Abstract
We report in this paper that the DNA-binding drug mithramycin is a potent inducer of γ-globin mRNA accumulation and fetal hemoglobin (HbF) production in erythroid cells from healthy human subjects and β-thalassemia patients. Erythroid precursors derived from peripheral blood were grown in 2-phase liquid culture. In this procedure, early erythroid progenitors proliferate and differentiate during phase 1 (in the absence of erythropoietin) into late progenitors. In phase 2, in the presence of erythropoietin, the latter cells continue their proliferation and mature into Hb-containing orthochromatic normoblasts. Compounds were added on days 4 to 5 of phase 2 (when cells started to synthesize Hb), and cells were harvested on day 12. Accumulation of mRNAs for γ-globin, β-globin, α-globin, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and β-actin were measured by real-time quantitative reverse transcription–polymerase chain reaction (RT-PCR); induction of HbF was analyzed by high-performance liquid chromatography (HPLC) and, at cellular level, by flow cytometry. We demonstrated that mithramycin was able to up-regulate preferentially γ-globin mRNA production and to increase HbF accumulation, the percentage of HbF-containing cells, and their HbF content. Mithramycin was more effective than hydroxyurea, being, in addition, not cytotoxic. This was shown by the lack of cytotoxicity on erythroid and myeloid in vitro primary cell cultures treated with mithramycin at concentrations effective for HbF induction. These results are of potential clinical significance because an increase of HbF alleviates the symptoms underlying β-thalassemia and sickle cell anemia. The results of this report suggest that mithramycin and its analogs warrant further evaluation as potential therapeutic drugs.
Introduction
Pharmacologically mediated regulation of the expression of the human γ-globin genes could be of interest as a potential therapeutic approach for hematologic disorders, including β-thalassemia and sickle cell anemia.1-8 It is well established, indeed, that increase of fetal hemoglobin (HbF) to 30% of the total hemoglobin (Hb) leads to a significant improvement of the clinical status of patients affected by these hematologic disorders.1-3 Therefore, current research has been focused on screening of various agents, such as hormones, cytotoxic drugs, hemopoietic cytokines, and short fatty acids as agents capable of augmenting HbF levels in humans.8-13
In this respect, DNA-binding drugs appear to be of great interest.14,15 These agents are known to modify the formation of DNA/nuclear protein complexes and thereby control gene expression.16-22 Our research group has recently demonstrated that tallimustine16 and some cisplatin analogs,15 as well as the guanosine-cytosine (GC)–rich binders chromomycin and mithramycin (MTH),14 are powerful inducers of erythroid differentiation of the human leukemic K562 cell line, suggesting that the pattern of erythroid differentiation and of γ-globin gene expression could be influenced by treatment with DNA-binding drugs. Interestingly, while chromomycin binding to DNA generates stable complexes, MTH-DNA complexes are highly unstable.23 This could explain the low toxicity of MTH as compared with chromomycin.24 For this reason, MTH was proposed as a therapeutic agent in several neoplastic diseases (such as chronic myelogenous leukemia and testicular cancer),25 in Paget disease,26 and in pathologies associated with hypercalcemia.27,28
The main issue of the present paper was to test whether MTH is able to augment HbF production in erythroid precursor cells from healthy human subjects as well as from β-thalassemia patients. This is a mandatory, preliminary step to evaluate MTH as a potential drug for the development of treatments of these diseases.
Materials and methods
DNA-binding drugs and inducers
Mithramycin (MTH), hydroxyurea (HU), and cytosine arabinoside (ara-C) were from Sigma Chemical (St Louis, MO).
K562 cells
Human erythroleukemia K562 cells29 were cultured in humidified atmosphere of 5% CO2 in RPMI 1640 (Sigma) supplemented with 10% fetal bovine serum (FBS; Celbio, Milano, Italy), 50 U/mL penicillin, and 50 μg/mL streptomycin.14 Treatment of K562 cells with inducers was carried out by adding the appropriate drug concentrations at the beginning of the cultures (cells were seeded at 30 000/mL). The medium was not changed during the induction period.14
Erythroid cell cultures from healthy donors and β-thalassemia patients
The 2-phase liquid culture procedure was employed as previously described.30-34 Following obtaining informed consent, peripheral blood samples were drawn from healthy donors and from patients with β-thalassemia. This study was approved by the Ferrara University and Hadassah University Hospital, Jerusalem, institutional review boards. Mononuclear cells were isolated by Ficoll-Hypaque density gradient centrifugation and seeded in α medium supplemented with 10% FBS (both from Biological Industries, Beit-Haemek, Israel), 1 μg/mL cyclosporine A (Sandoz, Basel, Switzerland), and 10% conditioned medium from the 5637 bladder carcinoma cell line.30-32 The cultures were incubated at 37°C, under an atmosphere of 5% CO2 in air, with extra humidity. After 7 days' incubation in this phase 1 culture, the nonadherent cells were harvested, washed, and recultured in fresh medium composed of α medium, 30% FBS, 1% deionized bovine serum albumin (BSA; Sigma), 10–5 M β-mercaptoethanol, 2 mM l-glutamine, 10–6 M dexamethasone, and 1 U/mL human recombinant erythropoietin (EPO; Ortho Pharmaceutical, Raritan, NJ). This part of the culture is referred to as phase 2.30,31 Compounds were added on days 4 to 5 of phase 2. Cell samples were analyzed on day 12 or 13 of phase 2. Hb-containing erythroid precursor cells were counted following staining by the benzidine:H2O2 procedure as previously described.30 The proportion of HbF out of the total Hb (%HbF) was determined by high-performance liquid chromatography (HPLC) as elsewhere described.10
Colony formation by normal erythroid and myeloid progenitors
Bone marrow–derived mononuclear cells from healthy donors were seeded in 35-mm culture dishes in premixed semisolid culture medium containing 1.2% methylcellulose (Fisher Scientific, Fair Lawn, NJ) in α medium, 30% FBS, 1% BSA, 10–5 M β-mercaptoethanol, and 2 mM l-glutamine in the presence of either 1 U/mL EPO or 10% 5637 cell–conditioned medium. After 14 days of incubation, colonies of 50 or more cells were counted under an inverted microscope. The erythroid nature of colonies was verified by staining with the benzidine:H2O2 procedure.
Immunofluorescence staining for HbF-containing cultured cells
Cells were washed with phosphate-buffered saline (PBS), fixed for 15 minutes with 4% paraformaldehyde (Biolab, Jerusalem, Israel), and permeabilized with methanol-acetone 1:4 (vol/vol). After washing in PBS, the cells were stained with phycoerythrin (PE)–conjugated antihuman HbF monoclonal antibodies (IQ Products, Groningen, The Netherlands) for 1 hour at room temperature. PE-conjugated antibodies of the same isotype were used for control. Cell distribution with respect to HbF was analyzed using the FACSCalibur (Becton Dickinson Immunofluorometry Systems, Mountain View, CA) flow cytometer. Cells are passed at a rate of 1000 cells per second using saline as the sheath fluid. A 488 nm argon laser beam served as the light source for excitation. Forward light scatter and fluorescence intensity of 10 000 cells were analyzed using the CellQuest software.
Real-time quantitative RT-PCR
Reverse transcription–polymerase chain reaction (RT-PCR) was performed as recently described.35 After production of cDNA 35,36 using 1 μg total RNA, a control PCR for γ-globin gene expression was performed using the γ-globin mRNA-specific primers 5′-ACTCGCTTCTGGAACGTCTGA-3′ and 5′-AGTGCCCTGTCCTCCAGATAC-3′. Quantitative real-time PCR assay37-40 of γ-globin mRNA, β-globin, and α-globin transcripts were carried out using gene-specific double fluorescently labeled probes in a 7700 Sequence Detection System version 1.6.3 (Applied Biosystems, Warrington Cheshire, United Kingdom). The following primer and probe sequences were used for real-time (RT) PCR: γ-globin forward primer, 5′-TGGCAAGAAGGTGCTGACTTC-3′; γ-globin reverse primer, 5′-TCACTCAGCTGGGCAAAGG-3′; γ-globin probe, 5′-FAM-TGGGAGATGCCATAAAGCACCTGG-TAMRA-3′; β-globin forward primer, 5′-CAAGAAAGTGCTCGGTGCCT-3′; β-globin reverse primer, 5′-GCAAAGGTGCCCTTGAGGT-3′; β-globin probe, 5′-FAM-TAGTGATGGCCTGGCTCACCTGGAC-TAMRA-3′; α-globin forward primer, 5′-TCCCCACCACCAAGACCTAC-3′; α-globin reverse primer, 5′-CCTTAACCTGGGCAGAGCC-3′; α-globin probe, 5′-FAM-TCCCGCACTTCGACCTGAGCCA-TAMRA-3′. The fluorescent reporter and the quencher were 6-carboxyfluorescein (FAM) and 6-carboxy-N,N,N′,N′-tetramethylrhodamine (TAMRA), respectively. For real-time PCR of the reference genes, we used the endogenous control human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and β-actin kits, where the probes were fluorescently labeled with VIC (Applied Biosystems).
Statistical analysis
All the data were normally distributed and presented as mean ± SD. Statistical differences between groups were compared using 1-way ANOVA (ANalyses Of VAriance between groups) software. Statistical significance was assumed at a P value less than .05.
Results
Mithramycin is a powerful inducer of γ-globin gene expression by human leukemic K562 cells and by normal human erythroid precursors: a real-time quantitative RT-PCR analysis
We first studied the expression of γ-globin mRNA by quantitative RT-PCR analysis performed in K562 cells. This rapid and sensitive technique allows precise quantitation of mRNA templates.38-40 The cells were induced to erythroid differentiation by 30 nM MTH and 0.5 μM ara-C, a well-known inducer of K562 cells.14,15 Complementary DNA was produced using total RNA as substrate. In agreement with our previous report,14 the results indicated that the kinetics of generation of γ-globin RT-PCR products are much faster using as substrate cDNA from MTH- and ara-C–treated cells as compared with control untreated cells. No major differences were observed in the kinetics of generation of GAPDH or β-actin RT-PCR products (data not shown). The data from 4 independent experiments were analyzed using the Sequence Detection Software System 1.6.3; the results indicated that in K562 cells treated with ara-C the fold induction of γ-globin mRNA compared with control untreated cells was 6.2 ± 1.6 (P < .001) using GAPDH mRNA as reference and 7.1 ± 2.1 (P < .001) using β-actin mRNA as reference. In MTH-treated K562 cells, a significantly higher γ-globin mRNA content was found compared with untreated cells (14.5-fold ± 1.5-fold and a 12.5-fold ± 1.1-fold increase using as reference genes GAPDH and β-actin, respectively; in both cases P < .001). In addition, the MTH-induced increase in γ-globin mRNA content was found to be significantly higher even compared with ara-C–treated cells (P < .002 and P < .02 using as reference genes GAPDH and β-actin, respectively). Taken together, these data give clear indication for an MTH-mediated induction of γ-globin gene expression in K562 cells.
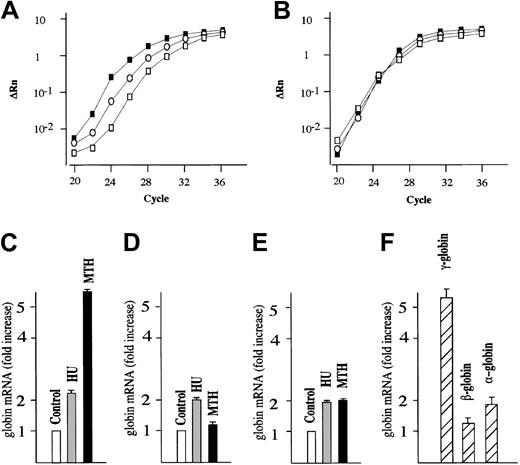
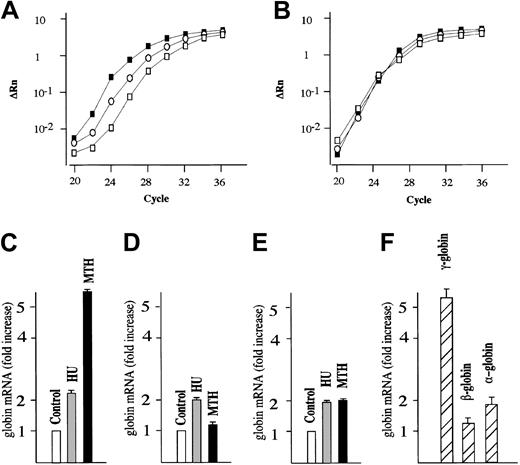
We next studied the effects of MTH on γ-globin mRNA accumulation in human erythroid precursor cells cultured according to the 2-phase liquid culture protocol.30-32 In this procedure, early erythroid committed progenitors derived from the peripheral blood proliferate and differentiate during phase 1 (in the absence of EPO) into late progenitors (erythroid colony-forming units [CFU-Es]). In phase 2, in the presence of EPO, the latter cells continue their proliferation and mature into Hb-containing orthochromatic normoblasts. MTH (20 nM) and HU (150 μM), a known stimulator of HbF production in vitro and in vivo,10 were added on day 4-5 of phase 2 (when cells started to synthesize Hb), and the cells were harvested on day 12. The effects of MTH treatment on γ-globin, β-globin, α-globin, and GAPDH mRNAs were studied by quantitative RT-PCR. Figure 1A shows a representative experiment indicating a significant higher γ-globin mRNA content in MTH- and HU-treated cells compared with untreated cells. No difference is observed among the cultures with respect to GAPDH mRNA content (Figure 1B). Figure 1C-E shows a representative experiment outlining the fold increase in the content of γ-globin, β-globin, and α-globin mRNAs in MTH- and HU-treated cultures compared with untreated cultures (in both cases P < .001). The results of 4 independent experiments are shown in Figure 1F and indicate a preferential increase in γ-globin mRNA compared with the other globin mRNAs in MTH-treated cells (P < .001). In all the experiments, MTH proved to be a more efficient inducer of γ-globin mRNA than HU (data not shown).
Quantitative RT-PCR analysis of globin mRNAs from normal human erythroid cultures. (A-B) Normal erythroid precursors were harvested from untreated control cultures or cultures treated with either 150 μM HU or 20 nM MTH. Total RNA was reverse transcribed and 50 ng used for PCR amplification. For each sample, the ΔRn for γ-globin (A) or GAPDH (B) is plotted against the cycle number. □ indicate RNAfrom control untreated cultures; ○, RNA from HU-treated cultures; and ▪, RNA from MTH-treated cultures. (C-E) The data obtained were analyzed using the Sequence Detection Software System 1.6.3, and the fold increase of γ-globin (C), β-globin (D), and α-globin (E) mRNA in cultures treated with HU or MTH compared with untreated cultures (taken as 1) was calculated. Data were derived from quantitative RT-PCR plots using GAPDH mRNA as reference and expressed as average ± SD of 4 independent RT-PCR analyses on a same donor sample (P < .001 when data from panel C of MTH-treated cells are compared with those of HU-treated or untreated control cells). (F) Fold increase of γ-globin, β-globin, and α-globin mRNA in cultures treated with MTH compared with untreated cultures. Data were derived from quantitative RT-PCR plots using GAPDH mRNA as reference and expressed as average ± SD of 5 independent experiments using different human donors (P < .001 when data of γ-globin mRNA content are compared with either β-globin mRNA or α-globin mRNA content).
Quantitative RT-PCR analysis of globin mRNAs from normal human erythroid cultures. (A-B) Normal erythroid precursors were harvested from untreated control cultures or cultures treated with either 150 μM HU or 20 nM MTH. Total RNA was reverse transcribed and 50 ng used for PCR amplification. For each sample, the ΔRn for γ-globin (A) or GAPDH (B) is plotted against the cycle number. □ indicate RNAfrom control untreated cultures; ○, RNA from HU-treated cultures; and ▪, RNA from MTH-treated cultures. (C-E) The data obtained were analyzed using the Sequence Detection Software System 1.6.3, and the fold increase of γ-globin (C), β-globin (D), and α-globin (E) mRNA in cultures treated with HU or MTH compared with untreated cultures (taken as 1) was calculated. Data were derived from quantitative RT-PCR plots using GAPDH mRNA as reference and expressed as average ± SD of 4 independent RT-PCR analyses on a same donor sample (P < .001 when data from panel C of MTH-treated cells are compared with those of HU-treated or untreated control cells). (F) Fold increase of γ-globin, β-globin, and α-globin mRNA in cultures treated with MTH compared with untreated cultures. Data were derived from quantitative RT-PCR plots using GAPDH mRNA as reference and expressed as average ± SD of 5 independent experiments using different human donors (P < .001 when data of γ-globin mRNA content are compared with either β-globin mRNA or α-globin mRNA content).
The increase in the ratio of γ-globin mRNA/GAPDH mRNA was MTH dose dependent; erythroid precursors treated with 100 to 200 nM MTH showed a 25.5-fold ± 3.5-fold increase with respect to control untreated cells (P < .001) in 4 different experiments. This was, however, associated with strong inhibition of cell proliferation; we therefore preferred to limit the MTH doses to low, noncytotoxic concentrations (10 to 20 nM).
Mithramycin-mediated induction of HbF in normal human erythroid precursors: HPLC and flow cytometric analyses
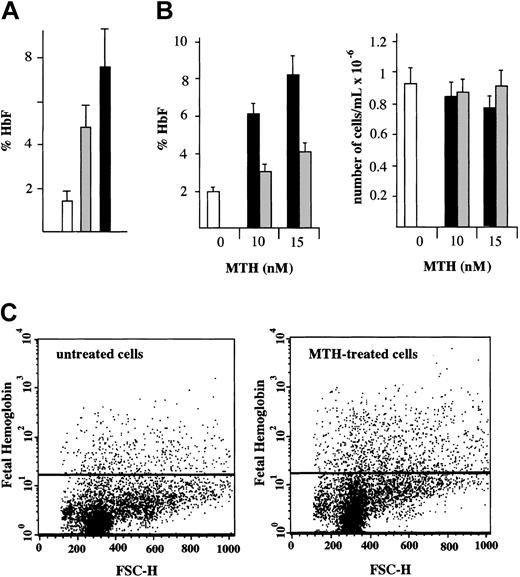
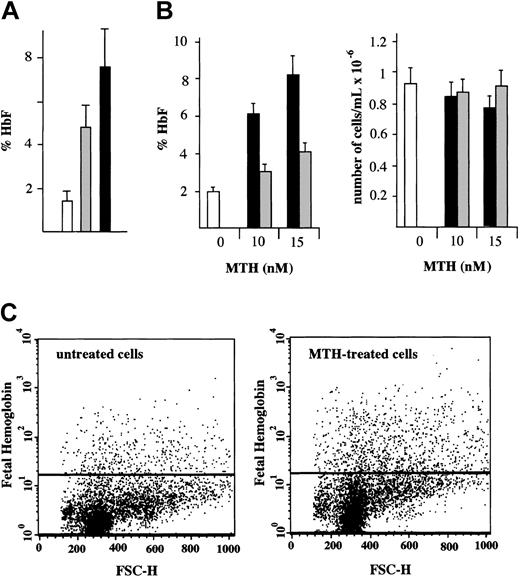
Determination of the HbF content in normal erythroid cultures treated with either MTH or HU from day 4 to day 12 of phase 2 was performed by HPLC.10 The results (Figure 2A) are presented as percentage of HbF with respect to total Hb (%HbF). The results of 9 independent experiments demonstrate that HbF increases from 1.5% ± 0.5% in control cells to 4.7% ± 1.3% (P < .001) in HU-treated cells and to 7.4% ± 1.8% (P < .001) in MTH-treated cells. The different levels of HbF production in HU- and MTH-treated cells were also significant (P < .005). The fold increase of HbF in MTH-treated cells (5.1-fold increase ± 0.5-fold) fairly correlates with the increase of γ-globin mRNA content (5.3-fold ± 0.3-fold). A similar correlation was found in HU-treated erythroid precursor cells (data not shown).
HbF in normal erythroid cultures: HPLC and fluorescence-activated cell sorter (FACS) analyses. (A) HPLC analysis. Cultures of cells derived from healthy individuals were either untreated (□) or treated with 150 μMHU(▦)or20nM MTH (▪). The drugs were added to the cultures on day 4 of phase 2 and the cells harvested on day 12. The cells were washed, lysed, and the hemolysate analyzed for hemoglobins by HPLC. The results present the %HbF (mean values ± SD of independent induction experiments performed on precursors from 5 healthy individuals; P < .001 when data from MTH- or HU-treated cell samples are compared with control; P < .005 when MTH-treated and HU-treated samples are compared). (B) Effects of timing of addition of MTH on cell growth and HbF production. MTH (at 0, 10, and 15 nM) was added to erythroid cultures on either the first (▪) or the fourth (▦) day of phase 2. □ indicate control untreated cells. On day 13, cells were harvested, an aliquot was counted by benzidine staining, and the rest of the cells were washed, lysed, and analyzed for hemoglobins by HPLC. Left panel: %HbF out of the total Hb produced. Right panel: number of erythroid cells per milliliter (× 10–6). The results represent mean values ± SD of 3 independent experiments. (C) FACS analysis. A culture aliquots were permeabilized, stained with antihuman HbF antibodies, and analyzed by flow cytometry. Dot plots of forward light scatter (FSC) and phycoerythrin (PE) fluorescence of 10 000 cells are shown. The horizontal lines denote the level of fluorescence of cells stained with an isotype control antibody.
HbF in normal erythroid cultures: HPLC and fluorescence-activated cell sorter (FACS) analyses. (A) HPLC analysis. Cultures of cells derived from healthy individuals were either untreated (□) or treated with 150 μMHU(▦)or20nM MTH (▪). The drugs were added to the cultures on day 4 of phase 2 and the cells harvested on day 12. The cells were washed, lysed, and the hemolysate analyzed for hemoglobins by HPLC. The results present the %HbF (mean values ± SD of independent induction experiments performed on precursors from 5 healthy individuals; P < .001 when data from MTH- or HU-treated cell samples are compared with control; P < .005 when MTH-treated and HU-treated samples are compared). (B) Effects of timing of addition of MTH on cell growth and HbF production. MTH (at 0, 10, and 15 nM) was added to erythroid cultures on either the first (▪) or the fourth (▦) day of phase 2. □ indicate control untreated cells. On day 13, cells were harvested, an aliquot was counted by benzidine staining, and the rest of the cells were washed, lysed, and analyzed for hemoglobins by HPLC. Left panel: %HbF out of the total Hb produced. Right panel: number of erythroid cells per milliliter (× 10–6). The results represent mean values ± SD of 3 independent experiments. (C) FACS analysis. A culture aliquots were permeabilized, stained with antihuman HbF antibodies, and analyzed by flow cytometry. Dot plots of forward light scatter (FSC) and phycoerythrin (PE) fluorescence of 10 000 cells are shown. The horizontal lines denote the level of fluorescence of cells stained with an isotype control antibody.
We next determined the effects of timing of MTH addition on HbF production (Figure 2B) and cell growth in normal erythroid cultures. MTH (at 0, 10, and 15 nM) was added on either the first or the fourth day of phase 2. On day 13, cells were harvested, an aliquot was counted by benzidine staining, and the rest of the cells were analyzed for hemoglobins by HPLC. The %HbF (Figure 2B, left side) and the number per milliliter of erythroid cells (Figure 2B, right side) are presented. The results indicate that the day of MTH addition did not affect the number of erythroid cells in the cultures (P > .6). However, addition of MTH on day 1 stimulated a higher proportion of HbF compared with when the addition was delayed until day 4 (P < .001), suggesting that, for maximal effect on HbF production, MTH should be present at the onset of EPO-mediated differentiation of erythroid progenitors.
To determine the erythroid cell distribution with respect to HbF, a flow cytometric analysis was performed. Erythroid cells were cultured with or without 15 nM MTH from day 4 to day 13 of phase 2. The cells were then harvested, washed, fixed, permeabilized, and stained with antihuman HbF antibodies. The cells were analyzed by flow cytometry. Dot plots of forward light scatter (FSC) and phycoerythrin (PE) fluorescence of 10 000 cells are shown in Figure 2C. The horizontal lines show the level of fluorescence of cells stained with an isotype control antibody. The results of this experiment demonstrate a 2.6-fold increase in the percentage of HbF-containing cells and 1.8-fold increase in the intensity of the fluorescence (mean fluorescence channel) of MTH-treated cells as compared with untreated cells. In this experiment the percentage of Hb-containing cells was 1.6% in the control and 4.1% in MTH-treated cells. The results of 5 independent experiments of cultures obtained from different donors showed a 3.1-fold ± 1.2-fold increase in the percentage of HbF-containing cells (P < .005) and 1.8-fold ± 0.4-fold increase in the mean fluorescence channel of MTH-treated cells versus untreated cells (P < .01). These results indicate that both the number of HbF-containing cells and their level of HbF content is increased following MTH treatment.
Mithramycin-mediated induction of HbF in human erythroid precursors from homozygous β-thalassemic patients
We next compared the effects of MTH and HU on cell growth and HbF production by thalassemic erythroid precursors. Table 1 shows the results obtained in cultures from 9 β-thalassemia patients listed in order of increasing proportion of constitutive (in untreated cultures) production of HbF. The %HbF in untreated cultures varied from 3.6% (patient 1) to 79.8% (the hereditary persistence of fetal hemoglobin [HPFH] patient 9). This variability was due to a different level of expression of both β-globin and γ-globin genes. MTH was able to increase HbF production in all cases, while HU was not effective in 2 cases (patients 3 and 6) and was toxic in 1 (patient 4). In some cases (patients 1, 2, and 8) the activity of MTH was similar to that of HU; in most cases (patients 3, 5, 6, 7, and 9) the activity of MTH was higher than HU. In all cases, HU was found to strongly inhibit cell proliferation, while, at concentrations able to induce HbF production, MTH was found to exert minimal effect on cell growth, in agreement with the results shown in Figure 2B.
In an attempt to have a general idea on the differential effects of HU and MTH on HbF production in erythroid precursors from β-thalassemia patients, we calculated the gain index as follows: (%HbF [MTH] – %HbF [control])/(%HbF [HU] – %HbF [control]). Results from patients 3, 4, and 6 were not considered, because no induction of HbF production was observed after HU treatment (patients 3 and 6) or HU was highly cytotoxic (patient 4). For the remaining patients, the average gain index value obtained was 1.73 ± 0.66 (P < .03), indicating more than 1.5-fold higher activity of MTH with respect to HU.
As far as the effect on cell growth is concerned, the cell number per milliliter of HU-treated cells for patients 1, 2, 5, 7, 8, and 9 is 24.4% ± 8.8% (P < .02) of control untreated cultures, while that of MTH-treated cells (exhibiting the highest induction of HbF) was found to be 75.2% ± 16.6% (P > .2) of control cells, indicating that, compared with HU, MTH is about 3-fold less inhibitory to β-thalassemia erythroid cell growth in the second phase of the culture protocol. The differential effects of HU and MTH on cell growth of erythroid precursors were found to be significant (P < .001).
Effects of mithramycin on proliferation of normal human erythroid and myeloid precursors cells
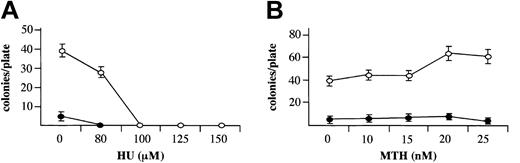
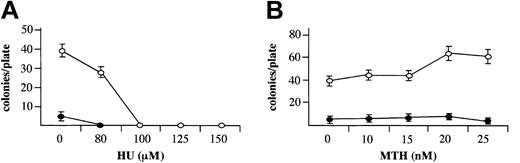
When MTH, at HbF-stimulating concentrations, was added to erythroid cultures from day 4 to day 13 of phase 2, it did not inhibit cell growth, in contrast to HU (Figure 2B and Table 1), in agreement with a previous report on HU.41 This period of the culture encompasses relative late stages of erythroid cell development (from proerythroblasts to orthochromatic normoblasts). To evaluate the effect of MTH on the entire erythroid as well as the myeloid development processes, bone marrow cells were cloned in semisolid medium in the presence of increasing concentrations of HU (Figure 3A) or MTH (Figure 3B), added at the onset of the cell culture period. In this system early committed progenitors proliferate and differentiate to develop by day 14 into either erythroid colonies (containing mainly orthochromatic normoblasts) in the presence of EPO or myeloid colonies (containing neutrophils or monocytes) in the presence of 5637 cell–conditioned medium. The results indicated that in this system HU, at HbF-stimulating concentrations (100 to 150 μM), completely abolished the development of erythroid and myeloid colonies. In contrast, MTH, at HbF-stimulating concentrations (10 to 25 nM), had no effect on colony development. These results indicate that HbF stimulation can be induced by MTH under conditions that do not inhibit cell proliferation.
Effects MTH on growth of erythroid and myeloid cultures. Mononuclear cells derived from normal bone marrow were cultured in semisolid medium in the presence of the indicated concentrations of HU (μM) (A) or MTH (nM) (B). The medium contained either 1 U/mL EPO for development of erythroid colonies (○) or 10% (vol/vol) 5637 cell–conditioned medium for development of myeloid colonies (▪). Colonies of 50 or more cells were counted after 14 days of incubation under an inverted microscope. The results represent the number of colonies per plate (average ± SD of 3 independent experiments).
Effects MTH on growth of erythroid and myeloid cultures. Mononuclear cells derived from normal bone marrow were cultured in semisolid medium in the presence of the indicated concentrations of HU (μM) (A) or MTH (nM) (B). The medium contained either 1 U/mL EPO for development of erythroid colonies (○) or 10% (vol/vol) 5637 cell–conditioned medium for development of myeloid colonies (▪). Colonies of 50 or more cells were counted after 14 days of incubation under an inverted microscope. The results represent the number of colonies per plate (average ± SD of 3 independent experiments).
Discussion
Mithramycin is a DNA-binding drug able to reversibly interact with GC-rich target DNA sequences.20,21,23,42 By virtue of this feature, MTH has been proposed as a gene expression modulator and is actually used, as plicamycin (Mithracin; Pfizer, New York, NY)25,26 for treatment of human pathologies.25-28,43-46
We have previously reported that MTH is a potent inducer of erythroid differentiation in K562 cells.14 Differentiation was found to be associated with increase in the synthesis of γ-globin mRNA and production of mostly Hb Portland, suggesting that this DNA-binding drug could be proposed as an inducer of human γ-globin genes.
In the present report we evaluated the effects of MTH on HbF production in healthy human and thalassemic-cultured erythroid precursor cells. In several independent experiments, using peripheral blood from different healthy donors, we reproducibly found an increase in HbF production (from 1.4% ± 0.4% of control cells in 20 independent experiments to 7.4% ± 1.8% of MTH-treated cells in 9 independent experiments; P < .001). This increase was found to be consistently higher than that induced by HU (HbF production = 3.5% ± 1.32% in 18 independent experiments; P < .05), a potent inducer of HbF both in vitro and in vivo.10 These data were fully in agreement with quantitative RT-PCR analysis, showing a preferential increase of γ-globin mRNA accumulation in MTH-treated erythroid precursors in comparison to α-globin and β-globin mRNA. In addition, the data demonstrate that MTH is a powerful inducer of HbF production in erythroid progenitors from β-thalassemia patients (both HPFH and non-HPFH). Unlike HU, the effect of MTH was not associated with inhibition of cell growth.
Therefore, our data strongly support possible clinical application of MTH for induction of HbF in patients affected by β-thalassemia or sickle cell disease, because it has been already demonstrated that an increase of HbF to 30% of total hemoglobins leads to a significant improvement of the clinical status of patients affected by these diseases. Thalassemic patients vary considerably with respect to HbF production.1-3 Our results suggest that those patients expressing low levels of HbF should benefit the most from such treatment. On the other hand, patients with high HbF (HPFH) are less likely to benefit from the treatment, because our results demonstrated a lower increase in HbF in erythroid precursors expressing constitutive high levels of HbF (Table 1). However, such HPFH patients are usually in a good clinical status.11-13
It should be underlined, however, that as with other DNA-binding drugs, mutagenic and carcinogenetic effects of MTH cannot be ruled out. We have, however, demonstrated,23 by surface plasmon resonance analysis, that the MTH-DNA interaction is highly unstable, unlike many other DNA-binding drugs, even those that are very similar in structure to MTH (such as chromomycin). This explains the finding that MTH is much less mutagenic than chromomycin.24
Clinical data with an MTH-based drug (plicamycin) demonstrated a variety of side effects, including immediate effects (such as vein irritation, nausea and vomiting, hypocalcemia, fever), early effects (such as depression of clotting factors; alterations of white blood cell, red blood cell, and platelet count; skin problems; azotemia; headache), and delayed effects (such as acute necrosis of the liver and tubular necrosis of the kidney).43-47 Serious side effects are present also during treatment with HU.48 Therefore, it is imperative to show that the concentrations of MTH and HU proven effective in vitro are attainable in vivo. Accordingly, HU at 100 μM, which demonstrated effectiveness in vitro, was maintained in vivo following continuous infusion of a therapeutic dose of 20 μg/kg HU.48 As for MTH, the concentrations that were found in the present study to increase HbF production (10 to 25 nM; Table 1) were lower than those found in vivo following treatment with plicamycin. Patients receiving a 2-hour continuous infusion of 25 μg/kg reached peak MTH plasma levels of 300 to 350 nM. However, the rate of the plasma half-life (clearance) of MTH was rapid—10.6 hours49 — indicating that protocols for MTH-based drug infusion should be carefully designed in order to maintain effective drug concentration in the hematopoietic sites. Efficient methods for pharmacokinetic analysis of plicamycin may be useful in this respect.49,50
We underline that these studies should be performed before treatment can be considered. However, because MTH is already used in therapy (for chronic myelogenous leukemia and testicular cancer, in Paget disease, and in pathologies associated with hypercalcemia), studying such patients (eg, with respect to HbF) could provide valuable, yet not conclusive, information on MTH activity in vivo, which could be related to data obtained in experimental animals. This latter strategy is highly warranted, as for other HbF inducers.51
In conclusion, we hereby report the activity of MTH as HbF inducer. Because many analogs of MTH have been recently described,52,53 these and structurally related agents should be screened in order to identify analogs exhibiting the lowest cytotoxic/mutagenic effects, the most favorable pharmacokinetics, and the highest ability to induce HbF. Further experiments, including in vivo tests on experimental animals, are still necessary to conclusively determine the potential use of such agents in the treatment of severe hematologic diseases, such as non-HPFH β-thalassemia and sickle cell anemia.
Prepublished online as Blood First Edition Paper, May 8, 2003; DOI 10.1182/blood-2002-10-3096.
Supported by CNR PF Biotecnologie and by Associazione Veneta per la Lotta alla Talassemia (Rovigo); the Ministry of Science and Technology, Israel; and Consiglio Nazionale delle Ricerche (CNR), Italy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank S.I.T. ULSS 18, Rovigo (Servizio di Immunoematologia e Trasfusione: Prof Rocco Potenza, Dr Francesco Chiavilli, Dr Stefano Modonesi) and Dr Ada Goldfarb (Hadassah University Hospital, Jerusalem) for clinical samples. The experienced technical assistance of Mrs Aliza Treves (Hadassah University Hospital, Jerusalem) is greatly appreciated.