Abstract
Binding of factor VIIa (FVIIa) to its cellular receptor tissue factor (TF) was previously shown to induce various intracellular signaling events, which were thought to be responsible for TF-mediated biologic effects, including angiogenesis, tumor metastasis, and restenosis. To understand the mechanisms behind these processes, we have examined the effect of FVIIa on apoptosis. Serum deprivation–induced apoptosis of BHK(+TF) cells was characterized by apoptotic blebs, nuclei with chromatin-condensed bodies, DNA degradation, and activation of caspase 3. FVIIa markedly decreased the number of cells with apoptotic morphology and prevented the DNA degradation as measured by means of TdT-mediated dUTP nick end labeling (TUNEL). The antiapoptotic effect of FVIIa was confirmed by the observation that FVIIa attenuated caspase 3 activation. FVIIa-induced antiapoptotic effect was dependent on its proteolytic activity and TF but independent of factor Xa and thrombin. FVIIa-induced cell survival correlated with the activation of Akt and was inhibited markedly by the specific PI3-kinase inhibitor, LY294002. Blocking the activation of p44/42 mitogen-activated protein kinase (MAPK) by the specific mitogen-induced extracellular kinase (MEK) inhibitor, U0126, impaired modestly the ability of FVIIa to promote cell survival. In conclusion, FVIIa binding to TF provided protection against apoptosis induced by growth factor deprivation, primarily through activation of PI3-kinase/Akt pathway, and to a lesser extent, p44/42 MAPK pathway.
Introduction
Blood coagulation occurs when coagulation factor VII(a) (FVIIa) binds to tissue factor (TF). FVIIa/TF-mediated blood coagulation is important in hemostasis after tissue injury and in the pathogenesis of many thrombotic disorders. In addition to triggering the coagulation cascade, investigations have implicated that TF is involved in various physiological and pathophysiological processes, including embryo development, angiogenesis, restenosis, inflammation, and tumor metastasis.1 The significance of TF in such varied biologic processes is unclear. Studies showing that FVIIa/TF induces intracellular signaling events have become central in further elucidation of possible links between TF function and the biologic effects.2-5
Observations suggest that FVIIa/TF is important in the delicate balance between cell proliferation and apoptosis. This applies to the developmental defects observed in TF knock-out mice,6 the increased metastasis of tumor cells overexpressing TF,7,8 the suppression of tumor metastasis by impairing FVIIa binding to TF,9,10 and the decrease in restenosis and neointimal hyperplasia by selective inhibition of FVIIa/TF.11 An imbalance between cell survival and apoptosis has been implicated as an essential part of the pathogenesis. A possible clue to the involvement of FVIIa/TF complex in regulation of cellular growth and survival comes from studies demonstrating that binding of FVIIa to cell surface TF induces a transient phosphorylation of p44/42 mitogen-activated protein kinase (MAPK)12,13 and Akt14 as well as downstream targets of these kinases.15 It also was shown that FVIIa/TF activates the translatory machinery15 and induces several growth factor–like genes.16,17 However, a link between FVIIa-induced signaling and its possible effect on cell growth and survival has not yet been established. A recent study suggests that cell proliferation could not be a plausible cause for FVIIa/TF-induced neovascularization since exposure of TF-expressing cells to FVIIa failed to increase cell proliferation.15 No information is currently available on whether FVIIa affects apoptosis.
Apoptosis can be triggered in vivo by various death insults such as hypoxia and growth factor deprivation or by activation of transmembrane death receptors of the FAS/TRAIL/TNF receptor 1 family.18-20 Both pathways cause activation of caspases, cysteine aspartyl proteases, which are involved in a cascade of cleavage events that result in apoptosis. Caspases are synthesized as latent zymogens that are activated by proteolytic cleavage.18 A diverse group of cell-signaling pathways, including MAP kinases and Akt, has been implicated to promote cell survival.21 Thus, the status of apoptotic machinery as well as the levels of activation of various signaling pathways at a given time influences cell fate. As mentioned earlier, FVIIa was shown to activate both p44/42 MAPK and Akt signaling pathways. However, it is unknown whether FVIIa-induced activation of these pathways affects apoptosis.
To elucidate the mechanism by which FVIIa/TF is involved in the pathogenesis of various diseases, we examined the effect of FVIIa on cell survival. The data presented show that FVIIa binding to TF provided protection against apoptosis induced by growth factor deprivation. The antiapoptotic effect of FVIIa was dependent on its binding to TF and on its protease activity. We also show FVIIa-induced protection to be dependent primarily on the activation of the Akt pathway. The implications of these observations for TF-related pathophysiology are discussed.
Materials and methods
Reagents
Recombinant human FVIIa22 and FFR-FVIIa23 were prepared as described earlier. Factors X, Xa, thrombin, and hirudin were from Enzyme Research Laboratories (South Bend, IN). The specific FXa inhibitor, recombinant tick anticoagulant protein (TAP), was a generous gift from Dr George Vlasuk (Corvas, La Jolla, CA). Antibodies against caspase 3, phosphorylated p44/42 MAPK, phosphorylated Akt were from Cell Signaling Technology (Boston, MA). β-actin antibody was from Abcam (Cambridge, United Kingdom). LY294002, U0126, and AG1478 were from Promega (Madison, WI).
Cell lines
The Baby Hamster Kidney cell line BHK-21 tk– (ATCC CRL1632) was cultured in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal calf serum (FCS), 100 IU/mL penicillin, 100 μg/mL streptomycin (complete DMEM medium), and stably transfected with human TF as described.13 Chinese hamster ovary (CHO-K1, ATCC CCL-61) cells were cultured in Ham-F12 medium supplemented with 10% FCS and 1% nonessential amino acids (complete Ham-F12 medium). CHO cells were transfected with human TF in pcDNA3 (Invitrogen, Carlsbad, CA) using FuGene (Roche Diagnostics, Indianapolis, IN). Stable cell lines were selected by resistance to geneticin (0.7 mg/mL). Clonal cell lines were tested for TF expression in an FXa generation assay23 employing intact monolayers of cells. The expression of functional TF in the 2 transfected cell lines, BHK(+TF) and CHO(+TF), were comparable as judged in the FXa formation assay.
LDH activity assay
Cell death was determined by measurements of lactate dehydrogenase (LDH) activity released from damaged cells. The cells (20 000 cells/dish) were seeded in 96-well plates and incubated for 24 hours in 200 μL complete DMEM. The cells were washed twice and incubated with 200 μL serum-free medium (SFM) for an additional 24 hours in the presence of 10 nM FVIIa, 10 nM FFR-FVIIa, or 10% vol/vol serum. After centrifugation at 250 × g for 10 minutes, 100 μL supernatant was transferred to a microtiter plate for analysis of LDH activity according to the manufacturer (Roche Diagnostics).
TUNEL/flow cytometry
The cells were seeded in 9-cm dishes (800 000 cells/dish) in complete DMEM. At 80% confluence, cells were washed and serum deprived in the presence and absence of experimental compounds. Briefly, the monolayers were washed 2 times with DMEM and overlaid with 6 mL DMEM containing experimental test compound. At the end of a 24- or 48-hour incubation period, the floating cells in the media were collected on ice and pooled with the trypsin-detached adhered cells, and centrifuged at 300 × g for 5 minutes. The cells were washed with phosphate-buffered saline (PBS) before fixation in 1% paraformaldehyde in PBS on ice for 15 minutes. After the fixation, the cells were washed in PBS and resuspended in 500 μL PBS before adding 5 mL of 70% EtOH. The cell suspension was analyzed by TdT-mediated dUTP nick end labeling (TUNEL) assay (Apo-BRDU; BD Biosciences Pharmingen, San Diego, CA) according to the manufacturer's instructions. Samples were analyzed by flow cytometry recording 10 000 events/sample using a FACScan (BD Biosciences, San Jose, CA). The samples were gated based on forward and side scatter profiles to exclude aggregates and cell debris. The gated population was presented in a histogram with fluorescein isothiocyanate (FITC)–BrdU on the X-axis and relative cell number on the Y-axis. In every experiment, the histograms from SFM-treated and 10% (vol/vol) FCS-treated cells were used to gate nonapoptotic cells. Cells to the right of this gate were taken as apoptotic cells.
Detection of caspase 3 activation
Cells were seeded in 9-cm dishes in complete medium. At 80% confluence cells were washed and serum deprived in the presence and absence of experimental test compound as described in “TUNEL/flow cytometry.” At the end of the indicated time interval, the dish was placed on ice, and floating cells were collected. The adhered cells were scraped from the bottom of the dish and pooled with the floating cells and centrifuged at 300 × g for 5 minutes at 4°C to obtain the cell pellet. The cells were lysed by adding 50 μL ice-cold Chaps (3-[(3-cholamidopropyl)dimethylammonio]-1-propane-sulfonic acid) cell extraction buffer (50 mM Pipes [piperazine diethanesulfonic acid]/KOH, pH 6.5, 2 mM EDTA (ethylenediaminetetraacetic acid), 0.1% Chaps, supplemented immediately prior to use with 20 μg/mL leupeptin, 10 μg/mL aprotinin, 5 mM DTT (dithiothreitol), 0.1 mM 4-(2-aminoethyl)-bezenesulfonylfluoride (AEBSF). The lysates were frozen and thawed 3 times, centrifuged at 15 000 × g for 5 minutes at 4°C. The supernatant was collected and total protein concentration was determined by Bio-Rad protein assay kit (Bio-Rad Laboratories, Hercules, CA). Approximately 10 μg protein was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions on 4%-12% gradient gel. Caspase 3 activation was evaluated by Western blot analysis using an antibody against caspase 3.
Determination of Akt and p44/42 MAP kinase activation
Cells were seeded in the complete medium in 6-well plates. At 80%-90% confluency, the cells were washed twice in SFM and left in the SFM for 2 hours to make the cells quiescent. Cells were stimulated with FVIIa for 10 minutes at 37°C unless stated differently. Where inhibitors were included, they were added to the cells 30 minutes prior to the addition of FVIIa. After treatments, cells were lysed in lysis buffer (20 mM Tris [tris(hydroxymethyl)aminomethane], 0.1% [vol/vol] Triton X-100, 1 mM EDTA, 1 mM EGTA [ethylene glycol diaminoethyl tetraacetic acid], 50 mM sodiumfluoride, 10 mM sodium β-glycerophosphate, 5 mM sodium pyrophosphate, 150 mM NaCl, 0.1 mM AEBSF, 1 mM benzamidine, pH 7.5), supplemented just prior to use with 1 mM sodium orthovanadate, 5 μg/mL leupeptin, and 10 μg/mL aprotinin. Ten micrograms of protein was loaded onto SDS-PAGE (4%-12% gradient gel, reducing condition) and subjected to Western blot analysis using specific antibodies against phosphorylated p44/42 MAPK and Akt.
Western blot analysis
After electroblotting, the nitrocellulose membranes were blocked in blocking buffer (Tris-buffered saline [TBS] containing 0.1% [vol/vol] Tween-20 and 5% [wt/vol] nonfat dry milk) for 1 hour and washed in TBS with 0.1% (vol/vol) Tween-20 (TBS/T) before adding the primary antibody (1:1000, and for β-actin 1:1 000 000) in the blocking buffer. After overnight incubation with the primary antibody at 4°C, the membranes were washed 3 times in TBS/T before adding the horseradish peroxidase (HRP)–conjugated secondary antibody (1:2000) in the blocking buffer. Membranes were incubated for 1 hour at room temperature before being washed thrice with TBS/T. Chemiluminescence substrate (Supersignal; Pierce, Rockford, IL) was added for 1-5 minutes, and the chemiluminescence was detected by a cooled CCD-camera (LAS1000; Fujifilm, Stamford, CT). These digital images were used to quantitate the intensity of the bands using Image Gauge version 4.0 (Fujifilm).
Immunocytochemistry
BHK(+TF) cells were seeded in complete DMEM in 8-chamber glass slides (Nalge Nunc International, Rochester, NY) and grown to 30% confluence. Cells were incubated with SFM supplemented with none, 20 nM FVIIa, 20 nM FFR-FVIIa, or 10% vol/vol FCS for 6 hours, whereafter they were fixed for 15 minutes at 4°C in 4% (vol/vol) paraformaldehyde in PBS, postfixed in 70% (vol/vol) ethanol for 15 minutes at room temperature, and air dried. Antigen retrieval was achieved by treating the cells with 10 mM citrate buffer, pH 6.0, preheated to 60°C (3 × 5 minutes cycles). The cells were then preincubated for 15 minutes in 5% (vol/vol) donkey serum (Jackson ImmunoResearch Laboratory, West Grove, PA) in TBS, followed by goat anti–human TF IgG (American Diagnostica, Greenwich, CT) overnight at 4°C. The cells were incubated with biotinylated donkey antigoat (Jackson ImmunoResearch Laboratory) for 1 hour, HRP-streptavidin (NEN Life Science Products, Boston, MA) for 30 minutes, and TSA-FITC for 10 minutes (Tyramide Signal Amplification Fluorescein system) (NEN Life Science Products, Boston, MA), followed by counter-staining with Hoechst (Molecular Probes, Leiden, The Netherlands) for morphological analyses of apoptotic cells and mounted with mounting medium fluorescence (DAKO, Glostrup, Denmark). The cells were photographed using an Olympus BX51 reflected fluorescence system microscope equipped with selective AMCA (aminomethylcoumarin acetate) and FITC filters, and a DP50 digital camera (all from Olympus Denmark, Albertslund, Denmark).
Statistical analysis
All experiments were repeated independently at least 3 times. Statistical analyses were performed with Excel (Microsoft, Redmond, WA) using 2-tailed Student t test.
Results
FVIIa promotes cell survival
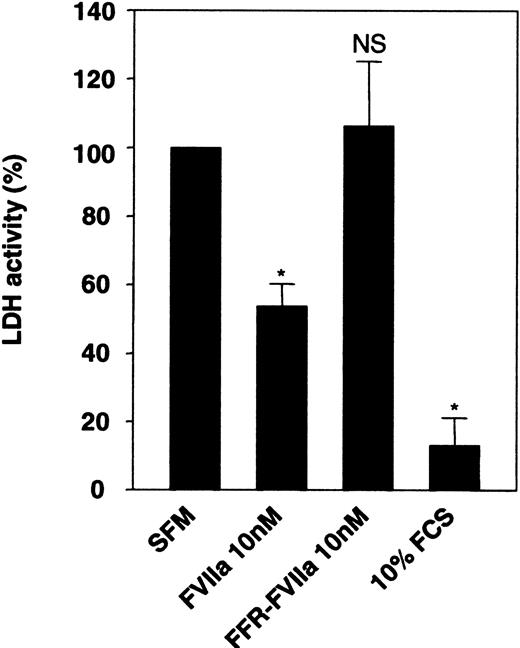
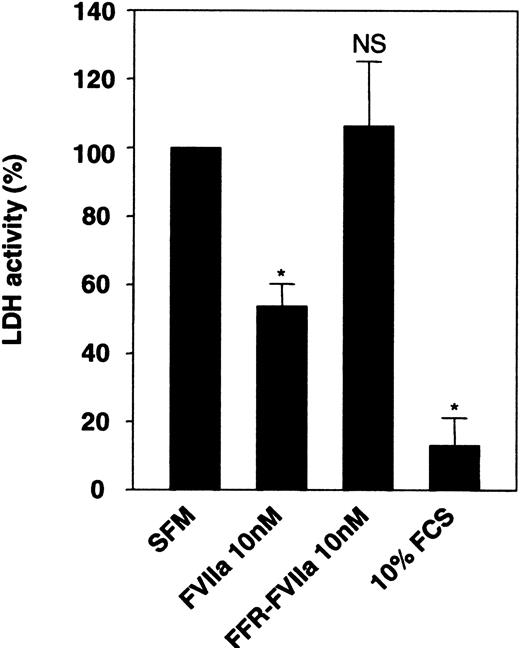
During our earlier investigation on FVIIa/TF-induced signaling,13,24 we noted that upon serum deprivation, BHK(+TF) cells tend to round up, loosely adhere to culture dishes (detach easily from the dish), and undergo subtle morphological changes. These morphological changes were less obvious when FVIIa was included in the serum-free medium. This observation, taken together with the observation that FVIIa induces phosphorylation of an antiapoptotic kinase, Akt,14,15 prompted us to examine the effect of FVIIa on cell viability and apoptosis. Monolayers of BHK(+TF) cells were incubated for 24 hours in serum-free medium alone or the serum-free medium supplemented with FVIIa (10 nM) or catalytically inactive FVIIa (FFR-FVIIa) (10 nM), and the cell viability was evaluated by measuring the release of LDH, which is associated with cell death, into the overlying medium. The conditioned medium from cells deprived of serum had 10-fold higher LDH activity compared with the conditioned medium of cells grown in serum-containing medium (Figure 1). If FVIIa was included in the serum-free medium, there was a substantial and significant reduction (53%) in LDH activity compared with the cells maintained in the serum-free medium alone (Figure 1), implying that cell death was reduced by FVIIa. This reduction was not observed if FFR-FVIIa, instead of FVIIa, was added to the medium, suggesting FVIIa's proteolytic activity was essential for its protective effect.
Effect of FVIIa on cell viability. Monolayers of BHK(+TF) cells cultured to 80% confluency were incubated for 24 hours in serum-free medium (SFM) alone or SFM supplemented with FCS (10% vol/vol), FVIIa (10 nM), or FFR-FVIIa (10 nM). At the end of 24 hours, the overlying conditioned medium was removed and assayed for LDH activity. LDH activity obtained with SFM was set to 100%. Data are presented as mean ± SD (n = 3). Statistically significant differences from the value obtained in SFM are indicated by an asterisk (P < .001). NS indicates no statistically significant difference.
Effect of FVIIa on cell viability. Monolayers of BHK(+TF) cells cultured to 80% confluency were incubated for 24 hours in serum-free medium (SFM) alone or SFM supplemented with FCS (10% vol/vol), FVIIa (10 nM), or FFR-FVIIa (10 nM). At the end of 24 hours, the overlying conditioned medium was removed and assayed for LDH activity. LDH activity obtained with SFM was set to 100%. Data are presented as mean ± SD (n = 3). Statistically significant differences from the value obtained in SFM are indicated by an asterisk (P < .001). NS indicates no statistically significant difference.
To examine whether the antiapoptotic effect observed with 10% FCS treatment stems from the potential activation of residual zymogen coagulation factors that could have been present in the serum, BHK(+TF) cells were preincubated with FFR-FVIIa or polyclonal anti–human TF antibodies before addition of 10% FCS or 50 nM FVIIa. LDH activity was measured after 24 hours. Neither 200 nM FFR-FVIIa nor 100 μg/mL anti–TF IgG was able to prevent the antiapoptotic effect of 10% FCS. As expected, FFR-FVIIa and anti–TF IgG inhibited the antiapoptotic effect of FVIIa (data not shown). Therefore, the antiapoptotic effect of FCS was not due to the residual coagulation zymogens in the serum.
FVIIa prevents nuclear condensation induced by serum deprivation
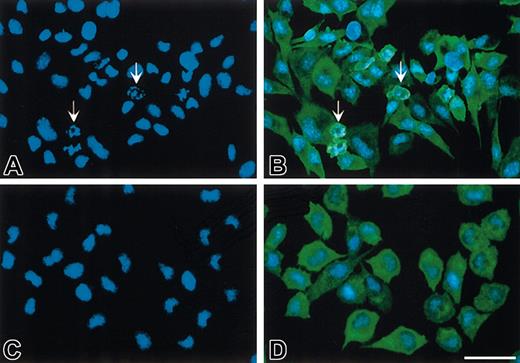
LDH release to the medium cannot distinguish between different causes of cell death. The cells were therefore examined further for possible apoptotic morphology changes. Fluorescence microscopy revealed that under serum-depriving conditions a considerable number of BHK(+TF) cells were characterized by apoptotic blebs and nuclei with chromatin-condensed bodies (Figure 2A-B). The presence of FVIIa during serum starvation markedly decreased the number of cells with apoptotic morphology (Figure 2C-D). Cells exposed to FVIIa had an appearance similar to those maintained in serum-containing medium (data not shown). In contrast to FVIIa, FFR-FVIIa failed to prevent nuclei condensation induced by serum deprivation (data not shown).
FVIIa prevents nuclei condensation in growth factor–deprived BHK(+TF) cells. Monolayers of BHK(+TF) cells were incubated in SFM for 6 hours (A-B) or treated with 20 nM FVIIa in SFM (C-D). At the end of the incubation, the cells were washed, fixed, permeabilized, and stained with Hoechst (A,C) or with goat anti–human TF IgG, followed by biotinylated donkey anti–goat IgG, HRP-streptavidin, and TSA-FITCH (B,D). Note the apoptotic cells (arrows) when BHK(+TF) cells were deprived of serum (A-B) compared with the inhibition of apoptosis seen by the absence of any apoptotic nuclei when treated with FVIIa (C,D). All fields are representative of multiple fields observed in 3 independent experiments. Scale bar indicates 50 μm.
FVIIa prevents nuclei condensation in growth factor–deprived BHK(+TF) cells. Monolayers of BHK(+TF) cells were incubated in SFM for 6 hours (A-B) or treated with 20 nM FVIIa in SFM (C-D). At the end of the incubation, the cells were washed, fixed, permeabilized, and stained with Hoechst (A,C) or with goat anti–human TF IgG, followed by biotinylated donkey anti–goat IgG, HRP-streptavidin, and TSA-FITCH (B,D). Note the apoptotic cells (arrows) when BHK(+TF) cells were deprived of serum (A-B) compared with the inhibition of apoptosis seen by the absence of any apoptotic nuclei when treated with FVIIa (C,D). All fields are representative of multiple fields observed in 3 independent experiments. Scale bar indicates 50 μm.
Prevention of DNA degradation by FVIIa under serum-deprived conditions
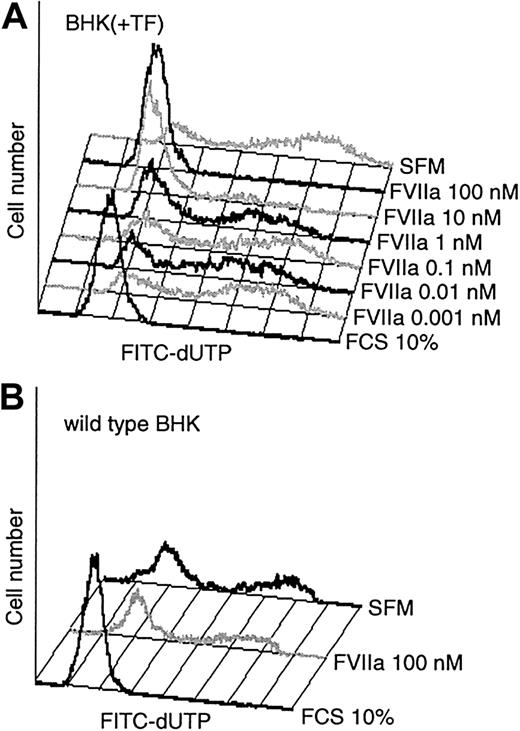
The cell morphology observations in Figure 2 suggested that exposure of FVIIa to serum-starved BHK(+TF) cells produces an antiapoptotic effect. To obtain a quantitative characterization of this effect, we examined the cells for apoptotic changes by measuring DNA degradation using TUNEL/flow cytometry. Compared with the serum control, culturing of BHK(+TF) cells in serum-free conditions for 24 hours markedly increased the fraction of apoptotic cells with advanced DNA degradation (Figure 3A). Addition of FVIIa, in a dose-dependent manner (1 pM to 100 nM FVIIa), to the serum-free medium rescued the cells from apoptosis. The antiapoptotic effect of FVIIa was evident at 1 nM with substantially increased cell survival at 10 nM FVIIa, a concentration equivalent to the FVII plasma level. An increase to 100 nM FVIIa slightly improved cell survival further (Figure 3A). A similar antiapoptotic profile of FVIIa was observed in cells exposed to FVIIa for 48 hours (data not shown).
Antiapoptotic effect of FVIIa. (A) Monolayers of BHK(+TF) cells were exposed to SFM or the SFM supplemented with varying concentrations of FVIIa or serum (10% vol/vol) for 24 hours. At the end of the incubation period, fragmented DNA was labeled with TUNEL and detected by flow cytometry. (B) Wild-type BHK cells were exposed for 48 hours to SFM alone or to the medium supplemented with serum (10% vol/vol) or FVIIa (100 nM). At the end of the incubation period, cells were processed for TUNEL assay as described for panel A. Figures show a representative experiment (n = 3).
Antiapoptotic effect of FVIIa. (A) Monolayers of BHK(+TF) cells were exposed to SFM or the SFM supplemented with varying concentrations of FVIIa or serum (10% vol/vol) for 24 hours. At the end of the incubation period, fragmented DNA was labeled with TUNEL and detected by flow cytometry. (B) Wild-type BHK cells were exposed for 48 hours to SFM alone or to the medium supplemented with serum (10% vol/vol) or FVIIa (100 nM). At the end of the incubation period, cells were processed for TUNEL assay as described for panel A. Figures show a representative experiment (n = 3).
Wild type nontransfected BHK cells do not express detectable levels of TF. Like TF-transfected cells, culture of these cells under serum-free conditions for 48 hours resulted in extensive apoptosis as demonstrated by the TUNEL assay. However, as shown in Figure 3B, in contrast to BHK(+TF) cells, wild-type BHK cells could not be rescued from apoptosis by FVIIa, which suggests that binding to TF is essential for FVIIa's antiapoptotic effect.
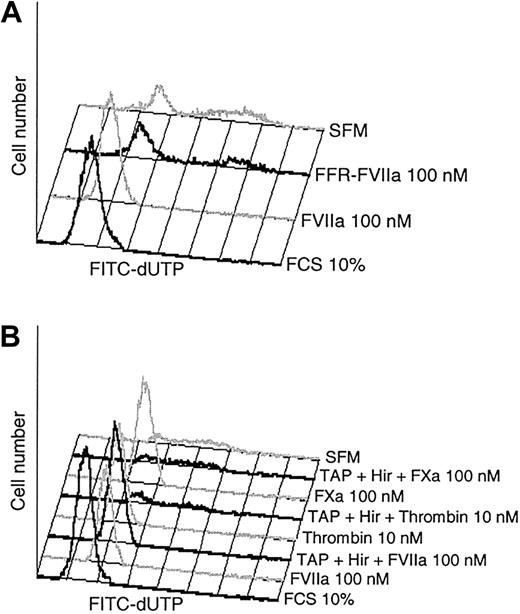
The observation that FVIIa but not FFR-FVIIa was able to preserve BHK(+TF) cells from the morphological changes induced by serum starvation prompted us to explore this further with the TUNEL assay. FVIIa/TF has been reported to induce cell signaling by a protease activity-dependent as well by an activity-independent mechanism,3 and it was therefore of interest to further establish whether or not FVIIa proteolytic activity was required for its antiapoptotic effect. The results shown in Figure 4A and Table 1 clearly indicate that the proteolytic activity is essential since FVIIa, but not FFR-FVIIa, protected BHK(+TF) cells from the apoptosis.
Proteolytic activity of FVIIa is mandatory for the antiapoptotic effect. (A) Monolayers of BHK(+TF) cells were incubated in SFM alone or the SFM supplemented with FCS (10% vol/vol), FVIIa (100 nM), or FFR-FVIIa (100 nM). At the end of the 24-hour incubation period, cells were processed for TUNEL assay. (B) Monolayers of BHK(+TF) cells were incubated in SFM medium alone or the SFM supplemented with FVIIa (100 nM), FXa (100 nM), or thrombin (10 nM) in the presence and absence of specific inhibitors, hirudin (25 U/mL), and TAP (200 nM). The inhibitors were added to the cells 30 minutes prior to the protease. At the end of 24 hours, the cells were processed for TUNEL assay. Each panel shows a representative experiment (n = 3).
Proteolytic activity of FVIIa is mandatory for the antiapoptotic effect. (A) Monolayers of BHK(+TF) cells were incubated in SFM alone or the SFM supplemented with FCS (10% vol/vol), FVIIa (100 nM), or FFR-FVIIa (100 nM). At the end of the 24-hour incubation period, cells were processed for TUNEL assay. (B) Monolayers of BHK(+TF) cells were incubated in SFM medium alone or the SFM supplemented with FVIIa (100 nM), FXa (100 nM), or thrombin (10 nM) in the presence and absence of specific inhibitors, hirudin (25 U/mL), and TAP (200 nM). The inhibitors were added to the cells 30 minutes prior to the protease. At the end of 24 hours, the cells were processed for TUNEL assay. Each panel shows a representative experiment (n = 3).
Like FVIIa, the downstream proteases of the TF coagulation pathway, FXa and thrombin, are known to induce intracellular signaling producing various cellular responses. It was therefore important to rule out that FVIIa exerted its effect through the generation of small amounts of downstream coagulation factors. Although we have used recombinant FVIIa in these studies and found no evidence for the generation of FXa or thrombin in our experimental system, we evaluated the antiapoptotic effect of FVIIa in the presence of specific inhibitors of both FXa and thrombin, that is, TAP and hirudin. As shown in Figure 4B, TAP and hirudin failed to prevent the antiapoptotic effect of FVIIa, whereas they completely abolished the antiapoptotic effect of FXa and thrombin. These data establish that the specific protease activity of FVIIa/TF is responsible for the reduction in apoptosis observed in TF-expressing cells exposed to FVIIa.
FVIIa suppresses caspase 3 activation
Caspases, a family of cysteine proteases, are central regulators of apoptosis. Caspases are synthesized as latent zymogens organized in cascade systems that upon activation stimulate apoptosis. Inhibition of apoptosis is accomplished either by inhibiting the activity of caspases or by preventing their activation. To obtain clues to the mechanism by which FVIIa inhibits apoptosis, we investigated the activation of the central apoptosis effector, caspase 3, by Western blot analysis using an antibody that recognizes the zymogen as well as the activated enzyme. Serum removal induced a time-dependent appearance of an 18 to 20–kDa caspase 3 band in BHK(+TF) cells (Figure 5A), which represents the activated caspase 3. Activation of caspase 3 was evident 2 hours after serum removal and reached a maximum at 3 hours. The activation was maintained for at least 5 hours (the duration of the experiment). However, the presence of 100 nM FVIIa in the serum-free medium clearly attenuated caspase 3 activation (70%-80% inhibition). In additional experiments (data not shown), FVIIa induced a dose-dependent inhibition of caspase 3 activation, which correlated with the dose-dependent inhibition of apoptosis measured in TUNEL assay. The FVIIa-induced prevention of caspase 3 activation was furthermore only observed in TF-transfected cell lines BHK(+TF) (Figure 5A) and CHO(+TF) (Figure 5C) but not in the wild-type BHK (Figure 5B) nor the wild-type CHO cells (Figure 5D). Serum abolished the activation of caspase 3 in both TF-transfected cells as well as in wild-type cells.
FVIIa prevents activation of caspase 3 in serum-deprived cells. (A) Monolayers of BHK(+TF) cells were incubated in SFM in the absence or presence of FVIIa (100 nM) for 0.5, 1, 2, 3, 4, or 5 hours. At the end of incubation, the cells were collected and lysed. Equal amounts of protein (10 μg) were subjected to SDS-PAGE followed by Western blot analysis with anti–caspase 3 antibody. The blots were stripped and reprobed with a β-actin–specific antibody as a measure of equal loading. (B) Wild-type BHK cells were treated with SFM or the SFM supplemented with FVIIa (100 nM) or serum (10% vol/vol) for 4 hours, and the cells were processed as described for panel A. (C) CHO(+TF) cells were incubated in SFM or the SFM supplemented with FVIIa (100 nM) or serum (10% vol/vol) for 4 hours, and the cells were processed as described for panel A. (D) CHO-K1 wild-type cells were incubated in SFM or the SFM supplemented with FVIIa (100 nM) or serum (10% vol/vol) for 4 hours, and the cells were processed as described for panel A. The figure shows a representative Western blot of 3 independent experiments.
FVIIa prevents activation of caspase 3 in serum-deprived cells. (A) Monolayers of BHK(+TF) cells were incubated in SFM in the absence or presence of FVIIa (100 nM) for 0.5, 1, 2, 3, 4, or 5 hours. At the end of incubation, the cells were collected and lysed. Equal amounts of protein (10 μg) were subjected to SDS-PAGE followed by Western blot analysis with anti–caspase 3 antibody. The blots were stripped and reprobed with a β-actin–specific antibody as a measure of equal loading. (B) Wild-type BHK cells were treated with SFM or the SFM supplemented with FVIIa (100 nM) or serum (10% vol/vol) for 4 hours, and the cells were processed as described for panel A. (C) CHO(+TF) cells were incubated in SFM or the SFM supplemented with FVIIa (100 nM) or serum (10% vol/vol) for 4 hours, and the cells were processed as described for panel A. (D) CHO-K1 wild-type cells were incubated in SFM or the SFM supplemented with FVIIa (100 nM) or serum (10% vol/vol) for 4 hours, and the cells were processed as described for panel A. The figure shows a representative Western blot of 3 independent experiments.
FVIIa/TF-mediated antiapoptotic effect depends on activation of the PI3K pathway
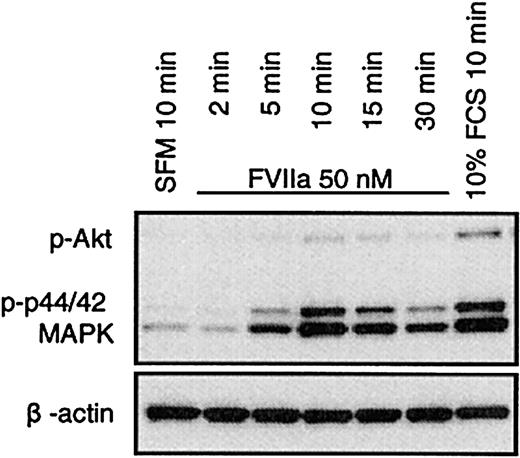
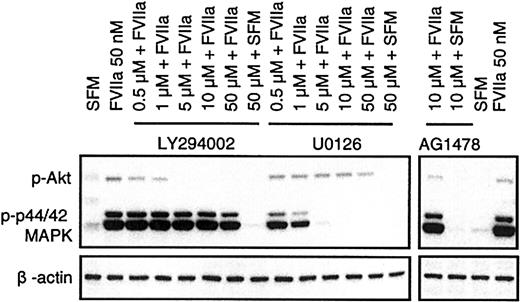
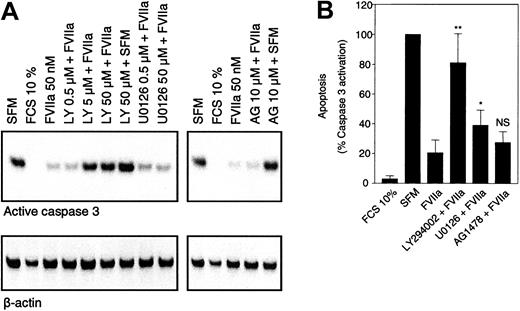
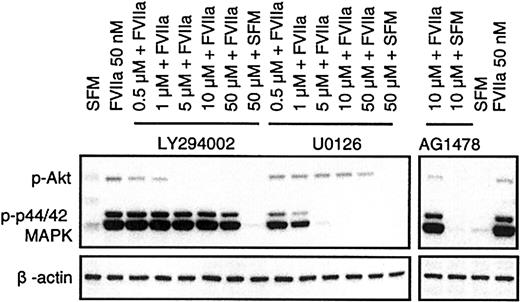
FVIIa was shown in BHK(+TF) cells to activate p44/42 MAPK12,13 as well as Akt.14,15 FVIIa induced a similar transient phosphorylation of p44/42 MAPK and Akt in CHO(+TF) cells (Figure 6). Both the kinases are considered important players in survival signaling. Whereas p44/42 MAPK is activated via mitogen-induced extracellular kinase 1/2 (MEK1/2), the activation of Akt is mediated via PI3K. Since specific inhibitors exist for both MEK1/2 (U0126) and PI3K (LY294002), it is possible to characterize the relative importance of the 2 pathways in FVIIa-induced antiapoptotic signaling. BHK(+TF) cells were treated with various concentrations (0.5 to 50 μM) of the MEK1/2 inhibitor U0126 or the PI3K inhibitor LY294002 before they were exposed to FVIIa. The efficiency of the inhibitors to suppress the FVIIa-induced antiapoptotic effect as monitored by caspase 3 activation was compared with the inhibition of Akt and p44/42 MAPK phosphorylation examined by Western blot analysis using phospho-specific antibodies. The FVIIa-dependent suppression of caspase 3 activation was significantly reduced at 5 μM (Figure 7) or higher concentrations of the PI3 kinase inhibitor LY294002. This corresponded closely to the concentration range at which FVIIa-induced phosphorylation of Akt was effectively inhibited by LY294002 (Figure 8). As expected, LY294002, even at 50 μM, did not inhibit FVIIa-induced phosphorylation of p44/42 MAPK. The MEK inhibitor U0126, on the other hand, had a minor but significant effect on the antiapoptotic effect of FVIIa (Figure 7), although it fully inhibited the FVIIa-induced p44/42 MAPK phosphorylation (Figure 8). These data suggest that FVIIa-induced activation of Akt via PI3K is primarily responsible for FVIIa's antiapoptotic effect. A specific inhibitor of epidermal growth factor (EGF) receptor tyrosine kinase (AG1478) had no effect on the FVIIa-induced p44/42 MAPK and Akt phosphorylation (Figure 8) and also failed to affect FVIIa's attenuation of caspase 3 activation (Figure 7). This rules out the involvement of the EGF receptor in FVIIa/TF-induced signaling. The EGF receptor previously has been shown to be involved in transactivation of a G-protein–coupled receptor.25
FVIIa induces phosphorylation of p44/42 MAPK and Akt in CHO(+TF) cells. Monolayers of quiescent CHO(+TF) cells were stimulated with SFM, FVIIa (50 nM), or serum (10% vol/vol) for the indicated times. Cell lysates were analyzed by Western blot analysis using phospho-specific antibodies against p44/42 MAPK and Akt. One representative experiment of 3 independent experiments is shown.
FVIIa induces phosphorylation of p44/42 MAPK and Akt in CHO(+TF) cells. Monolayers of quiescent CHO(+TF) cells were stimulated with SFM, FVIIa (50 nM), or serum (10% vol/vol) for the indicated times. Cell lysates were analyzed by Western blot analysis using phospho-specific antibodies against p44/42 MAPK and Akt. One representative experiment of 3 independent experiments is shown.
PI3K/Akt pathway is responsible for the antiapoptotic properties of FVIIa. Monolayers of BHK(+TF) cells were incubated in SFM alone or the SFM supplemented with FVIIa (50 nM) in the presence and absence of inhibitors. Inhibitors were added to the cells 30 minutes prior to the addition of FVIIa. After 4 hours, the cells were lysed and the cell lysates (10 μg) were subjected to Western blot analysis using anti–caspase 3 antibody. Panel A shows a representative Western blot analysis. Panel B represents quantification of band intensity obtained from digital pictures. Caspase 3 activation obtained with serum-free medium was set to 100% apoptosis. Concentration of inhibitors was LY294002 (5 μM), U0126 (5 μM), and AG1478 (10 μM). Data are presented as mean ± SD (n = 4). Statistically significant differences from FVIIa value are indicated. *P < .05; **P < .001; and NS indicates not significant.
PI3K/Akt pathway is responsible for the antiapoptotic properties of FVIIa. Monolayers of BHK(+TF) cells were incubated in SFM alone or the SFM supplemented with FVIIa (50 nM) in the presence and absence of inhibitors. Inhibitors were added to the cells 30 minutes prior to the addition of FVIIa. After 4 hours, the cells were lysed and the cell lysates (10 μg) were subjected to Western blot analysis using anti–caspase 3 antibody. Panel A shows a representative Western blot analysis. Panel B represents quantification of band intensity obtained from digital pictures. Caspase 3 activation obtained with serum-free medium was set to 100% apoptosis. Concentration of inhibitors was LY294002 (5 μM), U0126 (5 μM), and AG1478 (10 μM). Data are presented as mean ± SD (n = 4). Statistically significant differences from FVIIa value are indicated. *P < .05; **P < .001; and NS indicates not significant.
Specific action of PI3K and MEK1/2 pathway inhibitors on activation of Akt and p44/42 MAPK. Monolayers of BHK(+TF) cells were made quiescent by serum deprivation for 2 hours. Then the cells were treated with FVIIa in the presence and absence of inhibitors as described in Figure 7. After 10 minutes, the experiment was terminated by lysing the cells. The cell lysates (10 μg) were subjected to Western blot analysis using phospho-specific antibodies against p44/42 MAPK and Akt. The figure shows one representative Western blot of 4 independent experiments.
Specific action of PI3K and MEK1/2 pathway inhibitors on activation of Akt and p44/42 MAPK. Monolayers of BHK(+TF) cells were made quiescent by serum deprivation for 2 hours. Then the cells were treated with FVIIa in the presence and absence of inhibitors as described in Figure 7. After 10 minutes, the experiment was terminated by lysing the cells. The cell lysates (10 μg) were subjected to Western blot analysis using phospho-specific antibodies against p44/42 MAPK and Akt. The figure shows one representative Western blot of 4 independent experiments.
Discussion
The data presented in this work for the first time link FVIIa/TF signaling directly to regulation of cellular apoptosis. Two major pathways initiate apoptosis. One pathway is mediated through activation of the death receptors, resulting in activation of caspases 8, 10, and 12. The other pathway is initiated by extracellular stress conditions, such as oxidative stress and growth factor withdrawal, and acts through disruption of the mitochondrial membrane integrity, leading to cytochrome c release and activation of caspase 9. The two pathways merge in activation of the executioner caspase 3. The present work showed that serum withdrawal from BHK(+TF) and CHO(+TF) cells resulted in apoptosis as evidenced by caspase 3 activation, nuclear condensation, and DNA degradation. FVIIa/TF attenuated this response, suggesting that FVIIa promotes cell survival by inhibiting apoptotic reactions induced by cellular stress. The antiapoptotic effect of FVIIa was dependent on its binding to TF and on the proteolytic activity of FVIIa. Further, the effect was specific for FVIIa, as specific inhibitors of FXa and thrombin failed to inhibit the FVIIa-induced antiapoptotic effect.
Our earlier data show that stimulation of BHK(+TF) cells12,14 with FVIIa results in activation of both the p44/42 MAPK pathway and the Akt pathway. The data presented in the manuscript with CHO(+TF) cells confirm this finding. A similar simultaneous activation of Akt and p44/42 MAPK has been seen in several studies with growth factor stimulation.26,27 The Akt pathway has emerged as the major mechanism by which a long list of growth factors and other extracellular ligands promote cell survival.28 It also has been shown that activation of Ras and its downstream effectors Raf/MEK/ERK are critical for the promotion of cell survival under some conditions.29 The present data show that FVIIa failed to prevent apoptosis in the presence of the PI3K inhibitor LY294002, which suggests that FVIIa exerts its effect via stimulation of PI3K and its downstream effector Akt. In contrast, a complete blockade of p44/42 MAPK pathway with the MEK inhibitor U0126 resulted in only a modest, although statistically significant, decrease in FVIIa-dependent rescue from the apoptosis. The absence of cross-inhibition between the PI3K inhibitor and the MEK inhibitor suggests that the inhibitors are highly selective for the respective pathways at the concentrations applied. Based on these data, we conclude that FVIIa-induced activation of the PI3K/Akt signaling pathway is primarily responsible for mediating FVIIa's antiapoptotic effect.
The antiapoptotic function of FVIIa/TF described in the present study, in concert with FVIIa/TF-induced up-regulation of specific genes5,16,17 and cell migration,3,30,31 could contribute to tumor growth and metastasis. TF expression is shown to correlate closely with microvessel density, malignancy, and metastatic potential in many tumor types.32,33 In vivo experiments using tumor cells and cells transfected with TF have shown that the metastatic potential depends strongly on TF expression.7,8,10 Tumor cells transfected to overexpress TF also have a higher growth rate and establish larger and more vascularized tumors than the control transfectants.34 Administration of TF antibodies or active site-inactivated factor VIIa, both of which inhibit FVIIa binding to TF, were shown to attenuate TF-dependent tumor metastasis.10 Further studies showing that overexpression of TF up-regulates the proangiogenic factor vascular endothelial growth factor (VEGF)34,35 led to the suggestion that increased expression of VEGF is responsible for the angiogenic effect associated with TF-expressing cells. However, others found no correlation between TF and VEGF expression.9 Thus, the concept that FVIIa/TF promotes angiogenesis by direct elaboration of VEGF is debatable. Based on the present data, we propose that FVIIa/TF-induced cell survival, in addition to other FVIIa/TF-induced cellular processes, contributes to tumor growth and metastasis.
The progression of a variety of natural physiological processes such as embryo development, tissue remodeling, angiogenesis, and wound healing are closely regulated by a delicate balance between apoptotic and antiapoptotic reactions,21,36 and the pathogenesis of several diseased states involves impairment of this balance. Thus, it is conceivable that the antiapoptotic function of FVIIa/TF described in the present study may play a pivotal role in many of the above-described pathophysiological processes. However, it remains to be established whether FVIIa-induced signaling is potent enough to act as antiapoptotic stimulus in primary cells that express a much lower concentration of TF. Testing the effect of FVIIa in primary cells, such as endothelial cells and smooth muscle cells, and obtaining equivocal data could be somewhat difficult since not only is TF expression in these cells relatively low but also many stimuli that are used to induce TF expression in vitro could themselves induce myriad responses. It is possible that factor Xa or thrombin, the downstream coagulation factors generated by FVIIa/TF, may also be relevant as antiapoptotic molecules in vivo. Our present study shows that both factor Xa and thrombin also protect BHK(+TF) cells from apoptosis. However, one should note that thrombin exhibits a dual response in many cell types, including tumor cells, as a survival factor as well as a proapoptotic factor, depending on the concentration of thrombin and duration of the exposure.37-40 It is unlikely that FVIIa behaves similarly since the concentration of FVIIa/TF that can be generated on cell surfaces is limited to the amount of TF present on the cell surface.
At present, the mechanism by which FVIIa binding to TF leads to activation of PI3-kinase and MEK is unresolved. Since the proteolytic activity of FVIIa is essential for the activation of PI3 kinase,14,15 MEK,12 and antiapoptotic effect (present study), and the activators of protease activated receptors (PAR), such as thrombin37,38 and activated protein C,41,42 have been shown to induce antiapoptotic signaling, it is likely that FVIIa-induced antiapoptotic signaling may also be mediated via activation of a PAR. Conflicting data exist on whether FVIIa-induced signaling involves the activation of a known PAR. Heterologous desensitization studies indicate that FVIIa-induced signaling may not necessarily involve a known PAR.13,17,43 In an earlier study,24 based on FVIIa's inability to release intracellular Ca++ in BHK(+TF) and its failure to desensitize the cells to the subsequently added PAR activators, we have concluded that FVIIa-induced intracellular signaling is not mediated via known PARs. Although the above observation holds true and extends to CHO(+TF) and many other TF-expressing cells (B.B.S. et al, unpublished data, 2002), a number of recent studies, using other approaches, show that FVIIa/TF could signal through the activation of either PAR-1 or PAR-2.44-46 It is possible that slower kinetics of PAR activation by FVIIa, partly due to the need for FVIIa to bind TF before activating PARs, may be the reason why FVIIa fails to induce intracellular Ca++ release as does a typical PAR activator. Therefore, at present, we cannot exclude the possibility that the FVIIa-induced antiapoptotic effect is mediated via either PAR-1 or PAR-2. In this regard, one should note that both BHK(+TF)24 and CHO(+TF) (B.B.S. et al, unpublished data, 2002) cells express PAR-1 and PAR-2, as evidenced by the release of intracellular Ca++ upon stimulation with PAR-1 and PAR-2 peptide agonists. Studies by Versteeg et al14 showed that FVIIa binding to TF stimulates the signaling pathway involving the activation of Src-like kinases, subsequently PI3K, followed by c-Akt. Therefore, it is possible that FVIIa-induced activation of Src-like kinases contributes to the FVIIa-induced antiapoptotic signaling.
In summary, the present data provide evidence for the first time that FVIIa-induced signaling promotes cell survival by inhibiting apoptosis. FVIIa/TF-induced PI3K/Akt signaling pathway is primarily responsible for this antiapoptotic effect. The antiapoptotic effect of FVIIa/TF may explain how FVIIa/TF can influence various cellular processes. Further studies are needed to elucidate the mechanism by which FVIIa-induced signaling provides protection against apoptosis and to identify molecular components involved in cell survival mediated by FVIIa.
Prepublished online as Blood First Edition Paper, May 8, 2003; DOI 10.1182/blood-2003-01-0157.
B.B.S., D.T., and L.C.P. are employed by a company (Novo Nordisk) whose product (recombinant coagulation factor FVIIa) was studied in the present work.
Supported partly by National Institutes of Health grant HL 65500 (L.V.M.R.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Lone Langhoff, Berit Lassen, Steen Kryger, and Elke Gottfriedsen are thanked for excellent technical assistance.