Abstract
The cytokine tyrosine kinase receptors c-kit and flt3 are expressed and function in early mouse and human hematopoiesis. Through its ability to promote ex vivo expansion and oncoretroviral transduction of primitive human hematopoietic progenitors, the flt3 ligand (FL) has emerged as a key stimulator of candidate human hematopoietic stem cells (HSCs). However, recent studies in the mouse suggest that though it is present on short-term repopulating cells, flt3 is not expressed on bone marrow long-term reconstituting HSCs, the ultimate target for the development of cell replacement and gene therapy. Herein we demonstrate that though only a fraction of human adult bone marrow and cord blood CD34+long-term culture-initiating cells (LTC-ICs) express flt3, most cord blood lymphomyeloid HSCs capable of in vivo reconstituting nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice are flt3+. The striking difference in flt3 and c-kit expression on mouse and candidate human HSCs translated into a corresponding difference in flt3 and c-kit function because FL was more efficient than SCF at supporting the survival of candidate human HSCs. In contrast, SCF is far superior to FL as a viability factor for mouse HSCs. Thus, the present data provide compelling evidence for a contrasting expression and response pattern of flt3 and c-kit on mouse and human HSCs.
Introduction
All blood cells arise from a common hematopoietic stem cell (HSC),1 which, because of its self-renewal capacity, can maintain hematopoiesis throughout the lifespan of an individual.2,3 The molecular mechanisms regulating HSC fate decisions between self-renewal and lineage commitment remain largely unknown.2 However, evidence supports that blood-lineage maturation and HSC self-renewal are regulated in part by cytokines interacting with their specific surface receptors. Stem cell factor (SCF, or c-kit ligand) and thrombopoietin (TPO) have been shown to act directly on HSCs,4-9 and gene-targeting studies have demonstrated that SCF and TPO play important roles in sustaining HSC number and function, in contrast to other cytokines.10-13 Flt3 (or flk2) is, like c-kit, a cytokine tyrosine kinase receptor expressed primarily at very early stages of hematopoiesis.14-18 Mice deficient in the expression of flk2 or flt3 ligand (FL) show deficient lymphopoiesis.19,20 FL has also been implicated as a key and potent stimulator of candidate mouse and human HSCs and is frequently used to promote HSC ex vivo expansion and oncoretroviral-mediated gene transfer.21-25 However, it has not been established whether this reflects a role of flt3 in HSC generation, maintenance, homing, engraftment, or expansion after transplantation.
Surprisingly, recent studies revealed that mouse adult long-term reconstituting HSCs (LTR-HSCs) do not express flt3 during steady-state hematopoiesis and that the up regulation of flt3 expression within the HSC compartment is accompanied by loss of self-renewal capacity.26,27 In addition, SCF, but not FL, efficiently supports the survival of mouse LTR-HSCs,26,28 which correlates with high levels of expression of c-kit5 and lack of flt3 expression on HSCs.26,27 In contrast, SCF has limited capacity to support in vitro survival of human CD34+CD38- candidate HSCs,29 which have been shown to express low levels of c-kit.30,31
A number of studies have demonstrated that flt3 is expressed in the human CD34+ progenitor cell compartment,17,18 including a fraction of primitive hematopoietic progenitors capable of sustaining long-term in vitro cultures.18 However, to what degree flt3 is expressed on human HSCs capable of in vivo lymphomyeloid reconstitution has not been investigated. Such studies are important to address whether human HSCs might lack flt3 expression, as reported in the mouse.26,27 If so, it could obviously have important implications for the ongoing efforts of using FL to promote stem cell expansion and retroviral gene transfer.
In the present study, we evaluated the potential role of flt3 and its ligand in human HSC regulation by investigating the expression and function of flt3 within the CD34+ compartment in adult bone marrow and umbilical cord blood. In striking contrast to mouse HSCs, virtually all human multilineage reconstituting HSCs in CB were flt3+, and FL but not SCF efficiently promoted the survival of candidate HSCs, indicating perhaps distinct roles of FL and SCF in murine and human HSC regulation.
Materials and methods
Purification of CD34+progenitor/stem cell populations
Following informed consent and with the approval of the Ethics Committee at the University Hospital of Lund, placentas from normal full-term deliveries were obtained for umbilical cord blood (CB) collection. Posterior iliac aspirations were performed on healthy volunteers for adult bone marrow (BM) collection. Mononuclear cells (MNCs) were isolated by Ficoll-Hypaque gradient centrifugation (Lymphoprep; Nycomed Pharma AS, Oslo, Norway). After MNC isolation, CB samples were pooled. Positive selection of CD34+ cells was performed using a MACS CD34 isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions and as previously described.32 For purification of CD34+CD38-/low cells, CD34-enriched cells were incubated with anti-CD34–fluorescein isothiocyanate (FITC) and anti-CD38–phycoerythrin (PE) monoclonal antibodies or with isotype-matched control antibodies (all from Becton Dickinson [BD], San Jose, CA). To exclude dead cells, 7-AAD (Sigma, St Louis, MO) was added. Cell sorting, at a rate of 2000 to 8000 cells per second, was performed on a FACS Vantage (BD) equipped with a 488-nm argon, 633-nm He-Ne laser. As previously described,32 a conservative approach was taken so that only the 3% lowest CD38-expressing CD34+ BM cells were sorted to obtain highly purified CD34+CD38- cells (reproducibly greater than 95% purity).
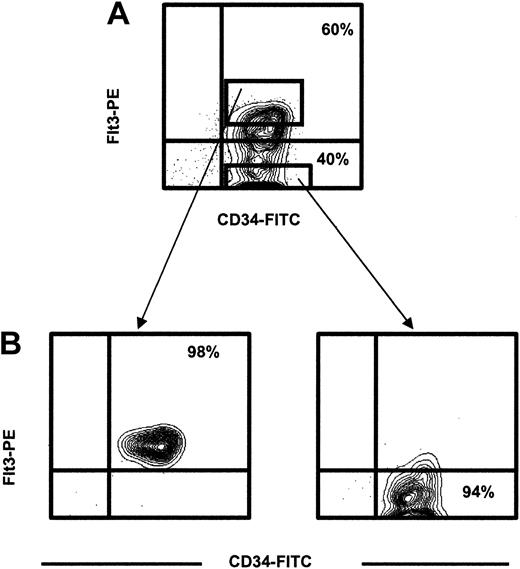
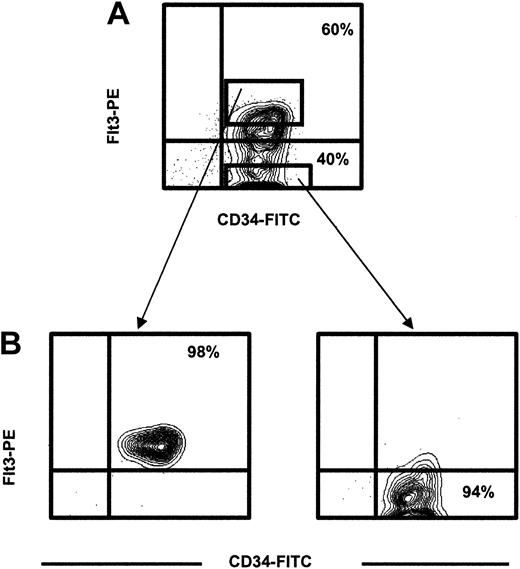
For the purification of CD34+flt3+ and CD34+flt3- cells, CD34-enriched cells were incubated with 7-AAD, anti-CD34-FITC; and anti–flt3-PE (4G8 clone; PharMingen, San Diego, CA; or SF1.340 clone; Immunotech, Marseilles, France) monoclonal antibodies or with isotype-matched control antibodies. Figure 1 illustrates the gating, sorting, and reanalysis. After gating on viable cells, the expression of flt3 was analyzed within the CD34+ cell population, and sorting gates were set to sort the CD34+flt3+ and CD34+flt3- populations (purity, 90%-98% for each population).
Identification and purification of CD34+flt3+and CD34+flt3-cells. (A) CD34-enriched cells were stained with an anti-flt3 or an irrelevant control antibody. CD34+flt3+and CD34+flt3-cells were sorted using the indicated sort gates. Figure shows representative profile for adult bone marrow. (B) Purity analysis of sorted populations.
Identification and purification of CD34+flt3+and CD34+flt3-cells. (A) CD34-enriched cells were stained with an anti-flt3 or an irrelevant control antibody. CD34+flt3+and CD34+flt3-cells were sorted using the indicated sort gates. Figure shows representative profile for adult bone marrow. (B) Purity analysis of sorted populations.
Hematopoietic growth factors
Recombinant human (rh) stem cell factor (rhSCF) and rh granulocyte–colony-stimulating factor (rhG-CSF) were generously supplied by Amgen (Thousand Oaks, CA). Rh flt3 ligand (rhFL), rh interleukin-3 (rhIL-3), and rh granulocyte macrophage–colony-stimulating factor (rhGM-CSF) were kind gifts from Immunex (Seattle, WA). Rh thrombopoietin (rhTPO) was generously provided by Genentech (San Francisco, CA), and rhIL-6 was supplied by Genetics Institute (Cambridge, MA). Rh erythropoietin (rhEPO) was supplied by Boehringer Mannheim (Mannheim, Germany).
Semisolid clonogenic assay
Freshly sorted or cultured CD34+flt3+ and CD34+flt3- cells were plated in 1 mL methylcellulose (MethoCult H4100; StemCell Technologies, Vancouver, BC, Canada) supplemented with 20% fetal calf serum (BioWhittaker, Walkersville, MD), 10-6 M 2-mercaptoethanol (Sigma Chemical), 100 U/mL penicillin (BioWhittaker), 100 U/mL streptomycin (BioWhittaker), and human cytokines (as specified) in 35-mm Petri dishes. Cultures were incubated at 37°C in a humidified atmosphere with 5% CO2 for 12 to 14 days, at which time colonies were visualized and scored with an inverted microscope.
Long-term culture–initiating cell assay
Long-term culture-initiating cells (LTC-ICs) were established with mouse stroma feeders engineered to produce human growth factors (M2-10B4 and SL/SL mixed at 1:1; kindly provided by Dr D. E. Hogge, Vancouver, BC, Canada).33 The mouse stroma irradiated with 8000 cGy was plated in 96-well collagen-coated microtiter plates (5000 cells/well of each cell line) and cultured in long-term culture medium (MyeloCult H5100; Stem Cell Technologies) supplemented with hydrocortisone 21–hemi-succinate (10-6 M).
The CD34+CD38-, CD34+flt3+, and CD34+flt3- BM or CB cells were plated at 150 to 2000 cells per well. Cocultures were maintained at 37°C in high humidity and with 50% medium exchange every week. After 6 weeks, nonadherent and adherent cells were plated in methylcellulose cultures supplemented with rhSCF, rhGM-CSF, rhG-CSF, rhFL (all at 10 ng/mL), rhIL-3 (5 ng/mL), and rhEPO (5 U/mL). Long-term culture colony-forming cells (LTC-CFCs; readout of LTC-IC assay) were scored after an additional 14 days of culture. To more specifically detect erythroid progenitors generated from LTC-ICs (LTC–burst-forming unit–erythroid [LTC–BFU-Es]), cells were plated in methylcellulose supplemented with 50 ng/mL SCF and 5 U/mL EPO, and colonies were scored after 14 days in culture. Stroma cells without hematopoietic cells were plated in methylcellulose with cytokines and used as a negative control for the colony assay (data not shown).
Viability assay
Sorted BM CD34+CD38- cells were plated at 500 to 2000 cells per well in 96-well plates in serum-free medium (X-Vivo 15; BioWhittaker) supplemented with 1% detoxified bovine serum albumin (BSA; StemCell Technologies) and 200 ng/mL SCF or 200 ng/mL FL. After 5 days of culture, cells were transferred to the pre-established stroma feeder layers, and they were evaluated for LTC-IC after 6 weeks, as described under “Long-term culture–initiating cell assay.”
NOD/SCID reconstituting assay
To perform the nonobese diabetic/severe combined immunodeficiency (NOD/SCID) reconstituting assay, NOD/LtSz-SCID mice (kindly provided by Dr L. D. Shultz, The Jackson Laboratory, Bar Harbor, ME) were bred and housed under sterile conditions in microisolator cages and were given autoclaved food and acidified drinking water. Mice were irradiated with 350 cGy from a cesium Cs 137 source at 8-12 weeks of age and thereafter were given prophylactic ciprofloxin (100 μg/mL) in the drinking water until analysis (6-13 weeks after transplantation). Tail-vein transplantation/injection of CD34+flt3+ and CD34+flt3- CB cells in 0.5 mL medium was performed within 4 hours of irradiation. Test cell populations were administered transplants together with 1 × 106 irradiated (1500 cGy) human MNCs as accessory cells. To investigate human cell engraftment at different times after transplantation, bone marrow aspiration was performed as previously described34 at 6 to 8 weeks after transplantation, and BM was harvested from the femora and tibiae of the same mice at 12 to 13 weeks. Mice were analyzed for human reconstitution as previously described.35 Briefly, cells were stained with antihuman CD45–allophycocyanin (APC), CD19-PE, CD66b/CD15/CD33-FITC antibodies (all from BD) and 7-AAD. Mice that did not undergo transplantation (negative controls) and mixtures of 0.1% human cells in mouse BM (positive controls) were always included. For each sample, a minimum of 100 000 viable BM cells were analyzed by a FACScalibur (BD). Mice were defined as positive for SCID repopulating cell (SRC) reconstitution if they showed at least 0.1% human reconstitution, including myeloid and lymphoid engraftment (minimum of 10 positive events each per 100 000 BM cells analyzed). Gates were set so that samples incubated with isotype-matched control antibodies had a maximum of 1 positive event per 100 000 analyzed BM cells. Flow-activated cell sorting (FACS) analysis was performed using CellQuest (Becton Dickinson) and FlowJo (Tree Star, San Carlos, CA) software.
If no engraftment was detected by FACS or if the myeloid engraftment was low, BM cells from NOD/SCID mice were plated in methylcellulose (1 × 106 cells/plate; 3-6 replicates) with human-specific cytokines (10 ng/mL rhGM-CSF, 5 ng/mL rhIL-3, and 25 ng/mL rhSCF) and 5 U/mL rhEPO. Granulocyte-macrophage CFC (CFC-GM) and erythroid colonies (BFU-Es) were scored after 10 to 14 days. No colonies were observed in the absence of cytokines or from the BM of NOD/SCID mice that did not undergo transplantation using the same cytokines (E.S. and S.E.W.J., unpublished observation, June 2002).
Statistics
All results are expressed as means and SEMs. Statistical significance of differences between groups was determined using the Student t test.
Results
In vivo multilineage reconstituting HSCs express flt3
CD34+ cells in human adult BM and CB are highly enriched for candidate human HSCs, as defined by their ability to reconstitute LTC-IC cultures in vitro and immunodeficient mice in vivo.3,33,36 In agreement with previous studies17,18 we found that 40% to 60% of BM CD34+ cells (Figure 1) and 30% to 40% of CB CD34+ cells (data not shown) were flt3+. CD34+flt3+ and CD34+flt3- cells were next sorted to high purity (greater than 94%) to investigate their content of candidate HSCs (Figure 1).
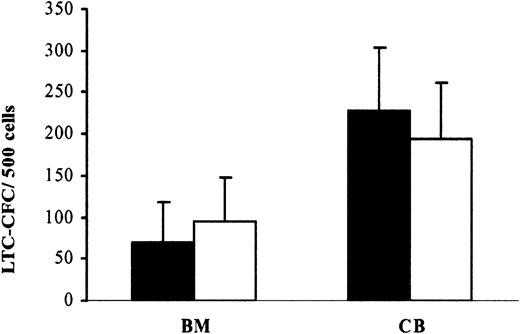
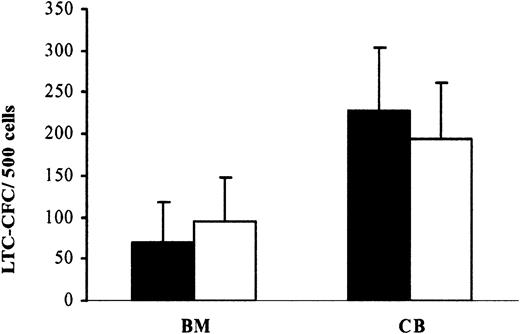
The LTC-IC assay represents the most stringent in vitro assay for evaluating candidate HSCs.33,37 After 6 weeks of coculture, the generation of LTC-CFCs per 500 adult BM cells was 70 ×49 and 95 ×53 from CD34+flt3+ and CD34+flt3- cell populations, respectively, whereas 500 CB CD34+flt3+ and CD34+flt3- cells produced 228 ×85 and 194 ×70 LTC-CFCs, respectively (Figure 2). Thus, adult BM and CB CD34+flt3+ and CD34+flt3- cells contain comparable numbers of human candidate HSCs capable of reconstituting long-term cultures in vitro.
CD34+flt3+BM and CB cells contain LTC-ICs. Sorted CD34+flt3+(▪) and CD34+flt3-(□) cells were plated on established stroma layers (“Materials and methods”). Six weeks after coculture, all cells were transferred to methylcellulose cultures, and the numbers of colonies were scored after 14 days. Results are expressed as number of colonies per 500 cells. Data represent mean values (SEM) from 3 adult BM and 4 CB experiments, with 4 to 6 replicates in each group. Differences between BM and CB CD34+flt3+and CD34+flt3-cells were not statistically significant.
CD34+flt3+BM and CB cells contain LTC-ICs. Sorted CD34+flt3+(▪) and CD34+flt3-(□) cells were plated on established stroma layers (“Materials and methods”). Six weeks after coculture, all cells were transferred to methylcellulose cultures, and the numbers of colonies were scored after 14 days. Results are expressed as number of colonies per 500 cells. Data represent mean values (SEM) from 3 adult BM and 4 CB experiments, with 4 to 6 replicates in each group. Differences between BM and CB CD34+flt3+and CD34+flt3-cells were not statistically significant.
The recent development of the xenotransplantation assay allows more careful evaluation of human HSCs capable of in vivo reconstitution of myelopoiesis and lymphopoiesis.36,38 In 3 experiments at 6 to 8 weeks after transplantation, 13 of 16 mice injected with 50 000 to 70 000 CD34+flt3+ cells isolated from CB showed human myeloid and B-lymphoid reconstitution, with an average 19.7% human cell engraftment. In striking contrast, only 7 of 17 mice that received 50 000 to 70 000 CD34+flt3- cells showed human myeloid and B-lymphoid reconstitution with an average of only 2.5% human cell engraftment (Table 1). To compare the durability of human reconstitution by CD34+flt3+ and CD34+flt3- cells and to determine whether the frequency of reconstituting SRCs is higher in CD34+flt3+ than in CD34+flt3- cells, additional experiments were performed in which mice underwent transplantation with more limiting numbers of CD34+flt3+ and CD34+flt3- CB cells and engraftment was analyzed at 6 and at 12 to 13 weeks after transplantation (Table 1). In 3 experiments, 13 of 18 (72%) mice that received only 2500 to 5000 CD34+flt3+ cells showed human myeloid and B-lymphoid reconstitution, with an average 3.9% human cell engraftment that persisted at 12 weeks after transplantation in 9 of 18 (50%) mice (Table 1; Figure 3). In contrast, at 6 weeks only 4 of 26 (15%) mice that received higher numbers (5000-10 000) of CD34+flt3- cells showed human reconstitution with an average engraftment of 0.13%, and only 2 of 26 (8%) mice were positive for human myeloid and B-lymphoid reconstitution at 12 weeks (Table 1). These results clearly demonstrate that most in vivo repopulating CB CD34+ HSCs express flt3.
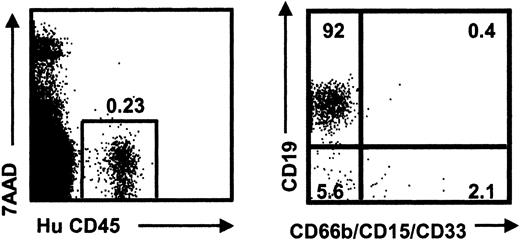
Human multilineage reconstitution of NOD/SCID mice with CD34+flt3+CB cells. Representative human reconstitution and lineage analysis of a mouse that received 2500 CD34+flt3+CB cells 6 weeks before analysis. Mouse shows low (0.23%) levels of human (CD45) engraftment but significant human B (CD19) and myeloid (CD66b/CD15/CD33) reconstitution.
Human multilineage reconstitution of NOD/SCID mice with CD34+flt3+CB cells. Representative human reconstitution and lineage analysis of a mouse that received 2500 CD34+flt3+CB cells 6 weeks before analysis. Mouse shows low (0.23%) levels of human (CD45) engraftment but significant human B (CD19) and myeloid (CD66b/CD15/CD33) reconstitution.
CD34+flt3+cells are devoid of erythroid-committed progenitors but contain multipotent progenitor/stem cells with erythroid reconstituting potential
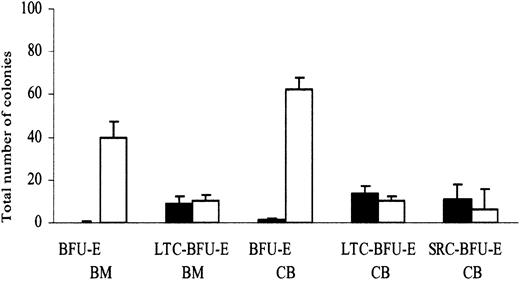
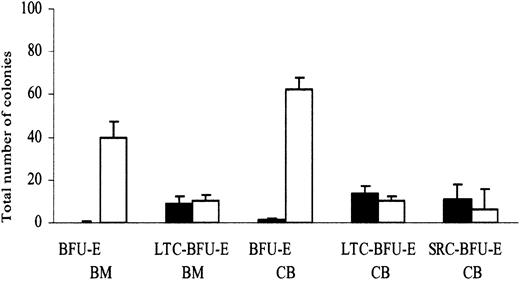
In agreement with previous studies,18 adult BM and CB CD34+flt3+ cells failed to display erythroid potential when plated directly in methylcellulose (0.1-1.3 BFU-E/500 cells), whereas CD34+flt3- cells were highly enriched in BFU-Es (40-62 BFU-E/500 cells) (Figure 4). We have recently found that flt3-expressing cells within the mouse Lin-Sca-1+c-kit+ HSC pool have reduced erythroid and megakaryocyte differentiation potential (S.E.W.J. et al, unpublished observation, June 2002). To investigate whether primitive human CD34+flt3+ cells also have restricted erythroid differentiation potential, CB and adult BM CD34+flt3+ and CD34+flt3- cells were cocultured on stroma cells for 6 weeks and then transferred into methylcellulose to detect BFU-E. Noteworthy, CD34+flt3+ and CD34+flt3- cells contained primitive hematopoietic progenitor/stem cells capable of producing erythroid progenitors in long-term culture (Figure 4). CB CD34+flt3+ cells also reconstituted erythroid progenitors in NOD/SCID mice (Figure 4). Taken together, these results demonstrate that though committed erythroid progenitors are all flt3-, CB in vivo reconstituting HSCs expressing flt3 have erythroid differentiation potential.
Committed erythroid progenitors are CD34+flt3-, but CD34+flt3+ reconstituting HSCs have erythroid differentiation potential. To evaluate the frequency of BFU-E, sorted CD34+flt3+(▪) and CD34+flt3-(□) cells from CB and adult BM were plated directly after sorting in methylcellulose cultures containing SCF+EPO. To measure the frequency of erythroid progenitors generated during long-term culture, sorted CD34+flt3+and CD34+flt3-cells from CB and adult BM were cocultured on stroma layers for 6 weeks and then were plated in methylcellulose cultures containing SCF+EPO (LTC–BFU-E). Finally, to evaluate the frequency of erythroid progenitors generated from SRC, sorted CD34+flt3+and CD34+flt3-cells from CB were transplanted into NOD/SCID mice; 6 to 8 weeks after transplantation, unfractionated BM cells harvested from mice were plated in methylcellulose cultures containing SCF+EPO (SRC–BFU-E). BFU-Es were scored after 14 days. Results are expressed as number of erythroid colonies per 500 CD34+flt3+and CD34+flt3-cells for BFU-E and LTC–BFU-E and per 1 × 106 total NOD/SCID BM cells for SRC-BFU-E. Data represent mean values from 3 experiments using cord blood and 2 experiments using adult bone marrow cells with 3 to 6 replicates in each group; error bars depict SEMs.
Committed erythroid progenitors are CD34+flt3-, but CD34+flt3+ reconstituting HSCs have erythroid differentiation potential. To evaluate the frequency of BFU-E, sorted CD34+flt3+(▪) and CD34+flt3-(□) cells from CB and adult BM were plated directly after sorting in methylcellulose cultures containing SCF+EPO. To measure the frequency of erythroid progenitors generated during long-term culture, sorted CD34+flt3+and CD34+flt3-cells from CB and adult BM were cocultured on stroma layers for 6 weeks and then were plated in methylcellulose cultures containing SCF+EPO (LTC–BFU-E). Finally, to evaluate the frequency of erythroid progenitors generated from SRC, sorted CD34+flt3+and CD34+flt3-cells from CB were transplanted into NOD/SCID mice; 6 to 8 weeks after transplantation, unfractionated BM cells harvested from mice were plated in methylcellulose cultures containing SCF+EPO (SRC–BFU-E). BFU-Es were scored after 14 days. Results are expressed as number of erythroid colonies per 500 CD34+flt3+and CD34+flt3-cells for BFU-E and LTC–BFU-E and per 1 × 106 total NOD/SCID BM cells for SRC-BFU-E. Data represent mean values from 3 experiments using cord blood and 2 experiments using adult bone marrow cells with 3 to 6 replicates in each group; error bars depict SEMs.
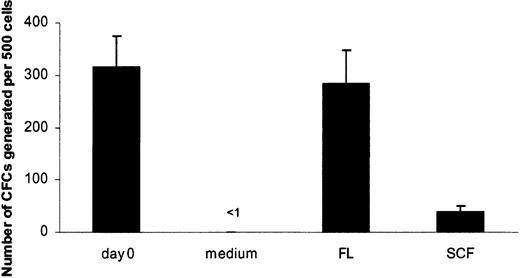
Flt3 ligand supports in vitro survival of candidate human HSCs
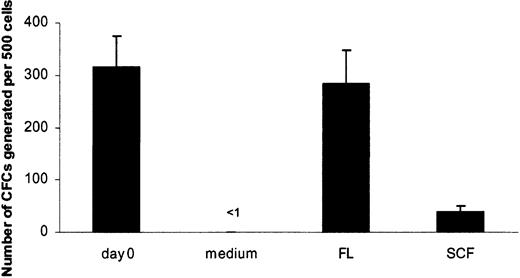
SCF has been demonstrated to efficiently support the survival of mouse HSCs, whereas FL has no such ability.26 Thus, we here investigated the survival-promoting activity of these 2 ligand receptor pairs on candidate human HSCs. Although LTC-ICs can be CD34+CD38+ and CD34+CD38-, the most primitive LTC-ICs are believed to have a CD34+CD38- phenotype.39 To investigate the ability of FL and SCF to support the survival of candidate human HSCs, we therefore used highly purified CD34+CD38- adult BM cells precultured for 5 days in the absence of cytokines or in the presence of FL or SCF. Cells were subsequently transferred to pre-established stroma layers and were cultured for 6 weeks in the absence of exogenous cytokines to assess the ability of FL and SCF to support the survival of LTC-ICs (Figure 5). As expected, after 5 days of culture in medium alone, no LTC-IC activity could be recovered from CD34+CD38- cells, whereas virtually all LTC-CFC activity could be recovered from FL-preincubated cultures (90% of freshly isolated cells; P = .37) (Figure 5). In contrast, though SCF promoted the survival of a low number (12% of freshly isolated cells; P < .01) of LTC-CFCs, 7-fold more LTC-CFCs were derived from FL-supplied cultures (P < .01) (Figure 5). These data demonstrate that FL is a potent survival factor for candidate human HSCs, whereas SCF is a less efficient viability factor than reported for mouse HSCs.28
Flt3 ligand supports in vitro survival of LTC-IC. Purified CD34+CD38- BM cells were placed on established stroma layers at 500 to 2000 cells per well, directly after sorting (day 0) or after 5 days of preculture in medium alone, SCF, or FL. After 6 weeks of culture on stroma, cells were transferred to methylcellulose to measure the generation of LTC-CFCs. Data from 4 experiments with 12 to 24 replicates per group are expressed as mean values; error bars depict SEMs.
Flt3 ligand supports in vitro survival of LTC-IC. Purified CD34+CD38- BM cells were placed on established stroma layers at 500 to 2000 cells per well, directly after sorting (day 0) or after 5 days of preculture in medium alone, SCF, or FL. After 6 weeks of culture on stroma, cells were transferred to methylcellulose to measure the generation of LTC-CFCs. Data from 4 experiments with 12 to 24 replicates per group are expressed as mean values; error bars depict SEMs.
Discussion
Through a number of recent observations, flt3 and its ligand have emerged as important regulators of the HSC pool in mice and humans.21-25 Although the flt3 (flk2) receptor was originally identified and cloned based on its preferential expression in highly enriched mouse HSCs,14,21 recent studies have surprisingly demonstrated that the up-regulation of flt3 expression within the Lin-Sca-1+c-kit+ HSC compartment in mouse BM is accompanied by a loss of self-renewal potential, the cardinal property of HSCs.26,27 Furthermore, mice deficient in FL expression have normal levels of HSCs.40 Thus, flt3 appears to be constitutively expressed on a population of multilineage short-term reconstituting HSCs in mouse bone marrow but not on long-term repopulating HSCs, the ultimate targets for the development of efficient cell replacement and gene therapy.
FL also acts on early progenitor cells in human BM and CB.21,22,24,25 Specifically, cobblestone-area–forming cells (CAFCs), representing primitive progenitors and HSCs,41 are distributed equally between CD34+flt3+ and CD34+flt3- cells.18 Whether flt3 is expressed on in vivo reconstituting human HSCs was addressed for the first time in the present studies. Whereas our data confirm that the frequencies of LTC-IC in CD34+flt3+ and CD34+flt3- cells are similar, CB SRCs capable of in vivo reconstituting myelopoiesis and lymphopoiesis were found to be almost exclusively CD34+flt3+. When transplanted at high numbers (50 000-70 000 cells), CD34+flt3+ cells reconstituted human lymphomyelopoiesis to much higher levels than CD34+flt3- cells. At limiting cell doses, the frequency of mice positive for human lymphomyeloid reconstitution was 5-fold higher with CD34+flt3+ than with CD34+flt3- cells, though they underwent transplantation at 50% lower cell doses (2500-5000 and 5000-10 000, respectively). Furthermore, CD34+flt3+ cells provided sustained lymphomyeloid reconstitution not only at 6 but also at 12 weeks after transplantation. Considering that the SRC assay probably detects an earlier HSC population than LTC-IC,38,39 the data presented herein suggest that flt3 is expressed on candidate human HSCs. It cannot be excluded that the difference in reconstituting ability of transplanted CD34+flt3+ and CD34+flt3- cells could in part be attributed to an accessory cell population, though high numbers of irradiated accessory cells were cotransplanted with progenitor/stem cell populations to enhance engraftment.
While this manuscript was under revision, another group of investigators published data further supporting that human CB cells expressing flt3 are capable of engrafting NOD/SCID mice.42
Previous studies had suggested that CD34+flt3+ cells have restricted erythroid differentiation potential.17,18 This could reflect that flt3 is down-regulated on erythroid commitment or that multipotent flt3+ progenitors are restricted in their lineage reconstitution potential. Our studies confirm that committed erythroid progenitors are CD34+flt3-, but they also demonstrate that CD34+flt3+ multipotent progenitors have erythroid differentiation potential, as expected by candidate HSCs.
The present findings and corresponding studies on mouse HSCs26,27 provide compelling evidence for a contrasting pattern of flt3 expression on mouse and human HSCs in that HSCs in mouse BM are exclusively flt3-, whereas human BM and CB candidate HSCs appear to be flt3+. This translates into a corresponding and striking difference in flt3 function because FL fails to promote the survival of mouse HSCs26 but efficiently supports the viability of candidate human HSCs. Conversely, c-kit shows an opposite expression pattern: in steady state, bone marrow mouse HSCs within the Lin-Sca-1+ population are almost exclusively found in the c-kithigh fraction, whereas candidate human HSCs appear to be predominantly c-kitlow.30,43 Although SCF and FL both are potent synergistic factors for the growth of primitive human progenitors,44 we here demonstrate that SCF is less efficient than FL at promoting the survival of CD34+CD38- candidate human HSCs, whereas SCF is far superior to FL as a viability factor for mouse HSCs.26,28 Thus, SCF and FL, considered key regulators of early hematopoiesis,21 might prove to have distinct roles in the regulation of HSCs and early hematopoietic development in mice and humans. If so, the present findings suggest that this can be explained by the different expression patterns of their corresponding receptors in the HSC compartment. However, an alternative explanation for the observed and other reported phenotypic differences between mouse and human HSCs26,39,45,46 could be that the SRC assay preferentially detects short-term rather than long-term HSC. Although this possibility appears less likely and is currently difficult to address, it nevertheless must be entertained because it could have major implications for ongoing efforts aimed at the identification, characterization, and clinical development of human HSCs.
Prepublished online as Blood First Edition Paper, April 3, 2003; DOI 10.1182/blood-2002-06-1694.
Supported by grants from ALF (Governmental Public Health Grant), Alfred Österlund Foundation, Funds of Lunds Sjukvårdsdistrikt, the Swedish Medical Research Council, Swedish Foundation for Strategic Research, Swedish Cancer Society, the Swedish Society of Pediatric Cancer, and the Tobias Foundation. E.S. is a senior scientist supported by the Swedish Gene Therapy Program. S.L. received an educational grant from AstraZeneca.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the staffs and donors at the Department of Obstetrics, University Hospital of Lund and Helsingborg Hospital for providing cord blood and the volunteers and staff at the Department of Hematology, University Hospital of Lund for bone marrow contributions and aspirations. We also thank Lilian Wittmann, Gunilla Gärdebring, Anna Fossum, Zhi Ma, Julia Menning, Eva Gynnstam, and Irene Person for their expert technical assistance.