Abstract
Protein S exhibits anticoagulant activity independent of activated protein C (APC). An automated factor Xa–based one-stage clotting assay was developed that enables quantification of the APC-independent activity of protein S in plasma from the ratio of clotting times (protein S ratio [pSR]) determined in the absence and presence of neutralizing antibodies against protein S. The pSR was 1.62 ± 0.16 (mean ± SD) in a healthy population (n = 60), independent of plasma levels of factors V, VIII, IX, and X; protein C; and antithrombin, and not affected by the presence of factor V Leiden. The pSR strongly correlates with the plasma level of protein S and is modulated by the plasma prothrombin concentration. In a group of 16 heterozygous protein S–deficient patients, the observed mean pSR (1.31 ± 0.09) was significantly lower than the mean pSR of the healthy population, as was the pSR of plasma from carriers of the prothrombin G20210A mutation (1.47 ± 0.21; n = 46). We propose that the decreased APC-independent anticoagulant activity of protein S in plasma with elevated prothrombin levels may contribute to the thrombotic risk associated with the prothrombin G20210A mutation.
Introduction
Protein S is a vitamin K–dependent anticoagulant protein that plays an important role in the regulation of blood coagulation. Several studies have indicated that a deficiency in protein S is a risk factor for venous thrombosis.1-3 Protein S regulates blood coagulation by various mechanisms. Protein S acts as a cofactor for activated protein C (APC) in the inactivation of factor Va4,5 and VIIIa6,7 and it exhibits anticoagulant activity in the absence of APC8-11 ; that is, protein S was shown to directly inhibit the activity of the prothrombin and intrinsic factor X–activating complexes on negatively charged phospholipids, platelets, and endothelial cells.10-14 In plasma, approximately 60% of protein S circulates in complex with C4b binding protein (C4BP), a regulator of the classical complement cascade.15 Binding of protein S to C4BP completely inhibits the APC-cofactor activity of protein S,16 but the complex still exhibits APC-independent anticoagulant activity.10
Binding of protein S to phospholipids and direct interactions with factors Va and Xa appear to be important for its inhibitory action.10,11,13 However, the precise molecular mechanisms by which protein S exerts APC-independent anticoagulant activity remain unknown.
It was previously shown that purified protein S preparations contain 2 fractions: a multimeric fraction having an unnaturally very high affinity for phospholipids and a normal (natural) fraction.17 Prothrombinase inhibition by the isolated multimeric fraction has been observed to be strongly phospholipid dependent. These protein-S multimers, although present in percentage amounts, can make up for more than 90% of the total inhibitory potential of purified protein S, depending on the phospholipid concentration. Because protein-S multimers are absent in plasma,17 the anticoagulant activity of protein S in plasma in the absence of APC can therefore be regarded as the natural activity not obscured by the in vitro–generated multimers. These observations emphasize the importance of studying unmodified protein S in its natural environment, that is, full plasma.
Several genetic defects are known that influence plasma protein S levels,18 APC cofactor activity of protein S,19 or both.20,21 However, no functional abnormalities have been described for the APC-independent activity of protein S. This may in part be due to the fact that it is difficult to measure the APC-independent activity of protein S in plasma. An assay for the APC-independent activity was developed in which coagulation in plasma was triggered with diluted tissue factor in the presence of low-molecular-weight heparin (LMWH; Fragmin).22 The ratio of clotting times measured in plasma in the absence and presence of a functional inhibitory antibody against protein S was used as a measure of the APC-independent activity of protein S. However, due to the presence of heparin and because plasma samples were diluted 1:1 with protein S–depleted plasma, the reaction conditions chosen in the LMWH-based assay22 may mask the influence of other plasma components on the APC-independent activity of protein S. In the present study we investigated the APC-independent anticoagulant activity of protein S in undiluted plasma without the use of heparin.
Patients, materials and methods
Materials
Protein S and factor Xa were purchased from Enzyme Research Laboratories (South Bend, IN). HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) was from ICN (Costa Mesa, CA). Bovine serum albumin (BSA; initial fractionation by heat shock) was purchased from Sigma (St Louis, MO). LMWH was obtained from Pharmacia and Upjohn (Woerden, the Netherlands). Human recombinant tissue factor (Innovin) was from Dade Behring (Marburg, Germany). Cyanogen bromide (CNBr)–activated Sepharose, QAE-Sephadex, and Mono Q resins were purchased from Pharmacia (Uppsala, Sweden). 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) and 1,2-dioleoyl-sn-glycero-3-phosphoserine (DOPS) were obtained from Avanti Polar Lipids (Alabaster, AL). Protein C–depleted plasma and its corresponding parent plasma, as well as factor II–, V–, VII–, VIII–, IX–, and X–depleted plasma and antithrombin-depleted plasma, were purchased from Affinity Biologicals (Hamilton, ON, Canada). Antibodies against protein S were obtained from Dako (Glostrup, Denmark). The REAADS Monoclonal Free Protein S Antigen Test Kit was purchased from Corgenix (Westminster, CO). Ecarin (prothrombin activator from the venom of the saw-scaled viper Echis carinatus) was obtained from Pentapharm (Basel, Switzerland). Other reagents were of the highest grade available. Human prothrombin was purified as previously described.7
Specificity of polyclonal antibodies against protein S
The polyclonal antibody against protein S was specific for protein S as determined by enzyme-linked immunosorbent assay (ELISA) using normal and protein S–deficient plasma. The polyclonal antibody was tested on other vitamin K–dependent coagulation factors (factor II, factor X, protein C) in an ELISA setup and cross-reactivity was absent. On sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) Western blots from plasma and purified protein S, only one band was visible when the polyclonal antibody against protein S was used. When tested on purified prothrombin on native and SDS-PAGE Western blots, no signal was obtained. Protein S–depleted plasma incubated with and without antibodies showed identical turbidity change (clotting) curves, indicating that the antibody against protein S does not functionally affect plasma coagulation proteins other than protein S.
Collection of plasma samples
After discarding the first 2 mL, 9 parts of venous blood from 80 healthy hospital workers, postthrombotic patients with protein S deficiency (n = 16), and prothrombin G20210A carriers (n = 46, 15 men and 31 women) were drawn from the antecubital vein via an open system and collected in 1 part 3.8% (0.13 M) trisodium citrate. The blood was centrifuged at 3000g to separate plasma from blood cells and centrifuged again at 19 000g to obtain platelet-poor plasma. Normal pooled plasma was prepared by pooling plasma from 80 healthy individuals (51 men and 29 women; mean age, 40 years). Individual normal plasma samples were from the last 60 individuals (40 men and 20 women; mean age, 40 years) who donated blood for the normal pool. The presence of factor V Leiden was determined using the prothrombinase-based APC resistance assay as previously described23 and via a polymerase chain reaction (PCR)–based method. None of the volunteers who participated in this study were using oral anticoagulants or oral contraceptives. Blood samples were taken from control subjects and patients who were enrolled in a large database and who were referred to our center for thrombophelia screening. Approval for the screening was obtained from the institutional board ethics committee of University Hospital Maastricht. Written informed consent was given by all participants.
Preparation of depleted plasmas
Fresh venous blood was drawn via an open system from 9 healthy and sober volunteers (6 men, 3 women) as described above. Protein S–depleted plasma was obtained by incubating the pooled plasma batchwise with rabbit polyclonal anti–protein S antibodies coupled to Sepharose CL-4B for 3 hours at room temperature, after which the resin was removed by centrifugation. Before use, both resins had been equilibrated in HEPES-buffered saline (HBS; 25 mM HEPES, pH 7.7, 175 mM NaCl). As a control, the parent plasma was incubated with Sepharose CL-4B. The protein-S total antigen levels of the plasmas, as determined by ELISA, were 85% of the levels of normal pooled plasma in the case of parent plasma and approximately 1% in the case of the protein S–depleted plasma.
Preparation of hemolytic and lipemic plasma
Fresh venous blood was drawn via an open system from a healthy male volunteer after overnight fasting as described above. The anticoagulated blood was divided into 2 portions. One portion was given an ultrasonic pulse of approximately 0.5 seconds, using a probe tip sonicator (set at 7 μm peak-to-peak amplitude). Platelet-poor plasma was prepared from both plasmas as described above. The sonicated plasma was red owing to hemolysis by sonication. Several hours later, the same volunteer was offered a meal consisting of chicken livers and French fries and blood was taken 2 hours after the meal. Platelet-poor lipemic plasma was prepared from the blood as described above.
Assays for the APC-independent anticoagulant activity of protein S
Heparin-based assay performed on microtiter plates. The heparin-based assay for the APC-independent anticoagulant activity of protein S was performed as described.22 Briefly, to 75 μL plasma (37.5 μL subject plasma and 37.5 μL protein S–depleted plasma) in a 96-well microtiter plate, LMWH was added to a final concentration of 1 U anti–factor Xa per milliliter. The plasma was incubated in the presence of 9 μL Dako rabbit polyclonal anti–protein S antibodies or buffer for 30 minutes at room temperature. Coagulation was initiated by addition of 25 μL human recombinant tissue factor diluted 1:200 in HBS containing 68 mM CaCl2 and 5 mg/mL BSA. The coagulation process was monitored at 37°C in a plate reader at 405 nm. The clotting time was defined as the time required to obtain half-maximal absorbance change at 405 nm.
Factor Xa–based assay performed on microtiter plates. Plasma (100 μL) was incubated in the presence of 12 μL Dako rabbit polyclonal anti–protein S antibodies (2.5 μM final concentration) or buffer for 30 minutes at room temperature. In a 96-well microtiter plate, 50 μL plasma was incubated for 3 minutes at 37°C and coagulation was triggered by the addition of 150 μL 25 pM factor Xa and 8 μM DOPS/DOPC (20/80, M/M) vesicles in HBS containing 11 mM CaCl2 and 5% BSA. This resulted in final concentrations of 20 pM factor Xa, 6 μM DOPS/DOPC (20/80, M/M) vesicles, 5 mM CaCl2 and 5% (wt/vol) BSA. Progress of coagulation was monitored at 37°C in a plate reader at 405 nm. The clotting time was defined as the time required to obtain half-maximal absorbance change at 405 nm. The APC-independent activity of protein S in normal pooled plasma was completely inhibited at an anti–protein S antibody concentration of more than 2.25 μM (data not shown).
Factor Xa–based assay. The factor Xa–based assay was adapted to be performed on an ACL-300 (Instrumentation Laboratory, Milan, Italy) with the following coagulation program parameters: sample position, 50 μL; reagent position 1, 150 μL; incubation time, 210 seconds; intermediate ramp time, 1 second; delay, 0 seconds; acquisition time, 600 seconds; rotation speed, 1200 rpm. A PT-FIB reagent container (Instrumentation Laboratory) was used at position 1 for the start reagent containing 25 pM factor Xa, 8 μM DOPS/DOPC (20/80, M/M) vesicles, 11 mM CaCl2, and 5% BSA in HBS. The clotting time was defined as the time needed for the absorbance to pass a threshold value of 0.06 optical density (OD) units above the initial absorbance of the sample (onset of coagulation).
Preparation of phospholipid vesicles
Phospholipid vesicles were prepared in glass test tubes by evaporating 400 μL 10 mM DOPS/DOPC (20/80, M/M) stock solution in chloroform/methanol under nitrogen flow. The resulting phospholipid film was suspended in 2 mL HBS and sonicated on ice to clarity, using a probe tip sonicator (set at 7.5 μm peak-to-peak amplitude). The resulting 2 mM DOPS/DOPC (20/80, M/M) vesicle suspension was diluted 10-fold and aliquots were stored under nitrogen at –80°C.
Purification of human C4BP containing the β-chain
The protein S–C4BP complex was isolated from 20 units of freshly frozen plasma (5 L) as previously described.7 Briefly, the plasma was subjected to barium citrate, ammonium sulfate precipitation, and QAE-Sephadex anion exchange chromatography. The protein S–C4BP complex was obtained as an early peak eluting from the QAE-Sephadex column. The pooled fraction containing the protein S–C4BP complex was dialyzed overnight against 25 mM Tris (tris(hydroxymethyl)aminomethane)–HCl, pH = 7.4, 150 mM NaCl, 2 mM EDTA (ethylenediaminetetraacetic acid), and loaded onto Mono Q resin (1 mL bed volume). A linear gradient of 25 mM Tris-HCl, pH = 7.4, 600 mM NaCl, 2 mM EDTA was applied and free C4BP was collected as a peak at approximately 250 mM NaCl. The C4BP obtained via this method contained approximately 5% (m/m) protein S.
Prothrombin assay
The concentration of prothrombin was determined using the purified prothrombin activator Ecarin from the venom of the saw-scaled viper (E carinatus) as described before.24
Determination of total and free protein-S antigen
Total protein-S antigen was determined using an ELISA with rabbit-polyclonal antibodies (Dako) against human protein S and was performed essentially as described,17 except that diluted plasma samples were incubated in the microtiter plate overnight at room temperature instead of 1 hour at room temperature to facilitate C4BP–protein S complex dissociation. Free protein-S antigen was determined using a REAADS monoclonal free protein-S antigen determination kit (Corgenix, Westminster, CO) according to the manufacturer's instructions. Samples were processed as described.25 All antigen determinations were performed using normal pooled plasma as the standard and antigen levels were expressed as percentages of levels present in normal pooled plasma.
Statistical analysis
Population means were compared using a t test. Correlations given in this report are represented as Pearson correlation coefficients.
Results
Comparison of factor Xa– and heparin-based assays for the APC-independent anticoagulant activity of protein S
In the reported assay for the measurement of APC-independent anticoagulant activity of protein S,22 plasma samples are diluted 1:1 in protein S–depleted plasma and LMWH is added to enhance the inhibition of coagulation by protein S. Although these assay conditions appeared to be necessary to effectively measure the anticoagulant effect of protein S, the addition of LMWH changes the milieu in which protein S normally acts and therefore may cause incorrect assessment of its in vivo activity.
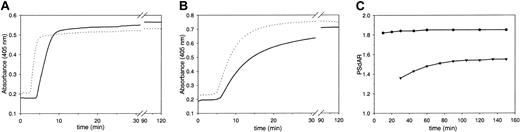
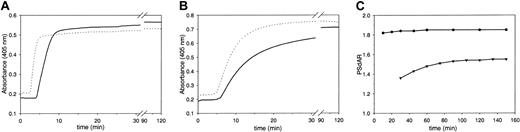
We have investigated the possibility of measuring the APC-independent activity of protein S in undiluted plasma in the absence of LMWH. In the test that we have developed, coagulation was initiated by the addition of factor Xa, phospholipids, and CaCl2 in plasma preincubated with and without inhibitory polyclonal antibodies against protein S. Clotting was monitored by following the turbidity change at 405 nm. Owing to inhibition of the anticoagulant action of protein S by the anti–protein S antibodies, the lag time that precedes clotting of plasma was shorter in antibody-treated plasma than in untreated plasma (Figure 1A). The protein S–dependent anticoagulant ratio (PSdAR), calculated from the time required to obtain the half-maximal turbidity change in the absence and presence of antibody, was approximately 1.8. To compare the performance of our test with that of the reported assay,22 normal pooled plasma was diluted 1:1 in protein S–depleted plasma in the presence of LMWH and coagulation was initiated with diluted tissue factor in plasma with and without anti–protein S antibody, as described.22 Again, plasma incubated with anti–protein S antibodies had a shorter clotting time than untreated plasma (Figure 1B). However, comparison of panels A and B in Figure 1 shows that the change in turbidity proceeded much slower in the LMWH-based assay than in the factor Xa–based assay. In order to accurately determine the PSdAR, coagulation must be allowed to reach an absolute end point, which in the LMWH-based assay took 1.5 to 2 hours. For both assays the relation between assay duration (ie, the time taken as end point of fibrin formation) and the PSdAR was investigated. The PSdAR of the factor Xa–based assay was virtually independent of the time at which the end point was taken, whereas in the LMWH-based assay the ratio varied significantly with the end-point time (Figure 1C).
Factor Xa– and LMWH-based assays for the APC-independent activity of protein S in plasma. Normal pooled plasma was incubated for 30 minutes with (dashed line) or without (solid line) anti–protein S neutralizing antibodies. (A) Coagulation was triggered with CaCl2 (5 mM), factor Xa (20 pM), and phospholipids (6 μM DOPS/DOPC, 20/80 M/M) at indicated final concentrations. (B) LMWH was added and coagulation was triggered with dilute human recombinant tissue factor as described in “Patients, materials and methods.” Fibrin formation was followed by measuring the change in turbidity at 405 nm. (C) Protein S–dependent anticoagulant ratio (PSdAR) of the times to half-maximal turbidity at 405 nm in the absence and presence of anti–protein S antibodies. The factor Xa–based assay (•) and LMWH-based assay (▾) are represented as a function of assay duration.
Factor Xa– and LMWH-based assays for the APC-independent activity of protein S in plasma. Normal pooled plasma was incubated for 30 minutes with (dashed line) or without (solid line) anti–protein S neutralizing antibodies. (A) Coagulation was triggered with CaCl2 (5 mM), factor Xa (20 pM), and phospholipids (6 μM DOPS/DOPC, 20/80 M/M) at indicated final concentrations. (B) LMWH was added and coagulation was triggered with dilute human recombinant tissue factor as described in “Patients, materials and methods.” Fibrin formation was followed by measuring the change in turbidity at 405 nm. (C) Protein S–dependent anticoagulant ratio (PSdAR) of the times to half-maximal turbidity at 405 nm in the absence and presence of anti–protein S antibodies. The factor Xa–based assay (•) and LMWH-based assay (▾) are represented as a function of assay duration.
Determination of the APC-independent anticoagulant activity of protein S from the onset of coagulation
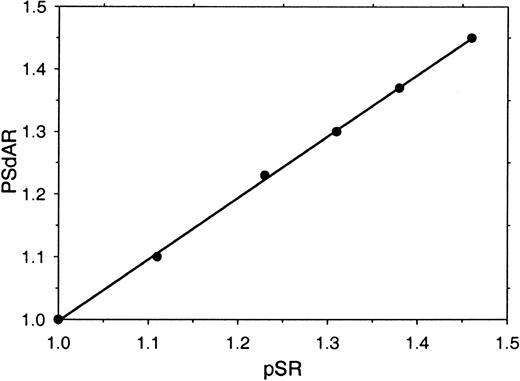
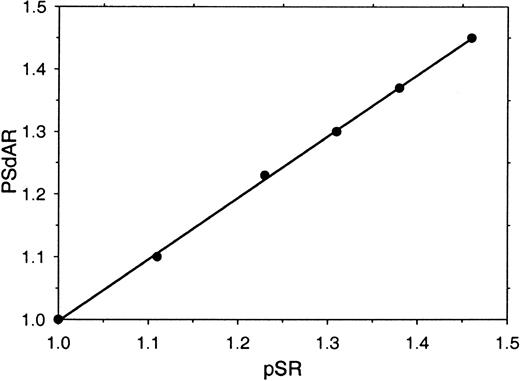
Visual inspection of the clotting curves (Figure 1A-B) indicates that, particularly in the factor Xa–based assay, there is a distinct difference between the lag times that precede the onset of absorbance change in antibody-treated and untreated plasma (Figure 1A). This prompted us to investigate whether this clotting lag-time ratio (protein S ratio [pSR]—the ratio of the times required to reach an absorbance change of 0.06 OD above base level in untreated and antibody-treated plasma) could be used as an alternative for the PSdAR. A high correlation between pSR and PSdAR (r2 = 0.998) was observed when the amount of protein S present in plasma was varied by mixing varying amounts of protein S–depleted plasma and parent plasma (Figure 2). When the pSR is used to measure the APC-independent anticoagulant activity of protein S, the assay is well suited to be performed on an ACL-300 autoanalyzer.
Relation between the PSdAR and the pSR. Protein S–depleted plasma was mixed with increasing amounts of its corresponding parent plasma (0%, 20%, 40%, 60%, 80%, and 100% parent plasma) and coagulation was triggered with CaCl2 (5 mM), factor Xa (20 pM), and phospholipids (6 μM DOPS/DOPC, 20/80 M/M) at indicated final concentrations. The PSdAR was plotted against the pSR.
Relation between the PSdAR and the pSR. Protein S–depleted plasma was mixed with increasing amounts of its corresponding parent plasma (0%, 20%, 40%, 60%, 80%, and 100% parent plasma) and coagulation was triggered with CaCl2 (5 mM), factor Xa (20 pM), and phospholipids (6 μM DOPS/DOPC, 20/80 M/M) at indicated final concentrations. The PSdAR was plotted against the pSR.
Effect of factor Xa and phospholipid concentrations on the protein S ratio (pSR)
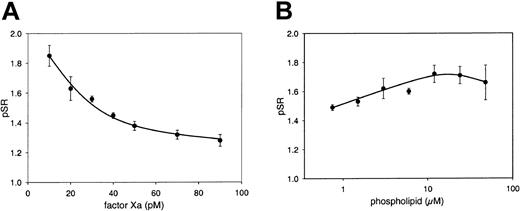
The influence of the amounts of factor Xa and phospholipid on the pSR was investigated by activating coagulation with various concentrations of factor Xa at a constant phospholipid concentration. At 6 μM phospholipid (DOPS/DOPC, 20/80, M/M), the pSR depended on the factor Xa concentration and increased with decreasing amounts of factor Xa (Figure 3A). Remarkably, at 20 pM factor Xa (final concentration), the pSR was only slightly dependent on phospholipid concentration (Figure 3B). This is in contrast to a strong phospholipid dependence of the APC-independent activity of protein S observed in purified systems,14 which most likely results from in vitro multimerization effects due to protein purification methods.17 Final concentrations chosen for the assay were 20 pM factor Xa and 6 μM DOPS/DOPC (20/80, M/M). Under these conditions the pSR could be sensitively measured while interassay variations remained within an acceptable low range.
Effect of the factor Xa and phospholipid concentration on the pSR. Normal pooled plasma was incubated for 30 minutes with and without antibodies against protein S. Coagulation was triggered with 5 mM CaCl2 and either 6 μM phospholipid vesicles (DOPS/DOPC, 20/80, M/M) and 10, 20, 30, 40, 50, 70, and 90 pM factor Xa (A), or 20 pM factor Xa and 0.75, 1.5, 3, 6, 12, 24, and 48 μM phospholipid vesicles (DOPS/DOPC, 20/80, M/M) (B). The pSR is indicated as a function of factor Xa (A) or phospholipid concentration (B). Means ± SDs of 3 measurements are shown.
Effect of the factor Xa and phospholipid concentration on the pSR. Normal pooled plasma was incubated for 30 minutes with and without antibodies against protein S. Coagulation was triggered with 5 mM CaCl2 and either 6 μM phospholipid vesicles (DOPS/DOPC, 20/80, M/M) and 10, 20, 30, 40, 50, 70, and 90 pM factor Xa (A), or 20 pM factor Xa and 0.75, 1.5, 3, 6, 12, 24, and 48 μM phospholipid vesicles (DOPS/DOPC, 20/80, M/M) (B). The pSR is indicated as a function of factor Xa (A) or phospholipid concentration (B). Means ± SDs of 3 measurements are shown.
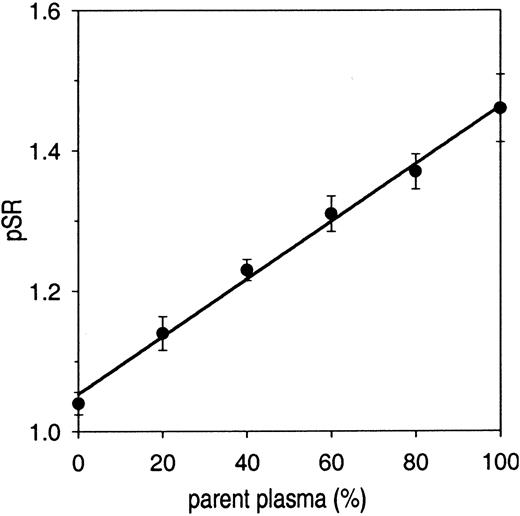
A calibration curve made by mixing protein S–depleted plasma with its corresponding parent plasma (Figure 4) showed that the pSR was linearly dependent on the amount of parent plasma and varied between 1.04 ± 0.02 (protein S–depleted plasma) and 1.46 ± 0.05 (parent plasma). The pSR of the parent plasma (1.46) was lower than that of normal pooled plasma (1.63) because the plasma was diluted (to 85%) during the batchwise depletion procedure (incubation with Sepharose CL-4B with or without anti–protein S antibodies).
pSR as a function of total protein S in plasma. Protein S–depleted plasma was mixed with parent plasma and the APC-independent activity of protein S (pSR) was determined with the factor Xa–based assay. pSRs are plotted as a function of percentage of parent plasma. Each data point represents the mean value ± SD of at least 3 measurements.
pSR as a function of total protein S in plasma. Protein S–depleted plasma was mixed with parent plasma and the APC-independent activity of protein S (pSR) was determined with the factor Xa–based assay. pSRs are plotted as a function of percentage of parent plasma. Each data point represents the mean value ± SD of at least 3 measurements.
Incubation of normal pooled plasma with 200 nM purified C4BP resulted in a minor decrease of the pSR (by 11%, from 1.59 to 1.41), confirming that the complex of protein S and C4BP still has APC-independent activity.10
Repeated determination showed that the pSR of normal pooled plasma was 1.63 ± 0.08 (mean ± SD, 57 measurements) yielding an interassay variance of 4.7%. The intra-assay variance of the pSR was 1.6% (9 measurements). The pSR was not significantly influenced by hemolysis (sonication) or high lipid content of plasma due to high-fat diet (data not shown).
The effect of plasma coagulation factor levels on the APC-independent anticoagulant activity of protein S
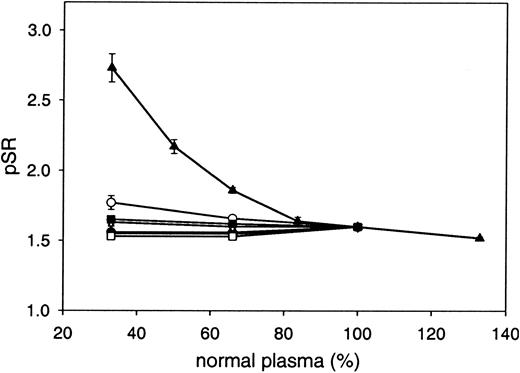
The influence of variation of the plasma levels of the coagulation proteins prothrombin; factors V, VII, VIII, IX, and X; and antithrombin was investigated by mixing plasma depleted of the respective coagulation protein with normal pooled plasma and determining the pSR. The pSR appeared to be dependent only on the prothrombin level in plasma and was increased almost 2-fold at a prothrombin level of 33% (Figure 5).
Influence of plasma coagulation proteins on the pSR. pSRs were plotted as a function of the concentration of prothrombin (▴), factor V (•), factor VII (○), factor VIII (▾), factor IX (▿), factor X (▪), or antithrombin (□). Coagulation factor–depleted plasmas were mixed with normal pooled plasma. Each data point represents the mean ± SD of at least 3 measurements. Purified human prothrombin was added to normal pooled plasma to obtain plasma with 133% prothrombin.
Influence of plasma coagulation proteins on the pSR. pSRs were plotted as a function of the concentration of prothrombin (▴), factor V (•), factor VII (○), factor VIII (▾), factor IX (▿), factor X (▪), or antithrombin (□). Coagulation factor–depleted plasmas were mixed with normal pooled plasma. Each data point represents the mean ± SD of at least 3 measurements. Purified human prothrombin was added to normal pooled plasma to obtain plasma with 133% prothrombin.
In order to exclude involvement of protein C and APC in the assay, the pSR of protein C–depleted plasma was compared with the pSR of its corresponding parent plasma. The pSR of protein C–depleted plasma (1.77 ± 0.02) was even slightly higher than that of its corresponding parent plasma (1.69 ± 0.01), while protein S levels were identical, indicating that the assay outcome is independent of (activated) protein C levels in plasma. In addition, polyclonal antibodies against protein C that completely inhibit APC anticoagulant activity were applied in the current test. Polyclonal antibodies against protein C did not affect the clotting times in the assay, either in the absence or presence of antibodies against protein S, yielding identical pSR values (1.66) when compared with the same plasma without antibodies against protein C. Therefore, the traces of APC that were reported to exist in blood in vivo26 are likely inhibited by serpins in plasma samples used in our study. Furthermore, the contribution of in situ activation of protein C during our assay duration is considered absent or too low to affect the clotting times and thus the pSR.
The effect of plasma protein S and prothrombin levels on the pSR
The APC-independent activity of protein S and the plasma levels of protein S (total and free) and prothrombin were measured in plasma of 60 of the 80 healthy individuals who contributed to the normal plasma pool, in plasma from 16 heterozygous protein S–deficient individuals, and in 46 carriers of the prothrombin G20210A mutation (Table 1 and Figure 6). The pSRs of factor V Leiden carriers are indicated by open circles (Figure 6).
APC-independent anticoagulant activity of protein S in plasma from healthy controls, protein S–deficient patients, and carriers of the prothrombin G20210A mutation. The APC-independent anticoagulant activity of protein S (pSR) was determined with the factor Xa–based assay in plasma from 60 healthy controls, 16 protein S–deficient patients, and 46 prothrombin G20210A carriers as described in “Patients, materials and methods.” Factor V Leiden carriers are indicated by open circles. The mean value, represented as a horizontal bar, was 1.62 for the control population, 1.31 for the protein S–deficient patients, and 1.47 for the prothrombin G20210A carriers.
APC-independent anticoagulant activity of protein S in plasma from healthy controls, protein S–deficient patients, and carriers of the prothrombin G20210A mutation. The APC-independent anticoagulant activity of protein S (pSR) was determined with the factor Xa–based assay in plasma from 60 healthy controls, 16 protein S–deficient patients, and 46 prothrombin G20210A carriers as described in “Patients, materials and methods.” Factor V Leiden carriers are indicated by open circles. The mean value, represented as a horizontal bar, was 1.62 for the control population, 1.31 for the protein S–deficient patients, and 1.47 for the prothrombin G20210A carriers.
pSR values of the healthy population were distributed symmetrically around an average of 1.62 ± 0.16 (which is very close to the pSR [1.63 ± 0.08] of normal pooled plasma), with values ranging between 1.35 and 2.24 (Figure 6).
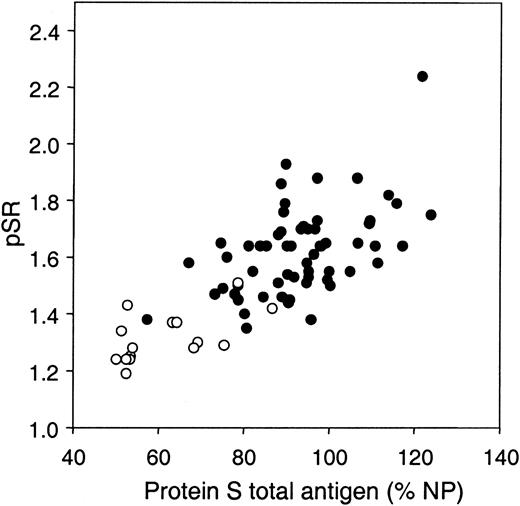
The pSR correlated well with protein S levels, and this correlation was stronger with total protein S (Figure 7; r = 0.520, P < .001) than with free protein S (data not shown; r = 0.365, P = .004). Considering the influence of prothrombin on the pSR (Figure 5), the prothrombin levels in the control population were measured. The average prothrombin level of the control population was 99% ± 13.0% (Table 1), which, together with the protein S total and free levels, was within normal range (Table 1), and the pSR did not significantly correlate with the plasma prothrombin concentration (r = –0.168, P = .20). However, in a regression model both variables were indicated as determinants of the pSR (r = 0.566, P < .001), with protein S having an up-regulating effect and prothrombin having a down-regulating effect on the pSR.
Correlation between APC-independent anticoagulant activity of protein S and plasma levels of protein S total antigen. The APC-independent anticoagulant activity of protein S (pSR) was determined with the factor Xa–based assay in plasma from 60 healthy controls (•) and 16 protein S–deficient patients (○).
Correlation between APC-independent anticoagulant activity of protein S and plasma levels of protein S total antigen. The APC-independent anticoagulant activity of protein S (pSR) was determined with the factor Xa–based assay in plasma from 60 healthy controls (•) and 16 protein S–deficient patients (○).
In the plasmas of 16 individuals with protein S deficiency, the mean pSR (1.31 ± 0.09; Table 1) was significantly lower (P < .001) than the pSR of the healthy population (Figure 6). This observation was in agreement with the decreased protein S levels but normal prothrombin levels in the protein S–deficient patients (Table 1). Unlike free protein S (r = 0.420, P = .105), only total protein S was found to correlate significantly with the pSR in the protein S–deficient patients (r = 0.586, P < .05). Interestingly, there was a significant negative correlation between prothrombin levels and the pSR in protein S–deficient patients (r = –0.605, P < .05). This indicates that the effect of prothrombin on the pSR is increased at low protein S concentrations.
To identify a modulatory effect of increased prothrombin levels associated with the prothrombin G20210A mutation on the APC-independent activity of protein S, the pSR of a group of 46 prothrombin G20210A carriers was determined (Figure 6). The average pSR of the prothrombin G20210A carriers was 1.47 ± 0.21, which was significantly lower (P < .001) than the mean pSR of the control group (1.62; Table 1). The prothrombin levels of the prothrombin G20210A carriers were significantly elevated (Table 1), whereas protein S total levels were similar to the protein S total levels of the control group. Protein S antigen values in men are significantly higher than in women. Since 40 of 60 in the control population and 15 of 46 of the prothrombin mutation carriers were men, it was important to know whether the results would change if only men or only women were considered. After stratification, a significant difference remained between the pSR of the control and prothrombin G20210A populations in men and women, respectively (P < .05). Taken together, increased prothrombin levels can explain the decreased APC-independent activity of protein S observed in carriers of the prothrombin G20210A mutation.
Discussion
In this paper we have described an automated one-stage coagulation assay that enables quantification of the APC-independent anticoagulant activity of protein S in plasma. In this assay, coagulation is initiated in plasma with low amounts of factor Xa, phospholipids, and CaCl2, and the time to onset of coagulation is compared with that of plasma in which protein S is inhibited by antibodies. Using this assay, the APC-independent activity of protein S could be effectively measured without the necessity of adding coagulation inhibitors like heparin or diluting plasma in protein S–depleted plasma as was reported previously.22
In the present assay a clotting time ratio (pSR), calculated from coagulation lag-times, was used to quantify the APC-independent activity of protein S. This is in contrast to the ratio of times required to reach half-maximal turbidity during the clotting of plasma, used in the previously reported heparin-based assay (PSdAR22 ). While the present assay has the advantage that clotting time ratios can be determined from the lag times within 5 minutes, stable turbidity end-point determinations in the heparin-based assay are only reached at approximately 60 minutes after initiation of coagulation. In the heparin-based assay, pSR measurements (ie, ratios calculated from clotting lag times) are not possible, owing to very small differences between the lag times of plasma treated with anti–protein S antibody and untreated plasma. The present pSR assay offers the advantage of quick measurements (< 5 minutes) and enables determination of the pSR on autoanalyzers.
The pSR assay was set up to directly determine the APC-independent anticoagulant activity of protein S without dilution of the test plasma in protein S–depleted plasma. Therefore, plasma components that might modulate the APC-independent activity of protein S are more likely to be unveiled. The APC-independent activity of protein S (pSR) was 1.63 in normal pooled plasma. The pSR was independent of plasma concentrations of factors V, VIII, IX, and X and was not affected by the presence of the factor V Leiden mutation. Factor VII and antithrombin had only a minor influence on the pSR, and a possible effect of (activated) protein C was excluded. Low levels of antithrombin (33%) resulted in a slightly decreased pSR (1.53 ± 0.01), and the pSR was slightly elevated at low levels of factor VII (pSR = 1.77 ± 0.05 at 33% factor VII), phenomena for which we have as yet no explanation. Prothrombin appeared to have a large effect on the APC-independent anticoagulant activity of protein S. The pSR increased from 1.52 ± 0.01 at 133% prothrombin to 2.73 ± 0.10 at 33% prothrombin. The enhancement of APC-independent activity of protein S at low prothrombin concentrations is most likely explained by decreased competition between prothrombin and protein S for binding to the prothrombinase complex components (factor Xa, factor Va, and phospholipid).
Modulation of protein S by prothrombin has already been observed in previous studies. It was reported that protein S was able to directly inhibit factor Xa in the absence of factor Va, and this inhibition could be counteracted by increasing amounts of prothrombin.8 Later it was shown that binding of prothrombin to immobilized factor Va was effectively inhibited by protein S.9 Interestingly, it was also shown that prothrombin was able to inhibit the APC-cofactor activity of protein S,27 and inhibition of APC-cofactor function as well as APC-independent activity of protein S by prothrombin may, at least in part, share a mechanistic basis. However, structural requirements for the APC-cofactor and APC-independent anticoagulant activities of protein S have been shown to be different.28 In addition, prothrombin has been shown to inhibit APC activity in the absence of protein S,29 and therefore establishment of the mechanism by which protein S inhibits prothrombinase will not be straightforward.
We observed only a modest effect of phospholipid concentration on the APC-independent activity of protein S in our assay. We believe that an effect of the phospholipid concentration on APC-independent activity of protein S is observed particularly when purified protein S preparations are used in reconstituted systems. It was reported that purified protein S preparations contain multimers as a result from in vitro purification procedures.17 These protein S multimers have a high phospholipid-binding affinity (Kd < 1 nM) and a 100-fold increased APC-independent anticoagulant activity due to the ability of multimers to inhibit phospholipid-dependent coagulation reactions.17 The overall APC-independent activity of a protein S preparation is therefore governed by this small percentage of protein S multimers that act via a different mechanism (direct competition for phospholipid binding sites), and that explains previously reported results in in vitro reaction systems.13,14 The fact that protein S multimers are absent in normal plasma17 explains why the APC-independent activity of protein S in plasma is independent of the phospholipid concentration; that is, the mechanism of competition of phospholipid surface is not the native mechanism by which protein S attenuates blood coagulation in absence of APC. The in vitro multimerization of purified protein S emphasized the need to study the APC-independent activity of native protein S in its native environment.
The pSR of normal pooled plasma (1.63 ± 0.08) corresponded well to the mean pSR of the healthy individuals (1.62 ± 0.16). However, there was considerable variation of the pSR within the healthy population, hinting at individual differences. The pSR correlated both with the free and total plasma levels of protein S. The better correlation of the pSR with total protein S than with free protein S supports the observation that the protein S–C4BP complex as well as free protein S contribute to the APC-independent inhibition of the prothrombinase complex.10 In the present assay this was confirmed by a small decrease in pSR after all protein S was complexed to exogenously added C4BP.
Considering the strong dependence of the pSR on plasma protein S levels, it is not surprising that in a group of protein S–deficient patients the pSR was significantly lower than that of the healthy population. At lower levels of protein S, factors other than protein S (eg, prothrombin) may contribute to the assay outcome. This idea is supported by the observation that a significant negative correlation was found between the pSR and plasma prothrombin levels (r = –0.605, P < .05) in protein S–deficient patients. In the healthy population, however, a poor correlation between prothrombin and the pSR was observed. This can be explained by opposite effects on the pSR by prothrombin and protein S of which the plasma levels are linked by their synthesis in the liver. We therefore subsequently performed a multivariate regression model in which prothrombin and protein S total and free levels were added as parameters. This model revealed prothrombin and protein S total levels as significant determinants of the pSR in plasma (data not shown).
Analogous to the uncoupling of protein S and prothrombin levels in the group of heterozygous protein S–deficient patients, increased plasma prothrombin levels may decrease the APC-independent activity of protein S in plasma when the prothrombin level in plasma changes independent of protein S. Indeed, carriers of prothrombin G20210A who have a mutation in the 3′ untranslated region of the prothrombin gene resulting in elevated plasma prothrombin30 (Table 1) were observed to have a significantly lower pSR (1.47 ± 0.21, P < .001) than the control group (1.62 ± 0.16), while total protein S levels were not significantly different. These observations may, at least in part, provide a possible rationale for the thrombotic risk associated with the prothrombin G20210A mutation.30
In conclusion, the assay presented in this report enables reproducible and efficient measurement of APC-independent activity of protein S in plasma and will contribute to a better understanding of the mechanism by which protein S attenuates blood coagulation. Measuring APC-independent anticoagulant activity of protein S in plasma may offer an additional tool to establish the physiologic importance of the anticoagulant activity of protein S in the absence of APC.
Prepublished online as Blood First Edition Paper, May 1, 2003; DOI 10.1182/blood-2003-02-0620.
Supported in part by a fellowship from The Royal Netherlands Academy of Arts and Sciences (KNAW) (T.M.H.) and by research grants from the Netherlands Organization for Scientific Research (NWO) (VIDI 917.36.372, T.M.H, and 902.26.210, R.R.K.)
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.