Abstract
Selectins recognize ligands containing carbohydrate chains such as sialyl Lewis x (sLex) that are mainly presented at the terminus of N-acetyl lactosamine repeats on core 2 O-glycans. Several glycosyltransferases act successively to extend the N-acetyl lactosamine repeats and to synthesize sLex, and β-1,4-galactosyltransferase (β4GalT) plays a key role in these processes. Recently isolated 6 β4GalT genes are candidates, but their individual roles, including those in selectin-ligand biosynthesis, remain to be elucidated. More than 80% of the core 2 O-glycans on the leukocyte membrane glycoproteins of β4GalT-I–deficient mice lacked galactose residues in β-1,4 linkage, and soluble P-selectin binding to neutrophils and monocytes of these mice was significantly reduced, indicating an impairment of selectin-ligand biosynthesis. β4GalT-I–deficient mice exhibited blood leukocytosis but normal lymphocyte homing to peripheral lymph nodes. Acute and chronic inflammatory responses, including the contact hypersensitivity (CHS) and delayed-type hypersensitivity (DTH) responses, were suppressed, and neutrophil infiltration into inflammatory sites was largely reduced in these mice. Our results demonstrate that β4GalT-I is a major galactosyltransferase responsible for selectin-ligand biosynthesis and that inflammatory responses of β4GalT-I–deficient mice are impaired because of the defect in selectin-ligand biosynthesis.
Introduction
The emigration of leukocytes to inflammatory sites during inflammation and infection, and the lymphocyte trafficking to lymph nodes (LNs) under normal circumstances progresses by means of sequentially acting cell adhesion molecules involved in leukocyte and endothelial cell interactions.1 The initial event in this process is leukocyte rolling on the endothelial surface, which is mediated by selectins and their oligosaccharide ligands. Leukocyte emigration is known to be mediated by E- and P-selectin on endothelial cells and sialyl Lewis x (sLex) on leukocytes.2,3 L-selectin on lymphocytes and sulfated sLex on the high endothelial venules (HEVs) of secondary lymphoid organs are involved in lymphocyte trafficking.4,5 P-selectin recognizes both sLex and a core protein, P-selectin glycoprotein ligand-1 (PSGL-1),6 whereas E- and L-selectin seem to bind oligosaccharide epitopes without a strict requirement for particular core proteins. However, the structure and biosynthesis of selectin oligosaccharide ligands remain to be elucidated.
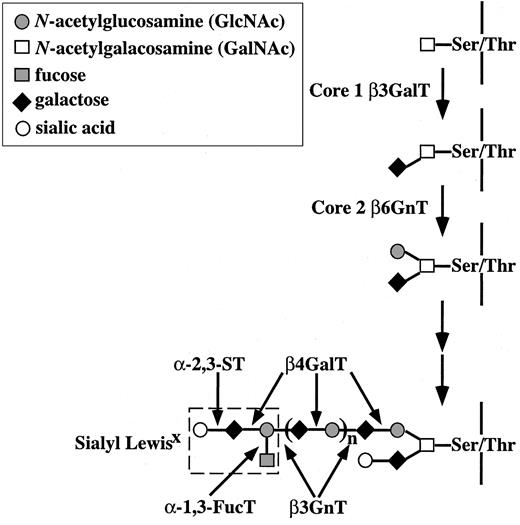
Selectin ligands such as sLex and sulfated sLex are mainly expressed at the terminus of N-acetyl lactosamine repeats on serine/threonine (O)–Linked oligosaccharides (O-glycans).7-9 In the biosynthesis of O-glycans, Galβ1→3GalNAcα1→Ser/Thr (core 1) is formed by the action of recently cloned core 1 β-1,3-galactosyltransferase10 (core 1 β3GalT). Core 1 is then converted to GlcNAcβ1→6(Galβ1→3)GalNAcα1→Ser/Thr (core 2) by core 2 β-1,6-N-acetylglucosaminyltransferase (core 2 β6GnT) (Figure 1). Studies of core 2 β6GnT-deficient mice have revealed that core 2 O-glycans are essential for leukocyte emigration during inflammation but not for lymphocyte trafficking under normal circumstances.11 These results suggest that most of the E- and P-selectin ligands are expressed on core 2 O-glycans. Recently, 6-sulfo sLex on extended core 1 O-glycans has been identified as one of the L-selectin ligands.12 β-1,4-Galactosyltransferase (β4GalT) and β-1,3-N-acetylglucosaminyltransferase (β3GnT) act alternatively to extend N-acetyl lactosamine repeats on core 2 O-glycans, and β4GalT is also required to synthesize the sLex epitope in collaboration with α-2,3-sialyltransferase (ST) and α-1,3-fucosyltransferase (FucT) (Figure 1). Among these glycosyltransferases, Fuc-TVII has been shown to be mainly responsible for the fucosylation of sLex.13,14 However, the contribution of the β4GalT genes in the biosynthesis of selectin ligands remains to be elucidated.
Biosynthesis of core 1 and core 2 O-glycans. Core 2 β6GnT generates a biantennary structure in O-glycans, and sLex is synthesized at the terminus of N-acetyl lactosamine disaccharide repeats on the core 2 branch. Core 1 β3GalT, core 1 β-1,3-galactosyltransferase; core 2 β6GnT, core 2 β-1,6-N-acetylglucosaminyltransferase; β4GalT, β-1,4-galactosyltransferase; α-1,3-FucT, α-1,3-fucosyltranferase; α-2,3-ST, α-2,3-sialyltransferase; β3GnT, β-1,3-N-acetylglucosaminyltransferase (i-extension enzyme).
Biosynthesis of core 1 and core 2 O-glycans. Core 2 β6GnT generates a biantennary structure in O-glycans, and sLex is synthesized at the terminus of N-acetyl lactosamine disaccharide repeats on the core 2 branch. Core 1 β3GalT, core 1 β-1,3-galactosyltransferase; core 2 β6GnT, core 2 β-1,6-N-acetylglucosaminyltransferase; β4GalT, β-1,4-galactosyltransferase; α-1,3-FucT, α-1,3-fucosyltranferase; α-2,3-ST, α-2,3-sialyltransferase; β3GnT, β-1,3-N-acetylglucosaminyltransferase (i-extension enzyme).
β4GalT is involved in the biosynthesis of biologically important galactose-containing oligosaccharides, such as stage-specific embryonic antigen-1 (SSEA-1 = Lex), sLex, and 6-sulfo sLex. SSEA-1, which is expressed specifically on preimplantation embryos, has been suggested to play a role in morula compaction and blastocyst formation.15 However, β4GalT-I–deficient embryos form normal compacted morulae and blastocysts. Polysialic acid (PSA) and human natural killer-1 (HNK-1) carbohydrate are expressed on the outer chains of the Galβ1→4GlcNAc-R backbone. These carbohydrates, which are expressed on neuronal cell adhesion proteins, are thought to be involved in neuronal cell recognition.16,17 In support of this idea, we and another group showed that mice deficient in the biosynthesis of HNK-1 and PSA exhibit impaired synaptic plasticity, respectively.18,19 To elucidate the biologic relevance of these galactose residues and their outer chains, we and another group previously generated β4GalT-I–deficient mice.20,21 These mice exhibit growth retardation and semi-lethality before weaning because of the augmented proliferation and abnormal differentiation of small intestinal epithelial cells.20 In contrast, PSA and HNK-1 are expressed normally, and no neuronal defects are detected in β4GalT-I–deficient mice,22 suggesting that a β4GalT gene(s) other than β4GalT-I is responsible for the biosynthesis of PSA and HNK-1. Although approximately half the β4GalT-I–deficient mice die before the weaning period, the rest can be used for further study. In the present study, selectin-ligand biosynthesis and the effect of selectin-ligand deficiency were examined in β4GalT-I–deficient mice.
Seven β4GalT genes (β4GalT-I to -VII) have been isolated thus far,23 and their individual roles, including those in selectin-ligand biosynthesis, remain to be elucidated. Because β4GalT-VII acts on xylose, β4GalT-I to –VI can catalyze the Galβ1→4GlcNAc linkage and thus are good candidates for the synthesis of selectin ligands such as sLex and 6-sulfo sLex.23 Here, to evaluate the contribution of β4GalT-I in selectin-ligand biosynthesis, the carbohydrate structures of leukocytes from β4GalT-I–deficient mice and the inflammatory responses of these mice were examined. More than 80% of the core 2 O-glycans on the leukocyte membrane glycoproteins of β4GalT-I–deficient mice lacked galactose residues in β-1,4 linkage, and the binding of soluble P-selectin to the neutrophils and monocytes of β4GalT-I–deficient mice was reduced. These results indicate that selectin-ligand biosynthesis was severely impaired in β4GalT-I–deficient mice. Furthermore, β4GalT-I–deficient mice exhibited peripheral blood leukocytosis and reduced acute and chronic inflammatory responses, including contact hypersensitivity (CHS) and delayed-type hypersensitivity (DTH) responses, indicating a selectin-ligand deficiency. Our results clearly demonstrate that among the β4GalT gene family,
β4GalT-I is a major galactosyltransferase responsible for selectin-ligand biosynthesis.
Materials and methods
β4GalT-I–deficient mice
The generation of β4GalT-I–deficient mice was described previously.20 β4GalT-I–/– mice on a mixed background between 129/Sv and C57BL/6 were used for the experiments, with β4GalT-I+/– littermates as controls. Carbohydrate structures of the serum glycoproteins between β4GalT-I+/+ and β4GalT-I+/– mice were indistinguishable,24 and no overt phenotypes were observed in the β4GalT-I+/– mice. The mice were kept under specific pathogen-free conditions in an environmentally controlled clean room at the Laboratory Animal Research Center, Institute of Medical Science, University of Tokyo and at the Institute for Experimental Animals, Kanazawa University. Experiments were conducted according to institutional ethical guidelines for animal experiments and safety guidelines for gene manipulation experiments.
Preparation of O-glycans from splenocytes
Splenocytes were prepared by grinding the spleen with the plunger of a disposable syringe, passing the ground spleen through nylon mesh, and suspending the cells in minimum essential medium (MEM). They were then treated twice with hemolysis buffer (17 mM Tris-HCl containing 140 mM NH4Cl, pH 7.2) to remove red blood cells and were passed through nylon mesh to remove debris. The cells were washed twice with phosphate-buffered saline (PBS), homogenized in 10 mM Tris-HCl containing 1 mM EDTA (ethylenediaminetetraacetic acid), pH 7.4 (TE buffer), and spun at 150g for 5 minutes. The supernatant was spun at 25 000g for 30 minutes, and the precipitate was washed twice with TE buffer and once with 10 mM ammonium acetate. The precipitated membrane fraction was lyophilized and delipidated as previously described.25 Delipidated ghosts were suspended in 1 mL 0.05 M NaOH containing 1 M sodium borohydride and were incubated at 45°C for 16 hours. Released glycans were purified according to the method described previously.26
Analysis of O-glycans by HPAEC-PAD
High-pH anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD) was performed using a Bio-LC system (Dinex, Sunnyvale, CA) equipped with a CarboPac PA-1 column (4 × 250 mm) and a pulsed amperometric detector. O-glycans were eluted with a linearly increasing concentration of 0 to 400 mM sodium acetate in 100 mM NaOH from 0 to 30 minutes. O-glycans were also desialylated by mild acid hydrolysis with 0.01 M HCl at 100°C for 20 minutes and analyzed by HPAEC-PAD using an isocratic elution with 22 mM NaOH.
Flow cytometric analysis
Splenocytes were prepared as described above except that they were treated with the hemolysis buffer only once. Splenocytes (2 × 106 cells) were blocked with antimouse CD16/CD32 for 15 minutes and were incubated with the first antibody or human–P-selectin immunoglobulin M (IgM) for 45 minutes. When antirat IgG2b was used as the second antibody, mouse serum was used for blocking instead of antimouse CD16/CD32. The splenocytes were washed, incubated with R-phycoerythrin (PE)– or fluorescein isothiocyanate (FITC)–conjugated second antibodies for 45 minutes, washed again, and incubated with 7-amino actinomycin D (Sigma, St Louis, MO). Incubations with antibodies were carried out on ice. Data were analyzed on a FACScan flow cytometer using Lysis II software (Becton Dickinson, Mountain View, CA).
A human P-selectin–IgM expression plasmid, human P-selectin–IgM/pcDNA1.1, which bears part of the human P-selectin cDNA containing the NH2-terminal lectin domain, the epidermal growth factor (EGF)–like domain, and the first 2 complement-binding protein-like domains (provided by Ono Pharmaceutical, Osaka, Japan), and the μ heavy chain of human IgM (provided by Dr A. Traunecker, Basel Institute for Immunology, Switzerland) was transfected into human embryonic kidney-derived 293T cells (provided by Dr Y. Kanakura, Osaka University Graduate School of Medicine, Japan). The pAdVAntage vector (Promega, Madison, WI) was cotransfected with human P-selectin–IgM/pcDNA1.1 to enhance protein expression levels. Four days after transfection, the culture medium containing the P-selectin–IgM was collected and stored at –80°C until use.
Antimouse CD16/CD32 (2.4G2), antimouse Gr-1 (RB6-8C5), and antimouse PSGL-1 (2PH1), were purchased from PharMingen (San Diego, CA). PE-antimouse F4/80 and FITC-antirat IgG were purchased from Caltag (Burlingame, CA), and FITC-antihuman IgM was purchased from American Qualex (San Clemente, CA). PE-antirat IgG was kindly provided by Dr T. Yoshimoto (Institute for Medical Sciences, University of Tokyo, Japan).
Colony-forming activity of bone marrow cells
Nonadherent mononuclear cells (1 × 104) were prepared from the bone marrow of β4GalT-I–/– (n = 5) and β4GalT-I+/– (n = 5) mice (2-3 months old) and plated into 0.45% methylcellulose in the presence of 50 ng/mL granulocyte–colony-stimulating factor (G-CSF) (provided by Chugai Pharmaceutical, Tokyo, Japan), 2 U/mL EPO (provided by Kirin Brewery, Tokyo, Japan), or 20 ng/mL interleukin-3 (IL-3; Amgen, Thousand Oaks, CA). Colonies were counted after 2 weeks.
Acute inflammation
Zymosan-induced inflammation was carried out as described previously.27 β4GalT-I–/– (n = 10) and β4GalT-I+/– (n = 8) mice were anesthetized with ketamine and xylazine, and 20 μL 2% zymosan A (Sigma) and saline were injected subcutaneously into the right and left earlobes, respectively, using a microsyringe (Hamilton, Reno, NV) with a 30-gauge needle. At 8 hours after the injection, when the swelling reached peak levels, the mice were killed, and a disk of each earlobe was removed using a 6-mm biopsy punch and weighed. Ear swelling was calculated as follows: [Increase of ear swelling (%)] = ([weight of challenged earlobe] – [weight of vehicle-treated earlobe]) × 100/[weight of vehicle-treated earlobe].
CHS response
β4GalT-I–/– (n = 11) and β4GalT-I+/– (n = 11) mice were sensitized by the epicutaneous application of 100 μL 7% 2,4,6-trinitrochlorobenzene (TNCB) (Tokyo Chemical, Tokyo, Japan) in acetone/olive oil (3:1, vol/vol) onto shaved abdomens. On day 5 after sensitization, 20 μL 1% TNCB solution and the solvent alone were applied epidermally onto the right and left earlobe, respectively. Mice were killed, and earlobe biopsies were taken as described above 24 hours after the second challenge, when the swelling reached peak levels. Each ear disk was weighed, and the ear swelling was calculated as described above.
DTH response
β4GalT-I–/– (n = 8) and β4GalT-I+/– (n = 8) mice were sensitized by the subcutaneous injection of 200 μL 1.25 mg/mL methylated bovine serum albumin (mBSA) (Sigma) in PBS/complete Freund adjuvant (1:1, vol/vol) into the tail roots. On day 7 after the sensitization, 20 μL 10 mg/mL mBSA in PBS and PBS alone were applied epidermally onto the right and left footpad, respectively. Mice were killed, and footpad thickness was measured 24 hours after the second challenge, when the swelling reached peak levels. Footpad thickness was calculated as follows: [Increase of footpad thickness (%)] = ([thickness of challenged footpad] – [thickness of vehicle-treated footpad]) × 100/[thickness of vehicle-treated footpad].
Histology
Tissues were fixed in 10% neutral formalin, dehydrated, and embedded in paraffin wax according to standard procedures. Footpads were defatted with methanol and chloroform (1:1) and were decalcified with 5% formic acid before they were embedded in paraffin wax. Sections of 6 μm were made and stained with Weigert hematoxylin-eosin.
Myeloperoxidase assay
Ear disks (obtained with a 6-mm punch) were homogenized in 400 μL PBS and sonicated for 30 seconds. The homogenates were frozen and thawed 3 times and again were sonicated for 30 seconds. After the debris was removed by centrifugation, myeloperoxidase (MPO) activity in the supernatants was measured as described.28 The MPO assay was carried out in 400 μL 50 mM potassium-phosphate buffer (pH 6.0) containing 0.4 mg/mL O-phenylenediamine (Sigma) and 0.05% H2O2 for 20 minutes. The reaction was stopped by the addition of 100 μL0.4 M H2SO4, and OD 490 nm was measured. MPO activity was calculated using commercial peroxidase (Sigma) as a standard.
Statistical analysis
Statistical evaluation was carried out by means of the Student t test or the Welch t test according to the Levene test for equality of variance between β4GalT-I–deficient and control mice. A 2-sided level of P < .05 was accepted as statistically significant. Data are presented as mean ± SEM.
Results
Biosynthesis of O-glycans on β4GalT-I–deficient leukocytes
Sialyl Lex is most abundantly expressed at the terminus of N-acetyl lactosamine disaccharide repeats on core 2 O-glycans.3 As shown in Figure 1, a β4GalT gene must be involved in the biosynthesis of the N-acetyl lactosamine disaccharide repeats and terminal sLex epitope on the core 2 branch. To clarify the contribution of the β4GalT-I gene in the core 2 O-glycan biosynthesis on leukocytes, the O-glycan structures of splenocyte membrane glycoproteins prepared from β4GalT-I+/– and β4GalT-I–/– mice were analyzed by HPAEC-PAD.
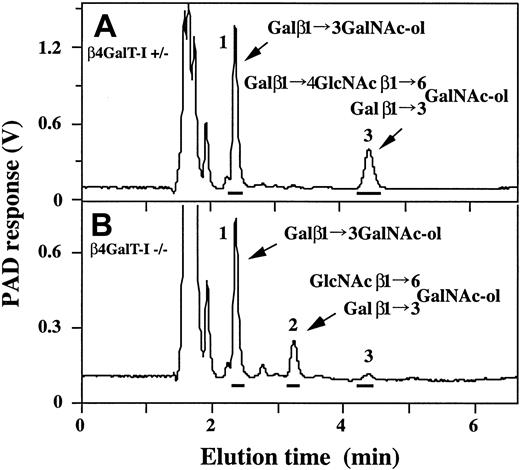
Eight major glycan peaks were detected in samples from the β4GalT-I+/– mice, and 6 major peaks were seen in samples from the β4GalT-I–/– mice (Figure 2A-B). Considering their elution positions compared with those of authentic O-glycan standards from fetuin, sheep erythrocytes, erythrocytes from β4GalT-I–/– mice,25,26 and their previous structural analysis,26 we deduced the structures giving rise to each peak to be those shown in Figure 2C. Thus, the glycans with β1,4-galactosylated core 2 branch structures corresponding to peaks 4, 6, and 8 in the β4GalT-I+/– sample were only faintly detected or not detected in the β4GalT-I–/– sample, and a glycan with an abnormal core 2 branch that lacked galactose residues in β-1,4 linkage (peak 2) was abundantly detected in the β4GalT-I–/– sample. In contrast, core 1 O-glycans were equally synthesized in both mice.
Analysis of O-glycans by HPAEC-PAD. Glycans released by alkaline-borohydride treatment from delipidated splenocyte membrane glycoproteins were analyzed by HPAEC-PAD. (A) β4GalT-I+/– splenocytes. (B) β4GalT-I–/– splenocytes. (C) Proposed structures of the O-glycans (A-B). The major glycan peaks are numbered in panels A and B, and the numbers in parentheses in panel C represent the peak numbers in panels A and B.
Analysis of O-glycans by HPAEC-PAD. Glycans released by alkaline-borohydride treatment from delipidated splenocyte membrane glycoproteins were analyzed by HPAEC-PAD. (A) β4GalT-I+/– splenocytes. (B) β4GalT-I–/– splenocytes. (C) Proposed structures of the O-glycans (A-B). The major glycan peaks are numbered in panels A and B, and the numbers in parentheses in panel C represent the peak numbers in panels A and B.
To further confirm the impaired β1,4-galactosylation of core 2 branches in the β4GalT-I–/– sample, the O-glycans of splenocyte membrane glycoproteins were mildly hydrolyzed to remove sialic acids and were analyzed by HPAEC-PAD. The resultant asialo O-glycans from the β4GalT-I+/– sample gave 2 peaks corresponding to a core 1 disaccharide, Galβ1→3GalNAc-ol (70%), and a core 2 tetrasaccharide, Galβ1→3(Galβ1→4GlcNAcβ1→6)GalNAc-ol (30%) (Figure 3A). In contrast, the similarly treated sample from β4GalT-I–/– mice was composed of the core 1 disaccharide (72%), a core 2 trisaccharide, Galβ1→3(GlcNAcβ1→6)GalNAc-ol (24%), and the core 2 tetrasaccharide (4.0%) (Figure 3B). The peak area ratio of the core 1 disaccharide was nearly the same for both mice. However, the core 2 tetrasaccharide was dramatically reduced for the β4GalT-I–/– mice. The ratio of core 2 trisaccharide (β1,4-ungalactosylated) to the tetrasaccharide (β1,4-galactosylated) revealed that most (86%) core 2 branch lacked galactose residues in β-1,4 linkage in the leukocytes of β4GalT-I–/– mice, as had also been observed in their erythrocytes.25 These results clearly indicate that β4GalT-I plays a central role in the formation of the galactosylated core 2 branch, which is a prerequisite for further elongation of the polylactosamine chains.
Analysis of desialylated O-glycans by HPAEC-PAD. Desialylated O-glycans from splenocyte membrane glycoproteins were analyzed by HPAEC-PAD. (A) β4GalT-I+/– splenocytes. (B) β4GalT-I–/– splenocytes. The major glycan peaks are numbered (A-B), and their proposed structures are given in the figure.
Analysis of desialylated O-glycans by HPAEC-PAD. Desialylated O-glycans from splenocyte membrane glycoproteins were analyzed by HPAEC-PAD. (A) β4GalT-I+/– splenocytes. (B) β4GalT-I–/– splenocytes. The major glycan peaks are numbered (A-B), and their proposed structures are given in the figure.
Selectin-ligand formation on β4GalT-I–deficient neutrophils and monocytes
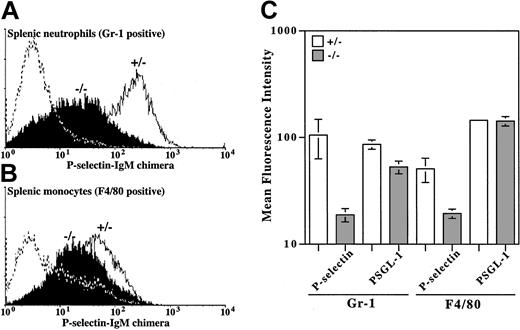
Selectin-ligand formation on the surface of neutrophils and monocytes from β4GalT-I–/– mice was analyzed by flow cytometry using a chimeric human P-selectin IgM molecule. The binding of P-selectin to splenic neutrophils (Gr-1–positive) and splenic monocytes (F4/80-positive) was markedly reduced in β4GalT-I–/– mice compared with β4GalT-I+/– mice (Figure 4). Because PSGL-1 is the predominant ligand for P-selectin on neutrophils,29 the expression of PSGL-1 on neutrophils and monocytes was compared between β4GalT-I–/– and β4GalT-I+/– mice and was found to be the same (Figure 4C). These data, together with the impaired core 2 O-glycan biosynthesis, indicate that the biosynthesis of the selectinligand oligosaccharide is severely reduced, though not completely abrogated, in β4GalT-I–deficient neutrophils and monocytes.
Binding of P-selectin and PSGL-1 to neutrophils and monocytes. Binding of the human–P-selectin IgM chimeric molecule and PSGL-1 to splenic neutrophils (Gr-1 gated) and splenic monocytes (F4/80 gated) was analyzed by flow cytometry. (A) Neutrophils from the spleens of β4GalT-I+/– (open histogram) and β4GalT-I–/– (filled histogram) mice. The dotted line shows negative control without the P-selectin–human IgM chimeric molecule. Results from 1 of 3 representative experiments are shown. (B) Monocytes from the spleens of β4GalT-I+/– and β4GalT-I–/– mice as in panel A. Results from 1 of 2 representative experiments are shown. (C) Mean fluorescence intensities of P-selectin binding and PSGL-1 expression in splenic neutrophils (Gr-1 gated; n = 3) and splenic monocytes (F4/80 gated; n = 2). Data are presented as means ± SEM.
Binding of P-selectin and PSGL-1 to neutrophils and monocytes. Binding of the human–P-selectin IgM chimeric molecule and PSGL-1 to splenic neutrophils (Gr-1 gated) and splenic monocytes (F4/80 gated) was analyzed by flow cytometry. (A) Neutrophils from the spleens of β4GalT-I+/– (open histogram) and β4GalT-I–/– (filled histogram) mice. The dotted line shows negative control without the P-selectin–human IgM chimeric molecule. Results from 1 of 3 representative experiments are shown. (B) Monocytes from the spleens of β4GalT-I+/– and β4GalT-I–/– mice as in panel A. Results from 1 of 2 representative experiments are shown. (C) Mean fluorescence intensities of P-selectin binding and PSGL-1 expression in splenic neutrophils (Gr-1 gated; n = 3) and splenic monocytes (F4/80 gated; n = 2). Data are presented as means ± SEM.
Leukocytosis and neutrophilia of β4GalT-I–deficient mice
Leukocytosis and neutrophilia are commonly observed in selectin-deficient mice and selectin ligand-deficient mice.11,13,14,30 Here, the total white blood cell count in the peripheral blood was elevated 2.2-fold in the β4GalT-I–/– mice compared with the β4GalT-I+/– mice (Figure 5A, Table 1). Neutrophil and lymphocyte counts were also increased 2.3-and 2.5-fold, respectively (Figure 5B, Table 1). Therefore, β4GalT-I–deficient mice also showed leukocytosis and neutrophilia.
Peripheral blood hematology and the colony-forming activity of bone marrow cells. (A) Total white blood cell counts, red blood cell counts, hematocrit values, and hemoglobin concentrations of the peripheral blood from β4GalT-I+/– (□;n = 6) and β4GalT-I–/– mice (▦; n = 6) at 2 to 3 months of age were measured by an automatic cell counter. (B) Numbers of neutrophils, lymphocytes, and monocytes from β4GalT-I+/– (□; n = 6) and β4GalT-I–/– (▦; n = 6) mice were measured manually. (C) CFUs of nonadherent mononuclear cells (1 × 104) prepared from the bone marrow of β4GalT-I+/– (□, n = 5) and β4GalT-I–/– (▦; n = 5) mice were measured in the presence of 50 ng/mL G-CSF, 2 U/mL EPO, or 20 ng/mL IL-3. (D) Weights of spleens, PLNs (cervical LN + axillary LN), and mesenteric (M) LNs were compared between β4GalT-I+/– (□;n = 9) and β4GalT-I–/– (▦; n = 8) mice at 2 to 4.5 months of age. #P < .005; *P < .01; +P < .05. Data are presented as means ± SEM.
Peripheral blood hematology and the colony-forming activity of bone marrow cells. (A) Total white blood cell counts, red blood cell counts, hematocrit values, and hemoglobin concentrations of the peripheral blood from β4GalT-I+/– (□;n = 6) and β4GalT-I–/– mice (▦; n = 6) at 2 to 3 months of age were measured by an automatic cell counter. (B) Numbers of neutrophils, lymphocytes, and monocytes from β4GalT-I+/– (□; n = 6) and β4GalT-I–/– (▦; n = 6) mice were measured manually. (C) CFUs of nonadherent mononuclear cells (1 × 104) prepared from the bone marrow of β4GalT-I+/– (□, n = 5) and β4GalT-I–/– (▦; n = 5) mice were measured in the presence of 50 ng/mL G-CSF, 2 U/mL EPO, or 20 ng/mL IL-3. (D) Weights of spleens, PLNs (cervical LN + axillary LN), and mesenteric (M) LNs were compared between β4GalT-I+/– (□;n = 9) and β4GalT-I–/– (▦; n = 8) mice at 2 to 4.5 months of age. #P < .005; *P < .01; +P < .05. Data are presented as means ± SEM.
Comparison of leukocytosis and neutrophilia between selectin-perturbed KO mice
. | Fold increase in cell numbers compared with control mice . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Blood cell types . | β4GalT-1 KO . | Core 2 β6GnT KO . | E/P-selectin KO . | Fuc-TVII KO . | Fuc-TIV/VII KO . | ||||
| Leukocytes | 2.2 | 2.4 | 3.9 | 3.2 | 2.9 | ||||
| Neutrophils | 2.3 | 4.3 | 16.6 | 7.4 | 18.4 | ||||
| Lymphocytes | 2.5 | 1.6 | 1.5 | 2.0 | 1.2 | ||||
| Monocytes | 1.6 | 1.4 | 9.8 | 2.9 | 4.2 | ||||
. | Fold increase in cell numbers compared with control mice . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Blood cell types . | β4GalT-1 KO . | Core 2 β6GnT KO . | E/P-selectin KO . | Fuc-TVII KO . | Fuc-TIV/VII KO . | ||||
| Leukocytes | 2.2 | 2.4 | 3.9 | 3.2 | 2.9 | ||||
| Neutrophils | 2.3 | 4.3 | 16.6 | 7.4 | 18.4 | ||||
| Lymphocytes | 2.5 | 1.6 | 1.5 | 2.0 | 1.2 | ||||
| Monocytes | 1.6 | 1.4 | 9.8 | 2.9 | 4.2 | ||||
To clarify whether leukocytosis was caused by abnormal hematopoiesis in the bone marrow, the colony-forming activity of bone marrow cells was measured. Colony-forming units (CFUs) in the presence of G-CSF or IL-3 were comparable between β4GalT-I–/– and β4GalT-I+/– mice, suggesting that the hematopoietic differentiation of β4GalT-I–deficient bone marrow cells into the leukocyte lineage was normal (Figure 5C). In contrast, CFUs in the presence of EPO were significantly increased in the β4GalT-I–/– mice (Figure 5C). Red blood cell counts, hematocrit values, and hemoglobin concentrations in the peripheral blood were slightly but significantly reduced in the β4GalT-I–/– mice (Figure 5A). The high response to EPO might have been attributed to the mild anemia observed in the β4GalT-I–/– mice.
In addition to peripheral blood leukocytosis, splenomegaly and extramedullary hematopoiesis in the spleen and liver were often observed in the β4GalT-I–deficient mice (Figure 5D and data not shown). Microscopic observation of spleen and liver sections showed extramedullary hematopoiesis, including the presence of erythroid precursors, granulocytes, and megakaryocytes, in the β4GalT-I–/– mice (data not shown).
Normal lymphocyte homing to peripheral lymph nodes
Lymphocyte homing to peripheral lymph nodes (PLNs) is mediated by L-selectin on lymphocytes and L-selectin ligands, such as 6-sulfo sLex on the HEVs of PLNs. β4GalT-I–/– mice had cervical, axillary, and mesenteric LNs that were comparable in weight to those of β4GalT-I+/– mice (Figure 5D). In addition, an in vivo lymphocyte homing assay using 5-chloromethylfluorescein diacetate (CMFDA)–labeled wild-type lymphocytes13 demonstrated that lymphocyte trafficking to PLNs was normal in β4GalT-I–/– mice (data not shown). These results suggest that lymphocyte homing to PLNs was not affected in the β4GalT-I–deficient mice.
Acute inflammation of β4GalT-I–deficient mice
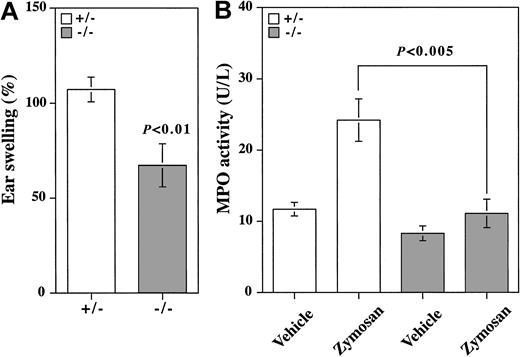
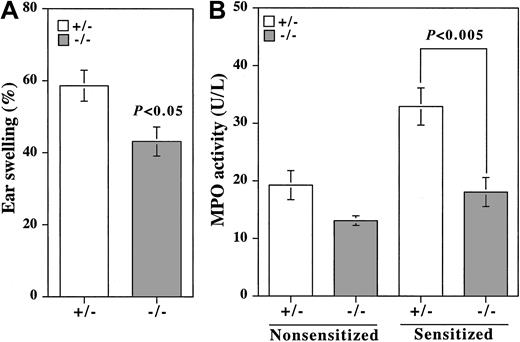
Cell adhesion through selectins and their ligands plays an important role in acute and chronic inflammation. Zymosan-induced dermatitis is a model in which acute inflammation is mediated by E- and P-selectins.27 Zymosan-induced ear swelling was significantly, but not completely, suppressed in β4GalT-I–/– mice compared with β4GalT-I+/– mice (Figure 6A). Neutrophil trafficking into the inflamed earlobe as measured by MPO activity was not induced by the zymosan treatment in β4GalT-I–/– mice, whereas in β4GalT-I+/– mice it was induced 2-fold (Figure 6B). Therefore, acute inflammation induced by zymosan was significantly suppressed in the β4GalT-I–deficient mice, probably because of a defect in neutrophil trafficking.
Neutrophil trafficking during acute inflammation. (A) Zymosan-induced dermatitis. Earlobes of β4GalT-I+/– (□; n = 8) and β4GalT-I–/– (▦; n = 10) mice were injected subcutaneously with zymosan (right ear) or saline (left ear), and the weight of each earlobe disk was measured after 8 hours. Ear swelling was calculated as described in “Materials and methods.” (B) MPO activity in the earlobe homogenates was measured. Data are presented as means ± SEM.
Neutrophil trafficking during acute inflammation. (A) Zymosan-induced dermatitis. Earlobes of β4GalT-I+/– (□; n = 8) and β4GalT-I–/– (▦; n = 10) mice were injected subcutaneously with zymosan (right ear) or saline (left ear), and the weight of each earlobe disk was measured after 8 hours. Ear swelling was calculated as described in “Materials and methods.” (B) MPO activity in the earlobe homogenates was measured. Data are presented as means ± SEM.
CHS and DTH responses of β4GalT-I–deficient mice
CHS is a typical chronic cutaneous inflammation in which selectins are known to be involved.31 TNCB was applied epidermally onto the earlobe 5 days after sensitization by the epicutaneous application of TNCB onto the abdomen. The increase in ear swelling 24 hours after the second challenge was significantly, but not completely, suppressed in β4GalT-I–/– mice compared with β4GalT-I+/– mice (Figure 7A). In addition, neutrophil trafficking as measured by MPO activity after the sensitization was also reduced in the β4GalT-I–/– mice (Figure 7B).
CHS response induced by TNCB. (A) CHS response was induced by TNCB in TNCB-sensitized β4GalT-I+/– (□;n = 11) and β4GalT-I–/– (▦; n = 11) mice. Ear swelling was calculated as described in “Materials and methods.” (B) MPO activity in the CHS-induced earlobe. TNCB-sensitized or nonsensitized β4GalT-I+/– (□) and β4GalT-I–/– (▦) mice were treated with TNCB, and the MPO activity in the earlobe homogenates was measured. Data are presented as means ± SEM.
CHS response induced by TNCB. (A) CHS response was induced by TNCB in TNCB-sensitized β4GalT-I+/– (□;n = 11) and β4GalT-I–/– (▦; n = 11) mice. Ear swelling was calculated as described in “Materials and methods.” (B) MPO activity in the CHS-induced earlobe. TNCB-sensitized or nonsensitized β4GalT-I+/– (□) and β4GalT-I–/– (▦) mice were treated with TNCB, and the MPO activity in the earlobe homogenates was measured. Data are presented as means ± SEM.
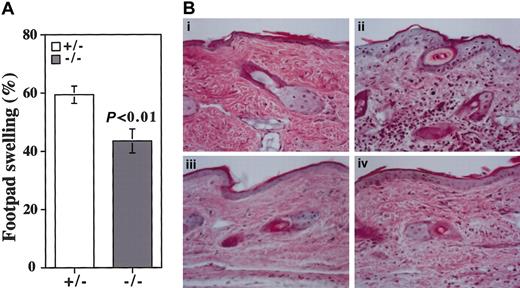
Although CHS was once thought to be a prototypic DTH response, recent reports indicate that CHS and DTH are mediated by major histocompatibility complex (MHC) class 1-restricted CD8+ T cells and MHC class 2-restricted CD4+ T cells, respectively.32 The mBSA-induced DTH responses were compared between the β4GalT-I–deficient and control mice. An increase in footpad thickness induced by the epicutaneous application of mBSA after sensitization was significantly, but not completely, suppressed in β4GalT-I–/– mice compared with β4GalT-I+/– mice (Figure 8A). Leukocyte infiltration into the dermis of the footpad was induced by the mBSA treatment in β4GalT-I+/– mice, but it was suppressed in β4GalT-I–/– mice (Figure 8B). Therefore, chronic inflammation such as CHS and DTH was also suppressed in the β4GalT-I–deficient mice.
DTH response induced by mBSA. (A) DTH response was induced by mBSA in mBSA-sensitized β4GalT-I+/– (□; n = 8) and β4GalT-I–/– (▦; n = 8) mice. The increase in footpad thickness was calculated as described in “Materials and methods.” (B) Weigert hematoxylin and eosin staining of footpad sections. (i) PBS-injected β4GalT-I+/– mice. (ii) mBSA-injected β4GalT-I+/– mice. (iii) PBS-injected β4GalT-I–/– mice. (iv) mBSA-injected β4GalT-I–/– mice. Original magnification, × 100.
DTH response induced by mBSA. (A) DTH response was induced by mBSA in mBSA-sensitized β4GalT-I+/– (□; n = 8) and β4GalT-I–/– (▦; n = 8) mice. The increase in footpad thickness was calculated as described in “Materials and methods.” (B) Weigert hematoxylin and eosin staining of footpad sections. (i) PBS-injected β4GalT-I+/– mice. (ii) mBSA-injected β4GalT-I+/– mice. (iii) PBS-injected β4GalT-I–/– mice. (iv) mBSA-injected β4GalT-I–/– mice. Original magnification, × 100.
Discussion
Biosynthesis of O-glycans and selectin ligands in β4GalT-I–deficient mice
β4GalT-I was thought to be mainly involved in the biosynthesis of Galβ1→4GlcNAc units in N-glycans and in lactose synthesis through binding with α-lactalbumin in the mammary gland.33 Our previous and present studies demonstrated that β4GalT-I is also responsible for the biosynthesis of core 2 O-glycans, given that core 2 O-glycan biosynthesis was reduced by more than 80% in the erythrocytes25 and leukocytes of β4GalT-I–deficient mice. Given that selectin ligands such as sLex and 6-sulfo sLex are mainly expressed on the core 2 branch of O-glycans,3 selectin ligand expression would be expected to be reduced in β4GalT-I–deficient mice. As shown in Figure 4, the binding of β4GalT-I–deficient neutrophils and monocytes to P-selectin was reduced in these mice, though PSGL-1 expression was not affected. Moreover, β4GalT-I–deficient mice showed selectin-deficient phenotypes, including peripheral leukocytosis and impaired acute and chronic inflammatory responses. These results clearly indicate that the biosynthesis of selectin ligands is greatly impaired in β4GalT-I–deficient mice.
Seven β4GalT genes have been isolated, and 6 of them are candidates for the biosynthesis of O-glycans and selectin ligands.23 Using purified β4GalT enzymes and synthetic oligosaccharide acceptors, Ujita et al demonstrated that β4GalT-IV synthesized the core 2 branch most efficiently, whereas β4GalT-I was involved in the biosynthesis of the core 4 branch in O-glycans biosynthesis.34,35 These findings are not consistent with our results. The N-acetyl lactosamine extension activity in core 2 and core 4 O-glycans was compared among the β4GalTs using various synthetic acceptors in their in vitro experiments. However, the in vivo situation could be different from that of the in vitro study, which used large amounts of purified β4GalTs and synthetic oligosaccharide acceptors instead of glycoproteins. Because the expression level of each β4GalT gene differs according to cell type, the biosynthesis of the core 2 branch of O-glycans could be carried out by different β4GalT genes in different cell types. We examined the biosynthesis of core 2 O-glycans in leukocytes, where selectin ligands are synthesized. Therefore, β4GalT-I definitely contributes to and plays a major role in the biosynthesis of the core 2 branch of O-glycans and selectin ligands in living leukocytes.
Given that approximately 20% of the core 2 branches were galactosylated by a β-1,4 linkage and that P-selectin bound weakly to neutrophils and monocytes in β4GalT-I–deficient mice, other β4GalT gene(s), such as β4GalT-IV, must be involved in the biosynthesis of the core 2 branch of O-glycans and selectin ligands. Recently, it has been reported that β4GalT-IV is involved in the biosynthesis of 6-sulfo sLex, an L-selectin ligand.36 To identify the other β4GalT gene or genes responsible for selectin-ligand biosynthesis, mice deficient in those genes will have to be analyzed.
Peripheral leukocytosis and normal lymphocyte homing to PLNs in β4GalT-I–deficient mice
β4GalT-I–deficient mice exhibited peripheral leukocytosis and neutrophilia similar to those seen in selectin- and selectin-ligand–deficient mice, strongly suggesting that β4GalT-I–deficient mice are impaired in selectin-dependent endothelial cell adhesion. A comparison of the β4GalT-I–deficient mice with selectin-deficient mice and other glycosyltransferase-deficient mice is shown in Table 1. E- and P-selectin double-deficient mice exhibit severe leukocytosis and neutrophilia,30 whereas P-selectin single-deficient mice show mild neutrophilia without leukocytosis37 and L-selectin and E-selectin single-deficient mice show normal hematology.38,39 Fuc-TIV/VII double-deficient mice also show severe leukocytosis and neutrophilia,14 but those in core 2 β6GnT-deficient mice are milder.11 The leukocytosis and neutrophilia of β4GalT-I–deficient mice were intermediate, comparable to those of core 2 β6GnT-deficient mice, which show a complete deficiency of core 2 O-glycans and a partial deficiency of selectin ligands.11 β4GalT-I–deficient mice also showed considerable deficiency in core 2 O-glycans biosynthesis and a partial deficiency of selectin ligands. Therefore, deficiency in the biosynthesis of core 2 O-glycans causes moderate leukocytosis and neutrophilia.
Normal hematopoietic activity of the bone marrow cells (Figure 5C) and extramedullary hematopoiesis in the spleen and liver (data not shown) suggest that leukocyte production was increased in the periphery in β4GalT-I–deficient mice, though increased hematopoiesis in the bone marrow cannot be ruled out completely. Subclinical infection will induce cytokine production and cause extramedullary hematopoiesis, similar to those observed in E- and P-selectin double-deficient mice.30,40 Our β4GalT-I–deficient mice, however, were kept under specific pathogen-free (SPF) conditions, and no pathologic features, including the ulcerative cutaneous infection seen in the E- and P-selectin double-deficient mice, were observed. Therefore, the basal expression of E- and P-selectins and their oligosaccharide ligands is indispensable for the regulated production of leukocytes in the periphery, independent of infection. Altered leukocyte homeostasis might be caused by several mechanisms, as discussed previously.30 One possibility is that tissue leukocytes are reduced because of a defect in selectin-dependent endothelial cell adhesion, causing an elevation of leukocytes in the blood. Another possibility is that neutrophilia may be caused by an increase in neutrophil half-life in the circulation, as reported in P-selectin–deficient mice.41 Homeister et al14 reported that extreme leukocytosis in Fuc-TIV/VII double-deficient mice is caused by decreased neutrophil turnover and increased neutrophil production in the periphery. Because epithelial cell proliferation is enhanced in β4GalT-I–deficient mice,20 leukocyte proliferation might be augmented.
Lymphocyte homing to PLNs is mediated by L-selectin on lymphocytes and L-selectin ligands on the HEVs of PLNs. Indeed, lymphocyte homing is impaired in L-selectin–deficient mice39 and in Fuc-TVII–deficient mice.13 However, the weight of the PLNs and the in vivo lymphocyte homing activity were comparable between β4GalT-I–deficient and control mice (Figure 5D and data not shown). These results suggest that lymphocyte homing to PLNs was not affected in β4GalT-I–deficient mice, as is also observed in core 2 β6GnT-deficient mice.11 Taken together, these observations indicate that core 2 O-glycans are dispensable for the biosynthesis of L-selectin ligands.
Reduced acute and chronic inflammatory responses in β4GalT-I–deficient mice
Cell adhesion through selectins and their oligosaccharide ligands is a prerequisite for leukocyte accumulation at inflammatory sites. Zymosan-induced inflammation is a model in which acute inflammation is mainly mediated by neutrophils. Zymosan-induced neutrophil recruitment is reduced in E- and P-selectin double-deficient mice27 and fully compromised in Fuc-TIV/VII double-deficient mice.14 These results indicate that E- and P-selectins and sLex play essential roles in zymosan-induced inflammation. In β4GalT-I–deficient mice, neutrophil recruitment was fully reduced, and ear swelling was partially reduced, suggesting that the selectin ligands on neutrophils were greatly compromised in β4GalT-I–deficient mice.
CHS and DTH responses are commonly used as a model of chronic inflammatory diseases.32 These responses consist of the initial sensitizing phase and the subsequent elicitation phase. A sensitizing allergen such as TNCB or mBSA applied epidermally is captured by Langerhans cells in the skin. Langerhans cells then migrate to draining lymph nodes and present the allergen to naive T cells in the sensitizing phase. The elicitation phase occurs when epidermal cells encounter the same allergen to which they have been previously exposed. The primed CD8+ Tc1 cells and CD4+ Th1 cells mount CHS and DTH responses, respectively, at the site of challenge and secrete cytokines and chemokines. These cytokines and chemokines recruit neutrophils, monocytes, and lymphocytes to the site of challenge.
CHS and DTH responses were partially, but significantly, suppressed in the β4GalT-I–deficient mice (Figures 7, 8). The β4GalT-I–deficient mice were impaired in neutrophil trafficking to the inflammatory sites in the CHS response (Figure 7B), probably because the amount of P-selectin ligands on neutrophils was reduced (Figure 4A). Therefore, the defect in neutrophil recruitment to the inflammatory sites during the elicitation phase might be the cause of the reduced CHS and DTH responses in the β4GalT-I–deficient mice. The oxazolone-induced CHS response is reduced in E- and P-selectin double-deficient mice, and neutrophil recruitment to the inflammatory sites is reduced during the elicitation phase by the P- or E/P-selectin deficiency.31,38,42 These reports are consistent with our results. The CHS response induced by 2,4-dinitrofluorobenzene (DNFB) is completely abrogated in Fuc-TIV/VII double-deficient mice.43 Fuc-TIV/VII double-deficient mice are impaired in L-selectin–dependent naive T-cell trafficking to PLNs and in E- and P-selectin–dependent Th1 and Tc1 T-cell trafficking to inflamed cutaneous sites. These defects result in a reduction of the CHS response in these mice. However, β4GalT-I–deficient mice showed normal lymphocyte homing to PLNs (Figure 5D), suggesting that the cause of the suppression of the CHS response is different between the β4GalT-I–deficient and Fuc-TIV/VII double-deficient mice.
The peripheral leukocytosis and reduced acute and chronic inflammatory responses observed in the β4GalT-I–deficient mice could be well explained by the deficiency of selectin-ligand biosynthesis in these mice. However, the carbohydrate structures of many glycoproteins are considerably changed, such as the shift of the outer chain moieties of the N-glycans from type 2 to type 1 chains, in β4GalT-I–deficient mice.24 We cannot rule out the possibility that other carbohydrate epitopes rather than selectin ligands caused the immunologic abnormalities we observed in the β4GalT-I–deficient mice.
Human congenital disorders of glycosylation and their animal models
A deficiency of glycosyltransferases might be the cause of some human congenital disorders of glycosylation (CDGs). The phenotype of β4GalT-I–deficient mice is similar to that found in patients with leukocyte adhesion deficiency 2 (LAD 2), who exhibit pronounced neutrophilia and have chronic severe periodontitis and mental and growth retardation.44 E- and P-selectin–deficient mice mimic the human syndrome given that they develop periodontal disease.45 LAD 2 is caused by selectin ligand deficiency, and a causal gene has been recently identified to encode a putative GDP-fucose transporter, designated CDG-IIc.46,47
A human mutation in the β4GalT-I gene has been recently identified in an 18-month-old boy with mental retardation, hydrocephalus, myopathy, and blood-clotting defects.48,49 This is a new type of congenital disorder of glycosylation, designated CDG-IId. Because only a single infant patient has been identified to date and only a few biochemical parameters were analyzed in the patient, it is still unknown whether the human mutation in the β4GalT-I gene causes the selectin-ligand deficiency and impaired inflammatory responses we observed in the mouse. In any case, our β4GalT-I–deficient mouse could be an animal model of CDG-IId syndrome. Further analysis of β4GalT-I–deficient mice may be of benefit to patients with CDG-IId and to our understanding of the syndrome.
Prepublished online as Blood First Edition Paper, April 24, 2003; DOI 10.1182/blood-2003-03-0836.
Supported in part by the Ministry of Education, Science, Sports and Culture of Japan (grants 10178104 and 13480280), the Inamori Foundation, and the Mochida Memorial Foundation for Medical and Pharmaceutical Research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr T. Nagata at the Hatano Research Institute, Food and Drug Safety Center for valuable comments for the histologic analysis. We also thank Drs A. Traunecker (Basel Institute for Immunology), Y. Kanakura (Osaka University Graduate School of Medicine), and T. Yoshimoto (Institute for Medical Sciences, University of Tokyo) for human IgM heavy chain, 293T cells, and PE-antirat IgG.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal