Abstract
Primary myelodysplasia (MDS) without an increased number of blasts is a rare finding in childhood. We performed a retrospective analysis of 67 children with a diagnosis of primary MDS to determine the clinical and hematologic course of the disease. The median age at diagnosis was 8.3 years (range, 0.3-18.1 years). In contrast to refractory anemia in adults, 44% of patients had hemoglobin levels greater than 10 g/100 mL. The median white blood cell count and the absolute neutrophil count were 3.6 × 109/L and 0.9 × 109/L, respectively. Seventy-five percent of patients had thrombocytopenia. Bone marrow was hypocellular in 43% of the patients. Results of cytogenetic analysis showed monosomy 7 in 49%, trisomy 8 in 9%, and other abnormalities in 9% of the patients. The probability of survival 10 years after diagnosis was 0.48 (standard error [SE] = 0.10). Patients with monosomy 7 had significantly higher estimated probabilities of progression to advanced MDS than did patients with other chromosomal anomalies or normal karyotype. Of the 67 children, 41 underwent allogeneic stem cell transplantation (SCT). Patients whose disease did not progress to advanced MDS before SCT had significantly greater probability of survival than patients who experienced progression (0.76 [SE = 0.09] vs 0.36 [SE = 0.16]). SCT improved the outcomes for patients with monosomy 7 and should be offered early in the course of the disease. Recommendations for best treatment options for children with other chromosomal abnormalities or normal karyotype may have to await results of prospective clinical trials.
Introduction
Myelodysplastic syndrome (MDS) is a heterogeneous group of acquired hematopoietic stem cell disorders characterized by peripheral blood cytopenia, ineffective and dysplastic hematopoiesis, and a varying propensity to leukemic transformation. The number of blasts in the bone marrow influences the natural history of the disease. In adults, MDS without an increased number of blasts and a low risk for leukemic progression is usually characterized by anemia and by normocellular or hypercellular marrow with erythroid hyperplasia. The French-American-British (FAB) group coined the term refractory anemia (RA) for these disorders.1 This nomenclature has been accepted in the recent World Health Organization (WHO) classification.2
In childhood, MDS with less than 5% blasts in the bone marrow is particularly difficult to diagnose because dysplasia of hematopoietic cells is frequently observed during infection, metabolic disorder, nutritional deficiency, and a variety of other diseases.3-6 After treatment of the underlying disorder, these dysplastic changes generally resolve. The presence of a cytogenetic abnormality in hematopoietic cells is helpful in confirming a diagnosis of MDS, but in the absence of such an abnormality the diagnosis is difficult. We have retrospectively reviewed the clinical and laboratory findings in a group of 67 children with refractory anemia to determine the natural history of the disease and to identify prognostic factors for disease progression and outcome.
Patients, materials, and methods
Data of 67 children younger than 19 years with a diagnosis of RA from Canada (n = 4), Czech Republic (n = 2), Denmark (n = 8), Germany (n = 18), Italy (n = 9), The Netherlands (n = 9), and the United Kingdom (n = 17) were analyzed. Diagnosis, clinical, and hematologic data of all the patients were reviewed nationally by the respective cooperative groups. Patients with a history of constitutional bone marrow failure disorders or constitutional chromosomal abnormalities and patients who underwent previous chemotherapy or radiotherapy were excluded. To rule out Fanconi anemia, hypersensitivity testing to DNA cross-linking agents was performed in most patients. Because of different national review structures, accrual time and age at diagnosis varied among nations. Overall accrual was from December 1979 to June 1998. Data were analyzed as of April 28, 2001. We previously reported data on 21 patients included in this series.7-11 Follow-up was available for all patients in the study.
Given the retrospective nature of the study, data were missing for several patients for some of the parameters. For patients with missing data for white blood count (WBC), platelet count, hemoglobin (Hb) differential count, or peripheral blood (PB) differential count at the time of diagnosis, values for later time points were reported. Normal ranges for mean corpuscular volume (MCV) of red blood cells according to age were modified according to Dallman and Siimes.12 BM biopsy findings for 2 patients without evaluable bone marrow (BM) aspirate at the time of diagnosis were indicative of RA. Biopsies were performed in 50 patients. BM cellularity was judged on biopsy. Of the 17 patients for whom biopsy was not performed, the aspirate showed a reduced cell number in 5. These 5 patients had been classified as having MDS because of the presence of dysplasia in at least 2 cell lines and because 4 of the 5 patients had abnormal karyotypes. Data on dysplasia scores for myelopoiesis, erythropoiesis, and megakaryopoiesis were available for 61, 64, and 58 patients, respectively. Chromosomal analyses of BM cells were performed by standard techniques by the national reference laboratories or by the local treatment center. For correlation between karyotype and clinical findings, patients were subdivided into 3 karyotype groups as follows: normal karyotype, monosomy 7, trisomy 8, or other cytogenetic abnormalities.
Overall survival was calculated from the date of diagnosis to the date of death or last follow-up. Advanced MDS was defined as a sustained increase in BM blasts of 5% or more. Time to progression to advanced MDS was defined as the time from date of the diagnosis of RA to the date of progression to more advanced MDS. To calculate the risk for progression, patients who underwent stem cell transplantation (SCT) before progression were censored at the time of allograft. Survival after SCT was calculated from the date of SCT to the date of death or last follow-up.
The Kaplan-Meier method was used to estimate survival rates. Standard errors were calculated using the Greenwood formula. The 2-sided log-rank test was used to test the equality of survivorship functions in different subgroups.13 To acknowledge the presence of competing risks, the incidence of progression to advanced MDS was expressed as cumulative hazard rates.14,15 The Kruskal-Wallis H test is used to compare the medians of 3 or more groups (Mann-Whitney U test).16 Spearman rank correlation (ordinal data) and Cramer V (categorical data) were used to analyze associations between prognostic factors.17,18
Results
Clinical presentation
Thirty boys and 37 girls in this study had RA. The median age at diagnosis was 8.3 years (range, 0.3-18.1 years). The most common presenting symptoms were malaise (60%), bleeding (45%), fever (22%), and infection (21%). For 19% of patients, no clinical signs or symptoms were reported. In 2 patients, both with monosomy 7, diagnoses were made during work-up for BM donation for sibling transplantation. Lymphadenopathy, most likely caused by infections, was seen in 4 patients; 1 patient had a slightly enlarged spleen. None of the patients had hepatomegaly. Congenital abnormalities were observed in 9 (13%) patients. They included Dandy Walker malformation (2 patients), von Willebrand disease type 2, dysmorphic features with mental retardation or with congenital heart disease, pes equino varus, hypermelanosis, platelet storage pool disorder, and factor XI deficiency. The median interval between the onset of symptoms and diagnosis was 3.5 months (range, 0-84 months).
Hematologic data
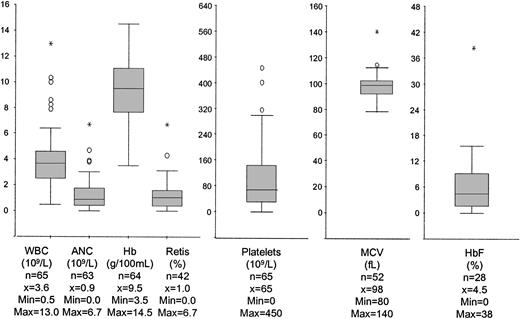
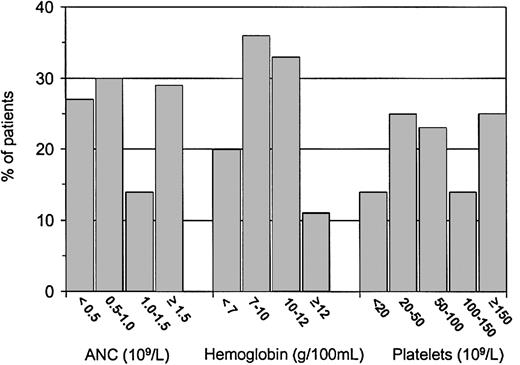
At diagnosis, the median WBC count was 3.6 × 109/L (Figure 1). Severe neutropenia was noted in 27% of patients (Figure 2). In 2 patients, PB monocytosis (> 1 × 109/L) developed in the absence of organomegaly or clinical course indicative of chronic myelomonocytic leukemia. An increase in PB eosinophils and basophils (more than 5%) was seen in 6 and 1 patients, respectively. Two patients had absolute eosinophil counts exceeding 1 × 109/L. Most patients had thrombocytopenia; 75% had platelet counts below 150 × 109/L (Figure 2). Hemoglobin concentrations and reticulocyte counts varied over a wide range. Fifty-six percent of patients had Hb levels below 100 g/L (10 g/100 mL), and 11% had Hb levels within the normal range (Figure 2). Macrocytosis of RBCs evaluated according to patient age was seen in 75% of children, and HbF level was elevated in 82% of the 28 patients in whom it was measured. There was no correlation between HbF and MCV, BM, cellularity, or karyotype.
Hematologic data in peripheral blood at diagnosis. Box plots graphically display the median (horizontal line), 1st quartile (25%), 3rd quartile (75%), nonoutliers, outliers (○), and extreme values (*).
Hematologic data in peripheral blood at diagnosis. Box plots graphically display the median (horizontal line), 1st quartile (25%), 3rd quartile (75%), nonoutliers, outliers (○), and extreme values (*).
BM cellularity was increased in 25% of patients, normal in 33% of patients, and reduced in 43% of patients. Within the 3 groups with hypercellular, normocellular, and hypocellular BM, the median WBC count was 4.7 × 109/L, 3.4 × 109/L, and 2.7 × 109/L, respectively (P < .01). There were no differences among the 3 groups with respect to other PB parameters, karyotype, or survival. Erythroid predominance with a ratio of myelopoiesis to erythropoiesis of less than 1 was noted in 45% of patients. Dysplasia of red cells, white cells, and megakaryocytes was reported in 85%, 58%, and 58% of cases, respectively. The presence of dysplasia did not correlate with progression to advanced MDS.
Chromosomal abnormalities
Data on the karyotypes of BM cells at diagnosis were available for 66 of the 67 patients. Karyotype was normal in 22 children; 32 patients had monosomy 7, 6 had trisomy 8, and 6 had other aberrations. Among the 6 children with a chromosomal abnormality other than monosomy 7 or trisomy 8, 2 had a loss of 7q material. Two of the patients with monosomy 7 and 3 of the patients with trisomy 8 had additional abnormalities. There were no differences among patients with normal karyotype, monosomy 7, or trisomy 8 and other abnormalities with respect to age, sex, median values for WBC count, ANC, platelet count, MCV, fetal hemoglobin (HbF), or BM cellularity. Patients with monosomy 7 had significantly higher Hb levels (median, 106 g/L [10.6 g/100 mL]; range, 41-126 [4.1-12.6]) than patients with normal karyotype (median, 85 g/L [8.5 g/100 mL]; range, 35-145 [3.5-14.5]) (P < .01).
Serial chromosomal studies were performed in 44 patients with a median of 4 (range, 2-14) analyses per patient. The original chromosomal aberrations persisted in all patients. Karyotypic evolution was noted in 5 patients (Table 1).
With the exception of children with normal karyotype, the original aberrations remained in all patients. In each patient, karyotypic evolution was accompanied by progression to advanced MDS. Abnormal karyotypes other than monosomy 7 alone are listed in Table 1.
Clinical course and progression to advanced stages of MDS
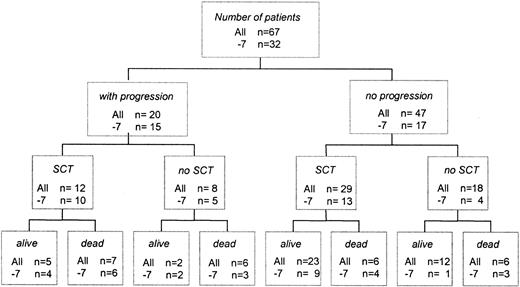
At the time of analysis, 25 patients had died and 42 were alive (Figure 3). Overall survival at 10 years was 0.48 ([SE] = 0.10). Four children died within 0.1 to 12 months from diagnosis because of complications from pancytopenia before any specific therapy or progression to advanced MDS. Two other children died as a result of intensive chemotherapy delivered for RA in the absence of disease progression. Twelve patients were alive with stable disease without SCT (Figure 3). Of the 29 children who underwent transplantation before the progression of MDS, 23 were alive. Twenty patients had progression to advanced MDS. Of those, 12 underwent transplantation and 8 had received intensive chemotherapy only. In total, 13 children received intensive chemotherapy. Granulocyte–colony-stimulating factor before intensive therapy or SCT had been administered to 3 patients; 4 children had been treated with immunosuppressive therapy consisting of cyclosporine and antithymocyte globulin.
Flow sheet indicating clinical course and outcome of children with refractory anemia. Numbers given refer to the total study population regardless of karyotype (all) and to patients with monosomy 7 (–7).
Flow sheet indicating clinical course and outcome of children with refractory anemia. Numbers given refer to the total study population regardless of karyotype (all) and to patients with monosomy 7 (–7).
For the 20 patients with progression to advanced MDS, the median time to progression was 1.7 years (range, 0.2-5.3 years). The cumulative incidence of progression was significantly higher for patients with monosomy 7 than for patients with other chromosomal abnormalities or with normal karyotype (Figure 4).
Cumulative incidence of progression to advanced MDS for patients with RA and either normal karyotype, monosomy 7, trisomy 8, or other abnormalities at the time of diagnosis. Patients who underwent SCT were censored at the time of transplantation.
Cumulative incidence of progression to advanced MDS for patients with RA and either normal karyotype, monosomy 7, trisomy 8, or other abnormalities at the time of diagnosis. Patients who underwent SCT were censored at the time of transplantation.
Stem cell transplantation
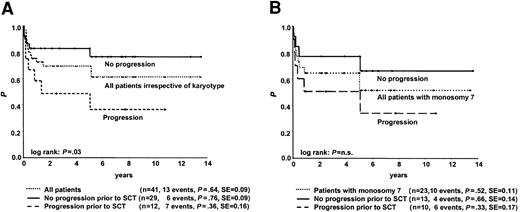
Of the 67 children, 41 underwent allogeneic SCT at a median time of 16 months (range, 1-124 months) from diagnosis. In 17 transplantations the donor was an HLA-compatible family donor (MFD), and in 24 transplantations the donor was an unrelated volunteer (MUD). Stem cell sources were bone marrow, peripheral blood, and umbilical cord in 34, 5, and 2 patients, respectively. Preparative regimens varied widely and included total body irradiation (TBI) in 21 patients and busulfan in the remaining 20. After SCT, 28 patients are alive, 2 are in relapse, and 11 died of transplantation-related causes. The latter included graft failure (n = 2), graft-versus-host disease (n = 4), infection (n = 4), and thrombocytopenic purpura (n = 1). Probability of survival at 6 years was 0.64 ± 0.18 for the 41 children (Figure 5), with results for patients grafted from either an MFD or an MUD 0.73 ± 0.24 and 0.55 ± 0.25, respectively (P = NS). There was no difference in outcome after SCT according to year of transplantation, though it must be considered that more MUD transplantations have been performed since 1995 than in the earlier years (P = .03).
Survival from the time of SCT for children with diagnoses of RA with or without progression to advanced MDS before SCT. (A) Data for all children irrespective of karyotype. (B) Data for children with monosomy 7. n.s. indicates not significant.
Survival from the time of SCT for children with diagnoses of RA with or without progression to advanced MDS before SCT. (A) Data for all children irrespective of karyotype. (B) Data for children with monosomy 7. n.s. indicates not significant.
Twelve patients experienced progression to advanced MDS before SCT at a median of 6 months (range, 1-27 months) from diagnosis. Patients without progression before SCT had significantly better probability of survival than patients who had disease progression (P = .03; Figure 5). Two patients, both of whom had monosomy 7, had relapses after SCT. One of these patients underwent transplantation with the diagnosis of RA, and the other had progression before SCT. There were no significant differences in survival after SCT according to karyotype.
Survival according to karyotype
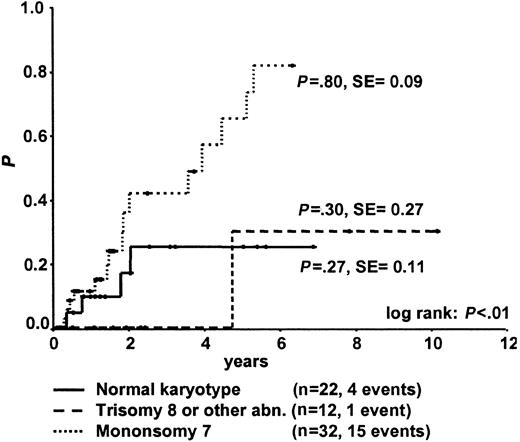
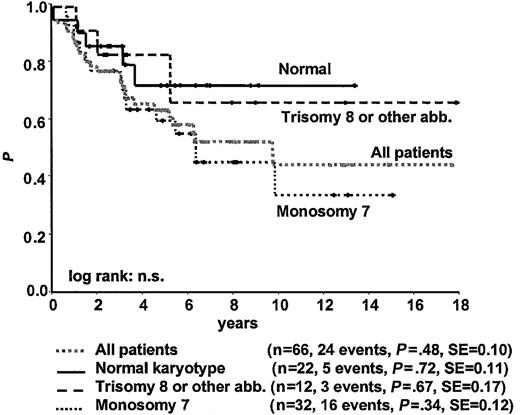
No statistically significant difference was observed in survival according to karyotype at time of diagnosis among patients with normal karyotype, monosomy 7, trisomy 8, or other karyotype (Figure 6; Tables 2, 3). However, including the 2 patients with normal karyotype who subsequently acquired a karyotypic abnormality in the respective karyotype group, survival in patients with persistently normal karyotype was significantly better than it was in patients with monosomy 7 (P = .02) but not in patients with other karyotypes. Without SCT, patients with monosomy 7 had poor survival (Tables 2, 3). Children with trisomy 8 or other karyotypes who underwent transplantation tended to have lower WBC counts, more platelet transfusions, and greater hypocellular BM than children who did not undergo transplantation. Four of the 12 patients with trisomy 8 or other abnormalities were alive with stable disease without SCT at 2.2, 2.3, 3.2, and 7.9 years after diagnosis.
Survival from the time of diagnosis for 66 children with RA according to karyotype.
Survival from the time of diagnosis for 66 children with RA according to karyotype.
Discussion
Patients with RA, as defined by the FAB cooperative group for myelodysplastic disorders, are usually older than 50; anemia is the main presenting symptom. Low hemoglobin level is associated with reticulocytopenia, dyserythropoiesis, and infrequently dysgranulopoiesis.19 Occasionally, other cytopenias are present at diagnosis.20 Children differ in their hematologic presentation because neutropenia and thrombocytopenia are more frequently observed at diagnosis. In our cohort, more than half the patients presented with an ANC less than 1.0 × 109/L or a platelet count less than 100 × 109/L; some had both. Although the term anemia, as in aplastic anemia or Fanconi anemia, is often used for pancytopenic states, we suggest that the term refractory cytopenia (RC) is more appropriate for this group of MDS patients and will be used from here on.21,22
Cytopenia from ineffective hematopoiesis in a hypercellular or normocellular bone marrow is a hallmark of MDS.23 Bone marrow cellularity is generally estimated on biopsy specimens.24 In the study presented here, biopsies had not been performed in all patients. Taking this limitation into account, 39% of children with RC had reduced cellularity, a percentage similar to that reported for RA in some of the adult series.25,26 However, we excluded from the study patients with RC that evolved from acquired or constitutional bone marrow failure disorders. Therefore, marrow hypocellularity may be more frequent among all patients with low-grade MDS in childhood. There was no correlation between cellularity and karyotype.
In the FAB classification, RA is defined as having dysplasia largely restricted to the erythroid line.1 The recently proposed WHO classification continued to define RA as a disorder involving the erythroid lineage only, but it separated patients with dysplastic features in the cells of 2 or more cell lines.2 In the latter, patients have worse prognoses because of marrow failure or leukemic progression.27,28 In our study, more than half the patients had granulocytic or megakaryocytic dysplasia or both. There was, however, no correlation between lineage dysplasia and disease progression. Prospective studies on childhood RC will clarify whether dysplasia in 2 or more cell lines is of prognostic relevance.
Although dysplastic features were most often observed in the red cell precursors, erythroid hyperplasia was present in 45% of children only. Macrocytosis of red cells, a finding characteristic of RA,29 was noted in 75% of the patients, whereas HbF levels were elevated in approximately 80% of the patients in whom they were measured. In contrast to juvenile myelomonocytic leukemia (JMML), there was no correlation between MCV or HbF and karyotype. Interestingly, children with RC and monosomy 7 had significantly higher Hb concentrations than patients with normal karyotype, a finding not observed in the JMML group.30
The pathogenesis of MDS remains largely obscure. Although chromosomal changes are not thought to be initiating events, they are probably involved in disease progression. Karyotypic evolution, noted here in 5 of the 44 patients with serial chromosomal studies, was accompanied by progression to more advanced forms of MDS. Of the 21 patients with normal karyotype at diagnosis and follow-up, 2 subsequently acquired chromosomal aberrations. Serial chromosomal analyses are helpful in the assessment of children with RC.
The frequency of chromosomal abnormalities varies between FAB types of MDS. In adult RA, karyotypic changes are seen in approximately 20% to 30% of patients.31,32 In this series, chromosomal aberrations were noted in 67% of children for whom results of standard cytogenetic studies were available. This result must, however, be interpreted with caution. The presence of a cytogenetic abnormality can be an important means to confirm the diagnosis of MDS. Because of the difficulty in establishing the diagnosis of childhood RC, it is likely that patients with chromosomal changes were preferentially reported in this retrospective study. Furthermore, patients with monosomy 7 may be overrepresented because data of some of these patients had previously been collected and analyzed.7 Nevertheless, our results confirm reports of other investigators5,33,34 indicating that monosomy 7 is the most common cytogenetic abnormality in childhood RC. In contrast, monosomy 7 is noted in less than 5% of adult RA.31,32,35
Although monosomy 7 is frequently accompanied by multiple karyotypic rearrangements in adult MDS, in childhood MDS it is often the sole abnormality; additional changes were noted in only 5 of 33 patients analyzed here. It is of interest that MDS was diagnosed in 2 children with monosomy 7 during the work-up for bone marrow harvest for sibling transplantation. Familial predisposition for MDS is not infrequent and may be associated with the loss of chromosome 7 or the partial deletion of its long arm.36 Morphologic and cytogenetic examinations of bone marrow cells are recommended for all potential sibling donors of MDS patients.
The second most frequent (9%) chromosomal abnormality in our series was trisomy 8, which may occur with similar frequency in children and adults with MDS.31 Loss of the long arm of chromosome 5 (5q–), the most frequent chromosomal aberration in adults with RA,31,35 is exceedingly rare in children with RA37 and was not observed in any of our patients.
Karyotype was the most important prognostic factor for progression to advanced MDS and survival. The estimated median time to progression for children with RC and monosomy 7 was 1.9 years. SCT in patients with RC and monosomy 7 early in the course of the disease and before progression was accompanied by better posttransplantation survival. Of the 13 children with monosomy 7 who received grafts without increased numbers of blasts, 1 had a relapse, 3 died of toxicity, and 9 are alive without disease. Although monosomy 7 is generally considered a poor prognostic predictor irrespective of therapy,35,38 our data indicate that monosomy 7 may not have a negative impact on survival after SCT for childhood RC. Two children with RC and monosomy 7 were treated with intensive chemotherapy in the absence of disease progression. Both died of therapy complications. There is no evidence that intensive chemotherapy has a role in the management of children with RC. Whether cytoreductive therapy before SCT for more advanced forms of MDS improves survival remains controversial.
In contrast to monosomy 7, only 4 of the 22 patients with normal karyotype had progression to more advanced MDS. This low rate of leukemic transformation may raise the question whether some of these children had an unrecognized constitutional disorder with dysplasia and marrow failure rather than acquired MDS. In the absence of chromosomal changes or other markers of clonality, the diagnosis of RC is difficult and it is important to define minimum diagnostic criteria in this group of patients.21 Twelve of the 22 children with RC and normal karyotype received grafts. Although we did not collect data on the clinical course of peripheral blood counts, one can assume that children who underwent SCT experienced more severe cytopenia. SCT might be the only curative therapy option for this patient group, but other treatment modalities such as immunosuppressive therapy39,40 and the use of hematopoietic growth factors41,42 have not been systematically studied in this pediatric patient cohort. It is notable that 4 of the 67 study patients died of pancytopenia within 1 year of diagnosis. In the absence of a suitable stem cell donor, these alternative therapy approaches might be offered to children with RC and severe cytopenia.
The overall survival of the 67 children was 0.48 ± 0.20 at 10 years. For patients with monosomy 7 or disease progression, survival largely depended on outcome after SCT. Similar to what has been observed for young patients in other series,43,44 the probability of survival of the 29 children who underwent transplantation before progression to advanced MDS was 0.76 ± 0.18. Because transplantation-related toxicity was the major cause of death,43,45 preparative regimens with reduced intensity are attractive but could increase the relapse rate.46
The results from this largest series of childhood RC clearly indicate that children with RC and monosomy 7 should be offered SCT early in the course of their disease. Spontaneous disappearance of monosomy 7 and cytopenia has been noted in some infants,47 but it remains a rare event. Karyotype analyses in our study and in previous reports were based on standard metaphase-banding cytogenetics. The significance of small monosomy 7 clones detected by fluorescence in situ hybridization (FISH) only remains to be determined.48 Because of small patient numbers and variable outcomes, recommendations regarding therapy for RC with chromosomal changes other than monosomy 7 cannot be drawn from this retrospective study. In the presence of a normal karyotype, a substantial proportion of children with RC will experience a long, stable disease course. An expectant approach with careful observation may be reasonable care for these patients in the absence of transfusion requirements, severe cytopenia, or infections.49 SCT early in the course of the disease has generally been reserved for patients with a matched sibling donor. Because results of MUD transplantations have improved and are becoming comparable to those reported for MFD transplantation, early referral for SCT before prolonged cytopenia or disease progression has been recommended recently.50,51 Future prospective multicenter trials will have to identify prognostic factors for disease progression and define the subgroup of these children with RC and normal karyotype who will benefit from early SCT.
Prepublished online as Blood First Edition Paper, May 22, 2003; DOI 10.1182/blood-2002-11-3444.
Supported in part by Deutsche José Carreras Stiftung e.V. and BMBF Compentence Net “Pediatric Oncology” and by grant IGA NE 5676-4 from the Ministry of Health of the Czech Republic.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the patients, families, and physicians who referred the patients. We also thank Charles Stiller, Pat Brownbill, and Janette Wallis for their help with the United Kingdom MDS registry.