment in tumor growth in Plg– mice. Indeed, footpad tumors from Plg–/Fib– mice were larger than those derived from control mice, though this difference was not statistically significant (P = .1). Plasminogen was not a significant determinant of tumor mass in the absence of fibrinogen (P = .7). Horizontal lines indicate median values. (B) Serial tumor width measurements demonstrated similar tumor expansion in fibrinogen-deficient mice with (▪, n = 7) and without (□, n = 7) plasminogen (P > .1 at each time point). All P values were established using the Mann-Whitney U test. Error bars indicate standard deviation from the mean.
Tumor location and hemostatic factors are linked determinants of fibrosarcoma tumor growth
To determine whether the apparent connection between tumor location and hemostatic factors in controlling tumor growth rate is a unique feature of LLC or a more general phenomenon, we performed similar analyses using the C57Bl/6-derived fibrosarcoma tumor cell line T241. Following a protocol similar to that used with LLC cells, T241 cells were injected into the right rear footpad or dorsal subcutis of control, Plg–, Fib–, and Plg–/Fib– mice, and tumor growth was tracked as described. Tumors in the dorsal subcutis became palpable approximately 4 days after T241 transplantation, regardless of genotype, and tumors grew at a similar rate in this location based on serial calipation (data not shown). Based on direct tumor weight measurements made at the time of killing 2 weeks after transplantation, dorsal skin tumors from plasminogen-deficient mice (median, 452 mg; range, 293-688 mg; n = 8) were comparable to those from control animals (median, 491 mg; range, 212-750 mg; n = 11) (P > .8; Mann-Whitney U test). Furthermore, the deletion of fibrinogen had no significant effect on tumor mass in the dorsal skin, regardless of plasminogen genotype (data not shown). In contrast, plasminogen-deficiency resulted in a significant diminution in T241 fibrosarcoma tumor growth when cells were transplanted into the footpad. At the time of killing, the median weight of footpad tumors from control mice was 170 mg (range, 101-493 mg; n = 10) compared with 65 mg in Plg– mice (range, 27-151 mg; n = 8) (P < .004; Mann-Whitney U test). Furthermore, the impediment to T241 fibrosarcoma tumor growth in the footpads of plasminogen-deficient mice was effectively lifted by concomitant fibrinogen deficiency (median weight, 214 mg; range, 84-285 mg; n = 8; P < .004 [cohort lacking plasminogen alone] and P > .3 [control cohort]; Mann-Whitney U test). The final weights of T241-derived footpad tumors in fibrinogen-deficient mice (median weight, 220 mg; range, 44-598 mg; n = 8) were not significantly different from those of control mice or animals with a combined deficit in plasminogen and fibrinogen (P > .3 for each comparison; Mann-Whitney U test). This experiment was repeated twice with similar results. Thus, the fibrinogen- and location-dependent impediment to tumor growth initially observed in plasminogen-deficient mice carrying LLC was also observed with a fibrosarcoma tumor model.
Microscopic analysis
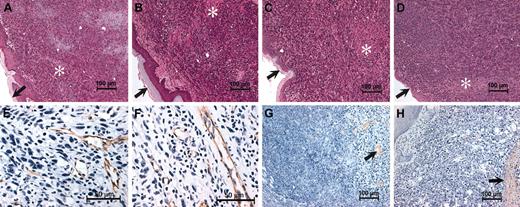
One inference from the location- and genotype-dependent differences observed in tumor growth is that the particular microenvironment of the footpad is somehow distinct. To gain a better understanding of the process underlying the specific impediment to tumor growth within the footpads of plasminogen-deficient mice, dorsal skin and footpad LLCGFP tumor tissue were collected from mice with single and combined deficits in plasminogen and fibrinogen (2 weeks after initial transplantation) and were processed for histologic analysis. As we reported earlier for mice with individual deficits in plasminogen42 and fibrinogen,47 the dorsal skin tumors collected here were microscopically unremarkable and had no genotype-dependent histologic differences (data not shown). More surprisingly, the histologic features of tumor tissue collected from the footpads of plasminogen-deficient mice were also not appreciably different than tumor tissue collected from control animals (Figure 4). LLCGFP tumors grew in sheets of monotonous, highly anaplastic cells regardless of genotype. Individual cells had large nuclei with prominent nucleoli and scant cytoplasm. Focal areas of necrosis and small areas of hemorrhage were also observed, but these features were not obviously different based on genotype. Neutrophils detected using Leder stain were extremely sparse within footpad tumors, regardless of genotype (data note shown). Macrophages highlighted with the Mac-3 antibody, F4/80, were more prevalent but also constituted a fraction of footpad tumor cells with no obvious genotype-dependent differences. F4/80 staining cells were quantitated in footpad tumors from 4 control and 4 Plg– mice by examining 10 fields magnified 1000 × from each tumor. Footpad tumors from Plg– mice had a median of 28 (range, 25-33) F4/80-staining cells per 1000 × field compared with a median of 27 (range, 26-36) in tumors derived from control mice (P > .6; Mann-Whitney U test). Like dorsal skin tumors,42,47 the footpad tumors collected from control and plasminogen-deficient mice were well vascularized and had a vascular density based on immunohistochemical staining of endothelium similar to that of an antibody to PECAM (Figure 4). This conclusion was affirmed by a formal quantitation of PECAM-stained vessels within random high-powered fields. Control mice had a mean of 22 (range, 18-27) vessels per 400 × field, compared with 23 (range, 19-32) in Plg–, 21 (range, 14-31) in Fib–, and 19 (range, 15-24) in Plg–/Fib– mice. None of these differences were statistically significant. Fibrin(ogen) deposition detected immunologically was similar and relatively sparse within footpad tumors of control and Plg– mice (Figure 4), a finding that was particularly surprising given the impressive acceleration in footpad tumor growth observed in plasminogen-deficient mice when fibrin(ogen) deficiency was superimposed. Furthermore, the histologically detectable fibrin(ogen) deposits within footpad tumor tissue (primarily peritumoral or associated with small areas of focal necrosis) were similar to those observed in tumors transplanted in the dorsal subcutis (data not shown), where no major impediment in tumor growth was found. Therefore, one could not immediately reconcile the limited apparent fibrin(ogen) deposition with the spectacular influence of fibrinogen on tumor growth in the footpads of plasminogen-deficient mice. As expected, there was no immunologically detectable fibrin(ogen) in footpad tumors from Fib– and Plg–/Fib– mice (data not shown).
Histologic features of footpad tumors are similar in plasminogen-deficient and control mice. Shown are representative hematoxylin-eosin–stained sections of footpad LLCGFP tumors collected from control (A), Plg– (B), Fib– (C), and Plg–/Fib– (D) mice 14 days after initial transplantation. Note that the overall microscopic features of the tumor tissue were unremarkable within animals of each genotype and suggest no obvious basis for the impediment in tumor growth in Plg– mice. LLCGFP tumors grew as sheets of anaplastic cells that often invaded normal structures, including adjacent muscle and bone. Small areas of necrosis (*) were also present, but these were not appreciably different between animals of each genotype. Dermal surfaces of the footpads are indicated by arrows in panels A to D. Immunohistochemical staining for the endothelial marker PECAM (brown stain) revealed abundant vessels in footpad tumors derived from control (E) and plasminogen-deficient mice (F). Fibrin(ogen) immunostains revealed relatively scant and focal fibrin(ogen) deposition in control (G) and Plg– (H) mice (brown staining highlighted by arrows). Sparse fibrin(ogen) deposition was generally peritumoral or was associated with small areas of necrosis. Sections used for immunohistology were counterstained with hematoxylin.
Histologic features of footpad tumors are similar in plasminogen-deficient and control mice. Shown are representative hematoxylin-eosin–stained sections of footpad LLCGFP tumors collected from control (A), Plg– (B), Fib– (C), and Plg–/Fib– (D) mice 14 days after initial transplantation. Note that the overall microscopic features of the tumor tissue were unremarkable within animals of each genotype and suggest no obvious basis for the impediment in tumor growth in Plg– mice. LLCGFP tumors grew as sheets of anaplastic cells that often invaded normal structures, including adjacent muscle and bone. Small areas of necrosis (*) were also present, but these were not appreciably different between animals of each genotype. Dermal surfaces of the footpads are indicated by arrows in panels A to D. Immunohistochemical staining for the endothelial marker PECAM (brown stain) revealed abundant vessels in footpad tumors derived from control (E) and plasminogen-deficient mice (F). Fibrin(ogen) immunostains revealed relatively scant and focal fibrin(ogen) deposition in control (G) and Plg– (H) mice (brown staining highlighted by arrows). Sparse fibrin(ogen) deposition was generally peritumoral or was associated with small areas of necrosis. Sections used for immunohistology were counterstained with hematoxylin.
Plasminogen is important in maintaining tumor vascular patency in the footpad
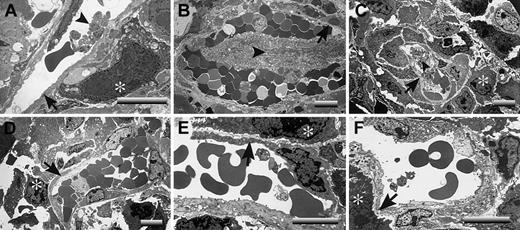
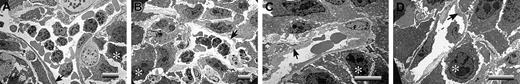
A critical question is raised by these studies: What is distinct about the footpad that makes LLC tumor growth profoundly plasminogen dependent, whereas tumor growth in the dorsal subcutis appears to be largely insensitive to plasminogen? One obvious difference is that footpad tumors are far more likely than dorsal skin tumors to sustain repeated mechanical trauma secondary to ambulation. Any such microvascular damage would also be expected to lead to a significant risk for local intravascular thrombosis. Therefore, one hypothesis that could explain the observed location- and plasminogen-dependent tumor growth is that, in the context of trauma, the formation and persistence of occlusive thrombi seriously compromise the tumor blood supply. This theory was particularly appealing in that it also provided a potential explanation for the seemingly conflicting findings that fibrin(ogen) deposition is relatively modest in LLC tumor tissue but that loss of fibrin(ogen) restores tumor growth. Relatively modest local intravascular fibrin-platelet deposits could result in a profound downstream restriction in the blood supply to tumor tissue that would substantially slow tumor growth. To test this hypothesis, tumor tissues were collected from the dorsal subcutis and footpads of control, Plg–, Fib–, and Plg–/Fib– mice and then processed for transmission electron microscopy. Tumor tissue sections were prepared from 3 mice of each genotype 14 days after initial transplantation of LLCGFP cells. The mass of the tumors chosen clustered around the median values shown in Figure 3A. At least 5 random vessels from each tumor sample were evaluated for patency by an investigator blinded to animal genotype. No obvious genotype-dependent differences were observed in vessel density or vessel architecture. All the vessels observed had a normal-appearing endothelium and were essentially unremarkable, with the exception of the footpad tumor vasculature derived from plasminogen-deficient mice. In the latter, microvascular thrombi were frequently encountered, and many of these lesions appeared highly occlusive (Figure 5; see representative data). Of 18 vessels examined in footpad tumor tissue from plasminogen-deficient mice, 11 contained obvious thrombi, whereas only 1 of 17 vessels viewed in the footpad tumors of control mice contained a microthrombus (P < .001; Fisher exact test). It is important to note that the thrombi observed in the footpad tumors of plasminogen-deficient mice were generally in areas of healthy-appearing tumor cells and were not simply a secondary consequence of local tumor necrosis (Figure 5). Furthermore, the thrombotic lesions were documented solely within the tumor vasculature of plasminogen-deficient mice that had circulating fibrinogen and were never encountered in animals with a combined deficit in plasminogen and fibrinogen (Figure 5; see representative data). None of 17 vessels examined by ultrastructural analysis in Fib– mice and none of 15 vessels examined in Plg–/Fib– mice contained microthrombi. Thus, the distinct absence of occlusive thrombi within the tumor vasculature of plasminogen-deficient mice lacking fibrinogen correlates with the restoration of tumor growth in this cohort. These findings strongly infer that the impediment to tumor growth in the footpads of plasminogen-deficient mice is a consequence of a restricted blood supply secondary to the formation, and persistence, of partially and fully occlusive thrombi. The minimal influence of plasminogen deficiency on tumor growth in the dorsal subcutis can also be easily understood based on the available electron microscopy data (Figure 6). None of the vessels examined from tumors grown in the dorsal subcutis had evidence of microthrombi (Figure 6), regardless of plasminogen or fibrinogen genotype. Thus, like tumor growth, the presence of microvascular thrombi within tumor tissue was plasminogen and location dependent.
Occlusive microvascular thrombi are prominent in LLC footpad tumors derived from plasminogen-deficient mice. Electron microscopic evaluation of footpad tumor vasculature revealed widespread microvascular thrombi within tumors derived from Plg– mice (A-C) but not tumors collected from control (D), Fib– (E), or Plg–/Fib– (F) animals 14 days after initial tumor cell transplantation. These representative sections from Plg– mice include examples of vessels with small platelet aggregates (A) and vessels with advanced, highly occlusive thrombi (B-C). Occlusive platelet thrombi (arrowheads) were found in areas populated with viable tumor cells (*) and where the endothelium appeared essentially intact (arrows). Note that occlusive intravascular thrombi were not a feature of the rapidly growing tumors of control mice and Fib– mice. More significantly, no such platelet aggregates were observed in plasminogen-deficient mice lacking fibrinogen (F), animals that exhibited no impediment in tumor growth. Horizontal bars in each micrograph indicate 8 μm.
Occlusive microvascular thrombi are prominent in LLC footpad tumors derived from plasminogen-deficient mice. Electron microscopic evaluation of footpad tumor vasculature revealed widespread microvascular thrombi within tumors derived from Plg– mice (A-C) but not tumors collected from control (D), Fib– (E), or Plg–/Fib– (F) animals 14 days after initial tumor cell transplantation. These representative sections from Plg– mice include examples of vessels with small platelet aggregates (A) and vessels with advanced, highly occlusive thrombi (B-C). Occlusive platelet thrombi (arrowheads) were found in areas populated with viable tumor cells (*) and where the endothelium appeared essentially intact (arrows). Note that occlusive intravascular thrombi were not a feature of the rapidly growing tumors of control mice and Fib– mice. More significantly, no such platelet aggregates were observed in plasminogen-deficient mice lacking fibrinogen (F), animals that exhibited no impediment in tumor growth. Horizontal bars in each micrograph indicate 8 μm.
Microvascular patency is maintained within LLC tumors of plasminogen-deficient mice when the tumors develop within the dorsal subcutis. Representative transmission electron micrographs illustrating dorsal skin tumor vasculatures of control (A), Plg– (B), Fib– (C), and Plg–/Fib– (D) mice. Note that in contrast to tumors grown in the footpad, tumors within the dorsal subcutis had no evidence of microvascular thrombus formation, regardless of animal genotype. Viable tumor cells are highlighted with asterisks, and endothelium is indicated with arrows. Horizontal bars in each micrograph indicate 8 μm.
Microvascular patency is maintained within LLC tumors of plasminogen-deficient mice when the tumors develop within the dorsal subcutis. Representative transmission electron micrographs illustrating dorsal skin tumor vasculatures of control (A), Plg– (B), Fib– (C), and Plg–/Fib– (D) mice. Note that in contrast to tumors grown in the footpad, tumors within the dorsal subcutis had no evidence of microvascular thrombus formation, regardless of animal genotype. Viable tumor cells are highlighted with asterisks, and endothelium is indicated with arrows. Horizontal bars in each micrograph indicate 8 μm.
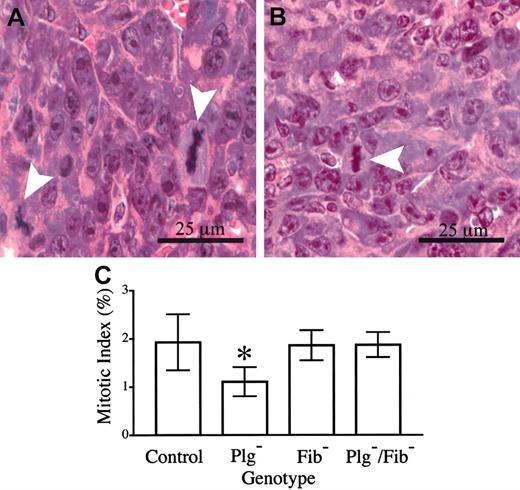
The presence of microvascular thrombi within footpad tumors of plasminogen-deficient mice and the secondary restriction of nutrient delivery and gas exchange are likely to result in a combination of increased tumor cell dropout (necrosis) and slowed tumor cell proliferation. Although a genotype-dependent difference in necrosis was not qualitatively apparent, a meaningful quantitative analysis could not be conducted because individual necrotic cells and small, focal areas of necrosis were occasionally interspersed among healthy cells. However, a direct quantitative measurement of mitotic indices within fields of viable footpad tumor tissue indicated that one factor contributing to the observed differences in tumor growth was a genotype-dependent difference in tumor cell doubling time. Here, mitotic figures and total cells were counted within 8 random, high-powered fields from each of 6 control mice and 6 mice with single and combined deficits in plasminogen and fibrinogen, and the mean mitotic index for each cohort was determined (Figure 7). Approximately 5000 total cells were evaluated within each genotype. The data reveal that the mitotic index in tumor tissue of control mice (1.9%) was significantly higher than that observed within tumor tissue of Plg– mice (1.1%). Assuming that the length of M phase is constant in cycling LLC tumor cells, this difference would translate to a 1.7-fold longer tumor cell cycle time in Plg– mice than in control animals. Although this difference may seem relatively small, given an exponential expansion of cells it would be expected to result in a 7- to 10-fold difference in the total of progeny cells produced in a period of 7 to 8 doublings for the faster dividing cells. It should also be noted that the genetic elimination of fibrinogen restored the mitotic index in footpad tumors of Plg– mice to that observed in control animals (Figure 7), consistent with the observed restoration of tumor growth.
Tumor cell mitotic index is diminished in footpad tumors derived from plasminogen-deficient mice relative to control animals. Mitotic figures (arrowheads) were easily discernible in hematoxylin-eosin–stained sections of footpad tumors from control (A) and plasminogen-deficient (B) mice. (C) Quantitative analysis of mitotic indices within footpad tumors of mice with single and combined deficits in plasminogen and fibrinogen. The mitotic index observed in plasminogen-deficient (*) mice was significantly less than that observed in control animals (P < .02). In contrast, no significant difference in mitotic index was observed in comparative analyses of control animals and plasminogen-deficient mice that also lacked fibrinogen (P > .8). Mitotic indices were also similar in control and fibrinogen-deficient mice (P > .9). All P values were established using the Mann-Whitney U test. Error bars indicate standard deviation from the mean value for 6 mice.
Tumor cell mitotic index is diminished in footpad tumors derived from plasminogen-deficient mice relative to control animals. Mitotic figures (arrowheads) were easily discernible in hematoxylin-eosin–stained sections of footpad tumors from control (A) and plasminogen-deficient (B) mice. (C) Quantitative analysis of mitotic indices within footpad tumors of mice with single and combined deficits in plasminogen and fibrinogen. The mitotic index observed in plasminogen-deficient (*) mice was significantly less than that observed in control animals (P < .02). In contrast, no significant difference in mitotic index was observed in comparative analyses of control animals and plasminogen-deficient mice that also lacked fibrinogen (P > .8). Mitotic indices were also similar in control and fibrinogen-deficient mice (P > .9). All P values were established using the Mann-Whitney U test. Error bars indicate standard deviation from the mean value for 6 mice.
Discussion
The long-standing hypothesis that fibrin(ogen) deposition and plasmin-mediated matrix remodeling may be important for developing a supportive tumor stroma and tumor growth was significantly challenged by the robust tumor growth reported in initial studies of gene-targeted mice with deficits in plasminogen and fibrinogen.40,42,47 However, the findings presented here demonstrate that plasminogen and fibrinogen can, in fact, be important determinants of tumor growth, at least in specific contexts. Consistent with earlier reports, we show that neither of these hemostatic factors was a significant determinant of LLC tumor growth after transplantation into the dorsal skin, whereas both were powerful determinants of tumor growth in another anatomic location, the footpad. Loss of plasminogen dramatically suppressed LLC tumor growth in the footpad through a mechanism that was shown to be fibrinogen dependent. The negative effect of plasminogen deficiency on footpad tumor growth was entirely abrogated by the concomitant genetic elimination of fibrinogen. This phenomenon was not merely an unusual feature of LLC tumors. T241 fibrosarcoma tumor growth in the footpad was also restricted by plasminogen deficiency in a fibrinogen-dependent manner. The likely mechanistic basis for these findings was revealed through detailed transmission electron microscopy studies of tumor tissues collected from the dorsal subcutis and footpad. Occlusive microvascular thrombi were a prominent feature within footpad tumors of plasminogen-deficient mice. On the other hand, no such lesions were observed in plasminogen-deficient mice carrying a superimposed genetic deficit in fibrinogen, a context in which tumor growth is similar to that of control animals. The data infer that tumor growth in the footpads of plasminogen-deficient mice is compromised as a function of mechanical stress (secondary to ambulation) and of the formation and persistence of vaso-occlusive thrombi that limit tumor blood supply. Direct quantitation of mitotic indices within footpad tumor tissues suggests that a significant lengthening of the tumor cell doubling time in plasminogen-deficient mice is one factor contributing to the slowed tumor growth relative to control animals. Quantitative comparison of individual tumor cell dropout and focal necrosis in control and plasminogen-deficient mice could not be made, but differences in rates of tumor cell necrosis may also contribute to the differences in tumor growth observed. Finally, the maintenance of vascular patency and the restoration of tumor growth in the absence of fibrinogen strongly suggest that a biologically relevant target of plasminogen in supporting tumor growth, at least in certain contexts, is intravascular fibrin.
The finding that fibrin(ogen) is a likely proteolytic target linking the plasminogen/plasmin system to tumor growth does not exclude the possibility that plasmin-mediated proteolysis of nonfibrin targets also contributes significantly to tumor progression. There are now well-established precedents for biologically important plasmin-mediated proteolysis of nonfibrin substrates in the context of pathologic processes (eg, excitotoxin-induced neurodegeneration in the hippocampus3 ) and physiologic processes (eg, hepatic repair after toxic injury2 ). The fact that both the growth and the microscopic features of LLC tumors are similar in control animals and plasminogen-deficient mice lacking fibrinogen argues that any benefits or liabilities associated with plasmin-mediated proteolysis of any nonfibrin substrate are likely to be subtle in this tumor model. However, given the diverse biologic characteristics of distinct tumor types and given the relatively broad substrate specificity of plasmin (eg, extracellular matrix components, matrix metalloproteinase proenzymes), an important role of plasmin-mediated proteolysis of nonfibrin targets in supporting tumor stroma formation, tumor growth, and tumor dissemination in other settings would not be surprising.
Our findings that tumor growth is plasminogen and “microenvironment” specific and is linked to vascular integrity may provide a partial explanation for the seemingly conflicting findings reported in earlier cancer studies in mice with deficits in plasminogen activator, plasminogen, and PAI-1. Gutierrez et al36 reported that subcutaneously transplanted T241 tumors grew more slowly in uPA-deficient mice relative to controls. However, a more recent study by Curino et al41 using the same tumor line demonstrated only a small difference in tumor growth in the skin in uPA-deficient mice. Yet, in an apparent conflict with the T241 findings presented in this report, these authors found that plasminogen deficiency resulted in a major diminution in tumor growth rate in the dorsal skin. Our finding that anatomic location is a significant variable for tumor growth in plasminogen-deficient mice may provide a reasonable explanation for this collection of apparently irreconcilable findings. Specifically, seemingly subtle differences in the site of transplantation among different studies might result in significant differences in mechanical insults to tumor tissue, either because of normal mouse movement or because of the accessibility of the tumor to physical manipulation by the mouse. Any such difference in mechanical challenge, in combination with a plasminogen activation defect, would potentially result in profound differences in tumor vascular patency and tumor growth. This hypothesis could be tested through follow-up electron microscopy studies focusing on tumor vasculature in various models and settings.
The specific impact of genetic alterations in PAI-1 levels on tumor growth has also been curiously inconsistent. One early comparative study of murine melanoma reported that tumor growth was similar in control, PAI-1–deficient, and PAI-1–overexpressing mice.39 However, other studies have indicated that PAI-1 is a determinant of tumor growth in at least some experimental settings.35-38 Recent extensions of these studies have suggested that PAI-1 influences tumor vasculature through a mechanism that depends, at least in part, on its serine protease inhibitor function.34,38 Taking these findings together, it is tempting to speculate that the varying importance of PAI-1 in tumor growth, like the findings reported here for plasminogen, may be in part related to differences in tumor microenvironment. Furthermore, an attractive extension of this theory is that the diminution in tumor growth observed in the absence of PAI-1 or the presence of excessive PAI-1 may be attributed to copious or insufficient plasmin-mediated proteolysis within the tumor vasculature. This concept could be readily explored through studies of tumor growth and vascular ultrastructure in mice by which PAI-1 expression is manipulated in parallel with genetic alterations in plasminogen activators or plasminogen. Of course, a contribution of PAI-1 to tumor development through mechanisms that are distinct from regulating plasmin-mediated proteolysis (eg, altering integrin engagement of vitronectin) also remains an attractive hypothesis that requires further evaluation.50,51
Given the prevalence of intravascular thrombotic lesions within footpad tumors of plasminogen-deficient mice, the occlusive restriction nutrient delivery and gas exchange within tumor tissues in these animals at this anatomic location seem to be the most likely impediments to tumor growth. Although this hypothesis may be largely correct, several additional biologic mechanisms have not yet been formally excluded that might also contribute to the diminution of tumor growth in the footpad. Other contributing factors may include diminished tumor cell retention after initial injection into plasminogen-deficient mice, genotype-dependent differences in the generation of inflammatory mediators that may control tumor or stromal cell growth or survival, and subtle differences in tumor vascular architecture. The possibility of diminished seeding or retention of tumor cells in the footpads of plasminogen-deficient mice has not been directly explored, but this concept is difficult to reconcile with the similar tumor growth, and presumably similar tumor cell seeding efficiency, observed in control and plasminogen-deficient mice after transplantation into the dorsal subcutis. Furthermore, it is difficult to imagine that the loss of a matrix and adhesion protein like fibrin(ogen) would improve tumor cell retention in the footpads of plasminogen-deficient mice and thereby restore tumor growth. Finally, the observed genotype-dependent differences in tumor growth can be largely accounted for by differences in the mitotic indices within established footpad tumors. Perhaps a more intriguing alternative concept is that local differences in the expression of inflammatory mediators may contribute to the observed differences in footpad tumor growth, particularly given that the generation of these factors may be in part dependent on genotype-related occlusive events and secondary tissue damage. Increasingly persuasive data point to a strong influence of inflammatory cells and inflammatory mediators on tumor biology, including tumor growth.52 Although we found no quantitative genotype-dependent difference in inflammatory infiltrates within footpad tumors, the available data do not exclude a potential genotype-dependent difference in inflammatory cell activity as a determinant of tumor growth. More detailed studies in this area may be revealing. Finally, the hypothesis that footpad tumor growth is sensitive to plasmin(ogen) through a mechanism coupled to stromal tissue formation or remodeling remains to be fully explored. However, no genotype-dependent difference in tumor vascular density was noted in either the footpad (this report) or the dorsal subcutis.42
The present findings illuminate one specific context in which fibrin(ogen) serves as a determinant of tumor growth, but multiple contexts may ultimately be identified apart from any defects in the plasminogen/plasmin system. For example, fibrinogen is likely to be a significant factor in tumor growth in any setting involving excessive local procoagulant activity (eg, high tissue factor expression or loss of local anticoagulant function). Furthermore, based on the present findings, platelet function is also expected to be a critical determinant of tumor growth within some, but not all, experimental settings. The concept that hemostatic factors can control tumor growth through a mechanism linked to vascular patency has been recognized earlier and viewed as a potential therapeutic opportunity. Multiple studies53-57 indicate that targeted thrombosis of tumor vasculature can be used to inhibit the growth of tumors in mice. For example, Nilsson et al55 demonstrate that tissue factor conjugated to an antibody recognizing a specific fibronectin splice variant prominent in tumor vasculature results in the infarction and involution of solid tumors in mice. Our data suggest that therapeutic strategies focusing on the plasminogen activation system may also be useful in suppressing tumor growth, or promoting tumor regression, under conditions in which intravascular thrombosis within tumor tissue can be imposed, pharmacologically or otherwise. Such an approach may limit the need for potentially morbid therapies such as radical surgery, intensive chemotherapy or radiotherapy to treat solid tumors.
Prepublished online as Blood First Edition Paper, June 26, 2003; DOI 10.1182/blood-2003-03-0881.
Supported in part by National Institutes of Health grants F32 CA83299 (J.S.P.), HL47826 (J.L.D.), and HL63194 (J.L.D.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Irene Hofmann for her expert technical assistance with electron microscopy. We also thank Keith Kombrinck and Amanda Hutchinson for their assistance with mouse genotyping and Dr Beth A. Myers for critically reviewing the manuscript.