Abstract
Immunoglobulin (Ig) and T-cell receptor (TCR) gene rearrangements provide clonal markers useful for diagnosis and measurement of minimal residual disease (MRD) in acute lymphoblastic leukemia (ALL). We analyzed the sequences of Ig and TCR gene rearrangements obtained at presentation and relapse in 41 children with ALL to study clonal stability, which has important implications for monitoring MRD, during the course of the disease. In 42%, all original Ig and/or TCR sequences were conserved. In 24%, one original sequence was preserved but the other lost, and in 14% the original sequences were conserved with new sequences identified at relapse. In 20% only new sequences were found at relapse. Using primers designed from the novel relapse sequences, the relapse clone could be identified as subdominant clones in the diagnostic sample in 8 of 14 patients. Alteration of these clonal gene rearrangements is a common feature in childhood ALL. MRD detection should include multiple gene targets to minimize false-negative samples or include also multicolor flow cytometry. In some cases the leukemic progenitor cell might arise earlier in lineage before DHJH recombination but retain the capacity to further differentiate into cells capable of altering the pattern of Ig and/or TCR rearrangements. (Blood. 2003;102:4520-4526)
Introduction
Advances in cytogenetic and immunophenotypic characterization have greatly improved the diagnostic accuracy and risk assignment of childhood acute lymphoblastic leukemia (ALL)1 resulting in cure rates approaching 80%,2,3 however relapse still occurs in about 20% of children. Monitoring of minimal residual disease (MRD) has proved useful for predicting which children will relapse.4-10 Most studies of MRD detection in pediatric ALL have used polymerase chain reaction (PCR)-based techniques.4,8,10,11 Immunoglobulin heavy chain (IgH) and T-cell receptor (TCR) gene rearrangements appear to be excellent patient-specific PCR targets for MRD detection, but, of concern, these rearrangements may be unstable due to clonal evolution during the course of the disease.12-17
Rearranged Ig and TCR genes have been used as molecular markers for characterization of clonality of lymphoid cell population.11,18,19 Generally, it is assumed that the relapses result from outgrowth of residual leukemic cells that persist in patients below the limits of detection of standard techniques.19,20 It has also been postulated that the “original” leukemia may in some cases be cured, but a “new,” therapy-related secondary leukemia can develop.21 Discrimination between those cases that relapse because of the re-emergence of the initial ALL and the occurrence of secondary ALL might be clinically important to guide subsequent therapy. A number of studies using Southern blotting or PCR amplification have previously demonstrated diverse patterns of the antigen receptor gene rearrangements in a larger proportion (10%-40%) of relapsed childhood ALL.22-24 Sequence analysis has provided some insight into the molecular mechanisms that account for altered rearrangement patterns, including ongoing rearrangements (eg, VH to DHJH joining, VH-VH replacement, and “open-and-shut” mechanism), disappearance of initial clone/subclones, and generation of new clone/subclones.13-15,24-26 TCR and Ig kappa delete element (Ig-κde) rearrangements have been reported to represent more stable molecular targets for MRD detection in comparison with the targets of IgH rearrangements, although only a subset of children with B-lineage ALL have detectable rearrangement at these loci.27,28
We prospectively attempted to sequence the antigen receptor gene rearrangements found at presentation in 491 children with ALL treated sequentially in a single protocol. We analyzed the clonal relationship of these rearrangements by comparing sequences obtained at the time of diagnosis and at the time of relapse in 41 of these children; 4 patterns emerged. In 17 children (41.5%) there was preservation of all original Ig and/or TCR sequences. In 10 children (24.4%) one original sequence was preserved but the other was lost. In 6 children (14.6%) original sequences were conserved although new sequences were identified at relapse. In 8 children (19.5%) only new sequences were found at relapse so that these children lacked a stable marker for MRD analysis. In the 14 children in whom sequences were found at relapse that were not identified at presentation, it was possible to PCR amplify these clones in the original diagnostic sample using primers designed from the relapse sequence in 8 cases, demonstrating that these were cases that did not represent emergence of a new leukemic clone. Real-time PCR quantification in the diagnostic tumor samples demonstrated frequencies of the minor clone ranging from 0.02% to 3.8%. The high frequency of ongoing antigen receptor rearrangements has implications not only for detection of MRD, but also for our understanding of the pathophysiology of ALL.
Patients, materials, and methods
Patients and samples
Bone marrow (BM) and/or peripheral blood (PB) samples were obtained at presentation and sequentially until relapse from children with ALL enrolled consecutively in Dana-Farber Cancer Institute (DFCI)/ALL Consortium Protocol 95-01. Institutional review board approval and informed consent according to the Declaration of Helsinki were obtained for treatment and for procurement of the samples in all cases.
DNA preparation
Mononuclear cells were isolated by Ficoll gradient centrifugation (Pharmacia, Uppsala, Sweden) and lysed, and DNA was extracted and purified according to the manufacturer's instructions using the NucleoSpin kit (BD Biosciences, Palo Alto, CA)
PCR analysis of Ig/TCR gene rearrangements
Sequences of all primers used in this study have been described previously.26,27,29-31 PCR for the detection of TCRγ and TCRδ gene rearrangement was performed as previously described.26,30 VH family-specific primers and a JH consensus primer were used for amplification of IgH genes.29 For patients who had different IgH and/or TCR sequences at relapse, we used 4 Vκ family primers and a kappa delete element (κde) primer to detect Ig-κde rearrangements.31 Primers were purchased from a commercial supplier (Invitrogen, Carlsbad, CA). The PCR conditions and methods used for detection of PCR products have also been previously described.32
Direct sequencing of PCR product
Clonal PCR products were excised and purified using QIAquick gel extraction kits (QIAGEN, Valencia, CA). Purified PCR fragments were sequenced directly by the Dana-Farber/Harvard Cancer Center Core Sequencing Facility (Boston, MA). Sequence reactions were analyzed on an Applied Biosystems 3700 capillary sequencer using Big Dye Terminator Chemistry version 2 (Applied Biosystems, Foster City, CA). The relevant consensus forward and reverse primers were used as sequence primers to obtain the sequence of both strands.
Nucleotide sequences were aligned using DNAstar software (DNASTAR, Madison, WI). TCR gene segments were identified using the Imunogenetics Database (http://imgt.cines.fr, IMGT; European Bioinformatics Institute, Montpellier, France), and all Ig rearrangements by comparison with known human Ig germ-line genes were obtained from the database of the IGBlast or the Blast (http://www.ncbi.nlm.nih.gov/igblast/; National Center for Biotechnology Information, Bethesda, MD) or VBASE (http://www.mrc-cpe.cam.ac.uk/dnaplot.php; Center for Protein Engineering, Cambridge, United Kingdom) directory.
ASO-PCR
For patients who had “new sequences” at relapse, allele-specific oligonucleotides (ASOs) based on sequences identified at relapse were designed to have annealing temperature of approximately 60°C for subsequent PCR amplification. For the IgH gene, an antisense clone-specific primer was designed in complementarity determining region 3 (CDR3) and amplified with VH family-specific primers. For the TCRδ gene, sense primers were designed and amplified with Dδ3 primer. For TCRγ genes, both sense- and antisense-specific primers were designed in CDR2 and CDR3. ASO-PCR amplification was then performed as previously described.33,34 A total of 15 μL ASO-PCR products were first electrophoresed on 3% agarose gels with ethidium bromide and visualized under ultraviolet (UV) light, and 1 μL was then analyzed on DNA500 chips (Agilent Technologies, Palo Alto, CA) to further characterize each band. Negative controls were included in each experiment: one sample containing the reaction mix without DNA and the other containing a DNA pool from peripheral blood mononuclear cells (PBMNCs) of at least 10 healthy donors as polyclonal control. DNAs obtained at presentation or at relapse were used as a positive control for each specific target.
Real-time quantitative PCR
Quantitative real-time PCR was used to determine the IgH minor level of new relapse clones in diagnostic samples. IgH TaqMan probes (Integrated DNA Technologies, Coralville, IA) were designed in framework (FR) 3 region as previously described.35 Based on the sequence, one patient-specific probe was designed (for case 472, 5′ CTGTGTCCTCGGTTTTCAGGC 3′) since this patient's sequence had 3 mismatches in the region of our consensus probe. These probes were synthesized by Integrated DNA Technologies and labeled with 6-carboxy fluorescein (FAM) at the 5′ end and with 6-carboxytetramethyl rhodamine (TAMRA) at the 3′ end. Real-time PCR amplification was carried out using the same ASO-PCR primers and universal PCR mixture (Applied Biosystems).35 Standard curves were prepared by diluting plasmid DNA containing the target gene in polyclonal DNA from healthy donors. Sample copy numbers were estimated from the standard curve. The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was used for normalization of DNA templates. Sequences of the probe and primers and real-time PCR analysis were previously described.35 The mean of triplicates of copy numbers of the target gene was divided by the mean of triplicates of copy numbers of GAPDH to obtain the value reported as target gene/GAPDH.
Statistical analysis
Descriptive statistics have been used to describe patients' characteristics and time to relapse for the 41 patients for whom we have information on clonal evolution. The Fisher exact test and the Wilcoxon rank-sum test were used to compare demographic and clinical characteristics at diagnosis for children with and without information on clonal evolution, and also to compare clinical characteristics at diagnosis and time to relapse between patients who had at least one original sequence conserved and patients who had only new sequences at relapse.36
Results
Between 1996 and 2000, 498 children with ALL were enrolled consecutively in DFCI/ALL Consortium Protocol 95-01. Of these patients, 3 were subsequently deemed to be ineligible, 3 refused further treatment, and 1 withdrew consent. Therefore, 491 patients were included. From the diagnostic samples, 389 IgH sequences and 164 TCR sequences were obtained. As of February 28, 2003, 64 relapses had occurred. No diagnostic sample was obtained from 4 children and the relapse sample was not available for analysis from 11 children. No sequences could be obtained by direct sequencing from 8 cases, so that sequences were available at both presentation and relapse from 41 children. From these 41 cases, 57 sequences (39 IgH and 18 TCR sequences) were obtained at the time of presentation and 55 sequences (42 IgH and 13 TCR sequences) were obtained at the time of relapse. Of the sequences, 33 were preserved between presentation and relapse, 24 found at presentation were lost at relapse, and 22 new were found at relapse. Clinical data for these 41 children are representative of all relapses in the treatment protocol and are shown in Table 1.
Preservation of sequences at presentation and relapse
In 17 (41.5%) of the 41 cases, the original 14 IgH sequences from 12 patients and 5 TCR sequences from 5 patients were fully conserved at relapse. In 2 cases, 2 unrelated IgH sequences were identified. For these 17 cases, the original sequences of antigen receptor gene rearrangements can be followed for analysis of MRD.
Loss of sequences at relapse
In 10 (24.4%) of the 41 cases, one of the original IgH and/or TCR gene rearrangements was conserved at relapse with loss of another rearrangement as shown in Figure 1. In 3 patients, 2 different IgH rearrangements were identified at presentation, and only 1 of these sequences was preserved at relapse (cases 62, 125, and 130). In 5 patients (cases 51, 174, 253, 318, and 332) an IgH target was conserved, but there was loss of a TCR rearrangement target. Case 489 had 2 TCR gene targets at presentation but only 1 was found at relapse. In these cases, MRD monitoring could be achieved successfully only if all of the original targets found at presentation were followed.
Schematic illustration of the clonal pattern of IgH and TCR gene rearrangements in 10 children with ALL in whom one original target was conserved but the other was lost. Germ-line gene segments are presented with more than 98% identity. IgH gene targets are shown in white and TCR gene targets are shown in gray.
Schematic illustration of the clonal pattern of IgH and TCR gene rearrangements in 10 children with ALL in whom one original target was conserved but the other was lost. Germ-line gene segments are presented with more than 98% identity. IgH gene targets are shown in white and TCR gene targets are shown in gray.
Appearance of new sequence at relapse
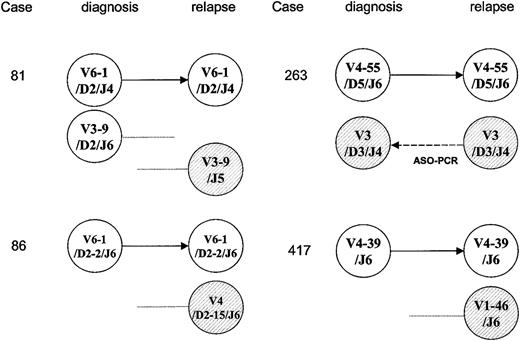
In 4 cases (10%), one original IgH target was conserved but new unrelated IgH sequences were also identified at relapse as shown in Figure 2. In case 81, 2 unrelated IgH rearrangements were present at presentation. One of these rearrangements was conserved at relapse but the other was lost; in addition, a new IgH rearrangement was identified at relapse. This new sequence of the IgH gene used the same VH3-9 germ-line gene segment as the original clone, but no DH gene segment was discernible and N sequences (12 base pair [bp]) and JH5 gene segments were used, differing from the original clone. These results indicated that although new clones were identified at relapse, at least one of the original clones was conserved. Again, in these cases MRD monitoring could be achieved successfully only if all of the original targets found at presentation were followed.
Schematic illustration of the clonality pattern of the antigen receptor gene rearrangements, demonstrating that one original target was conserved but new sequences were identified in 4 children with relapsed ALL. Germ-line gene segments are presented with more than 98% identity. IgH gene targets are shown in white and new sequences are represented as hatched circles. In case 263, the “new” relapse IgH target could be detected by ASO-PCR using a combination of VH3 family-specific primer and clone-specific primer based on the sequence at relapse.
Schematic illustration of the clonality pattern of the antigen receptor gene rearrangements, demonstrating that one original target was conserved but new sequences were identified in 4 children with relapsed ALL. Germ-line gene segments are presented with more than 98% identity. IgH gene targets are shown in white and new sequences are represented as hatched circles. In case 263, the “new” relapse IgH target could be detected by ASO-PCR using a combination of VH3 family-specific primer and clone-specific primer based on the sequence at relapse.
Taken together, these data demonstrate that in 76% of these children MRD could be followed using IgH/TCR gene rearrangements as molecular targets.
Relapse with new sequences only
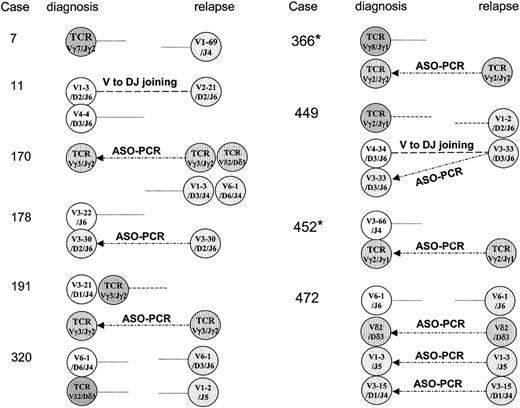
In 10 (24%) of the 41 cases, only new sequences of IgH and/or TCR gene rearrangements were identified and all original sequences were lost at relapse as shown in Figure 3.
Schematic illustration of alteration of clonal pattern of Ig and TCR gene rearrangements between presentation and relapse in 10 children with ALL. Germ-line gene segments are presented with more than 98% identity. Original IgH targets are shown in white; new IgH targets are shown as hatched circles. TCR targets identified at diagnosis are shown in gray, and new TCR targets are shown as dotted circles. Ongoing rearrangements (VH-VH replacement and VH to DJH joining) were found in 2 cases (11 and 449). It is possible to amplify the clones identified at relapse using ASO-PCR approaches on diagnostic samples in 7 patients with ALL, as shown here with arrows for cases 170, 178, 191, 366, 449, 452, and 472. In 2 cases identified with an asterisk (*), Ig-κde rearrangement was later identified at presentation and preserved at relapse.
Schematic illustration of alteration of clonal pattern of Ig and TCR gene rearrangements between presentation and relapse in 10 children with ALL. Germ-line gene segments are presented with more than 98% identity. Original IgH targets are shown in white; new IgH targets are shown as hatched circles. TCR targets identified at diagnosis are shown in gray, and new TCR targets are shown as dotted circles. Ongoing rearrangements (VH-VH replacement and VH to DJH joining) were found in 2 cases (11 and 449). It is possible to amplify the clones identified at relapse using ASO-PCR approaches on diagnostic samples in 7 patients with ALL, as shown here with arrows for cases 170, 178, 191, 366, 449, 452, and 472. In 2 cases identified with an asterisk (*), Ig-κde rearrangement was later identified at presentation and preserved at relapse.
In these 10 cases, 13 sequences found at presentation were lost at relapse and 19 new sequences were identified at the time of relapse. The 2 cases that involved ongoing IgH rearrangement mechanisms and the sequences at presentation and relapse showing VH-VH replacement (case 11) and VH to DHJH joining (case 449) are shown in Table 2. In case 11, the IgH rearrangement used VH 1-2, DH 2-2, and JH 6 gene segments at presentation. At relapse, the identical DJH region was found but VH 2-21 replaced the initial VH1-2 segment and 7 additional N sequences were introduced in the VH-D junction, indicating the VH-VH replacement mechanism involved in this case. In case 449, the diagnostic sequence of IgH rearrangement used VH 4-34, DH 3-9, and JH 6 gene segments. At relapse, the VH 3-33 gene segment attached to a common initial DJH gene complex, suggesting that VH to DJH joining was involved in this case.
To determine whether addition of Ig-κde would have been a useful addition as a clonal marker that could be used as a stable molecular target in those 10 patients shown in Figure 3, PCR amplification of Ig-κde rearrangements was performed using 4 sets of primers.31 There were 5 patients who demonstrated what appeared to be a clonal PCR product, but sequences were identified in only 3 of these cases. In case 366, sequence VκIII and κde segments were identified at diagnosis and conserved at relapse. In case 452, identical sequences using Vκ3 (A29) and JL7 segments were found both at presentation and at relapse. A sequence using Vκ2 (A18) and κde segments was identified only at relapse and was not found at presentation in case 11. The results indicate that although in 2 patients Ig-κde can alternatively be applied for MRD detection, the variety of pattern and low frequency of detection can still be limited. Therefore even with the use of IgH, TCR, and Ig-κde markers, we identified 8 children (19.5%) in whom only new sequences were identified at relapse.
There was a trend toward a shorter time to relapse (P = .07 by Wilcoxon rank-sum test) in patients with clonal evolution compared with children in whom at least one of the original sequences was preserved. The median time to relapse for the 8 children in whom only new sequences were identified at relapse was 10.5 (range, 6.0 to 54.5) months, and the median time to relapse for the 33 children who had at least one original sequence preserved at relapse was 29.9 (range, 7.4 to 68.5) months.
Identification of relapse clone as a minor clone present at the time of diagnosis
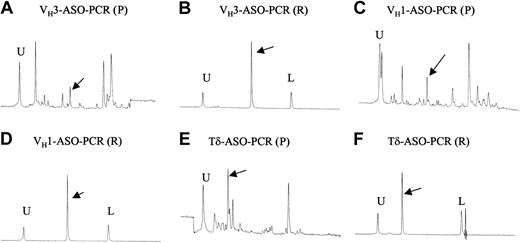
To determine whether the clones found at relapse represented emergence of a new leukemic clone or were due to emergence of a minor clone present below the level of detection for sequencing at the time of presentation, we performed PCR analysis of diagnostic samples using ASO primers designed upon the novel sequences obtained at relapse. In 8 of the 14 children (case 263, Figure 2; and cases 170, 178, 191, 366, 449, 452, and 472, Figure 3) in whom new rearrangements were found at relapse we could detect the presence of that clone in the diagnostic sample. Examples of results obtained by ASO-PCR are shown in Figure 4, demonstrating that identification of this minor clone at presentation can be difficult, even when using ASO primers from the relapse sequence.
ASO-PCR analysis using DNA 500 chip in case 472. U indicates upper marker; L, lower marker; P, presentation sample; and R, relapse sample. (A) IgH (VH3) gene rearrangement from diagnostic sample using a combination of VH3 family-specific primer and clone-specific primer based on sequence identified at relapse. The peaks indicated by an arrow show the PCR product. (B) ASO-PCR result of IgH-VH3 gene rearrangement from relapse tumor sample as a positive control (280 bp). Distinctive sharp peak of the ASO-PCR product is indicated by arrows. The PCR primers were the same as in panel A. (C) The IgH (VH1) gene rearrangement from the diagnostic sample using combination of VH1 family-specific primer and clone-specific primer based on sequence identified at relapse. The peak indicated by an arrow shows the PCR product. (D) ASO-PCR result of IgH-VH1 gene rearrangement from relapse tumor sample as a positive control (271 bp). Distinctive sharp peak of the ASO-PCR product is indicated by an arrow. The PCR primers were the same as in panel C. (E) ASO-TCRδ gene rearrangement from diagnostic sample using combination of relapse clone-specific primer and Dδ3 primer. The peak indicated by an arrow shows the PCR product. (F) ASO-PCR result of TCRδ gene rearrangement from relapse tumor sample as a positive control (100 bp). The distinctive sharp peak of the ASO-PCR product is indicated by an arrow. The PCR primers were the same as in panel E.
ASO-PCR analysis using DNA 500 chip in case 472. U indicates upper marker; L, lower marker; P, presentation sample; and R, relapse sample. (A) IgH (VH3) gene rearrangement from diagnostic sample using a combination of VH3 family-specific primer and clone-specific primer based on sequence identified at relapse. The peaks indicated by an arrow show the PCR product. (B) ASO-PCR result of IgH-VH3 gene rearrangement from relapse tumor sample as a positive control (280 bp). Distinctive sharp peak of the ASO-PCR product is indicated by arrows. The PCR primers were the same as in panel A. (C) The IgH (VH1) gene rearrangement from the diagnostic sample using combination of VH1 family-specific primer and clone-specific primer based on sequence identified at relapse. The peak indicated by an arrow shows the PCR product. (D) ASO-PCR result of IgH-VH1 gene rearrangement from relapse tumor sample as a positive control (271 bp). Distinctive sharp peak of the ASO-PCR product is indicated by an arrow. The PCR primers were the same as in panel C. (E) ASO-TCRδ gene rearrangement from diagnostic sample using combination of relapse clone-specific primer and Dδ3 primer. The peak indicated by an arrow shows the PCR product. (F) ASO-PCR result of TCRδ gene rearrangement from relapse tumor sample as a positive control (100 bp). The distinctive sharp peak of the ASO-PCR product is indicated by an arrow. The PCR primers were the same as in panel E.
Quantitative real-time PCR was performed to analyze the level of expression of the different clones in these cases. The minor clones that were not identified by sequencing at the time of initial presentation accounted for 0.02% to 3.8% of the diagnostic samples demonstrating that the subdominant clones were found at presentation at low frequency. It is unlikely that at these low levels of expression that these sequences could have been obtained at presentation even by cloning and sequencing, unless a very large number of clones were sequenced at presentation. Table 3 shows results of quantitative real-time PCR analysis of the clones identified at presentation and relapse for case 472. In this case, 2 novel sequences were found at relapse. Quantitative PCR analysis,35 using primers from the relapse sequence, demonstrated that these clones accounted for 0.9% and 0.02% of the diagnostic sample.
Quantitative PCR analysis was also used to study the dynamics of the appearance and disappearance of these clones during the course of the disease. Representative results are shown in Figure 5 for patient 449. In this case the sequence identified at presentation was no longer detectable by day 30, whereas the clone found at relapse remained detectable until day 180. However, even the relapse clone was not detectable at all time points and re-emerged at detectable levels at day 600, and further increased at day 690, while clinical relapse occurred on day 760.
Real-time quantitative PCR analysis in case 449. The x-axis presents the time during the course of the disease. The patient relapsed at day 760. The y-axis shows percent MRD level at different time points. Circles indicate the IgH-VH4 original clone, and this initial clone was undetectable from day 30 to relapse. The IgH-VH3 relapse clone is shown by triangles. This clone existed as a minor clone at presentation, persisted at detectable levels until day 180, and was detectable from day 600 to relapse. The limit of detection of the assay is represented by a dotted line.
Real-time quantitative PCR analysis in case 449. The x-axis presents the time during the course of the disease. The patient relapsed at day 760. The y-axis shows percent MRD level at different time points. Circles indicate the IgH-VH4 original clone, and this initial clone was undetectable from day 30 to relapse. The IgH-VH3 relapse clone is shown by triangles. This clone existed as a minor clone at presentation, persisted at detectable levels until day 180, and was detectable from day 600 to relapse. The limit of detection of the assay is represented by a dotted line.
Discussion
In almost half of the relapsed patients, there was full conservation of the original clone(s), indicating that MRD detection could be successfully achieved for these patients using a single marker. Despite the heterogeneity of the antigen receptor gene rearrangement patterns that emerged, MRD could be followed to potentially predict relapse in 80% of these pediatric ALL cases if all original Ig/TCR gene targets were followed. Previous studies have demonstrated that even late relapses are due to re-emergence of the original clone.37,38 The reason for the late regrowth of the original leukemic clone is unknown, but may be due to diminished immune surveillance for maintaining MRD or acquisition of new genetic mutations within a previously dormant leukemic cell.37,38
In 20% of our cases relapse occurred with novel sequences that were not found at the time of original diagnosis. This suggests that these cases lacked stable antigen receptor gene targets and were unsuitable for prospective MRD detection. This frequency is similar to that described in other studies using Southern blot analysis or PCR analysis without detailed sequence analysis.23,39 However, comparative sequencing of the Ig and TCR rearrangements allows us to postulate that different molecular mechanisms account for the altered patterns of the antigen receptor gene rearrangement at relapse in ALL that is due to ongoing antigen receptor re-arrangements during the course of the disease.13-15,17,24,25,40,41 Ongoing IgH rearrangement mechanisms (VH-VH replacement or VH to DHJH joining rearrangements) have been reported,13,14,24,25 but these accounted for IgH modification in only 2 of our cases. The etiology of this phenomenon remains unclear. It might partly be explained by a low frequency of recombination activating gene (RAG)-mediated hybrid VH gene formation.42 Another possibility is that the clonal evolution is driven by genomic instability affecting the Ig and TCR gene loci.43
There are cases in which the new relapse sequences cannot be explained by ongoing editing of the IgH or TCR loci.14,25 This has been suggested to represent development of a secondary new leukemia.12 This would be clinically important to detect since treatment and outcome for relapse versus secondary leukemias may differ. To examine whether the relapse clone represented a new event, or whether this was present as a subdominant clone at the time of initial diagnosis, we used relapse clone-specific primers for PCR amplification and demonstrated the emergence of a minor clone present at the time of presentation in 8 of 14 children. Quantitative real-time PCR analysis demonstrated that those IgH minor clones existed with a range from 0.02% to 3.8% in the diagnostic samples. Demonstration of the dynamics of elimination of these clones, as shown in the example in Figure 5, shows the differential sensitivity to chemotherapy of these clones, giving rise to a selective growth advantage of the original subdominant clone, perhaps by subsequent acquisition of further mutations.13,41
There were 8 cases that relapsed with new clones that could not be detected at the time of presentation, even by nested PCR using primers derived from the relapse sequences. These cases might represent emergence of subdominant clones that were present below the limit of detection of ASO-PCR at the time of presentation. Another intriguing hypothesis is that the relapses with completely novel clones represent cases in which the leukemic progenitor cells arise at a stage of differentiation before DHJH joining. Under these circumstances, the leukemic cell must retain the potential for some degree of subsequent differentiation to generate novel antigen receptor rearrangements. Although we have no direct evidence to support this hypothesis, it is of interest to note that we observed a trend (P = .07) toward a shorter time to progression for children having completely different antigen receptor gene sequences at relapse, further suggesting that clonal diversity represents a mechanism of disease progression.44 Further studies with a larger number of cases of relapse will be required to validate this observation.
The stability of IgH gene targets (17/31, 55%) found here was similar to TCR gene targets (7/13, 54%) in contrast to previous reports using Southern blot and PCR analysis.28 The use of a small set of primers will detect only a proportion of the potential TCR gene rearrangements and this might result in missing additional stable TCR targets. In addition, previous studies using Southern blot and PCR analysis have suggested that Ig-κde may be the most stable molecular target for MRD detection in ALL.28 Although we did not study this marker prospectively, the additional use of this marker reduced the frequency of cases in which there was the detection of entirely new clones at relapse from 24% to only 19.5% of relapses. Although Ig κde has been previously shown to be the most stable marker for PCR detection of MRD, even this marker is conserved in only 90% of cases.28
In summary, we demonstrate that MRD monitoring using PCR analysis can be successfully achieved in 80% of cases of childhood ALL if all initial molecular targets of the antigen receptor gene rearrangements are used. New rearrangement sequences were found at relapse in 20% of children, indicating that these patients lacked stable antigen receptor gene molecular targets. This finding has obvious implications in a situation when routine MRD screenings are included as a stratifying parameter in the therapy protocols. One important question to answer is if this high fraction of cases unsuitable for PCR-based MRD detection can be tolerated in the clinical setting. This study also demonstrated that subclone(s) and/or minor clone(s) selection is a source of relapse in patients with clonal heterogeneity, indicating that relapse can occur from relatively resistant subclones. The finding that 20% of relapse could not be predicted using molecular assessment strongly suggests that complementary studies, such as flow cytometric analysis, will be required to predict all relapses. A number of groups assess MRD in ALL using flow cytometric-based rather than molecular-based analyses,5,45-48 and studies from St Jude Children's Cancer Research Hospital currently use both flow cytometric- and PCR-based analyses.49,50 Ongoing studies will be required to determine whether the flow cytometric pattern of the relapses occurring with novel sequences would allow prospective prediction of relapse or whether there would also be a change in the immunophenotype of such cases.
Prepublished online as Blood First Edition Paper, August 28, 2003; DOI 10.1182/blood-2003-05-1455.
Supported by P01 CA68484 from the National Cancer Institute, Bethesda, MD.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank all members of the DFCI ALL consortium for providing samples for this study: Dana-Farber Cancer Institute/Boston Children's Hospital, Boston, MA; University of Rochester Medical Center, NY; McMaster University Medical Center, Hamilton, ON, Canada; San Jorge Children's Hospital, Santurce, Puerto Rico; Maine Children's Cancer Program, Portland; Hospital Saint Justine, Montreal, QC, Canada; Le Centre Hospitalier de L'Université Laval, QC, Canada; Oschner Clinic, New Orleans, LA; and Mount Sinai Medical Center, New York, NY.