Abstract
Human leukocyte elastase (HLE) interacts with HIV-1 glycoprotein (gp)41, suggesting a nonenzymatic receptor function for HLE in the context of HIV-1. HLE is found localized to the cell surface, but not granules in HIV permissive clones, and to granules, but not the cell surface of HIV nonpermissive clones. Inducing cell-surface HLE expression on HLE null, HIV nonpermissive clones permits HIV infectivity. HIV binding and infectivity diminish in proportion to HLE RNA subtraction. HIV binding and infectivity show dose dependence for the natural HLE ligand α1 proteinase inhibitor (α1antitrypsin, α1PI). Chemokines prevent, whereas α1PI promotes, copatching of HLE with the canonical HIV receptors. Recent demonstration that decreased viral RNA is significantly correlated with decreased circulating α1PI in HIV seropositive individuals is consistent with a model in which HLE and α1PI can serve as HIV coreceptor and cofactor, respectively, and potentially participate in the pathophysiology of HIV disease progression. (Blood. 2003;102:4479-4486)
Introduction
HIV-1 entry and fusion are thought to involve an initial interaction between the envelope glycoprotein, gp120, and cell-surface CD4. This interaction exposes a site within gp120 that then interacts with a coreceptor (ie, CCR5 or CXCR4), inducing a conformational change within the gp41 portion of the viral envelope; this set of events results in insertion of the fusion domain of gp41 into the cell membrane. Cell-surface expression of specific chemokine receptors and CD4 is necessary, but not sufficient, to confer HIV-1 permissivity.1,2 Cellular resistance to HIV-1 infectivity can be due to fusion/entry failure, suggesting this differentiation-associated restriction is due to a positive factor or negative factor.
Comparison of HIV-1 nonpermissive and permissive U937 subclones revealed relatively equivalent cell-surface CD4 and CXCR4 and a lack of CCR5.1 A notable difference was that nonpermissive, but not permissive, subclones expressed detectable granule-associated proteinases, cathepsin G (CatG) and human leukocyte elastase (HLE).3 These same proteinases are known to be cell-surface-associated in certain situations and to bind HIV-1 envelope proteins,4,5 but in association with the lipid bilayer, enzymatic activity and antigen detection are absent or compromised.6 This suggests that plasma membrane-associated proteinases may exhibit nonenzymatic receptor functions and that location, rather than gene expression, might impact HIV-1 permissivity.
Traditionally, the proteinase activity of HLE has been characterized in aqueous environments, and cell-surface lipids are known to negatively influence its catalytic activity6 further supporting a nonenzymatic function for plasma membrane HLE.7 In fact, the primary actions of cell-surface HLE and CatG involve adhesion, chemotaxis, and stem-cell mobilization.8-10 Granule-associated HLE rapidly translocates to the cell surface in response to many agonists including the bacterial endotoxin lipopolysaccharide (LPS),11 suggesting these activation signals rapidly mobilize HLE to the cell surface in the absence of protein synthesis. The precise domains in HLE that allow its association with the plasma membrane are not completely known; however, the α1antitrypsin (α1PI) domain that initiates chemotaxis has been identified as a hydrophobic pentapeptide concealed within its C-terminal region.9 The corresponding hydrophobic pentapeptides found in various other serine proteinase inhibitors contain a pair of phenylalanine residues that share the motif FXFXX or FXXFX, where X represents the hydrophobic amino acids V, L, I, or M.9 The identification of a pentapeptide having a similar motif within the fusion domain of HIV-1 gp41 (FLGFL) and other human viruses led to the discovery of a ligand-receptor interaction between gp41 and the α1PI receptor HLE.5
In HIV-1-seropositive patients, viral load is correlated with circulating levels of α1PI.12 In this study, 100% of patients in the asymptomatic category of disease manifested deficient levels of α1PI. Further, in vitro infectivity was shown to be highly correlated with cell-surface HLE (r2 = 0.81, P = .01).13 Individuals with the inherited form of α1PI deficiency, especially males, are notably susceptible to respiratory infections.14 Thus, α1PI deficiency acquired during HIV-1 infection may be a mechanism responsible in part for the attendant emphysema, pulmonary pathology, and onset of opportunistic infections as HIV-1 disease progresses. The discovery that defective chemokine expression corresponded to HIV-1 resistance15 precipitated the identification of chemokine receptors during HIV-1 entry.16 The relationship between α1PI and HIV-1 disease suggested the potential participation of an additional coreceptor cognate for α1PI. The physical relationship between proteinases, proteinase inhibitors, and chemokine receptors has not previously been examined.
Materials and methods
Cells and reagents
U937 subclones were generously provided by the Laboratory of Immunoregulation, National Institute for Allergy and Infectious Diseases, National Institutes of Health (NIH), and maintained using RPMI-1640 containing 10% fetal bovine serum (FBS) determined to have endotoxin levels less than 0.3 endotoxin units (EU)/mL. Cells were harvested during exponential growth and were more than 95% viable as determined by trypan blue exclusion. Endotoxin stimulation was performed by incubation with Escherichia coli strain O26:B6 LPS (Sigma Chemical, St Louis, MO) at a concentration of 5 μg/μL/2 × 105 cells in the presence or absence of 0.5 μg recombinant LPS-binding proteins (LBPs; Xoma, Berkeley, CA) in AIM V (R) serum-free media (Gibco Invitrogen, Carlsbad, CA) for 60 minutes at 37°C in a humidified chamber containing 5% CO2. Cells in AIM V (R) were examined by flow cytometry to exhibit CD4 and CXCR4 levels not different from cells in RPMI-1640, 10% FBS (data not shown). The functionally active concentrations of 2 preparations of α1PI (cat. no. A6150, lot no. 82H9323 and cat. no. A9024, lot no. 115H9320; Sigma) were determined using active-site titrated porcine pancreatic elastase, type 1 (PPE; EC 3.4.21.36; Sigma) as previously described.17
Immunocytochemical transmission electron microscopy (TEM)
TEM was performed by Wallace Ambrose, Microscopy Laboratory, Dental Research Center, University of North Carolina-Chapel Hill. Grids were stained using rabbit anti-HLE (Biodesign, Kennebunkport, ME) or anti-CatG (Biodesign) at concentrations of 1 mg/mL in 0.01 M phosphate, 0.15 M NaCl, pH 7.2 (PBS). Antibody binding was detected using Protein A polygold (0.23 μg Protein A/mL; Sigma) and photographed using a Philips CM/12 TEM/STEM transmission electron microscope (FEI, Hillsboro, OR). Positive-control grids were stained with rabbit anti-nuclear factor kappa-B (NFkB) p50 (Biodesign) and negative-control grids were stained without primary antibody.
Receptor/ligand binding analysis
Preparation of solubilized clone 10 membrane extract, the synthetic pentapeptide FLGFL representative of the HIV-1 fusion domain, and receptor/ligand binding analysis was performed as previously described.5 Binding by 125I-U937 clone 10-solubilized membranes to FLGFL was performed in hypertonic phosphate buffer, pH 7.4, 0.5 M NaCl, 0.05% tween-20 (HPBS) containing 2.5% lipid-free bovine serum albumin (BSA) to limit nonspecific ionic binding. The bound and unbound (U) fractions were counted. Specific binding (B) was determined as the difference between binding to coated and uncoated wells.
Immunofluorescent receptor staining and flow cytometric analysis
Three-parameter flow cytometric analysis was performed on a FACScan flow cytometer (Becton Dickinson, San Jose, CA). Cells were washed twice in PBS and resuspended at 1 × 106 cells/mL. Monoclonal antibodies included anti-CXCR4-phycoerythrin (PE) (PharMingen, San Diego, CA), anti-CD4-peridinin chlorophyll-alpha protein (PerCP) (clone SK3; Becton Dickinson), and isotype-matched controls. To detect cell-surface HLE, cells were incubated with polyclonal sheep anti-HLE-fluorescein isothiocyanate (FITC) (Biodesign, Kennebunkport, ME) or negative-control sheep immunoglobulin G (IgG)-FITC (Cappel, Aurora, OH). Alternatively, cell-surface HLE was detected using polyclonal rabbit anti-HLE (Biodesign) or negative-control rabbit IgG (Chemicon, Temecula, CA), and binding was detected using biotinylated antirabbit IgG (Amersham, Amersham, United Kingdom) followed by streptavidin-FITC (PharMingen). To investigate the influence of alternate receptor ligation, whole blood was stained by incubating 100 μL cells with 10 μL of each antibody stepwise for 15 minutes each at 20°C in the order anti-HLE-FITC, anti-CXCR4-PE, anti-CD4-PerCP, or in the order anti-CD4-PerCP, anti-HLE-FITC, anti-CXCR4-PE. Cells were washed in 2 mL PBS between each staining step. After the final wash, the cell pellet was resuspended and fixed in 0.5 mL 1% paraformaldehyde in PBS. For each analysis, at least 10 000 to 30 000 events were acquired. List mode multiparameter data files were analyzed using CellQuest Software (Becton Dickinson). Median fluorescence intensity (MFI) for each coreceptor was detected, and relative fluorescence intensity (RFI) was determined as MFI of coreceptor minus MFI of isotype control.
Receptor patching and colocalization
U937 cells (1 × 106 cells/mL) were incubated with various receptor ligands for 60 minutes in humidified 5% CO2 at 37°C. Ligands included polyclonal rabbit anti-HLE (1.4 mg/mL; Biodesign), monoclonal murine anti-HLE (65 μg/mL; Dako, Carpinteria, CA), 1.25 nM α1PI (A9024), 1.25 nM stromal-derived factor 1α (SDF-1α; Peprotech, Rocky Hill, NJ), 10 μM FLGFL solubilized in 9.5% ethanol vehicle (0.95% ethanol final concentration), vehicle only, or buffer only (Hanks balanced salt solution [HBSS]). To examine receptor localization, cells were incubated at 37°C, 5% CO2, with each ligand to determine optimal ligand concentrations, and cells were observed at 60-minute intervals for 24 hours to determine optimal detection time (data not shown). To demonstrate colocalization induced by receptor ligation, 1 × 106 cells/mL HBSS were incubated at 37°C, 5% CO2, first with 1 ligand at its optimal concentration for 15 minutes and subsequently with a second ligand at its optimal concentration for 60 minutes in sterile polypropylene microfuge tubes with caps loosened.
After washing 3 times in HBSS, aliquots (30 μL) were applied to the sample chambers of a cytospin apparatus (ThermoShandon, Pittsburgh, PA), and slides were centrifuged at 850 rpm for 3 minutes. Slides were fixed by application of 50 μL 10% formalin in PBS to the sample chambers of the cytospin apparatus followed by an additional centrifugation at 850 rpm for 5 minutes. Slides were sequentially incubated for 90 minutes at 20°C with a monoclonal antibody having specificity for human α1PI (5 μg/mL; Zymed Laboratories, South San Francisco, CA), biotinylated sheep antimouse immunoglobulin (100 μg/mL; Amersham, Arlington Heights, IL), FITC-conjugated streptavidin (80 μg/mL; Jackson Laboratories, West Grove, PA), rabbit antihuman SDF-1α (5 μg/mL; Peprotech), biotinylated donkey antirabbit immunoglobulin (100 μg/mL; Amersham, Arlington Heights, IL), and Texas Red-conjugated streptavidin (80 μg/mL; Jackson Laboratories). Slides were washed once between each incubation.
To demonstrate receptor copatching induced by HIV, an inoculum of 1 × 106 infectious units of HIV-1 T-tropic strain NL4-3 (HIVNL4-3, a generous contribution from Dr W. Resch, Lineberger Comprehensive Cancer Center, University of North Carolina, Chapel Hill) was incubated with 1.5 × 107 cells in 1 mL RPMI-1640 containing 10% FBS at 37°C in humidified 5% CO2. Aliquots of 250 μL were removed at 15-minute intervals for 90 minutes. Cells were washed 3 times in fresh medium and fixed using 1% paraformaldehyde. Fixed cells were sequentially incubated for 60 minutes at 20°C with rabbit polyclonal anti-HLE (1 mg/mL; Biodesign), biotinylated donkey antirabbit immunoglobulin (100 μg/mL; Amersham, Arlington Heights, IL), FITC-conjugated streptavidin (50 μg/mL; Becton Dickinson), monoclonal anti-gp120 (approximate epitope V3 loop, 10 μg/mL; Research Diagnostics, Flanders, NJ), biotinylated sheep antimouse immunoglobulin (100 μg/mL; Amersham), gold-conjugated streptavidin (1/50; Nanoprobes, Yaphank, NY), and either PerCP-conjugated monoclonal anti-CD4 (clone SK3; Becton Dickinson) or Per-CP-conjugated CXCR4 (Becton Dickinson). Bound anti-gp120 was detected using silver enhancement as recommended by the manufacturer (LI Silver; Nanoprobes). Antibody controls were purified rabbit IgG (1 mg/mL; Chemicon) and murine IgG (10 μg/mL; Jackson Laboratories). Cells were washed once between each staining step.
Slides were examined by epi-illumination UV microscopy on a Zeiss Axioskop (Thornwood, NY) equipped with filters for excitation/emmission wavelengths (nm) 546/590 and 450/520 to 490/520. Optical sections of intact cells were examined by confocal laser scanning microscopy (CLSM) performed by Dr C. R. Bagnell Jr, Confocal Microscopy Facility, Department of Pathology and Laboratory Medicine, UNC-Chapel Hill. Digital images were artificially colorized using Adobe Photoshop software (San Jose, CA).
Antisense oligomers
Morpholino antisense oligomers (Gene-Tools, Corvallis, OR) were 22- or 25-mers, targeted respectively to the HLE start codon (5′-CCGAGGGTCATGGTGGGGCTGGG) or to the 3′ splice site of HLE exon 4 (5′-CGACCCGTTGAGCTGTGGCGGTGGG). Anti-β-globin oligonucleotides were used as negative controls as described previously.18 Cellular delivery was achieved by passing 2.5 × 105 cells suspended in 250 μL culture media containing 10 μM individual oligonucleotides through a syringe fitted with a 25-gauge needle.19 Oligonucleotide-loaded cells were incubated for 60 minutes at 37°C in wells of a tissue-culture plate followed by addition of 250 μL fresh media. Cells were maintained in culture for 36 hours, 48 hours, or 66 hours, monitored for viability, and resuspended at 5 × 106 cells/200 μL for flow cytometry or HIV-1 infectivity assays. Optimal mRNA inhibition was determined to occur at 48 hours. All treatments yielded more than 99% viability.
HIV-1 infectivity
To demonstrate the influence of α1PI on infectivity, clone 10 cells were resuspended at 2 × 105 cells/mL in AIM V(R) serum-free medium (Gibco) containing varying concentrations of α1PI (Sigma; cat. no. A9024) either in 96-well tissue-culture plates (Costar, Cambridge, MA) or in microfuge tubes (Sarstedt, Newton, NC). Cells infected in tissue-culture plates were directly washed free of virus with PBS without transferring from wells and resuspended in AIM V(R) serum-free medium containing the original concentration of α1PI present during infection. Cells infected in microfuge tubes were washed free of virus with PBS, resuspended at 1 × 106 cells/mL in AIM V(R) serum-free medium containing the original concentration of α1PI present during infection, and 0.2 mL (2 × 105 cells) were transferred to wells in tissue-culture plates. To demonstrate infectivity of nonpermissive clones, LPS-stimulated clone 17 cells in the presence or absence of LBP were resuspended in serum-free medium with or without 30 μM α1PI. HIVNL4-3 viral stocks were propagated as previously described,1 and incubated with cells at a multiplicity of infection (MOI) of approximately 1 × 10-2, 1 × 10-3, and 1 × 10-4 for 3.5 hours at 37°C in a humidified chamber containing 5% CO2. Culture supernatants were collected and replaced with the corresponding medium every other day for 2 weeks. Culture supernatants were stored at -20°C for analysis of reverse transcriptase (RT) activity. RT activity was detected as previously described.20
To demonstrate the influence of antisense oligonucleotides, antisense-treated clone 10 cells were enumerated and resuspended at 5 × 105 cells/200 μL/well in 96-well tissue-culture plates in RPMI-1640 containing 10% FBS. HIVNL4-3 (4.9 × 105 IU/mL MAGI cells) was incubated at a concentration of 1 × 105 IU/well for 2 hours at 37°C in a humidified chamber containing 5% CO2. Cells were washed free of virus and resuspended in RPMI-1640 containing 10% FBS. Cells were harvested at 48 hours and analyzed by flow cytometry for cell-surface expression of HLE and by polymerase chain reaction (PCR) for the presence of HIV-1 minus-strand, strong-stop DNA. Enumeration of viable cells in suspension at 48 hours revealed 1.7 × 105 cells/well to 3.2 × 105 cells/well that were 99% to 100% viable.
HIV-1 DNA PCR
An equivalent number of cells from each treatment were harvested from tissue culture and washed once in PBS. Lysis, PCR amplification, and γ32P-labeled R/U5 primer pairs for HIV-1 minus-strand, strong-stop DNA were as previously described.21 β-actin primer pairs were 5′-AGGCCAACCGCGAGATGACC-3′ and 5′-GAAGTCCAGGGCGACGTAGCAC-3′. Amplified products were identified and pixelated for quantitation using 6% nondenaturing polyacrylamide gel electrophoresis.
Results
Different subcellular compartments occupied by HLE in HIV-1 permissive and nonpermissive U937 subclones
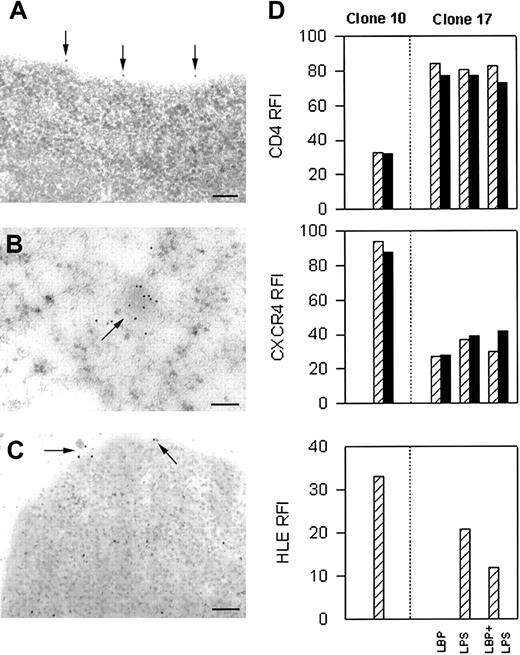
Immunocytochemical TEM examination of U937 subclones revealed HLE associated with the plasma membrane in HIV-1 permissive clone 10 (Figure 1A). Plasma membrane-associated HLE was never seen in unstimulated HIV-1 nonpermissive clone 17 (data not shown). Instead, HLE was found in clusters in clone 17 associated with intracytoplasmic, electron-dense, irregular-shaped bodies resembling granules (Figure 1B). Localization of HLE to an internal compartment in clone 17 was confirmed by CLSM (data not shown). Intracytoplasmic clusters of HLE were never seen in permissive clone 10. Positive-control protein CatG was found to localize to the same structures as HLE in clones 10 and 17, whereas NFκB was found to localize to the cytoplasm and mitochondria in both clones equivalently (data not shown). That HLE was found to reside in granule-like structures in nonpermissive cells, but on the cell surface in permissive cells suggested these clones had become arrested at disparate stages of differentiation.
Immunolocalization of HLE in HIV-1 permissive and nonpermissive clones. By TEM, HLE was found only on the cell surface of HIV-1 permissive clone 10 (A), and only in the intracytoplasmic compartment of unstimulated HIV-1 nonpermissive clone 17 (B). HLE was found on the cell surface and in the cytoplasm when clone 17 was stimulated by LPS (C). Homogeneous receptor density at equilibrium was achieved by exposing cells to α1PI by culturing in medium containing 10% FBS prior to staining. Cells were prepared for TEM in 3 separate experiments, examined independently by 2 investigators, and representative images are presented. Each bar represents 0.1 μm. (D) HIV-1 permissive and nonpermissive subclones were gated for live cells and were analyzed for coreceptor levels using 3-color flow cytometry to detect PerCP-conjugated anti-CD4, PE-conjugated anti-CXCR4, and FITC-conjugated anti-HLE. Cells were first interacted with anti-CD4 and second with anti-HLE and anti-CXCR4 (▨). Alternatively, cells were first interacted with anti-HLE and second with anti-CD4 and anti-CXCR4 (▪). HLE was detected on the surface of nonpermissive clone 17 after stimulating with LBP and LPS to induce granule release. CD4, CXCR4, and HLE expression on clone 17 in the presence of LBP was indistinguishable from medium alone (data not shown). Relative fluorescence intensity (RFI) were calculated as receptor MFI minus isotype control MFI. Flow cytometric analysis was performed on 10 000 to 30 000 events in 4 separate experiments. Data represent one analysis.
Immunolocalization of HLE in HIV-1 permissive and nonpermissive clones. By TEM, HLE was found only on the cell surface of HIV-1 permissive clone 10 (A), and only in the intracytoplasmic compartment of unstimulated HIV-1 nonpermissive clone 17 (B). HLE was found on the cell surface and in the cytoplasm when clone 17 was stimulated by LPS (C). Homogeneous receptor density at equilibrium was achieved by exposing cells to α1PI by culturing in medium containing 10% FBS prior to staining. Cells were prepared for TEM in 3 separate experiments, examined independently by 2 investigators, and representative images are presented. Each bar represents 0.1 μm. (D) HIV-1 permissive and nonpermissive subclones were gated for live cells and were analyzed for coreceptor levels using 3-color flow cytometry to detect PerCP-conjugated anti-CD4, PE-conjugated anti-CXCR4, and FITC-conjugated anti-HLE. Cells were first interacted with anti-CD4 and second with anti-HLE and anti-CXCR4 (▨). Alternatively, cells were first interacted with anti-HLE and second with anti-CD4 and anti-CXCR4 (▪). HLE was detected on the surface of nonpermissive clone 17 after stimulating with LBP and LPS to induce granule release. CD4, CXCR4, and HLE expression on clone 17 in the presence of LBP was indistinguishable from medium alone (data not shown). Relative fluorescence intensity (RFI) were calculated as receptor MFI minus isotype control MFI. Flow cytometric analysis was performed on 10 000 to 30 000 events in 4 separate experiments. Data represent one analysis.
The endotoxin LPS, a glycolipid found on Gram-negative bacteria, produces multiple effects on cells including release of granule-associated HLE, which becomes bound to the plasma membrane.11 Granule release is mediated by the interaction of LPS with 2 different signaling receptors including CD14 and β2-integrins CD11a/CD18, CD11b/CD18, and CD11c/CD18. In plasma, LBP facilitates receptor binding to LPS monomers by its action as a lipid transfer protein. When nonpermissive clone 17 was stimulated in the presence of LPS and LBP, HLE was visualized by TEM to translocate from granules to the cell surface, the cytoplasm, and the extracytoplasmic space (Figure 1C). These results suggest that in response to LPS, granule release allows plasma membrane association of HLE in the absence of protein synthesis.
Analysis of HIV-1 coreceptors by flow cytometry in the presence or absence of LPS stimulation was compared using 3-color fluorescence. Because HLE density was expected to be low or not detectable, HIV-1 permissive clone 10 was examined with or without anti-CD4 as a staining control. Surprisingly, HLE was greatly increased when clone 10 was interacted with antibodies specific for CD4 first and specific for HLE secondarily; HLE was undetectable when the order of addition was reversed (Figure 1D). The explanation for differences in detection of surface HLE on unfixed cells in the presence or absence of anti-CD4 is not known, but evidence suggests a potential role for membrane remodeling22 or autodegradation.23 In contrast, the order of coreceptor ligation had negligible influence on the fluorescence intensity of CD4 or CXCR4. LPS stimulation of HIV-1 nonpermissive clone 17 was found to have little or no influence on CD4 or CXCR4 density; however, HLE expression was found to increase. Further, as with clone 10, the order of receptor ligation influenced detection of HLE, but not detection of CD4 or CXCR4. The relatively identical HLE expression induced by LPS with or without LBP is interpreted to result from factors in the serum-containing tissue-culture medium. Results suggest a dynamic functional association between membrane-associated HLE and the HIV-1 coreceptor CD4.
HLE binding to the HIV-1 fusion domain
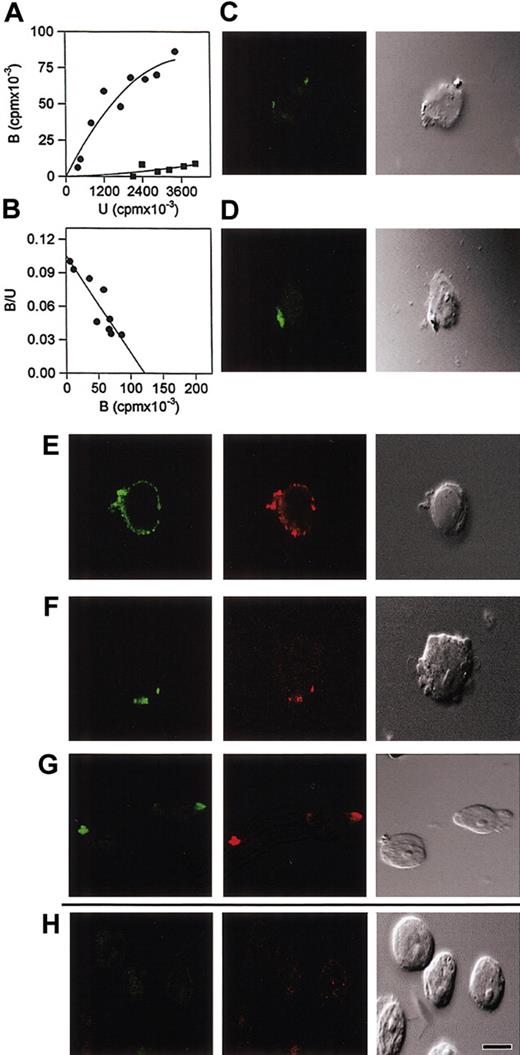
Association constants for soluble proteins are conventionally measured in 0.15 M NaCl, pH 7.2, to accommodate comparisons between proteins competing for the same soluble ligand and yield values in the range of 106 M-1. To optimize binding of nonsoluble proteins, association constants may be determined by modifying salt, pH, or temperature. Although a constant determined in 0.5 M NaCl at 0°C is not directly comparable to a constant determined in 0.15 M NaCl at 25°C, each constant is valid and definitive for the conditions under which affinity is measured. We have previously found that hypertonic saline (0.5 M NaCl) can be used to limit nonspecific ionic interactions between cell-surface receptors and the hydrophobic pentapeptide FLGFL, representative of the HIV-1 fusion domain.5 Using these conditions for Scatchard analysis, we demonstrated the existence of a single receptor (Kassoc = 1 × 103 M-1) on CEM T-lymphoblastoid cells for FLGFL. By competitive inhibition, the receptor was identified as HLE (HLE IC100 = 8.0 mM, α1PI IC100 = 8.1 mM with a ratio of HLE/α1PI = 0.99 at IC100). These results can be interepreted to mean that HLE and α1PI competitively inhibit the same molecular interaction involving FLGFL, but by different mechanisms. FLGFL was further shown to bind purified HLE, further supporting the identity of the FLGFL receptor as HLE. To compare whether membrane-associated HLE might also act as a receptor for the fusion domain on U937 cells, clone 10 plasma membranes were isolated, solubilized, and iodinated (125I-U937) as previously described.5 Saturable binding of 125I-U937 to immobilized FLGFL was detected (Figure 2A), and Scatchard analysis at saturation revealed linearity (Figure 2B). As we found using CEM cells, these data suggest the existence of a single proteinaceous receptor.5,24 Competitive inhibition in hypertonic saline using FLGFL solubilized in 9.5% ethanol revealed 100% inhibition when the peptide was more than 1.25 mM (data not shown). Competitive inhibition using HLE (IC100 = 77 mM) and α1PI (IC100 = 91 mM) under these conditions (data not shown) revealed virtually identical affinities (Kassoc = 1 × 103 M-1) and competitive binding ratios (HLE/α1PI = 0.85) as determined using CEM, and this strongly suggests that during unfolding of the HIV-1 envelope and exposure of the hydrophobic fusion domain, the HIV-1 fusion domain and HLE participate in a single molecular interaction.
Binding and patching of HLE in response to the fusion domain of HIV. (A) Wells in a microtiter plate were uncoated (▪) or were coated (•) with 10 mM HIV-1 fusion peptide (FLGFL) and incubated with 125I-U937 clone 10 solubilized cytoplasmic membrane proteins. Hypertonic saline (0.5 M NaCl) was used to limit nonspecific ionic interactions between solubilized membranes and the hydrophobic peptide. Maximum bound cpm/well at saturation was 1.3 × 105 (r2 = 0.94), Binding to uncoated wells was 9.0 ± 0.3 × 103 cpm. Specific binding to fusion peptide is represented as the difference between coated and uncoated wells, and binding to uncoated wells is depicted for comparison. (B) Scatchard analysis revealed a linear character (r2 = 0.81) to the interactions and association constant 1 × 103 M-1 in hypertonic saline. Interaction of cell-surface HLE with FLGFL (C) or α1PI (D) stimulated patching. Bound monoclonal antibody specific for HLE was detected using biotinylated anti-IgG and FITC-conjugated streptavidin. Significantly, HLE (green) was found to copatch with CXCR4 (red) in response to crosslinked polyclonal (E) or monoclonal (F) anti-HLE. Cells were stimulated, fixed, stained, and examined by suspension under coverslip. Negative-control cells stained for HLE in the absence of HIV-1 fusion peptide, α1PI, or other HLE ligands depicted no detectable staining (not shown). (G) Colocalization of α1PI (green) and SDF-1α (red) on the attached surface of clone 10 was detected when cells were stimulated by α1PI and postincubated with SDF-1α. (H) In contrast, when cells were first stimulated with SDF-1α and postincubated with α1PI, SDF-1α was detected in an internal section, but α1PI was not detected in any section. In the cell represented, SDF-1α was detected 2.4 μm from the attached surface of clone 10. Cells were stimulated, slides were prepared by cytospin, fixed, and stained. Colocalization by CLSM was analyzed using 8-step 0.6-μm sectional scanning from the attached surface toward the unattached surface of the cells. Patching and colocalization were performed at least 3 times and examined by 2 investigators independently. A representative cell is depicted. All photomicrographs were magnified to the same scale; bar represents 25 μm. Homogeneous receptor density at equilibrium was achieved by exposing cells to α1PI by culturing in medium containing 10% FBS prior to staining.
Binding and patching of HLE in response to the fusion domain of HIV. (A) Wells in a microtiter plate were uncoated (▪) or were coated (•) with 10 mM HIV-1 fusion peptide (FLGFL) and incubated with 125I-U937 clone 10 solubilized cytoplasmic membrane proteins. Hypertonic saline (0.5 M NaCl) was used to limit nonspecific ionic interactions between solubilized membranes and the hydrophobic peptide. Maximum bound cpm/well at saturation was 1.3 × 105 (r2 = 0.94), Binding to uncoated wells was 9.0 ± 0.3 × 103 cpm. Specific binding to fusion peptide is represented as the difference between coated and uncoated wells, and binding to uncoated wells is depicted for comparison. (B) Scatchard analysis revealed a linear character (r2 = 0.81) to the interactions and association constant 1 × 103 M-1 in hypertonic saline. Interaction of cell-surface HLE with FLGFL (C) or α1PI (D) stimulated patching. Bound monoclonal antibody specific for HLE was detected using biotinylated anti-IgG and FITC-conjugated streptavidin. Significantly, HLE (green) was found to copatch with CXCR4 (red) in response to crosslinked polyclonal (E) or monoclonal (F) anti-HLE. Cells were stimulated, fixed, stained, and examined by suspension under coverslip. Negative-control cells stained for HLE in the absence of HIV-1 fusion peptide, α1PI, or other HLE ligands depicted no detectable staining (not shown). (G) Colocalization of α1PI (green) and SDF-1α (red) on the attached surface of clone 10 was detected when cells were stimulated by α1PI and postincubated with SDF-1α. (H) In contrast, when cells were first stimulated with SDF-1α and postincubated with α1PI, SDF-1α was detected in an internal section, but α1PI was not detected in any section. In the cell represented, SDF-1α was detected 2.4 μm from the attached surface of clone 10. Cells were stimulated, slides were prepared by cytospin, fixed, and stained. Colocalization by CLSM was analyzed using 8-step 0.6-μm sectional scanning from the attached surface toward the unattached surface of the cells. Patching and colocalization were performed at least 3 times and examined by 2 investigators independently. A representative cell is depicted. All photomicrographs were magnified to the same scale; bar represents 25 μm. Homogeneous receptor density at equilibrium was achieved by exposing cells to α1PI by culturing in medium containing 10% FBS prior to staining.
In support of this hypothesis, HLE visualized by CLSM using a monoclonal antibody was found to patch in response to soluble FLGFL or α1PI (Figure 2C-D), but not in response to SDF-1α or vehicle (data not shown). These results support the specificity of binding of the fusion peptide to HLE and support a role for HLE during HIV-1 binding to cells of either monocytic and lymphocytic lineage. Receptor patching is known to be induced by crosslinking. Since receptor crosslinking by the HIV-1 fusion pentapeptide seems unlikely to have occurred, anti-HLE-induced patching was examined by CLSM for comparison. HLE was found to copatch with CXCR4 on uropods in response to crosslinking by anti-HLE (Figure 2E-F). Nystatin is a cholesterol-binding antibiotic that selectively disrupts HIV-1 receptors residing on cholesterol-containing platforms25 and has been demonstrated to interfere with in vitro HIV-1 infectivity (IC50 = 4 μM) in H9, HUT-78, and U937 cells, but has little effect in vivo except against candidiasis.26 HLE and CXCR4 copatching stimulated by α1PI was found to be completely abrogated by pretreating for 60 minutes with 50 μM nystatin (data not shown).
Immunolocalization of receptors on cells sequentially incubated with α1PI and SDF-1α was also examined by CLSM. When clone 10 was stimulated first by α1PI and second by SDF-1α, α1PI and SDF-1α were found copatched on the cell surface (Figure 2G). Copatching appeared not to resemble classic capping induced by crosslinking,27 but appeared as protrusions of the plasma membrane. When clone 10 was stimulated first by SDF-1α and second by α1PI, α1PI was not detectable, and SDF-1α was detected in an internal section of the cell but not on the cell surface (Figure 2H). These results support the cell-surface coassociation of receptors for SDF-1α and α1PI in HIV-1 permissive clones. Each preparation of proteinase inhibitor is composed of a unique ratio of active and inactive protein. To determine whether receptors recognize active or inactive forms of α1PI, 2 preparations of active-site standardized α1PI were compared which were 32.7% and 8.3% active. When clone 10 was stimulated by these 2 preparations at equivalent protein concentrations, a greater concentration of the 8.3% active preparation was required than the 32.7% active preparation (data not shown) suggesting the active fraction, and not the inactive fraction, of α1PI was recognized by the receptor. That ligation of cell-surface HLE is sufficient to induce colocalization of HLE with CXCR4 suggests that receptor copatching may be integral to HIV-1 entry.
Enhanced HIV-1 infectivity in the presence of exogenous α1PI
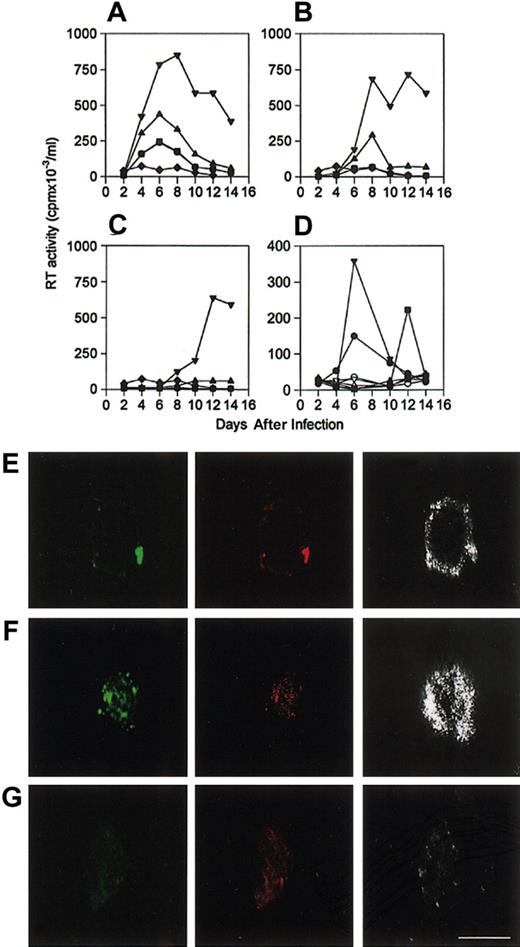
It has previously been shown that HIV-1 gp120 induces colocalization of CD4 and CXCR4.28 That α1PI induced copatching of HLE and CXCR4 suggested that α1PI might also influence infectivity. HIV-1 permissive clone 10 and nonpermissive clone 17 were infected with HIV-1NL4-3 using varying MOI in the absence or presence of various physiologic concentrations of α1PI in serum-free medium. It is known that α1PI is a chemoattractant that induces adherence. To examine the influence of α1PI without biasing the outcome against adherent cells, cells were infected and cultured in the same wells of a 96-well tissue-culture plate. For comparison, cells were infected in microfuge tubes and transferred to clean wells of a 96-well tissue-culture plate. When cells were infected, washed free of virus, and cultured in the same wells of a tissue-culture plate, peak RT activity was found to increase in direct relation to the culture concentration of α1PI (Figure 3A-C). When cells were infected in microfuge tubes, washed free of virus, and transferred to wells, there was little detectable RT activity after 12 days of culture in serum-free medium containing any concentration of α1PI (Figure 3A). The HIV-1 nonpermissive cells remained nonpermissive in all conditions tested. The loss of HIV-1 infectivity at the lowest concentration of α1PI even in the presence of the highest MOI demonstrates α1PI dose-dependence and suggests that cells infected in microplates were not prejudiced by residual inoculum. Further, that α1PI dose-dependence is identically replicated at all 3 MOIs and that decreasing MOI produce the expected decrease in infectivity suggests that α1PI is necessary for successful HIV-1 infectivity in serum-free medium and may stimulate adherence and selection of significant cell populations and influence subsequent detection of infectivity outcome using traditional infectivity assays. Further, these results suggest the hypothesis that α1PI might influence HIV-1 infectivity by its influence on receptor colocalization.
Influence of α1PI on HIV-1 infection kinetics in serum-free medium. To examine whether α1PI might exert its influence on virus or cells, cells were cultured overnight in serum-free medium and infected with varying doses of virus in the presence of varying concentrations of α1PI. To avoid biasing the outcome against adherent cells, U937 clones were infected directly in microplates. For comparison, cells were infected in microtubes, washed to remove free virus, and transferred to clean wells of a microplate. In vitro infectivity of HIV-1 permissive clone 10 was significantly increased in the presence of increasing concentrations of α1PI. Clone 17 failed to be infected at any viral dose or any concentration of α1PI. Cells were infected with HIV-1NL4-3 using (A) 1 × 10-2 MOI, (B) 1 × 10-3 MOI, or (C) 1 × 10-4 MOI. Clone 10 infected in the absence of α1PI (•) was equivalent to infectivity in the presence of 0.3 μM α1PI (▪) and increased in the presence of 3 μM (▴), or 30 μM (▾) active α1PI. Clone 10 exhibited low RT activity when cells were infected in microfuge tubes and transferred to wells of a tissue-culture plate in any concentration of α1PI (♦). Infectivity experiments were confirmed independently by the Laboratory of Immunoregulation, NIAID, NIH. A representative set of results is depicted. Infectivity outcome was determined in duplicate by measuring RT activity of cell-free supernatants. Mean values are depicted. (D) RT activity produced by clones 10 and 17 infected simultaneously in the presence (closed symbols), but not in the absence (open symbols), of α1PI is represented. RT activity was produced by HIV-1 nonpermissive clone 17 following pretreatment with LPS and LBP (▾). Cells infected in the presence of LPS and α1PI and the absence of LBP (▪) produced diminished peak RT activity and prolonged time to peak activity. RT activity was not produced by clone 17 in the absence of LPS and LBP even though α1PI was present (♦). Peak RT activity produced by clone 10 infected in parallel is represented for comparison (•). To examine colocalization of HIV-1 with receptors, clone 10 was incubated with HIV-1NL4-3 in serum as a source of α1PI. At 15-minute intervals, cells were washed free of virus, fixed, and slides were prepared. Following incubation for 15 minutes with HIV-1NL4-3, (E) HLE (green), CD4 (red), and HIV-1 (silver) were copatched, and (F) HLE (green), CXCR4 (red), and HIV-1 (silver) were copatched. (G) Copatching of HLE (green), CD4 (red), and HIV-1 (silver) on clone 10 was not detected in the absence of HIV-1NL4-3. HIV-1 coreceptors were detected by CLSM using polyclonal anti-HLE, monoclonal anti-CD4, and monoclonal anti-HIV specific for epitopes proximal to the V3 loop. Bar represents 30 μm. HIV-1 infectivity and CLSM were performed at least 3 times and representative data are presented.
Influence of α1PI on HIV-1 infection kinetics in serum-free medium. To examine whether α1PI might exert its influence on virus or cells, cells were cultured overnight in serum-free medium and infected with varying doses of virus in the presence of varying concentrations of α1PI. To avoid biasing the outcome against adherent cells, U937 clones were infected directly in microplates. For comparison, cells were infected in microtubes, washed to remove free virus, and transferred to clean wells of a microplate. In vitro infectivity of HIV-1 permissive clone 10 was significantly increased in the presence of increasing concentrations of α1PI. Clone 17 failed to be infected at any viral dose or any concentration of α1PI. Cells were infected with HIV-1NL4-3 using (A) 1 × 10-2 MOI, (B) 1 × 10-3 MOI, or (C) 1 × 10-4 MOI. Clone 10 infected in the absence of α1PI (•) was equivalent to infectivity in the presence of 0.3 μM α1PI (▪) and increased in the presence of 3 μM (▴), or 30 μM (▾) active α1PI. Clone 10 exhibited low RT activity when cells were infected in microfuge tubes and transferred to wells of a tissue-culture plate in any concentration of α1PI (♦). Infectivity experiments were confirmed independently by the Laboratory of Immunoregulation, NIAID, NIH. A representative set of results is depicted. Infectivity outcome was determined in duplicate by measuring RT activity of cell-free supernatants. Mean values are depicted. (D) RT activity produced by clones 10 and 17 infected simultaneously in the presence (closed symbols), but not in the absence (open symbols), of α1PI is represented. RT activity was produced by HIV-1 nonpermissive clone 17 following pretreatment with LPS and LBP (▾). Cells infected in the presence of LPS and α1PI and the absence of LBP (▪) produced diminished peak RT activity and prolonged time to peak activity. RT activity was not produced by clone 17 in the absence of LPS and LBP even though α1PI was present (♦). Peak RT activity produced by clone 10 infected in parallel is represented for comparison (•). To examine colocalization of HIV-1 with receptors, clone 10 was incubated with HIV-1NL4-3 in serum as a source of α1PI. At 15-minute intervals, cells were washed free of virus, fixed, and slides were prepared. Following incubation for 15 minutes with HIV-1NL4-3, (E) HLE (green), CD4 (red), and HIV-1 (silver) were copatched, and (F) HLE (green), CXCR4 (red), and HIV-1 (silver) were copatched. (G) Copatching of HLE (green), CD4 (red), and HIV-1 (silver) on clone 10 was not detected in the absence of HIV-1NL4-3. HIV-1 coreceptors were detected by CLSM using polyclonal anti-HLE, monoclonal anti-CD4, and monoclonal anti-HIV specific for epitopes proximal to the V3 loop. Bar represents 30 μm. HIV-1 infectivity and CLSM were performed at least 3 times and representative data are presented.
Since HLE is not constitutively expressed on the cell surface of clone 17, it was considered that LPS-induced cell-surface expression of HLE might confer permissivity. Clone 17 was cultured in serum-free medium and stimulated with LPS in the presence or absence of LBP prior to addition of HIV. Cells were subsequently infected in the presence or absence of physiologic concentrations of α1PI in serum-free medium. In the presence of LPS and LBP, the kinetics of appearance and disappearance of RT activity in nonpermissive cells were similar to those of permissive cells (Figure 3D). The delayed appearance of RT activity when cells were stimulated with LPS in the absence of LBP is consistent with previous evidence of the diminished level of LPS monomer formation and receptor recognition of LPS in the absence of LBP.29 These results suggest membrane expression of an additional HIV-1 cofactor in response to LPS. In the absence of α1PI, detectable RT activity occurred neither in permissive nor nonpermissive cells under any condition examined. These results suggest that α1PI and a cell-surface receptor are HIV-1 cofactors in these promonocytic cells.
To examine the possibility that patching might occur during HIV-1 entry, clone 10 was incubated at 37°C with infectious HIV-1NL4-3, and cells were fixed and examined at various time points by CLSM for colocalization of HIV-1 and coreceptors. HIV-1 was detected using a monoclonal antibody with specificity for epitopes proximate to the V3 loop (Research Diagnostics). This antibody has recently been shown not to recognize α1PI in contrast to a different monoclonal antibody (1C1; Repligen, Cambridge, MA) with specificity for epitopes proximate to the HIV-1 fusion domain.12 After incubation of cells with virus for 15 minutes, HIV-1 was detected in several patches on the cell surface colocalized with CD4, CXCR4, and HLE (Figure 3E-G). HIV-1 appeared to patch (Figure 3F) and coalesce with these receptors on extensions of the plasma membrane (Figure 3E).
Requirement for cell-surface HLE during HIV-1 infection
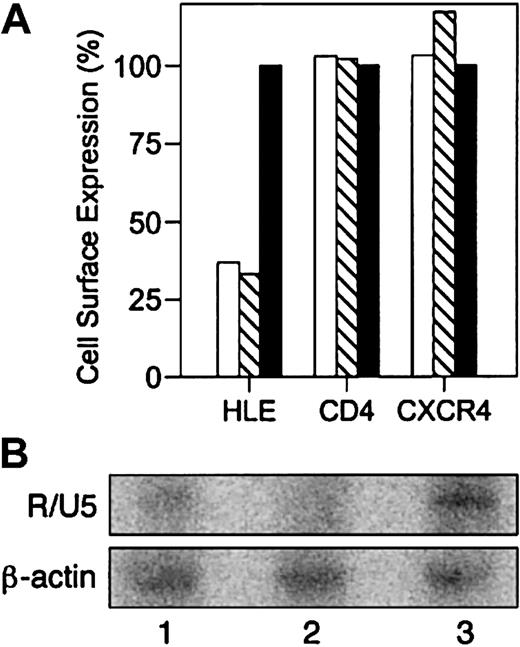
The data presented thus far suggest the participation of HLE as a receptor during HIV-1 capture and infection. Linking HIV-1 entry with the novel expression of CD4 and chemokine receptor cDNA in cells lacking their expression has established this method as the gold standard for identifying HIV-1 receptors. On the other hand, silencing gene expression has proven to be an equally powerful tool for demonstrating dependence of HIV-1 entry on CD4 expression.30 Since novel expression of HLE cDNA is technically not possible at this time, the importance of HLE during HIV-1 entry and infectivity was examined by inhibiting HLE expression. Morpholino antisense oligonucleotides complementary to HLE mRNA and pre-mRNA were introduced into HIV-1 permissive clone 10 and cultured for 48 hours. Antisense oligonucleotides complementary to β-globin were used as a negative control. Analysis by flow cytometry showed that cells transfected with antisense oligonucleotides specific for the start codon or for the 3′ splice site of HLE exon 4 diminished surface expression of HLE to approximately 30% relative to the control β-globin oligonucleotide (Table 1 and Figure 4A). Neither antisense oligonucleotide influenced cell-surface expression of CXCR4 or CD4.
Inhibition of HIV-1 binding with antisense oligonucleotides complementary to HLE mRNA. HIV-1 permissive clone 10 was transfected with antisense oligonucleotides complementary to the HLE start codon (▧), 3′ splice site of HLE exon 4 (□), or β-globin (▪). Homogeneous receptor density at equilibrium was achieved by exposing cells to α1PI by culturing in medium containing 10% FBS throughout transfection. (A) Cell-surface expression of HLE, CD4, and CXCR4 was determined by flow cytometric analysis 48 hours after transfection (Table 1). MFI of cells transfected with β-globin antisense was used to estimate baseline expression. Results are expressed as percent baseline expression and were calculated as 100 × (MFI after HLE antisense/MFI after β-globin antisense). Cell viability was determined prior to staining. (B) PCR amplification of HIV-1 minus-strand, strong-stop DNA using R/U5 primer pairs resulted in a minimally detected product in lysates from cells transfected with antisense complementary for the 3′ splice site of HLE exon 4 (lane 1), and slightly less product in lysates from cells transfected with antisense complementary to the HLE start codon (lane 2), and strongly detected product in lysates from cells transfected with negative control antisense oligomer complementary for β-globin (lane 3). Transfection and PCR amplification were repeated twice and representative data are presented.
Inhibition of HIV-1 binding with antisense oligonucleotides complementary to HLE mRNA. HIV-1 permissive clone 10 was transfected with antisense oligonucleotides complementary to the HLE start codon (▧), 3′ splice site of HLE exon 4 (□), or β-globin (▪). Homogeneous receptor density at equilibrium was achieved by exposing cells to α1PI by culturing in medium containing 10% FBS throughout transfection. (A) Cell-surface expression of HLE, CD4, and CXCR4 was determined by flow cytometric analysis 48 hours after transfection (Table 1). MFI of cells transfected with β-globin antisense was used to estimate baseline expression. Results are expressed as percent baseline expression and were calculated as 100 × (MFI after HLE antisense/MFI after β-globin antisense). Cell viability was determined prior to staining. (B) PCR amplification of HIV-1 minus-strand, strong-stop DNA using R/U5 primer pairs resulted in a minimally detected product in lysates from cells transfected with antisense complementary for the 3′ splice site of HLE exon 4 (lane 1), and slightly less product in lysates from cells transfected with antisense complementary to the HLE start codon (lane 2), and strongly detected product in lysates from cells transfected with negative control antisense oligomer complementary for β-globin (lane 3). Transfection and PCR amplification were repeated twice and representative data are presented.
Oligonucleotide-treated cells were incubated with HIV-1NL4-3, washed free of virus, and cultured for 48 hours. The presence of minus-strand, strong-stop DNA, the earliest RT product following HIV-1 entry, was determined by PCR amplification of cell lysates using R/U5 primer pairs.21 As was found with HLE expression, minus-strand, strong-stop DNA was diminished in cells transfected with antisense oligonucleotides complementary to the HLE start codon or the 3′ splice site of exon 4 (Figure 4B). In contrast, this DNA was easily detected in cells transfected with antisense oligonucleotide complementary to β-globin. We conclude that in addition to CD4 and CXCR4, cell-surface HLE is necessary for HIV-1 binding and infection.
Discussion
Cell-surface HLE ligation by α1PI, anti-HLE, and HIV-1 fusion peptide was shown here to induce copatching of HLE, CD4, and chemokine receptors. A model is proposed in which HLE ligation induces clustering of HIV-1 receptors, and that this dense focus of receptors increases the odds of the dense focus of HIV-1 envelope proteins to interact with remaining free receptors. Ligand-occupied receptors thereby produce a tangible increase in proximal receptors rather than producing steric interference and decreased accessibility of HIV-1 receptors. Consistent with this model, SDF-1α was found to prevent receptor clustering, and this suggests that chemokines may suppress HIV-1 infectivity by decreasing HIV-1 receptor clusters rather than by directly blocking receptors as previously believed.15 Increased circulating α1PI is correlated with increased viral load in the HIV-1 population, suggesting the hypothesis that α1PI might facilitate HIV-1 binding and infectivity. Here we have shown that RT activity produced by HIV-1 infectivity is dependent on α1PI in a dose-dependent manner, that α1PI facilitates copatching of HLE with the canonical HIV-1 receptors, that live HIV-1 preferentially binds copatched receptors, and that binding of virus to copatched receptors does not occur in the absence of α1PI. We have further demonstrated that translocation of HLE from granules to the cell surface conveys HIV-1 permissivity to nonpermissive cells and that inhibiting HLE mRNA proportionally diminishes detection of the earliest RT product, minus-strand, strong stop DNA. In support of these findings, previous studies have demonstrated that in contrast to permissive clones, nonpermissive clones are unable to efficiently form complexes between gp120, CD4, and CXCR4.31
The critical parameters determining the kinetics of HIV-1 infectivity have been defined as virus concentration, number of virions produced by one cell, and the time for one complete cycle of infection.32 In a homogeneous cell population, the time to peak RT activity has been shown to be directly related to virus concentration. We found that time to peak RT activity produced by clone 10 was diminished by 2 days for each logarithmic dilution of HIV-1NL4-3, and these results are consistent with previous kinetic studies.32 However, clone 10 produced negligible RT activity when cells were infected in serum-free medium lacking α1PI, and RT activity increased in a dose-dependent manner as α1PI increased suggesting α1PI is necessary for infectivity. That time to peak RT activity was not increased by α1PI suggests that the influence of α1PI on peak RT levels was not related to viral inoculum or time of infection. Rather, these results suggest that increased peak RT levels resulted either from an increased number of virions produced by each cell or from an increased number of infected cells.
The dose-dependent manner in which α1PI influenced HIV-1 infectivity suggested a receptor-dependent mechanism. HIV-1 permissive and nonpermissive U937 clones differ with respect to cell-surface localization of HLE. We found that HIV-1 infectivity was permitted when granule-released HLE was bound to the cell surface of HIV-1 nonpermissive clone 17. Significantly, HIV-1 infectivity of clone 17 also required the participation of α1PI, and this supports the identity of the responsible granule-released component as HLE. During the acute phase of inflammation coincident with LPS-stimulated HLE release, the concentration of α1PI increases as much as 4-fold. In this scenario, the acute phase of opportunistic infection would produce the phenomenon of HIV-1 receptor copatching and thereby facilitate increased viral load.
Evidence that HIV-1 gp120 binds CD4 includes the demonstration that binding is reversible and saturable,33 that introducing CD4 cDNA expression in CD4 null cells results in a corresponding increase in HIV-1 infectivity,34 and that silencing CD4 mRNA expression blocks HIV-1 infectivity.30 Since HLE may reside either on the cell surface or in granules, and since HLE localization is dependent neither on DNA, mRNA, nor protein structure, but on whether the cell is producing granules, an as-yet-undefined process, introduction of HLE mRNA into HLE-negative cells is not a good test of its receptor function. The true test is to identify cells lacking HLE on the cell surface; test whether these cells are not susceptible to HIV-1 entry in the presence of CD4, CCR5, and CXCR4; and determine whether coexpression specifically of HLE on the cell surface now rescues HIV-1 entry into these cells. Reversible, saturable binding of the HIV-1 fusion domain to HLE has been shown here and elsewhere.5 We have demonstrated here that inhibiting HLE mRNA expression blocks HIV-1 infectivity and that favoring HLE association with the plasma membrane favors HIV-1 infectivity. Clark's receptor occupancy model24 states that in addition to reversible, saturable binding of the ligand to the receptor, binding must produce a biologic response proportional to the number of receptors bound. Evidence presented suggests that suppression of HLE expression proportionally suppresses the earliest HIV-1 RT product, and this supports a requirement for HLE during HIV-1 binding and the earliest steps of infection consistent with a model of HIV-1 entry that requires HLE as a fusion receptor. We have previously found that in vitro viral infectivity outcome correlates with HLE, but not CD4, CXCR4, or CCR5,13 and this suggests HLE is a rate-limiting receptor for viral entry potentially by a mechanism involving the phenomenon of receptor copatching.
Prepublished online as Blood First Edition Paper, August 21, 2003; DOI 10.1182/blood-2003-05-1635.
Supported by grants from the Center for AIDS Research, University Research Council of the University of North Carolina-Chapel Hill, and The Rockefeller Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors wish to gratefully acknowledge D. Irlbeck, F. DiMeo, and Drs A. S. Fauci, H. Moriuchi, M. Moriuchi, M. Pope, W. Resch, E. Miller, M. Gonzales-Gronow, S. V. Pizzo, and R. R. Arnold for reagents and technical assistance; and Drs O. J. Cohen, R. W. Doms, H. Moriuchi, R. R. Arnold, H. Patel, and M. Pope for critically reviewing the manuscript. Special thanks to Drs J. D. Folds, K. A. Jacobson, and H. H. Fudenberg for advice and encouragement.