Abstract
Multiple myeloma (MM) and primary systemic amyloidosis (AL) remain incurable disorders, and new treatments targeted to the malignant plasma cells are needed. Alemtuzumab is a humanized monoclonal antibody to CD52 and has activity in chronic lymphocytic leukemia. We examined the CD52 expression on CD45+ and CD45- plasma cell populations to evaluate the potential for using alemtuzumab for these disorders. Bone marrows from 61 patients (29 AL, 23 MM, and 9 MGUS [monoclonal gammopathies of undetermined significance]) were studied using 3-color (CD38/45/52) flow cytometry. Among those with MGUS, MM, and AL, 67%, 52%, and 35%, respectively, were positive for CD52 expression. The CD52 expression was predominantly confined to the clonal CD38+/CD45+ plasma cell fraction with median expression of 68%, 88%, and 82% in MGUS, MM, and AL, respectively, compared with 18%, 6%, and 9% among the CD45- plasma cell population. Clinical trials are warranted in these diseases to learn the therapeutic benefit of anti-CD52 immunotherapy.
Introduction
Multiple myeloma (MM) is a clonal plasma cell (PC) proliferative disorder that accounts for nearly 10% of all hematologic neoplasms.1 It is estimated that there will be 14 600 new cases in this country in 2002 alone and that 10 800 will die of the disease.2 Although autologous stem cell transplantation improves survival, most patients eventually relapse, and salvage therapy options remain limited.3
Primary systemic amyloidosis (AL), another plasma cell disorder, is characterized by relentless progression of tissue deposition of immunoglobulin light chain–derived amyloid protein in different organs.4 Although improvement is seen in some patients with chemotherapy, the prognosis remains poor.5
CDw52, originally characterized as a human leukocyte differentiation antigen, is present on the surface of most peripheral blood lymphocytes, macrophages, and monocytes at relatively high density.6 It is absent from myeloid cells, platelets, and erythroid cells as well as hematopoietic stem cells.7 It is a small peptide attached to the cell membrane by a glycosylphosphatidylinositol anchor. Unlabeled antibodies directed against this antigen are very effective in destroying CD52+ cells by antibody-mediated cellular cytotoxicity as well as through complement fixation and activation.8-10 The humanized form of alemtuzumab (Campath-1H) is currently approved by the Food and Drug Administration (FDA) for therapy of chronic lymphocytic leukemia (CLL).11-14 Despite widespread use of rituximab (anti-CD20) and alemtuzumab for lymphoma and CLL, respectively, monoclonal antibodies have not been extensively tested for plasma cell disorders. The goal of this study was to examine CD52 expression on malignant marrow PCs to evaluate the potential for using alemtuzumab as a therapeutic agent for these disorders.
Study design
We studied the expression of CD52 on PCs from the bone marrow (BM) of 80 unselected patients with MM, monoclonal gammopathy of undetermined significance (MGUS), or AL who were undergoing BM examinations for clinical purposes. Seventy-six percent (61 of 80) of the cases had documented monoclonal CD38+ PCs and were studied for CD52 expression. All patients had given permission for use of samples for research and the study approval was obtained from the Institutional Review Board of the Mayo Clinic.
For CD52 expression, 1 million lysed whole BM cells were incubated with CD38-phycoerythrin (PE), CD45–peridinin chlorophyll protein (PerCP) (BD Immunocytometry Systems, San Jose, CA), and CD52–fluorescein isothiocyanate (FITC; Serotech, Raleigh, NC) or immunoglobulin G1 (IgG1)–FITC (control). For determination of clonality, 2 tubes were stained with CD38-PE and CD45-PerCP and then processed for cytoplasmic staining of kappa-FITC or lambda-FITC (Biosource International, Camarillo, CA) using a fixation and permeabilization kit (Caltag, AnDer Grub, Austria). After staining, cells were washed, resuspended in 1.0% paraformaldehyde, and run on the FACSCalibur flow cytometer (BD Biosciences, San Jose, CA). Fifty thousand mononuclear cell (MNC)–gated (forward scatter [FSC] versus side scatter [SSC]) events were collected from each tube (Figure 1A). The PCs for each case were gated according to the characteristic CD38bright/CD45 staining patterns. The expression of CD52 in the clonal PC populations was measured as the percentage positive according to gates set using the IgG1-FITC staining in the control tube. Positive expression was defined as 20% or more of the respective PC population expressing CD52. The intensity of CD45 and CD52 expression on PCs was assessed by comparison of the fluorescence channel numbers on the flow cytometry plots. The mean fluorescence intensity for the CD45 expression on PCs was calculated as the ratio between the fluorescence channels for CD38+/CD45+ and CD38+/CD45- PCs. The intensity of CD52 expression in the CD45+ and CD45- PC subsets was also calculated in a similar fashion, using the ratio between CD38+/CD52+/CD45+ and CD38+/CD52+/CD45- cell populations.
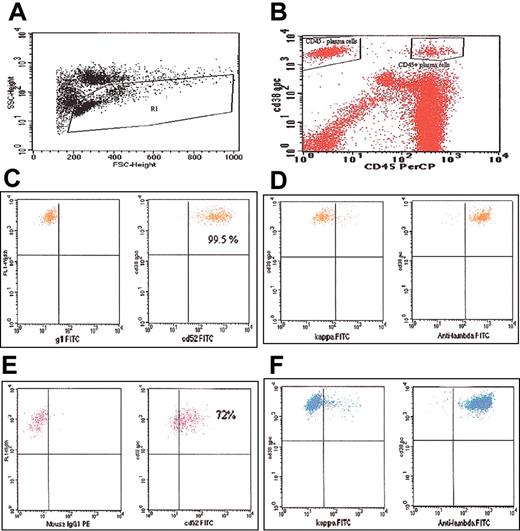
Flow cytometry plots. (A) Plot showing gating of the lymphocyte-monocyte population. (B) Plot showing the CD45+ and CD45- fractions of the CD38+ PCs gated separately. (C) Plot showing CD52 expression on the CD45+/CD38+ cells. (D) Plot demonstrating monoclonality of the CD45+ cells; kappa or lambda staining is on the x-axis. (E) Plot showing CD52 expression on the CD45-/CD38+ cells. (F) Plot demonstrating monoclonality of the CD45- cells; kappa or lambda staining is on the x-axis.
Flow cytometry plots. (A) Plot showing gating of the lymphocyte-monocyte population. (B) Plot showing the CD45+ and CD45- fractions of the CD38+ PCs gated separately. (C) Plot showing CD52 expression on the CD45+/CD38+ cells. (D) Plot demonstrating monoclonality of the CD45+ cells; kappa or lambda staining is on the x-axis. (E) Plot showing CD52 expression on the CD45-/CD38+ cells. (F) Plot demonstrating monoclonality of the CD45- cells; kappa or lambda staining is on the x-axis.
Results and discussion
Sixty-one patients with AL, MM, and MGUS had documented monoclonal CD38+ PCs in their BM and were studied. The results of CD52 expression on all PCs and the CD45+ and CD45- PC subsets are summarized in Table 1.
CD52 expression on healthy PCs was examined in 9 morphologically healthy marrows (3 with no hematologic problems, 6 performed for lymphoma staging). The median expression of CD52 on these polyclonal PCs was 37% (mean, 45%; range, 22%-75%). All healthy marrows had more than 20% of PCs expressing CD52.
CD52 expression was first studied on all the PCs (CD38+ cells, irrespective of CD45 expression). Among those with MGUS, MM, and AL, 67%, 52%, and 35% of samples, respectively, were positive for CD52 expression (Table 1). The CD52 expression on the PCs were comparable across the 3 diagnoses (P = .11). CD52 expression on CD38+/CD45- and CD38+/CD45+ PC populations was then determined separately (Figure 1). The median percentage of all CD38+ PCs that were CD45+ was 18%, 8%, and 20%, respectively, for those with MGUS, MM, and AL. The median percentage of CD38+/CD45+ PCs expressing CD52 in these 3 groups was 73%, 88%, and 80%, respectively (Table 1). When the analysis was confined to the CD38+/CD45- population, the CD52 expression was found in 18%, 7%, and 9%, respectively, of the PCs from those with MGUS, MM, and AL (Table 1). The CD45 expression on the plasma cells was usually bright (Figure 1B) with a median fluorescence ratio of 15 (mean, 20; range, 3-94). The CD52 expression was generally confined to the CD45+ population and brighter compared with CD45- plasma cells (Figure 1); with a median fluorescence ratio of 3.5 (mean 4; range 0.1-17).
We also examined the expression of CD52 on several different myeloma cell lines by flow cytometry using similar methods. Variable expression of CD52 was found on the My-5 and ANBL-6 (dim) cell lines. In contrast, no expression could be detected in U266, MM, KP6, KAS6/1, and the JJN3 myeloma cell lines.
In this study we demonstrate the presence of CD52 on malignant PCs and that the expression is more common on the CD45+ PC compartment. We have noted similar expression of CD52 on PCs from healthy bone marrow, highlighting the fact that this expression seen in the PC proliferative disorders is not aberrant. The presence of CD52 on PCs in MM has been reported previously,15,16 and our results are comparable to these reports. In addition, we have demonstrated the presence of CD52 on clonal PCs from other PC disorders, including MGUS and AL.
Two separate populations of PCs have been reported in MM on the basis of CD45 expression.17,18 Unlike the previous reports, we analyzed the CD38+/CD45+ and the CD38+/CD45- cell populations separately for their CD52 expression. We also confirmed the clonal nature of the PC subsets using kappa/lambda staining. The relative importance of these 2 PC fractions from a pathogenetic perspective remains unclear. CD45 is present on the surface of the early B cells and is classically absent from mature PCs. Activated T cells are capable of expressing CD38 antigen and can be detected as a CD38+/CD45+ cell population; however, we restricted our gates to monoclonal CD38+/CD45+ PCs and confirmed the clonal nature of the PC subsets. Other studies have indicated that the CD45+ PC population is more sensitive to interleukin 6 (IL-6)–induced proliferation, and IL-6 is capable of inducing CD45 expression on PCs.19
It has been shown, in flow cytometry–based studies, that the CD45+/CD38+ cells have a higher proliferative rate compared with the CD45- fraction.17 It is likely that the CD45+ PC is important in the pathogenesis and progression of MM. Animal studies also suggest an important role for CD45+ PCs in MM.20 Comparable data from patients with AL are lacking. Given the striking expression of CD52 on these CD45+ PCs, alemtuzumab provides us with a unique opportunity to target the malignant PCs, especially the CD45+ PCs.
We conclude that, given these findings, alemtuzumab might have therapeutic potential in MM and AL and needs further evaluation.
Prepublished online as Blood First Edition Paper, April 24, 2003; DOI 10.1182/blood-2002-12-3784.
Supported in part by grants from the National Cancer Institute (CA62242 and CN65125).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.