Abstract
Inflammatory and procoagulant host responses are closely related in sepsis. The protein C pathway serves as a regulatory pathway with anti-inflammatory and anticoagulant properties. Recently, recombinant human activated protein C (rhAPC) was shown to reduce mortality in severe sepsis. Nevertheless, the effects of rhAPC in humans are still ill defined. The infusion of low endotoxin doses into humans provides a standardized model to study inflammatory and hemostatic mechanisms. Thus, we investigated whether rhAPC acts as an anticoagulant or anti-inflammatory drug in human endotoxemia. There were 24 volunteers randomized to receive either 24 μg/kg per hour rhAPC or placebo intravenously for 8 hours. Lipopolysaccharide (LPS, 2 ng/kg) was administered 2 hours after starting the infusions. rhAPC decreased basal tissue factor (TF)–mRNA expression, and thrombin formation and action. In contrast, rhAPC did not significantly blunt LPS-induced thrombin generation. Consistently, rhAPC did not reduce LPS-induced levels of TF-mRNA or D-dimer and had no effect on fibrinolytic activity or inflammation. Finally, endogenous APC formation was enhanced during endotoxemia and appeared to be associated with inflammation rather than thrombin formation. In conclusion, even low-grade endotoxemia induces significant protein C activation. Infusion of rhAPC decreases “spontaneous” activation of coagulation but does not blunt LPS-induced, TF-mediated coagulation in healthy volunteers, which is in contrast to a number of anticoagulants.
Introduction
Sepsis is still a major challenge in medicine—the incidence of severe sepsis doubled during the last 10 years, and despite advances in intensive care medicine and antimicrobial treatment it carries a high mortality rate.1
Inflammatory and procoagulant host responses are closely related.2 Infectious agents and inflammatory cytokines induce coagulation by stimulating the expression of tissue factor (TF) on monocytes and endothelium, which leads to thrombin formation.3 Inflammatory cytokines and thrombin can impair the endogenous fibrinolytic potential by enhancing the release of plasminogen activator inhibitor 1 (PAI-1) from platelets and the endothelium. In addition, thrombin stimulates multiple inflammatory pathways. This coagulation-inflammation cycle can result in diffuse endovascular injury.
The protein C pathway serves as a regulatory pathway. It controls the conversion of prothrombin to thrombin through a feedback inhibition mechanism,4 and activated protein C (APC) can intervene in the systemic inflammatory and procoagulant response to infections at multiple points3 : it exerts antithrombotic effects by inactivating factors Va and VIIIa, which results in decreased thrombin generation.5 Additionally, it may increase the fibrinolytic response by inhibiting PAI-1.5 In vitro data also indicate that APC inhibits the production of proinflammatory cytokines and therefore potentially has anti-inflammatory effects.6
Recently, recombinant human activated protein C (rhAPC) was shown to reduce mortality in severe sepsis.3 Although rhAPC reduced d-dimer levels and increased the relative drop in interleukin-6 (IL-6) levels by 30%, the effects of rhAPC on coagulation and inflammation in humans are still ill defined: a variety of anticoagulant and anti-inflammatory properties of APC has been reported only in in vitro studies.7 These include suppression of TF expression in a monocyte cell line8 and E-selectin expression on endothelial cells by suprapharmacologic concentrations of rhAPC.9
Thus, we hypothesized that rhAPC may act as an anticoagulant in human endotoxemia, and may decrease TF-mRNA expression in vivo and E-selectin release into circulation based on these in vitro studies.
The infusion of low doses of endotoxin into humans provides a standardized model to study the pharmacodynamics of drugs with supposed anticoagulant or anti-inflammatory properties in vivo.10-12 Thus, we used this model to further characterize rhAPC effects in humans.
Patients and methods
Study design
The trial was approved by the Ethics Committee of the University of Vienna (July 2002) and all participants gave written informed consent. The study was performed at a single center (Department of Clinical Pharmacology).
We enrolled 25 healthy male volunteers (aged 18-39 years) in a 3:2 randomized double-blind, placebo-controlled trial, consisting of 2 parallel groups (n = 15 in the treatment and n = 10 in the placebo group). Basic examination included medical history, physical examination, laboratory parameters, and virologic and drug screening. Additionally, study subjects were tested for hereditary thrombophilia (protein C and S, antithrombin, and APC resistance).
Human endotoxemia is a well-standardized model of systemic inflammation13,14 and TF-induced coagulation activation. Detailed study procedures of the lipopolysaccharide (LPS) model have been outlined in other trials previously.11,14 In the current study, volunteers received either rhAPC (drotrecogin alfa [activated], Xigris; Eli Lilly, Vienna, Austria) for 8 hours or 0.9% NaCl as placebo. The rhAPC dosage of 24 μg/kg per hour was based on a dose-finding phase 2 study and the PROWESS trial.3,15 A bolus of 2 ng/kg National Reference Endotoxin (LPS, Escherichia coli; USP, Rockville, MD) was infused in all subjects 2 hours after the start of the rhAPC or placebo infusion, as steady-state plasma levels of rhAPC are achieved within 2 hours.16
NaCl (200 mL/h) was administered to all subjects during the whole study period in order to maintain adequate hydration.
Blood sampling and analyses
Blood samples were collected into citrated or EDTA (ethylenediaminetetraacetic acid)–anticoagulated tubes (Vacutainer; Becton Dickinson, Vienna, Austria) by venipuncture shortly before and 2, 3, 4, 5, 6, 7, 8, and 24 hours after starting rhAPC or placebo infusion. Plasma was obtained by centrifugation at 2000g (15 minutes at 4°C) and stored in 0.5-mL aliquots at –80°C until batch analysis.
Most coagulation and inflammatory parameters were measured by enzyme immunoassays. Plasma levels of prothrombin fragment (F1 + 2; Behring, Marburg, Germany) and thrombin-antithrombin complexes (TATs, Enzygnost TAT micro; Behring) were used as markers of in vivo thrombin generation, d-dimer (Boehringer Mannheim, Mannheim, Germany) as indicator for fibrin formation and plasmin activator inhibitor (PAI-1, STA-analyzer; Diagnostica Stago, Parsippany, NJ), and plasmin-antiplasmin complexes (PAPs, Enzygnost PAP micro; Behring) and tissue plasminogen activator (tPA; Chromogenix, Milano, Italy) as markers for endogenous fibrinolytic capacity. Activated partial thromboplastin time (aPTT) was measured immediately from fresh plasma samples with the STA-analyzer.17
Plasma protein C activity (PC:Act) was measured with a chromogenic assay (ACL 3000 plus; Instrumentation Laboratory, Lexington, MA), protein C antigen (PC:Ag) with ELISA (Asserchrom; Boehringer Mannheim, Vienna, Austria), and APC with a slightly modified method according to Gruber and Griffin.18
Tumor necrosis factor-α (TNF-α) and IL-6 (high-sensitivity TNF-α and IL-6; R&D Systems, Minneapolis, MN), soluble E-selectin and P-selectin (R&D Systems), and elastase (PMN-Elastase; Immundiagnostik, Bensheim, Germany) were measured to determine inflammatory responses, activation of endothelium and platelets, and degranulation of neutrophils, respectively.14
Blood counts were performed with a cell counter (XE-2100; Sysmex, Milton Keynes, United Kingdom).
Real-time quantitative reverse transcriptase–polymerase chain reaction (RT-PCR)
All blood samples were immediately processed to avoid storage-induced alterations in mRNA levels.19 After preparing total RNA with the Amp RNA easy kit (Qiagen, Valencia, CA), mRNA was directly transcribed into cDNA using the RT-Reagent kit (Applied Biosystems, Foster City, CA) and stored at –80°C until analysis.
For TF-mRNA quantification the Abi Prism 7700 (Applied Biosystems) was used. Primers were designed by Primer Express Software (Applied Biosystems) and synthesized based on the human TF cDNA sequence: 680 forward (F), 5′-CCCGAACAGTTAACCGGAAGA-3′ and 773 reverse (R), 5′-GCTCCAATGATGTAGAATATTTCTCTGA-3′, and TaqMan probe 711T FAM, CTCCTGGCCCATACACTCTACCGGG TAMRA (6-carboxytetramethylrhodamine). Because of its stable expression under endotoxemia (B.J., unpublished data, January 2003), 18s was used as a housekeeping gene for multiplexing (Applied Biosystems). TF-mRNA was normalized against the reference gene (18s) according to the cycle threshold (CT) method,19 and data are expressed as fold increase over baseline values. Dilution curves of TF-mRNA obtained from LPS-incubated blood samples revealed linearity (r = 0.999) of the assay up to 37.5 cycles, which was set as the limit of sensitivity.
In vitro incubation experiments with rhAPC
In order to clarify whether the prolongation in aPTT could be a direct effect of rhAPC, we spiked fresh citrated plasma (n = 4) with increasing concentrations of rhAPC (12.5, 25, 50, 100, 200, and 400 ng/mL), that is, the concentration range found in the PROWESS study.16 aPTT measurements were repeated after freezing and thawing once.
Data analysis
Data are expressed as mean and 95% confidence interval (CI) or median and interquartile ranges (IQRs) in case of highly skewed distribution. All statistical comparisons of continuous variables were made with nonparametric tests. After 2-way analysis of variance (ANOVA), the Friedmann ANOVA and the Wilcoxon test for post hoc comparisons were used to assess time-dependent chances in outcome variables within groups. Differences between groups were confirmed by the U test, which included one U test for the area under the curve (AUC) calculated for the primary outcome variable F1 + 2 (using Origin 7.0; Origin Lab, Northampton, MA). The Spearman correlation was used to measure correlation between parameters. A 2-tailed P < .05 was considered significant. All statistical calculations were performed using commercially available statistical software (Statistica Vers. 5.0; Stat Soft, Tulsa, OK). Power calculation was done as previously described.20
Results
There was no difference in baseline parameters between groups (n = 15 in the rhAPC group, n = 9 in the placebo group; Table 1). No severe, serious, or unexpected adverse effects were observed after LPS infusion, and infusion of rhAPC produced no untoward effects. From 25 randomized healthy volunteers, the last dropped out because he presented to the ward with a broken toe on the study day.
Pharmacokinetic/pharmacodynamic (PK/PD) effects of rhAPC on APC, PC:Act, PC:Ag, and aPTT
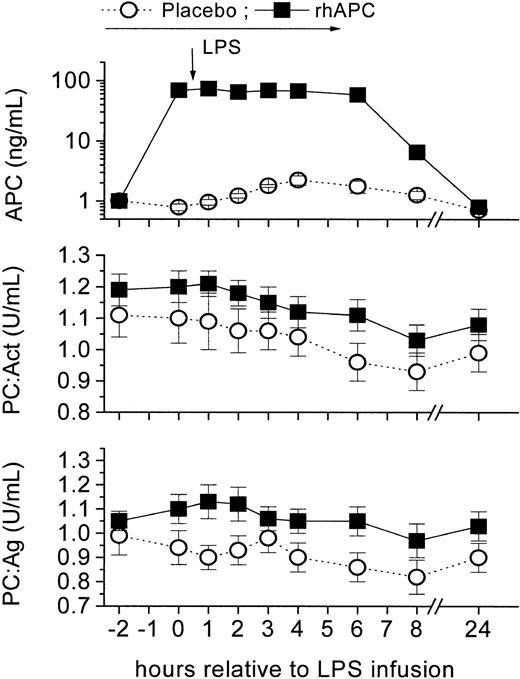
rhAPC infusion significantly increased APC levels 60- to 70-fold throughout the infusion period and steady-state APC concentrations of 60 to 80 ng/mL were obtained, comparable with the maximal effects observed in the PROWESS trial16 (P < .001 vs baseline, Figure 1). In contrast, APC levels increased 2.25-fold (CI, 1.41-3.06; P < .01) in the placebo group 4 hours after LPS infusion. Protein C activity (PC:Act) decreased by approximately 13% in the rhAPC group and by approximately 16% in the placebo group (Figure 1). Similarly, protein C antigen (PC:Ag) levels decreased on average by 8% and by 17% in the rhAPC and placebo groups, respectively. Thus, only APC levels but not PC:Act or PC:Ag were consistently different between treatment groups (from 0-8 hours; P < .001).
Pharmacokinetics of recombinant human activated protein C (rhAPC) in the human endotoxin (LPS) model. Healthy male volunteers received rhAPC (24 μg/kg per hour for 8 hours; ▪), which increased APC levels 70-fold (top graph), or placebo (○) intravenously starting 2 hours before LPS bolus infusion (2 ng/kg), which enhanced endogenous protein C activation (2.3-fold; 95% CI, 1.4-3.1). Friedman ANOVA for PC:Ag and PC:Act in both groups: P < .001; P = ns between groups; mean ± SEM.
Pharmacokinetics of recombinant human activated protein C (rhAPC) in the human endotoxin (LPS) model. Healthy male volunteers received rhAPC (24 μg/kg per hour for 8 hours; ▪), which increased APC levels 70-fold (top graph), or placebo (○) intravenously starting 2 hours before LPS bolus infusion (2 ng/kg), which enhanced endogenous protein C activation (2.3-fold; 95% CI, 1.4-3.1). Friedman ANOVA for PC:Ag and PC:Act in both groups: P < .001; P = ns between groups; mean ± SEM.
rhAPC prolonged aPTT by 20% (P < .001 between groups, Figure 2), while aPTT decreased by 9% at 6 hours in the placebo group, which is consistent with previous observations.17 In order to clarify whether the prolongation in aPTT could be a direct effect of rhAPC, we spiked plasma with increasing concentrations of rhAPC (12.5-400 ng/mL), that is, the concentration range found in the PROWESS study.16 Indeed, rhAPC dose dependently prolonged aPTT values in fresh plasma (by 10% and 20% at concentrations of 50 and 100 ng/mL, respectively). Thus the magnitude of aPTT prolongation observed in vivo was nicely reproduced by our in vitro incubation experiments, an effect that was lost after freeze-thawing. This indicates a direct effect due to the presence of APC in plasma.
Recombinant human activated protein C (rhAPC) prolongs activated partial thromboplastin time (aPTT) but decreases tissue factor (TF)–mRNA before LPS infusion. Healthy male volunteers received rhAPC (24 μg/kg per hour for 8 hours; ▪) or placebo (○) intravenously starting 2 hours before LPS bolus infusion (2 ng/kg). rhAPC increased aPTT levels (top graph) by 20% (P < .001 between groups). rhAPC decreased only basal TF-mRNA (bottom graph) by – 32% (CI, – 49 to – 14%; *P < .05 between groups at 0 hour). P < .001 versus baseline in both groups; mean ± SEM.
Recombinant human activated protein C (rhAPC) prolongs activated partial thromboplastin time (aPTT) but decreases tissue factor (TF)–mRNA before LPS infusion. Healthy male volunteers received rhAPC (24 μg/kg per hour for 8 hours; ▪) or placebo (○) intravenously starting 2 hours before LPS bolus infusion (2 ng/kg). rhAPC increased aPTT levels (top graph) by 20% (P < .001 between groups). rhAPC decreased only basal TF-mRNA (bottom graph) by – 32% (CI, – 49 to – 14%; *P < .05 between groups at 0 hour). P < .001 versus baseline in both groups; mean ± SEM.
Effects of rhAPC on LPS-induced coagulation
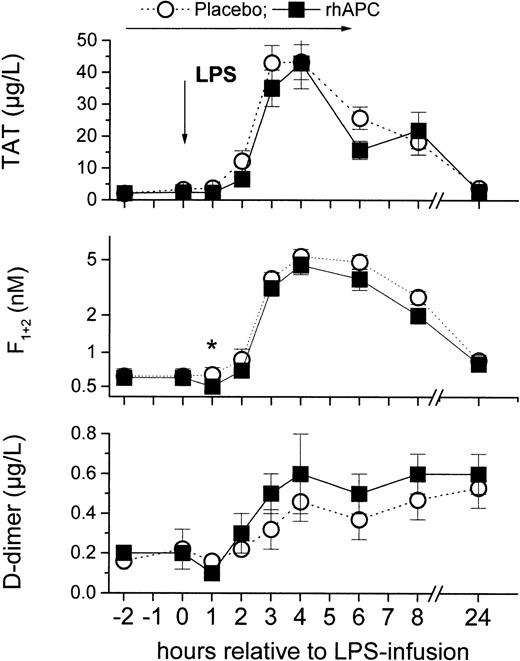
The primary finding of this randomized controlled trial with rhAPC was that rhAPC did not significantly blunt LPS-induced thrombin generation (F1 + 2 and TAT levels; Figure 3): LPS infusion enhanced TAT levels 20- and 21-fold in the rhAPC and in the placebo group, and F1 + 2 levels increased on average 7- to 8-fold in either group at 4 hours (P = ns [not significant] between groups). The AUCF1 + 2 was only trendwise lower in the rhAPC group (44; 95% CI, 33-56) compared with the placebo group (56; 95% CI, 45-67; P = .09).
Effects of recombinant human activated protein C (rhAPC) on endotoxin (LPS)–induced coagulation. Healthy male volunteers received rhAPC (24 μg/kg per hour for 8 hours; ▪) or placebo (○) intravenously starting 2 hours before LPS challenge (2 ng/kg). rhAPC slightly reduced thrombin formation and action during the first 3 hours of infusion (indicated by asterisk), but LPS-induced thrombin generation was not blunted: thrombin antithrombin complexes (TAT; top graph), prothrombin fragment (F1 + 2; middle graph), or d-dimer (bottom graph). *P < .005 versus baseline in the rhAPC groups and for relative change between groups, P < .001 for Friedman ANOVA for both groups, and P = ns between groups; mean ± SEM.
Effects of recombinant human activated protein C (rhAPC) on endotoxin (LPS)–induced coagulation. Healthy male volunteers received rhAPC (24 μg/kg per hour for 8 hours; ▪) or placebo (○) intravenously starting 2 hours before LPS challenge (2 ng/kg). rhAPC slightly reduced thrombin formation and action during the first 3 hours of infusion (indicated by asterisk), but LPS-induced thrombin generation was not blunted: thrombin antithrombin complexes (TAT; top graph), prothrombin fragment (F1 + 2; middle graph), or d-dimer (bottom graph). *P < .005 versus baseline in the rhAPC groups and for relative change between groups, P < .001 for Friedman ANOVA for both groups, and P = ns between groups; mean ± SEM.
Consistently, rhAPC did not alter LPS-induced levels of upstream TF-mRNA (Figure 2) and downstream d-dimer (Figure 3). TF-mRNA was detectable at very low levels in all subjects at baseline (Table 1), and increased 16- to 17-fold in either treatment group (P = .01 vs baseline; Figure 2) at 4 hours. Similarly, plasma levels of the fibrin split product d-dimer increased to a similar extent in both groups (P = ns between groups).
Currently, we can only speculate about the reasons why rhAPC has been ineffective in reducing LPS-induced, TF-mediated coagulation in healthy volunteers. First, as clinically relevant APC plasma levels were reached, we recalculated the power based on the variability observed in the current trial: we had an 80% power to detect a 40% lower F1 + 2 generation in the rhAPC group. This is deemed adequate in view of the 5- to 10-fold difference in the F1 + 2 formation between various anticoagulants and placebo observed previously in this model.10-12,21-23
Importantly, we would like to emphasize that our model is certainly not a sepsis model: based on our results we would not anticipate that rhAPC could effectively down-modulate sepsisinduced disseminated intravascular coagulation (DIC), whereas tissue factor pathway inhibitor (TFPI)12 was effective in this model but failed in a phase 3 trial in septic patients.24
In our model a true difference between placebo and rhAPC may be masked by the normal protein C levels in healthy subjects, whereas most septic patients are protein C–deficient.3 Similarly, our volunteers all had normal global coagulation tests at baseline, suggestive that they were having normal baseline levels of coagulation factors in the circulation. This is also in contrast to septic patients25 and levels of coagulation factors FII and FX have been reported to inhibit the anticoagulant potency of APC.26 As FII and FX levels drop during sepsis-induced DIC,27 these endogenous inhibitors may lose relative importance.
Finally, it has been shown that during meningococcemia the endothelial protein C receptor (EPCR) may be down-regulated on the microvasculature.28 Thus, one may assume that high concentrations of exogenous APC may partly compensate for this endothelial loss of EPCR and the concomitant loss in protein C activation.
However, we should recognize that the anticoagulant potency of rhAPC appears to be moderate even in septic patients: d-dimer levels decreased from approximately 4.2 μg/mL by only 30% to approximately 3 μg/mL.3 This is still 6-fold higher than the upper-normal limit of d-dimer levels in healthy subjects.
Effects of rhAPC on “spontaneous” coagulation activation
The second major finding of interest was that our results are compatible with an anticoagulant effect of rhAPC under basal conditions in healthy volunteers. While TAT levels increased 2-fold in the placebo group at 1 hour, the increase of TAT levels was delayed in the rhAPC group until 2 hours after LPS infusion. Consistently, rhAPC decreased F1 + 2 to 0.54 nM (CI, 0.50-0.59) at one hour, while no decrease was observed in the placebo group (P < .05 for relative change between groups for both parameters).
While basal TF-mRNA levels decreased by –32% (CI, –49% to –14%) in the rhAPC group, they increased slightly by 21% (CI, –36% to 77%) in the placebo group at 0 hour (P < .05 between groups). Similarly d-dimer levels decreased about one third to 0.14 μg/L (CI, 0.07-0.2; P < .01 vs baseline) in the rhAPC group at one hour, whereas no early decrease was observed in the placebo group.
Thus, rhAPC decreased “spontaneous” generation of TF-mRNA (Figure 2), F1 + 2, and d-dimer at 0 hour (Figure 3) and delayed the early LPS-induced increase in TAT observed at 1 hour in the placebo group (Figure 3). It remains to be determined whether the decrease in TF-mRNA was a direct consequence of rhAPC binding to monocytes, which are known to express a protein C receptor,29 or an indirect effect due to decreased thrombin generation. Overall, the consistent decrease in spontaneous thrombin generation and action is in line with the protective effects of endogenous protein C levels against “spontaneous” thrombotic disease30 and its higher prevalence in APC-resistant subjects.31
Effect of rhAPC on inflammatory markers, and endothelial and platelet activation
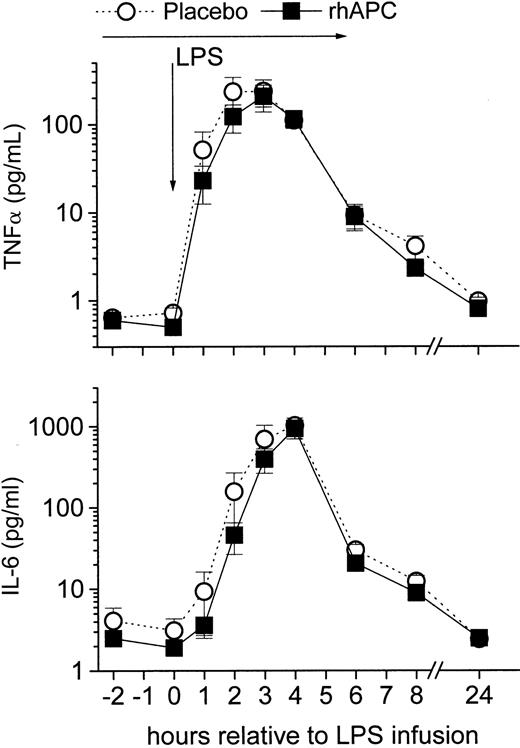
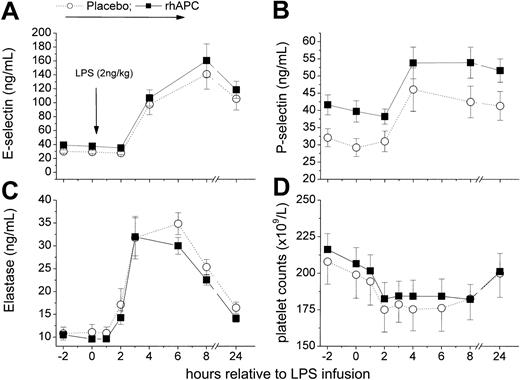
rhAPC did not alter systemic inflammation as measured by TNF-α and IL-6 release (Figure 4). Consistently, rhAPC did not alter plasma levels of markers of fibrinolysis (tPA, PAI-1, and PAP; Figure 5), of endothelial activation (E-selectin), and leukocyte degranulation (elastase; Figure 6), which are all dependent on TNF-α action in this model.32 Soluble E-selectin increased on average 4- to 5-fold in either group (P = ns between groups). The lack of effect on E-selectin release is in contrast to an in vitro study in which 181 nmol rhAPC decreased endothelial E-selectin expression. This discrepancy between results is possibly due to the suprapharmacologic rhAPC concentrations used for the in vitro experiment.9
Effects of recombinant human activated protein C (rhAPC) on endotoxin (LPS)–induced cytokine release. Healthy male volunteers received rhAPC (24 μg/kg per hour for 8 hours; ▪) or placebo (○) intravenously starting 2 hours before LPS challenge (2 ng/kg). rhAPC did not significantly reduce LPS-induced inflammation: tumor necrosis factor alpha (TNF-α; top graph) or interleukin-6 (IL-6; bottom graph) levels. P < .001 versus baseline for both groups, and P = ns between groups; mean ± SEM.
Effects of recombinant human activated protein C (rhAPC) on endotoxin (LPS)–induced cytokine release. Healthy male volunteers received rhAPC (24 μg/kg per hour for 8 hours; ▪) or placebo (○) intravenously starting 2 hours before LPS challenge (2 ng/kg). rhAPC did not significantly reduce LPS-induced inflammation: tumor necrosis factor alpha (TNF-α; top graph) or interleukin-6 (IL-6; bottom graph) levels. P < .001 versus baseline for both groups, and P = ns between groups; mean ± SEM.
Activation and subsequent inhibition of fibrinolysis by endotoxin. Healthy male volunteers received rhAPC (24 μg/kg per hour for 8 hours; ▪) or placebo (○) intravenously starting 2 hours before LPS challenge (2 ng/kg). Plasmin antiplasmin complexes (PAP; top graph), tissue plasminogen activator (tPA; middle graph), or plasminogen activator inhibitor (PAI-1; bottom graph). P < .001 versus baseline for both groups, and P = ns between groups; mean ± SEM.
Activation and subsequent inhibition of fibrinolysis by endotoxin. Healthy male volunteers received rhAPC (24 μg/kg per hour for 8 hours; ▪) or placebo (○) intravenously starting 2 hours before LPS challenge (2 ng/kg). Plasmin antiplasmin complexes (PAP; top graph), tissue plasminogen activator (tPA; middle graph), or plasminogen activator inhibitor (PAI-1; bottom graph). P < .001 versus baseline for both groups, and P = ns between groups; mean ± SEM.
Effects of recombinant human activated protein C (rhAPC) on endotoxin (LPS)–induced endothelial, platelet, or neutrophil activation. (A) E-selectin. (B) P-selectin. (C) Elastase. (D) Platelet counts. Healthy male volunteers received rhAPC (24 μg/kg per hour for 8 hours; ▪) or placebo (○) intravenously starting 2 hours before LPS challenge (2 ng/kg). P < .001 versus baseline for both groups, and P = ns between groups; mean ± SEM.
Effects of recombinant human activated protein C (rhAPC) on endotoxin (LPS)–induced endothelial, platelet, or neutrophil activation. (A) E-selectin. (B) P-selectin. (C) Elastase. (D) Platelet counts. Healthy male volunteers received rhAPC (24 μg/kg per hour for 8 hours; ▪) or placebo (○) intravenously starting 2 hours before LPS challenge (2 ng/kg). P < .001 versus baseline for both groups, and P = ns between groups; mean ± SEM.
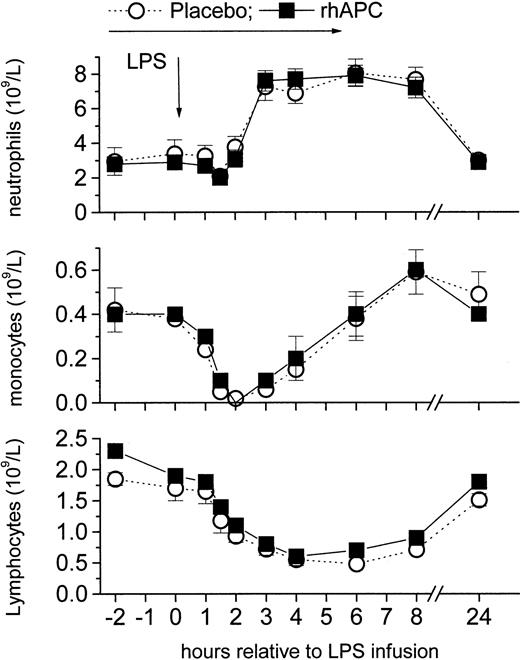
Similarly, rhAPC infusion did not alter the LPS-induced changes in the platelet activation marker P-selectin (Figure 6) and blood counts (Figure 7), which all changed by the expected order of magnitude.
Effects of recombinant human activated protein C (rhAPC) on endotoxin (LPS)–induced changes in white blood cell counts. Healthy male volunteers received rhAPC (24 μg/kg per hour for 8 hours; ▪) or placebo (○) intravenously starting 2 hours before LPS challenge (2 ng/kg). rhAPC did not alter white blood counts of neutrophils (top graph), monocytes (middle graph), or lymphocytes (bottom graph). P < .001 versus baseline for both groups; and P = ns between groups; mean ± SEM.
Effects of recombinant human activated protein C (rhAPC) on endotoxin (LPS)–induced changes in white blood cell counts. Healthy male volunteers received rhAPC (24 μg/kg per hour for 8 hours; ▪) or placebo (○) intravenously starting 2 hours before LPS challenge (2 ng/kg). rhAPC did not alter white blood counts of neutrophils (top graph), monocytes (middle graph), or lymphocytes (bottom graph). P < .001 versus baseline for both groups; and P = ns between groups; mean ± SEM.
Effect of rhAPC on hemodynamics
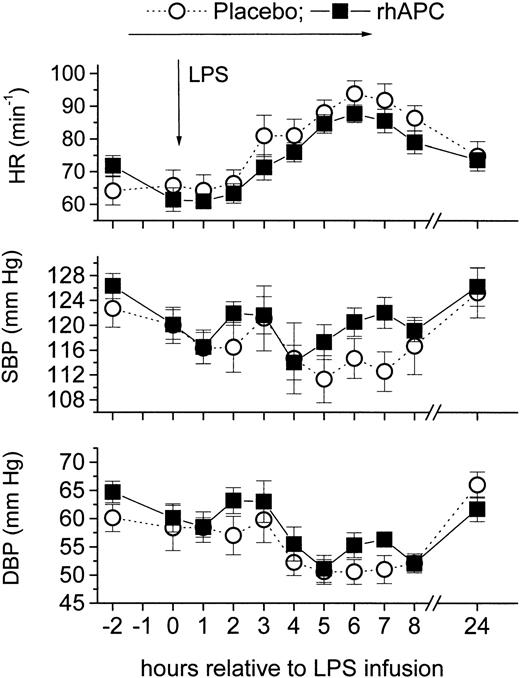
LPS infusion decreased systolic and diastolic blood pressure without any differences between the 2 groups. Mean arterial blood pressure decreased by approximately 10% after LPS infusion. In both groups heart rate increased to a maximum of 90 minutes-1 (CI, 85-95) at 5 hours (P = ns between groups; Figure 8).
Effects of recombinant human activated protein C (rhAPC) on endotoxin (LPS)–induced changes in vital parameters. Healthy male volunteers received rhAPC (24 μg/kg per hour for 8 hours, ▪) or placebo (○) intravenously starting 2 hours before LPS challenge (2 ng/kg). rhAPC did not significantly alter vital parameters: heart rate (HR; top graph), systolic blood pressure (SBP; middle graph), and diastolic blood pressure (DBP; bottom graph). P < .001 for Friedman ANOVA for both groups, and P = ns between groups; mean ± SEM.
Effects of recombinant human activated protein C (rhAPC) on endotoxin (LPS)–induced changes in vital parameters. Healthy male volunteers received rhAPC (24 μg/kg per hour for 8 hours, ▪) or placebo (○) intravenously starting 2 hours before LPS challenge (2 ng/kg). rhAPC did not significantly alter vital parameters: heart rate (HR; top graph), systolic blood pressure (SBP; middle graph), and diastolic blood pressure (DBP; bottom graph). P < .001 for Friedman ANOVA for both groups, and P = ns between groups; mean ± SEM.
Correlation between APC and coagulation or inflammation markers
To our knowledge, this is the first study that exactly characterizes endogenous activation of protein C in low-grade endotoxemia: our data show a more than 2-fold increase in endogenous APC levels 4 hours after LPS infusion (Figure 1). Further studies are necessary to prove the mechanism of protein C activation in this model. However, correlation analyses were performed to characterize potential associations between peak APC generation and markers of coagulation or inflammation. A good correlation was found between TNF-α (r = 0.68-0.95 for 0-8 hours; P < .001) and peak APC levels (at 4 hours) in the placebo group, but no correlation with coagulation markers.
This could possibly reflect an effect of TNF-α on the activation of APC or its rate of clearance (eg, by down-modulation of EPCR on endothelial cells). Conversely, it is very unlikely that endogenous APC decreased TNF-α generation in the placebo group, because we observed no effects of pharmacologic concentrations of rhAPC on TNF-α release (Figure 4).
There was no correlation between those parameters in the rhAPC group, but a moderate inverse correlation between elastase release and rhAPC levels (r = –0.53 to –0.59; P < .05) and PC:Act (r = –0.68 to –0.81; P < .01) in this group. This observation is in line with the report that APC is degraded by elastase in vitro.33 However, this correlation was moderate in our study and is not proof for a cause-effect relationship.
Discussion
As discussed above, our model is evidently not a sepsis model and we do not know whether another innate immunity pathway, not involving APC, may be the dominant factor in this setting. Alternatively, rhAPC could potentially act as antiapoptotic drug, and therefore counteract the presumed deleterious consequences of apoptosis in septic patients.32-37 Nonetheless, our data showing that rhAPC had only limited effect on any of the parameters measured during low-grade endotoxemia are in line with the current strategy and FDA recommendations to administer rhAPC only to patients with severe sepsis, where APC may act as an endothelial function modulator.
In summary, even low-grade endotoxemia induces significant protein C activation. Infusion of rhAPC (24 μg/kg per hour) decreases “spontaneous” activation of coagulation but has only minimal effects on LPS-induced, TF-mediated coagulation in healthy volunteers, which is in contrast to a number of anticoagulants.
Prepublished online as Blood First Edition Paper, May 15, 2003; DOI 10.1182/blood-2003-02-0416.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.