Abstract
Several bone marrow cells and lymphocyte subpopulations, known as “veto cells,” were shown to induce transplantation tolerance across major histocompatibility antigens. Some of the most potent veto cells are of T-cell origin, and in particular a very strong veto activity was documented for cytotoxic T-lymphocyte (CTL) lines or clones. However, these cells also possess marked graft-versus-host (GVH) reactivity. In the present study we evaluated a new approach to deplete CTLs of antihost clones by stimulating the donor T cells against third-party stimulators in the absence of exogenous interleukin 2 (IL-2). We demonstrate that such CTLs are depleted of GVH reactivity while maintaining marked veto activity in vitro. Furthermore, marked synergism was exhibited between the veto CTLs and rapamycin when tested in a murine model, which measures T-cell–mediated bone marrow allograft rejection, or in sublethally irradiated allogeneic hosts.
Our results suggest that engraftment of early progenitors could be enhanced by using host-nonreactive anti–third-party CTLs, in conjunction with nonmyeloablative rapamycin-based conditioning protocols, thereby significantly reducing the toxicity of allogeneic transplantation.
Introduction
Bone marrow transplantation following supralethal radiochemotherapy is associated with dangerous infections due to the slow immune reconstitution during the first year after transplantation.1-6 Thus, the use of reduced-intensity conditioning, associated with less severe immune ablation, could have remarkable potential in the treatment of a variety of nonmalignant diseases or for the induction of “mixed chimerism” as a prelude for cell therapy in cancer or in organ transplantation. However, the marked level of host hematopoietic and immune cells surviving mild preparatory regimens represents a difficult barrier for the engraftment of donor cells.
In patients with advanced hematologic malignancies who cannot withstand myeloablative conditioning because of age and/or performance status, recent attempts were made to develop low-toxicity conditioning protocols in conjunction with human leukocyte antigen (HLA)–matched transplants.7-10 Potent posttransplantation immunosuppression and the presence of large numbers of alloreactive T cells in the graft enabled a high rate of engraftment. However, graft-versus-host disease (GVHD), particularly lethal chronic GVHD, remains a major obstacle.9,11-13 While in high-risk leukemia such transplant-related mortality is acceptable, it would be totally intolerable if applied to patients with long life expectancy. Thus, the use of purified allogeneic stem cells, which do not pose any risk for GVHD and which can continuously present donor-type antigens in the host thymus, thereby inducing durable tolerance to donor cells or tissues, represents one of the most desirable goals in transplantation biology.
One approach to overcoming immune rejection of incompatible stem cells rigorously depleted of T cells made use initially of increased doses of T-cell–depleted bone marrow in mice14-17 and rats.18 Subsequently the cell-dose escalation concept was also shown with purified stem cells.19-22 However, although this modality has become a clinical reality in the treatment of patients with leukemia conditioned by intensive chemotherapy, it has been suggested in studies in mice21 and nonhuman primates (X. Yao, unpublished data, July 2001) that the number of hematopoietic precursors required to overcome the immune barrier in hosts pretreated with sublethal regimens cannot be attained with the state-of-the-art technology for stem cell mobilization. Rachamim et al23 demonstrated that when purified CD34+ cells were added to bulk mixed-lymphocyte reaction (MLR) they suppressed cytotoxic T lymphocytes (CTLs) against matched stimulators but not against stimulators from a third party.23 These results strongly indicated that cells within the human CD34+ population are endowed with potent veto activity. Veto activity was defined in 1980 by Miller24 as the capacity to specifically suppress cytotoxic T-cell precursors (CTL-p) directed against antigens of the veto cells themselves but not against third-party antigens.
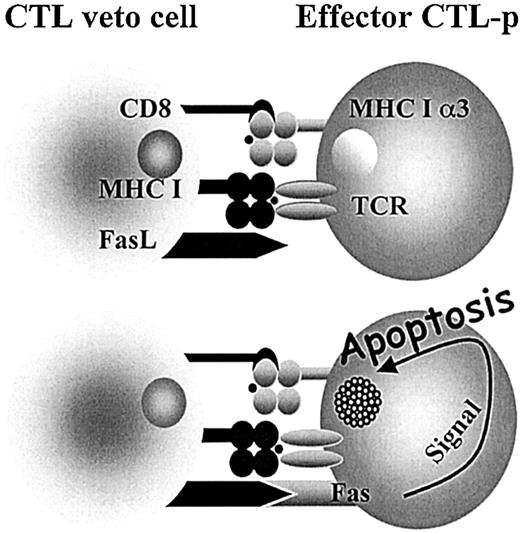
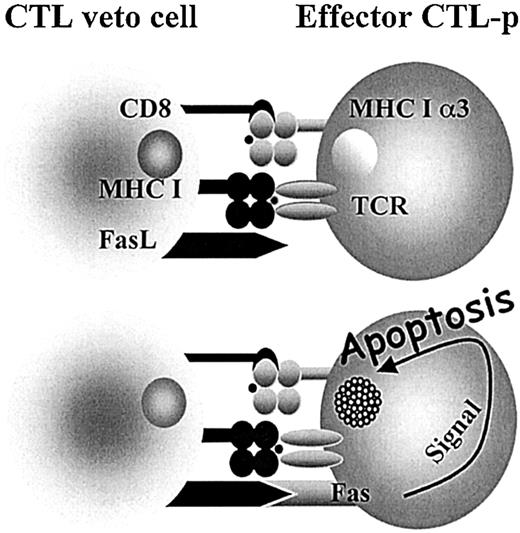
Interestingly, it has been shown that some of the most potent veto cells are of T-cell origin and, in particular, a very strong veto activity was documented for CD8+ CTL lines or clones.25-31 The specificity of the veto effect mediated by CTL clones was shown by several studies to be unrelated to their T-cell–receptor specificity.32-34 The suppression of effector CTL-p directed against the veto cells is both antigen specific and major histocompatibility complex (MHC) restricted, resulting from the unidirectional recognition of the veto cell by the responding CTLs, but not vice versa.33 Furthermore, it has been shown that this suppression is mediated by apoptosis33,35 and that coexpression of both CD8 and Fas ligand (FasL) is a prerequisite for the veto reactivity of the CTLs36 (Figure 1).
Veto CTLs induce apoptosis in the effector T cells by a Fas-FasL–mediated mechanism. Upon engagement between the TCR of the effector cell and class I (MHCI) of the veto cell, the effector cell is activated and Fas is up-regulated, allowing for the FasL on the veto CTL to induce apoptosis. However, inhibitors such as FLIP (FADD-like interleukin 1–converting enzyme-inhibitory protein) protect the activated effector T cell. The high affinity afforded by the interaction between CD8 on the veto cell and class I (MHCIα3) on the effector cell enables prolonged association until FLIP is down-regulated and apoptosis can take place. 36
Veto CTLs induce apoptosis in the effector T cells by a Fas-FasL–mediated mechanism. Upon engagement between the TCR of the effector cell and class I (MHCI) of the veto cell, the effector cell is activated and Fas is up-regulated, allowing for the FasL on the veto CTL to induce apoptosis. However, inhibitors such as FLIP (FADD-like interleukin 1–converting enzyme-inhibitory protein) protect the activated effector T cell. The high affinity afforded by the interaction between CD8 on the veto cell and class I (MHCIα3) on the effector cell enables prolonged association until FLIP is down-regulated and apoptosis can take place. 36
Clearly, the limitations of CD34+ collection in humans might be overcome if it was possible to supplement these cells with other veto cells provided the latter also lack GVH reactivity. Despite their remarkable potent veto activity, CD8 CTLs could not be used for tolerance induction in allogeneic stem cell transplantation due to their marked GVH reactivity. To address this problem we developed a new approach for the generation of host-nonreactive CTLs, based on stimulation of donor CD8 T cells against third-party stimulators under interleukin 2 (IL-2) deprivation. This approach is based on the observation that only the activated CTLs are capable of surviving the IL-2 starvation in the primary culture.36
In the present study we optimized the conditions that afford large numbers of anti–third-party CTLs under IL-2 deprivation, and we tested the efficacy of such cells to induce specific transplantation tolerance without GVHD.37 Furthermore, considering that previous studies documented the role of the immunosuppressive drug rapamycin in GVHD prevention38 and in overcoming rejection,39 the potential synergy between rapamycin and veto CTLs was evaluated.
Materials and methods
Experimental procedures
Animals. We used 6- to 12-week-old female mice. Balb/c, Balb/c-Nude, FVB, SJL, F1(C3HxBalb), and C57BL/6 mouse strains were obtained from the Weizmann Institute Animal Breeding Center (Rehovot, Israel). C3H/HeJ mice were obtained from the Roscoe B. Jackson Memorial Laboratory (Bar Harbor, ME). A breeding pair of transgenic (Tg) H-2b mice expressing the T-cell receptor (TCR) from the CTL clone 2C with specificity for H-2Ld was kindly provided by Janko Nikolic-Zugic (Sloan-Kettering, New York, NY). Progeny of these Tg mice was bred at the Weizmann Institute Animal Breeding Center. All mice were kept in small cages (5 animals in each cage) and fed sterile food and acid water containing cyprofloxacin (20 μg/mL).
Preparation of donor nonhost reactive CTLs. Spleen cells from Balb/c mice (donor origin), 6 to 16 weeks of age were harvested, lysed in (0.5 mL/spleen) cold ACK buffer (0.15 M potassium-ammonium chloride buffer) to remove red blood cells, and brought to a concentration of 2 × 106 cells/mL in RPMI 1640 supplemented with 15 mm HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid; pH 7.4), 10% heat-inactivated fetal calf serum, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, 2 mM l-glutamine, 5 × 10–5 2-β-mercaptoethanol (2-ME), 100 U/mL penicillin, and 0.1 mg/mL streptomycin. This splenic single-cell suspension was cocultured at a ratio of 1:2 with irradiated (20 Gy) ACK-treated spleen cells from C57BL/6 or FVB mice (third-party origin) and cultured at 37°C in a humidified 5% CO2 95% O2 atmosphere.
Six days later the cocultures were fractionated on Ficoll and the lymphoid fraction was enriched for CD8+ cells by positive selection using magnetically labeled anti-CD8 antibodies and a magnetic cell sorting (MACS) system (Miltenyi Biotec, Bergisch Gladbach, Germany). The CD8-enriched fraction was then resuspended at 0.5 × 106 cells/mL and cocultured at a ratio of 1:4 with the same irradiated (20 Gy) third-party stimulators in the presence of 40 IU/mL recombinant human IL-2 (rhIL-2). The CTL cultures were restimulated weekly and rhIL-2 was added every 3 days for 2 more weeks.
In vitro assay of anti–third-party veto CTLs: deletion of 2C effector CD8 T cells. To determine whether nonalloreactive donor anti–third-party CTLs possess veto activity, spleen cells of 2C transgenic H-2b mice expressing the TCR-αβ with specificity for H-2Ld mice40 (kindly provided by Janko Nikolic-Zugic, Sloan-Kettering), were prepared as described in “Preparation of donor nonhost reactive CTLs.” The cells (2 × 106/mL) were then stimulated with irradiated (20 Gy) Balb/c splenocytes (2 × 106/mL) in the presence of 10% cells of the veto anti–third-party CTLs from Balb/c origin while anti–third-party CTLs of C57BL/6 and/or SJL background served as nonspecific controls. Cultures containing 10% veto CTLs were cultured for 72 hours in 6-well plates. The deletion of the transgenic T cells was monitored by cytofluorimetric analysis measuring the level of 2C transgenic cells specifically stained by the 1B2 antibody40 directed against the clonotypic anti–H-2Ld TCR.
Evaluation of apoptosis by flow cytometry using Cy 5–annexin V
Cells (1-2 × 105) were stained with Cy 5–annexin V (MBL Medical & Biological Laboratories, Naka-ku Nagoya, Japan) according to manufacturer's protocol. Briefly, the cells were suspended in 100 μL binding buffer (10 mM HEPES/NaOH, pH 7.4; 140 mM NaCl; 2.5 mM CaC12) and incubated for 10 minutes. The cells were then washed with binding buffer and analyzed by flow cytometry.
Bone marrow transplantation. For GVH reactivity determination, C3H/HeJ mice were exposed to a single dose of 11 Gy (lethal conditioning) total body irradiation (TBI) from a Gamma beam 150-A 60Co source (produced by the Atomic Energy of Canada, Kanata, ON, Canada) with focal skin distance of 75 cm at a 0.65 Gy/minute dose rate. The following day the mice intravenously received an optimal dose (2 × 106) of Balb/c-Nude bone marrow (BM) supplemented with 2 × 106 anti–third-party CTLs of specific (Balb/c) and nonspecific (SJL) origin and unmanipulated donor splenocytes.
In the experiments studying the synergistic effect between veto CTLs and rapamycin under reduced conditioning, C3H/HeJ mice were sublethally irradiated (7 Gy) and 24 hours later inoculated with Balb/c-Nude BM (4-5 × 106) with or without veto CTLs (10 × 106). Chimerism was determined 30 days after transplantation.
Chimerism analysis. Chimerism was determined by cytofluorimetry. Peripheral blood cells were fractionated on Ficoll-Paque plus, and the isolated mononuclear cells of each mouse were double-stained by direct immunofluorescence with fluorescein isothiocyanate (FITC) anti-H2d monoclonal antibody specific for the donor and phycoerythrin (PE) anti-H2k for the specific host. The antibodies were obtained from Pharmingen (San Diego, CA): FITC–H-2Dd (clone 34-2-12) and PE–H-2Kk (clone 36-7-5).
T-cell–mediated allograft rejection model. C3H/HeJ female mice (8-12 weeks of age) were exposed to a single dose of 11Gy (supralethal conditioning) TBI on day –2. The following day the mice intravenously received 1 × 104 unseparated host T cells. Transplantation of 2 × 106 allogeneic Balb/c–Nude BM cells was performed on day 0 in conjunction with the veto cells to be evaluated. The weight of the mice and the survival rate were monitored twice a week.
Host T-cell preparation. Splenocytes of host C3H/HeJ mice were fractionated on Ficoll/Paque and the isolated mononuclear cells were subjected to a positive selection of T cells (CD4 plus CD8) by magnetic cell sorting (MACS). Cytofluorimetric analysis of the fractionated cells is carried out by triple immunofluorescent staining using the following directly labeled antibodies (Pharmingen): FITC-CD4/L3T4 (clone H129.19), PE-CD3e (clone 145-2C11) and Cy-Chrome–CD8a/Ly-2 (clone 53-6.7).
Cytofluorimetric analysis. Fluorescence-activated cell sorter (FACS) analysis was performed using a modified Becton Dickinson FACScan (Temse, Belgium). Fluorescence data were collected using 3-decade logarithmic amplification on 25 × 103 to 50 × 103 viable cells as determined by forward–light-scatter intensity. Cells were stained with a CD8a (Ly-2)–FITC, CD4 (L3T4)-CyChrome, CD8a (Ly-2)–allophycocyanin (APC), CD3ϵ-PE (Pharmingen), CD4-Qantum Red (Sigma, St Louis, MO), 1B2 biotinated (kindly provided by Janko Nikolic-Zugic, Sloan-Kettering), R-PE streptavidin (Jackson Immuno Research Lab, West Grove, PA).
Results
Optimization of an IL-2 deprivation protocol for generating anti–third-party veto CTLs devoid of GVH reactivity
Cell recovery and purity. We have shown in vitro that host-nonreactive anti–third-party CTLs generated under IL-2 deprivation for 5 days are endowed with marked veto activity.36 The IL-2 deprivation period was established based on the maximal time that mouse spleen T cells can be maintained in MLR culture without IL-2 (data not shown). However, attempts to grow large cell numbers for the investigation of these veto cells in vivo revealed irregularities in the cell composition of the harvested cells due to difficulties in controlling outgrowth of lymphokine-activated killer (LAK) cells and/or CD4 T cells (data not shown). Thus, this problem was addressed by removal of CD4+ and natural killer (NK) cells at the end of the IL-2 deprivation, prior to the addition of IL-2.
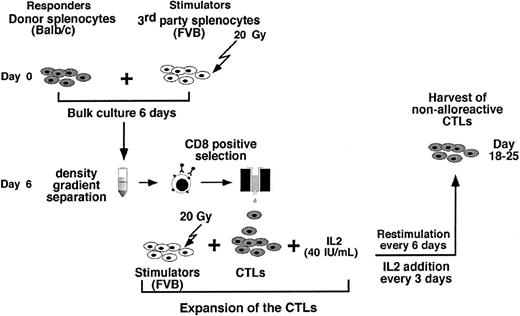
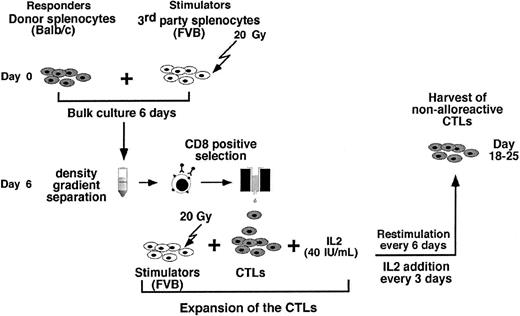
As can be seen in Figure 2 the preparation of anti–third-party CTLs comprises 3 major steps: (1) anti–third-party stimulation under IL-2 deprivation for 6 days; (2) positive selection of CD8+ T cells (at day 6); and (3) CTL expansion under restimulation in the presence of IL-2 (day 6 to 25).
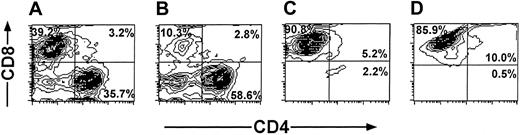
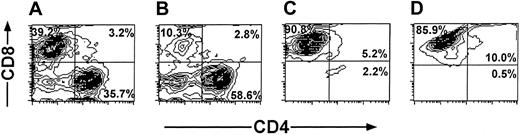
The cell recoveries obtained in 5 experiments from Balb/c donors and in 1 experiment from FVB mice are shown in Table 1. The average number of the splenocytes in the initial cell preparation was 2023 × 106 ± 579 × 106. Following 6 days of incubation with irradiated (20 Gy) third-party splenocytes at an effector-stimulator ratio of 1:2, live mononuclear cells were harvested by Ficoll density separation (316 × 106 ± 213 × 106) and the number of CD8 T cells recovered after positive selection with magnetic beads was further reduced to 69 × 106 ± 50 × 106 cells. These anti–third-party CTLs were then restimulated and cultured in the presence of IL-2 (40 IU/mL), affording 437 × 106 ± 116 × 106 cells within 18 to 25 days. FACS analysis of the cells that were positively selected for CD8 at day 6, as well as the cells harvested at the end of the culture period, showed that more than 90% of the cells are CD8+CD3+ T cells (Figure 3).
Purification profile of anti–third-party CTLs detected by FACS analysis. Anaylsis was carried out at day 6 (A-C) and at the end of the culture period (D). Unseparated cells (A). CD8– fraction (B). CD8+fraction (C-D).
Purification profile of anti–third-party CTLs detected by FACS analysis. Anaylsis was carried out at day 6 (A-C) and at the end of the culture period (D). Unseparated cells (A). CD8– fraction (B). CD8+fraction (C-D).
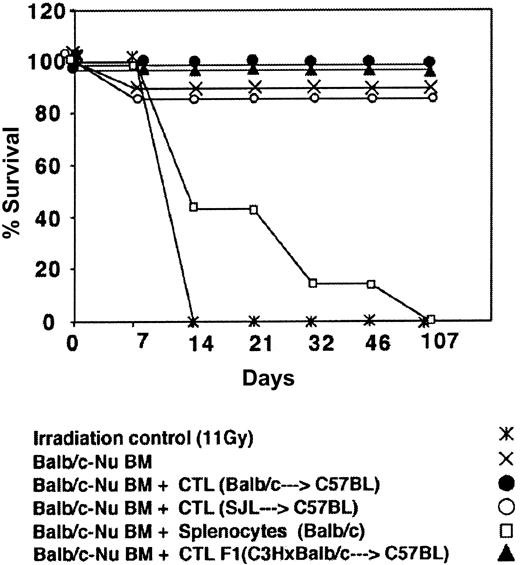
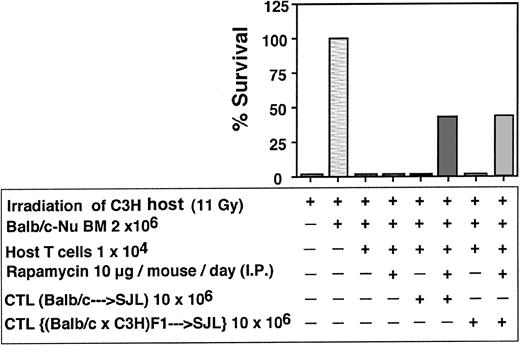
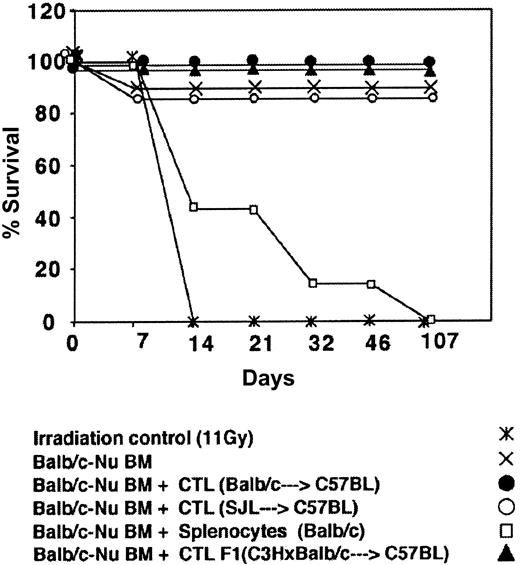
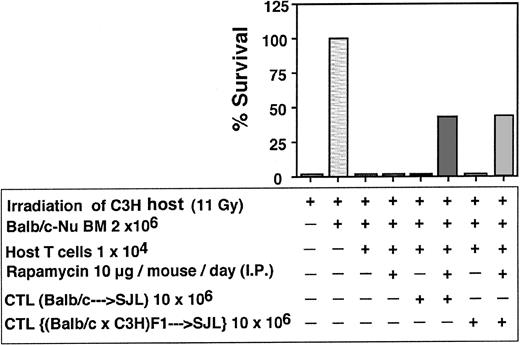
Depletion of GVH reactivity by IL-2 deprivation of anti–third-party CTLs. The CTLs recovered by the above protocol were routinely tested for GVH reactivity in fully mismatched hosts, lethally irradiated, and radio-protected with BM from Balb/c-“Nude” donors. As can be seen in Figure 4 showing a typical experiment, when C3H/HeJ hosts were conditioned by 11 Gy TBI all the mice died within 10 days while more than 80% of the mice receiving 2 × 106 BM cells from Balb/c-Nude donors survived. Infusion of 2 × 106 veto CTLs originating from Balb/c, F1(C3HxBalb), or SJL donors, together with the BM, was associated with 0%, 0%, and 15% mortality, respectively, in contrast to transplantation of 2 × 106 Balb/c splenocytes together with the BM cells that led to more than 80% mortality within the first month. Thus, the IL-2 deprivation procedure for the preparation of anti–third-party CTL culture is associated with marked depletion of GVHD reactivity.
Donor (Balb/c) anti–third-party (C57BL/6) CTLs are depleted of GVH reactivity against the host (C3H/HeJ). Host mice were conditioned with 11 Gy TBI and radio-protected with 2 × 106 Balb/c-Nude BM cells. The GVH reactivity of the CTLs or the unseparated spleen cells is reflected by the percent survival following infusion of 2 × 106 cells.
Donor (Balb/c) anti–third-party (C57BL/6) CTLs are depleted of GVH reactivity against the host (C3H/HeJ). Host mice were conditioned with 11 Gy TBI and radio-protected with 2 × 106 Balb/c-Nude BM cells. The GVH reactivity of the CTLs or the unseparated spleen cells is reflected by the percent survival following infusion of 2 × 106 cells.
In vitro determination of veto activity of anti–third-party CTLs in the 2C TCR transgenic mouse model. Considering that the limit dilution analysis assay used to monitor inhibition of CTL-p by veto cells is lengthy and cumbersome, we tested whether effector T cells from a TCR transgenic mouse model could afford a more convenient assay for veto activity, allowing the use of FACS for monitoring deletion of effector T cells by the veto CTLs. For this purpose, spleen cells of the 2C TCR transgenic mouse, in which the CD8 T cells express a TCR transgene directed against H-2d class I,40 were used as effector cells. The transgene can be stained by a clonotypic monoclonal antibody (1B2 kindly provided by Janko Nikolic-Zugic, Sloan-Kettering) allowing to monitor expansion or deletion of the effector cells upon stimulation in the presence or absence of veto cells.
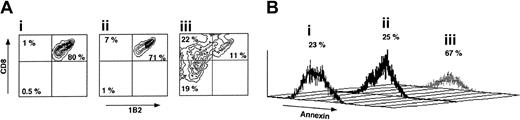
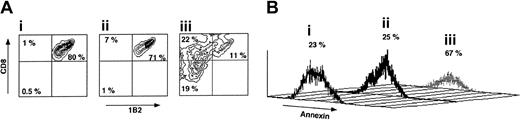
As can be seen in Figure 5, following a short incubation for 72 hours with veto CTLs the percentage of CD8+1B2+ cells is reduced from 80% to 11% if the CTLs are of H-2d origin, compared with reduction from 80% to 71% upon incubation with CTLs generated from spleen cells of H-2s background. Furthermore, annexin staining of the CD8+1B2+ showed that marked apoptosis is induced by the veto cells between 48 and 72 hours of incubation with the veto cells, in agreement with previous reports suggesting that veto CTLs exert their inhibitory effect by a deletion based mechanism.36 Along with the depletion of CD8+1B2+ cells by the specific veto cells, the CD8–1B2– and CD8+1B2– cell subpopulations were notably increased from 0.5% and 1%, respectively, in Figure 5Ai and to 19% and 22%, respectively, in Figure 5Aiii.
The veto activity of third-party CTLs. (A) Specific deletion of 2C effector CD8 T cells by veto CTLs following 72 hours of incubation. (i) 2C levels in the absence of veto CTLs. (ii) 2C levels in the presence of nonspecific veto CTLs of C57BL/6 background. (iii) 2C levels in the presence of veto CTLs of Balb/c background (recognized by the 2C TCR transgene). (B) Annexin staining demonstrating the induction of apoptosis by the specific veto CTLs described in Ai-iii.
The veto activity of third-party CTLs. (A) Specific deletion of 2C effector CD8 T cells by veto CTLs following 72 hours of incubation. (i) 2C levels in the absence of veto CTLs. (ii) 2C levels in the presence of nonspecific veto CTLs of C57BL/6 background. (iii) 2C levels in the presence of veto CTLs of Balb/c background (recognized by the 2C TCR transgene). (B) Annexin staining demonstrating the induction of apoptosis by the specific veto CTLs described in Ai-iii.
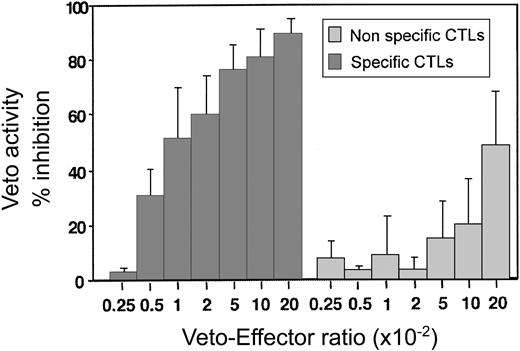
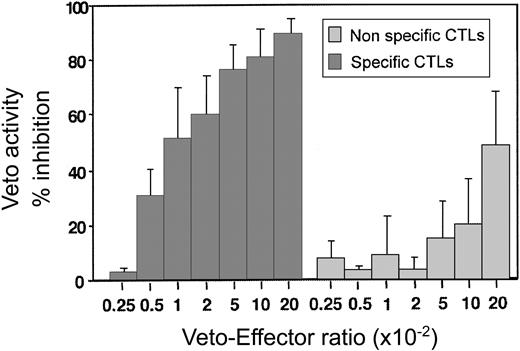
By using this assay the optimal effector–veto cell ratio was found to be between 1:20 and 1:50 (Figure 6). At this ratio the nonspecific veto activity by veto cells generated from mice of SJL origin (H-2s) is minimal. Likewise, as expected from studies based on the LDA of CTL-p, resting CD8 T cells of H-2d background, which can be recognized by the effector T cells but which have not been activated, lack veto activity and do not delete the 2C effector cells (data not shown.) Taken together, these results show that upon generation of host-nonreactive CTLs as described in this paragraph, it is possible to assess by a short and simple FACS assay the veto activity of the generated line prior to further in vivo experiments.
A dose-response curve comparing the inhibitory effect of specific anti–third-party CTLs recognized by the 2C effectors (origin of H-2d) and nonspecific CTLs (origin of H-2s). Average ± standard deviation of 5 different experiments.
A dose-response curve comparing the inhibitory effect of specific anti–third-party CTLs recognized by the 2C effectors (origin of H-2d) and nonspecific CTLs (origin of H-2s). Average ± standard deviation of 5 different experiments.
In vivo determination of tolerance induction by veto CTLs: synergy between veto CTLs, bone marrow cell dose, and rapamycin
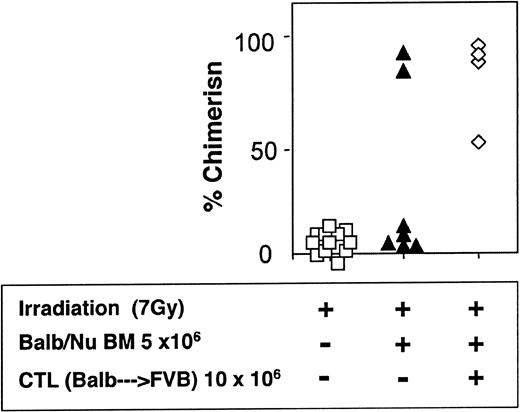
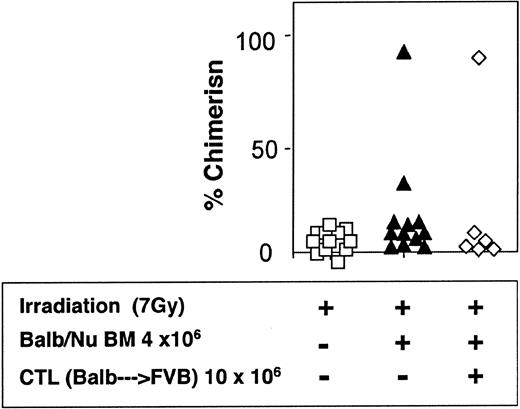
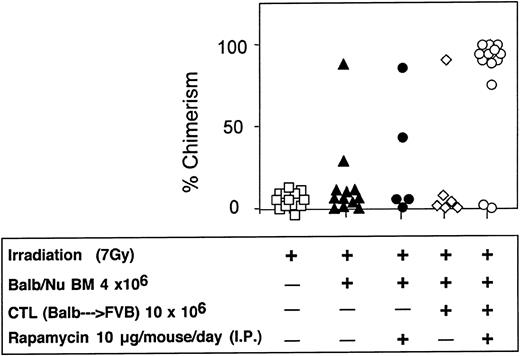
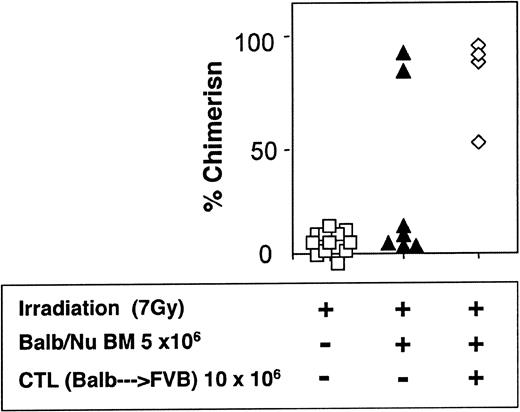
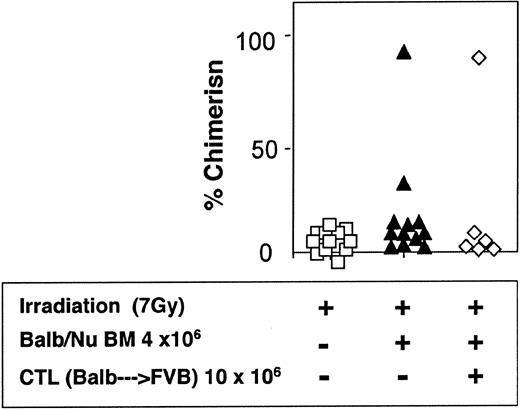
The veto activity of the CTLs was tested initially in sublethally irradiated (7 Gy) mice. As expected, following infusion of 5 × 106 Balb/c-Nude BM cells the level of chimerism was very low (2/7). However, it was markedly enhanced when veto CTLs were added (4/4) (Figure 7). The importance of stem cell dose in this equation was emphasized again in these experiments. Thus, upon reducing the BM cell dose to 4 × 106 cells, a high level of engraftment was not achieved even upon the infusion of the CTLs (1/6) (Figure 8).
Donor-type chimerism in sublethally (7 Gy) irradiated hosts (C3H/HeJ) of 5 × 106 allogeneic BM (Balb/c-Nude): enhancement by veto CTLs.
Donor-type chimerism in sublethally (7 Gy) irradiated hosts (C3H/HeJ) of 5 × 106 allogeneic BM (Balb/c-Nude): enhancement by veto CTLs.
Reduced donor-type chimerism in sublethally (7 Gy) irradiated hosts (C3H/HeJ) of 4 × 106 allogeneic BM (Balb/c-Nude) and veto CTLs.
Reduced donor-type chimerism in sublethally (7 Gy) irradiated hosts (C3H/HeJ) of 4 × 106 allogeneic BM (Balb/c-Nude) and veto CTLs.
The insights gained on the mechanism of action of veto CTLs are important for the selection of additional agents that could be synergistic with the veto cells. Based on previous work of Miller24 and Sambhara and Miller33,34 on the importance of CD8 on the veto cell and our more recent work36 showing the role of FasL, it has been established that deletion of effector T cells by the veto CTL is mediated through Fas up-regulation on the effector cells. Considering that rapamycin blocks IL-2 signaling that occurs after Fas up-regulation in the T-cell activation process, it could be predicted that rapamycin should not interfere with the veto activity. Moreover, cells that escape deletion by the veto cells could still be eliminated by rapamycin.
Thus we chose to test the potential synergy between rapamycin and veto CTLs. Since graft failure might reflect not only immune rejection but also stem cell competition, we tested the efficacy of the veto CTLs in a model, which adequately measures the isolated effect of our interventions on immune rejection. In this model (Figure 9), host mice are conditioned by supralethal TBI and radio-protected with Nude BM as a source of T-cell–depleted BM. Graft rejection is induced by infusion of 1 × 104 purified host T cells. Thus, all the mice die in the radiation control group while the mice that received BM transplants survive the radiation. As previously shown, the addition of host T cells in this model leads to graft rejection and consequently to lethal aplasia as evidenced by the blood cell counts.14
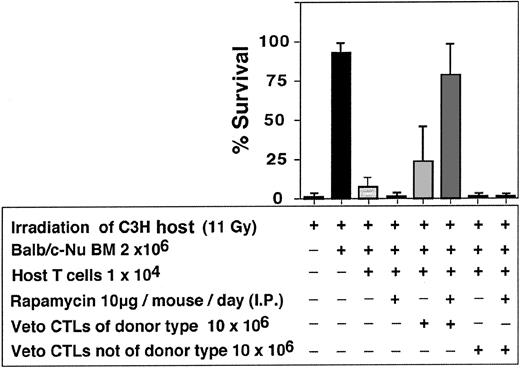
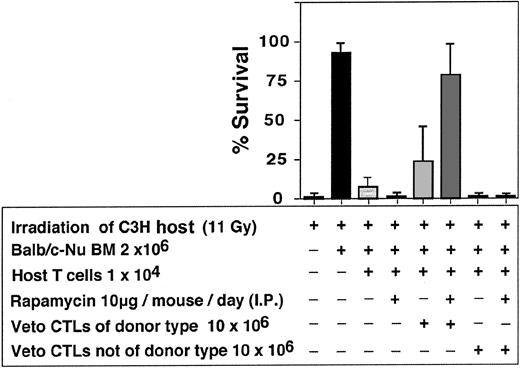
As can be seen in Table 2, a marked synergism between veto CTLs and rapamycin was recorded. In 7 independent experiments only 8 of 48 or 2 of 46 mice that were infused with 10 × 106 CTLs or treated with rapamycin alone were engrafted respectively, whereas treatment with rapamycin, together with CTL infusion, resulted in marked survival (34/47). The failure of rapamycin as a single agent to ameliorate graft rejection and prevent aplasia under these conditions could not be associated with bone marrow toxicity, as it did not affect adversely the survival of mice receiving Balb/c-Nude BM in the absence of host T cells (6/7). A similar synergistic effect between veto CTLs and rapamycin was observed when anti–third-party CTLs were generated from F1(C3H/HeJx Balb/C) origin, suggesting that the veto activity is not mediated by residual antihost reactivity (Figure 10). Furthermore, as illustrated in Figure 11, the veto effect was found to be H-2 specific, since a CTL line originating from a strain other than that of the BM donor, failed to prevent graft rejection.
Enhancement of engraftment by anti–third-party CTLs generated from (host x donor) F1 mice compared with enhancement induced by CTLs of donor type.
Enhancement of engraftment by anti–third-party CTLs generated from (host x donor) F1 mice compared with enhancement induced by CTLs of donor type.
Enhancement of engraftment of veto CTLs of the BM donor background (Balb/c) vs veto CTLs of a different background (STL//J).
Enhancement of engraftment of veto CTLs of the BM donor background (Balb/c) vs veto CTLs of a different background (STL//J).
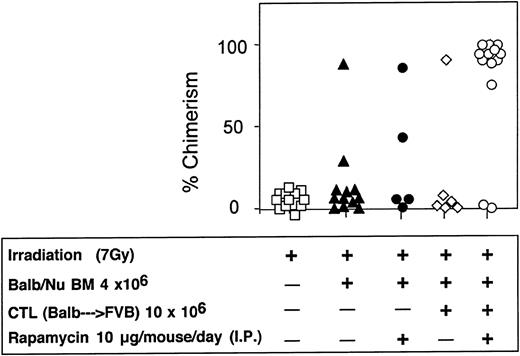
Considering that in sublethally irradiated mice infusion of 10 × 106 veto CTLs could effectively enhance engraftment of 5 × 106 Nude BM cells, but only marginally facilitated engraftment of 4 × 106 BM cells, the potential synergy with rapamycin was further evaluated in this model. As can be seen in Figure 12, anti–third-party CTLs and rapamycin by themselves exert a marginal effect on chimerism in hosts of 4 × 106 BM cells (1/6 and 2/5 mice were engrafted, respectively). However, when treated with the 2 agents together, 11 of 13 mice were found to be donor type chimera at 4 months after transplantation.
Synergistic enhancement of BM allografting of anti–third-party CTLs and rapamycin in sublethally irradiated mice.
Synergistic enhancement of BM allografting of anti–third-party CTLs and rapamycin in sublethally irradiated mice.
Discussion
Our results clearly show that anti–third-party CTLs generated under IL-2 deprivation afford a suitable source for effective veto cells that can enhance BM allografting without GVHD. In particular, these cells are effective if applied in conjunction with rapamycin. This synergism is compatible with the recent demonstration that veto CTLs operate via Fas-FasL triggering upstream of rapamycin inhibition of IL-2R signaling. Thus, rapamycin does not block the veto activity of CTLs but rather enhances their tolerizing effect so that effector T cells that might have escaped deletion by the veto cells could still be eliminated by rapamycin. To analyze the relative contribution of each agent to BM allografting we used a murine model that enables distinguishing between T-cell–mediated immune rejection and stem cell competition. In this model, rejection of T-cell–depleted BM allografts from Nude donors is induced in lethally irradiated mice by adoptive transfer of graduated numbers of purified host-type T cells. Stem cell competition is minimal under this conditioning and, in the absence of T-cell infusion, the host mice uniformly accept the allogeneic BM without GVHD. Thus, the observed graft rejection can be attributed to the adoptive transfer of purified host T cells.
It could be argued that the facilitating activity of the anti–third-party CTLs is associated with a possible contamination of host-reactive T-cell clones, which may not be sufficient to induce GVHD but could potentially eliminate the infused host-type T cells. This possibility is ruled out by 2 experiments: (1) anti–third-party CTLs that are not of donor origin, prepared by the same procedure used to generate the CTLs of donor origin, do not enhance engraftment of BM allografts; and (2) anti–third-party CTLs of (host x donor) F1 origin, lacking alloreactivity due to their genetic background, enhance BM allografting similarly to the CTLs generated from the donor genetic background.
The effective number of the veto CTL that was found to synergize with rapamycin in the graft rejection model is rather high. Although state-of-the-art technology enables large-scale expansion of T cells, it is likely that the potential synergy between the combined administration of veto CTLs and other tolerizing cells such as CD4+CD25+ and/or Sca-1+Lin– cells along with the use of maximal tolerable doses of rapamycin might further reduce the effective CTL dose.
An alternative approach to allow the use of anti–third-party CTLs has been suggested recently by Fowler et al41 who showed that TC2 CTLs are more effective veto cells compared with TC1 CTLs, and the former cells are also depleted of GVH reactivity.42
While we favor depletion of host-reactive CTL-p by IL-2 starvation, the 2 approaches might be combined in the future to further enhance veto potency and reduce host reactivity of anti–third-party CTLs. As could be anticipated from the studies in the graft rejection model, marked synergism between rapamycin and veto CTLs was also observed upon transplantation of Nude Balb/c BM into sublethally irradiated C3H hosts. In this model we have previously shown that purified Sca-1+Lin– stem cell transplants can overcome rejection if very large numbers are administered. Thus, in evaluating the results in this model, it is important to note the contribution of veto cells within the stem cell compartment or other facilitating cells within the non–T-cell compartment. Indeed, while administration of both rapamycin and veto CTLs was required to secure engraftment upon transplantation of 4 × 106 Nude BM cells, a high level of chimerism could be obtained by using CTLs without rapamycin when the BM cell dose was increased by 20%. These results suggest a quantitative relationship between the residual host antidonor CTL-p pool on one hand and the BM veto CTLs and rapamycin on the other. Preliminary results using (host x donor) F1 CTLs to distinguish between the infused CTLs and host- or naive donor–derived T cells, suggest that the infused CTLs survive for at least a few weeks after transplantation. In principle, when an adequate number of CTLs are infused and engraftment of donor cells prevails, it can be assumed that the majority of alloreactive host CTL-p has been eliminated. If the latter cells are not eliminated and are allowed to mature, graft rejection is more likely to take place.
This quantitative relationship between BM dose, veto CTLs, and rapamycin could have immediate implications to allogeneic BM transplantation (BMT), not only under reduced-intensity conditioning but also in the treatment of leukemia patients undergoing full myeloablation. Presently, the conditioning of hosts of T-cell–depleted BM or of purified CD34 cells, includes antithymocyte globulin (ATG), the serum levels of which remain high for a prolonged period of time. While this agent might reduce graft rejection and potential GVHD it can also interfere with immune reconstitution during the early period after transplantation. Considering the documented effect of short-term treatment with rapamycin on GVHD38 and on graft rejection39 in murine models, we anticipate that the combination of anti–third-party CTLs with rapamycin might successfully replace ATG in the conditioning of leukemia patients, even in the context of haploidentical transplants in which the risk for graft rejection and GVHD is significantly higher compared with HLA-identical transplants.
Clearly, achieving engraftment under reduced-intensity conditioning represents a major challenge due to the relatively large number of residual host T cells that might survive the conditioning. At present, such transplantations are carried out predominantly in elderly patients who have an HLA-identical sibling or an unrelated donor. Engraftment in these patients is greatly enhanced by the presence of a large number of alloreactive T cells in the graft, which are also associated with more than 20% GVHD lethality. The latter problem is even more pronounced in elderly patients with multiple myeloma or with B-cell chronic lymphocytic leukemia (B-CLL). Thus, the use of purified CD34 cells in conjunction with anti–third-party CTLs and rapamycin may prove beneficial for such patients. If this approach will indeed prove to be associated with low transplant-related mortality, it could be used as a prelude for cell therapy with donor lymphocyte infusions or with cell lines or clones directed against specific host-type minor hematopoietic antigens43-45 or against tumor-specific antigens.46,47 In this context, it is assumed that the CTLs are depleted of GVL reactivity and they are predominantly used to induce tolerance for donor cells and, subsequently, other more specific cell lines or clones might be needed to eradicate disease. However, it is possible that antitumor or antiviral clones within the infused CD8 cells are not completely diminished and might be expended upon proper stimulation in vivo. Further quantitative studies using the TcLandscape method developed by Guillet et al48 to define the repertoire of these anti–third-party CTLs are in progress. In addition, we found recently that anti–third-party human CTLs generated by a similar approach are endowed with an effective capacity to eradicate tumor cells from patients with B-CLL. This TCR-independent recognition between veto CTLs directed against third-party HLA and the B-CLL cells was shown to be mediated by lymphocyte function–associated antigen-1 (LFA1)–intracellular adhesion molecule-1 (ICAM1) (F. Arditti and Y. L., unpublished results, 2003). Finally, if successful, this approach could be further extended to treat diseases that are lethal in the course of years but do not present an immediate threat, such as nonmalignant hematologic diseases or in the induction of tolerance for organ transplantation.
Prepublished online as Blood First Edition Paper, May 29, 2003; DOI 10.1182/blood-2003-03-0759.
Supported in part by National Institutes of Health grant CA49369 and grants from Mrs E. Drake and the Gabriella Rich Center for Transplantation Biology Research. Y.R. holds the Henry H. Drake Professorial Chair in Immunology.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.