Abstract
Inosine is an endogenous nucleoside with immunosuppressive properties that is known to inhibit the accumulation of proinflammatory cytokines and protect mice from endotoxin-induced inflammation and lung tissue damage. There are no known receptors specific for inosine, but A3 adenosine receptors (A3Rs) have been shown to bind inosine, resulting in mast cell degranulation and increased vascular permeability. The present study specifically addresses the requirement for A2aR and/or A3R for the protective effect of inosine in 2 experimental in vivo models of inflammatory disease. The data show that A3R is essential for protection against ConA-induced fulminant hepatitis since only A3R-expressing mice were protected by inosine whereas wild-type and A2aR-deficient mice exhibited severe liver damage even after administration of inosine. In addition, we show in a model of LPS-induced endotoxemia that inosine protected both A2aR-/- and A3R-/- mice from inflammation, but not A2aA3R double-null mice, indicating that in this model both A2aR and A3R were used by inosine. Thus, we demonstrate that A2a and A3 adenosine receptors are differentially utilized by inosine for the down-regulation of tissue damage under different inflammatory conditions in vivo. (Blood. 2003;102:4472-4478)
Introduction
Inflammatory processes are crucial for defense against pathogens, but inappropriate and/or prolonged inflammation contributes to the pathogenesis of major diseases.1 Therefore, identification of endogenous molecules that are capable of inhibiting activated immune cells is important in order to better understand the physiologic mechanisms that regulate inflammation2 and to develop novel targets for anti-inflammatory drugs. The demonstration that the extracellular adenosine A2aR participates in an endogenous immunosuppressive loop that serves to down-regulate inflammation in vivo by a nonredundant mechanism3 heightened the interest in naturally occurring purines in the regulation of inflammation and tissue protection.
It is known that adenosine possesses anti-inflammatory properties and that adenosine analogs can protect mice from a variety of inflammatory diseases such as septic shock4,5 and rheumatoid arthritis6 by inhibiting the release of free radicals and proinflammatory cytokines from different immune cells. Relevantly, the selective A2aR agonist CGS21680 was shown to protect liver from ischemia/reperfusion injury in rats and from ConA-induced tissue damage in mice.3,53 The attractiveness of an adenosine-mediated anti-inflammatory pathway has been enhanced by the realization that widely used anti-inflammatory drugs, such as methotrexate and sulfasalazine, seem to exert their effects by inducing the release of endogenous adenosine, which then signals through adenosine receptors.7-10 Thus, the triggering of a “natural” anti-inflammatory pathway by ligands of adenosine receptors represents an attractive immunomodulation strategy for the treatment of inflammatory diseases.
Inosine is an anti-inflammatory endogenous nucleoside that can be considered a natural trigger of adenosine receptors. It is formed as a product of adenosine deamination11 and is released into the extracellular space at times of cellular stress (eg, systemic inflammation12 and hypoxia13 ) when metabolism of adenosine is high. Indeed, while normal interstitial concentrations of inosine have been reported to be in the micromolar range, concentrations of more than 1 mM in ischemic tissues and in patients with sepsis have been documented.14-18 Inosine is now known to exert wide-ranging anti-inflammatory effects that include inhibition of proinflammatory cytokine and chemokine production, enhancement of anti-inflammatory cytokine interleukin-10 (IL-10) production, and protection from endotoxin-induced inflammation and lung tissue damage19-22 as well as skeletal muscle reperfusion injury23 in mice. In humans, inosine has shown promise as a therapeutic agent against multiple sclerosis (MS)24 and Tourette syndrome,25 perhaps by indirectly increasing uric acid levels and acting as a dopamine agonist, respectively. Thus, inosine may have wide-ranging biologic effects with potentially beneficial implications in humans, underscoring the need to understand the molecular mechanisms of inosine action in vivo.
Importantly, inosine has no known specific receptors(s); however, it has been shown to utilize cyclic adenosine monophosphate (cAMP)-inhibiting Gi-coupled A3Rs. In functional and radioligand-binding in vitro assays, Jin et al26 showed that inosine in the range of 10 μM to 50 μM binds to and activates A3Rs on stably transfected HEK-293 cells, RBL-2H3 rat mastlike cells, perivascular mast cells of hamster cheek pouch arterioles, and in guinea pig lung membranes. In addition, Tilley et al27 have used mice lacking either A3Rs or mast cells to show that A3Rs are required for the inosine-mediated potentiation of bone marrow mast cell degranulation and promotion of plasma protein extravasation.
The question, however, remained as to whether the anti-inflammatory effect of inosine was mediated by A3R activation alone or if inosine also signaled through other subtypes of adenosine receptors. Indeed, while the competitive binding studies with recombinant rat and guinea pig adenosine receptors subtypes have indicated that inosine does not bind A1Rs or A2aRs on mast cells,26 it was suggested by Hasko and colleagues19 that inosine-mediated suppression of tumor necrosis factor α (TNFα) production from lipopolysaccharide (LPS)-stimulated peritoneal murine macrophages was significantly abrogated in the presence of pharmacologic antagonists of A1 or A2a receptors. Although it was not determined in this study if inosine acted directly or indirectly by altering adenosine levels, these data supported the possibility that inosine could trigger immunosuppressive signaling through A1 or A2a receptors.
To determine which adenosine receptors, A2aR or A3R, were responsible for the effects of inosine, we studied acute inflammatory damage in in vivo models of autoimmune and viral hepatitis and endotoxin-induced sepsis using mice genetically deficient in A2aR or A3R and A2aA3R double-deficient mice. In addition, studies of these acute inflammation models allowed us to compare the effect of inosine on the pathogenesis of diseases with very different initiating stimuli (polyclonal T-cell receptor [TCR] activator ConA vs Toll-like receptor activating LPS) and different types of immune cells.
In mice, ConA-induced liver damage in mice is a well-described in vivo inflammation model of viral and autoimmune hepatitis that is mediated by T cells, natural killer (NK) T cells, macrophages, neutrophils, and a variety of cytokines and chemokines including TNFα, IL-4, interferon γ (IFNγ), and macrophage inflammatory protein-2 (MIP-2),28-34 whereas endotoxin-induced shock involves mostly the activation of toll-receptors on macrophages.35-37 Importantly, these different cells may differ in their repertoire of expressed adenosine receptors.
The experimental data provided in this study show that A3R was essential for the inhibition of ConA-induced liver injury by inosine since the accumulation of proinflammatory cytokines (eg, TNFα) and alanine transaminase was inhibited only in A3R-expressing mice, whereas A2aR-deficient mice had elevated levels. This was supported by histologic evidence of inosine inhibiting hepatocyte apoptosis in A3R+/+ but not A3R-/- mice. In addition, the data show that LPS-induced TNFα production was inhibited by inosine in wild-type, A2aR-/-, and A3R-/- mice. However, inosine was ineffective in blocking TNFα production in LPS-challenged A2aA3R-/- double-knockout mice, indicating that both A2aR and A3R were used by inosine to inhibit endotoxin-induced inflammation.
These data provide the first genetic evidence that inosine differentially utilizes A2a and A3 adenosine receptors to exert its anti-inflammatory properties, and indicate that different adenosine receptors are employed by inosine in T-cell-dependent versus T-cell-independent acute inflammation. Accordingly, we propose that use of A2a and A3 adenosine receptors by the endogenous nucleoside inosine should be considered in comparative studies of mechanisms of acute inflammation with different etiology. The A2a adenosine receptors have been conclusively implicated in the down-regulation of inflammation in vivo3 and the data reported here point to the need to include inosine among those endogenous molecules that influence inflammatory responses by directly or indirectly triggering A2a and/or A3 adenosine receptors in vivo.
Materials and methods
Mice
Eight- to twelve-week-old, age-matched, male mice were used. All mice were on a C57BL/6 background. The A2aR-/- and A3R-/- mice were backcrossed at least 10 times and the A2aA3R-/- double-knockout mice were backcrossed 2 times. A2aR and A3R gene-deficient mice are described in Chen et al38 and Salvatore et al,39 respectively. A2aA3R double-deficient mice were generated by A2aR-/- × A3R-/- breeding. IL-10+/+ and IL-10-/- mice were purchased from Taconic (Germantown, NY). All mice were maintained under specific pathogen-free conditions at the National Institutes of Health (NIH) animal care facilities.
Reagents and drugs
Inosine, LPS (from Escherichia coli, serotype 055:B5), and ConA were purchased from Sigma (St Louis, MO). The A2aR agonist CGS21680 and A3R and A1R agonist 2-Cl-IB:MECA were purchased from Tocris Cookson (Ballwin, MO).
LPS study
Mice were injected intraperitoneally with LPS (10 mg/kg) with or without inosine (100 mg/kg) in sterile phosphate-buffered saline (PBS). Inosine was injected 10 minutes before LPS. For pharmacologic activation of A2aR or A3R, the agonists CGS21680 and 2-CL-IB:MECA were injected intraperitoneally at 0.5 mg/kg 10 minutes before injection of LPS. Blood was collected from the retro-orbital vein 1 hour after LPS injection and TNFα levels were determined from the isolated serum.
Liver injury study
Mice were injected intravenously (tail vein) with the indicated dose of ConA (15 mg/kg or 20 mg/kg), inosine (100 mg/kg), or a mixture of ConA plus inosine in sterile PBS. Blood was collected at 1 hour and 24 hours from the retro-orbital vein and TNFα and alanine transaminase (ALT) levels were determined from the isolated serum. Mice were killed 24 hours after injection and liver samples were taken for tissue histopathologic evaluation.
TNFα and ALT determination
TNFα was measured in the serum collected 1 hour after the indicated challenge using enzyme-linked immunosorbent assay (ELISA) kits purchased from R&D Systems (Minneapolis, MN). Detection limits were less than 5.1 pg/mL for TNFα. Serum ALT was measured from serum collected 24 hours after challenge with ConA, inosine, or ConA plus inosine using kits purchased from Sigma. The assays were performed according to the manufacturer's instructions.
Histology
Samples of liver tissue from dead mice were taken and fixed in formalin. In situ staining of single-strand breaks in nuclear DNA to detect apoptotic cells (TdT assay) and haematoxylin and eosin (H&E) staining were done at Molecular Histology (Montgomery Village, MD).
Results
Inosine utilizes only A3 adenosine receptors to inhibit ConA-induced liver damage in a fulminant hepatitis model in vivo
To determine whether A2a or A3 receptors or both are required for the inosine-mediated inhibition of inflammation in vivo, we compared the effect of inosine on ConA-challenged wild-type, A2aR-/-, A3R-/-, and A2aA3R-/- mice. Given that inosine had been shown in published studies to inhibit inflammatory responses in vivo when administered at 100 mg/kg, we chose this concentration of inosine in these and subsequent experiments. Following injection of ConA and/or inosine the levels of ALT and of the proinflammatory cytokine TNFα in the serum were analyzed as a measure of intensity of the inflammatory process and of liver damage.
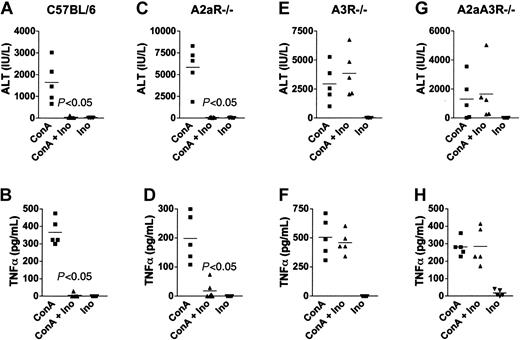
Many different cytokines (eg, IFNγ, IL-4, TNFα) were shown to be involved in ConA-induced liver injury in experiments using different gene-deficient and transgenic mice.28,31,40 As shown in Figure 1A-B, ConA challenge resulted in severe liver damage in wild-type mice as assessed by high levels of ALT and TNFα in the serum. Inosine, which alone did not induce liver damage, completely inhibited ALT and TNFα accumulation in wild-type mice when coinjected with ConA. The observed inhibition of liver damage by inosine was dependent on the presence of A3 adenosine receptors as evidenced by experiments where the effect of inosine on ConA-induced liver damage and inflammation was compared between A2aR-/-, A3R-/-, and A2aA3R-/- mice. It was found that both the extent of liver damage (ALT levels) and TNFα levels were inhibited by inosine regardless of the absence of the A2aR (Figure 1C-D), indicating that A2a receptors were not required for inhibition by inosine in this model. In contrast, mice deficient in A3R were resistant to the anti-inflammatory effect of inosine, and high levels of ALT and TNFα were detected in these mice after challenge with ConA (Figure 1E-H), thus indicating that A3Rs were necessary and required for inhibition of liver damage by inosine.
A3 adenosine receptors are required for the inhibition of ConA-induced ALT and TNFα by inosine. C57BL/6 (A-B), A2aR-/- (C-D), A3R-/- (E-F), and A2aA3R-/- (G-H) mice were injected with ConA, inosine (100 mg/kg), or both, and the level of TNFα and ALT in the serum at 1 hour and 24 hours, respectively, after challenge was determined. Because excessive inflammation is observed in the absence of the A2aR,3 A2aR-/- and A2aA3R-/- mice were injected with 15 mg/kg, whereas the C57BL/6 and A3R-/- mice were injected with 20 mg/kg ConA. This figure shows that inosine inhibited ConA-induced hepatitis only in mice expressing the A3R. Representative results from one of 3 separate experiments.
A3 adenosine receptors are required for the inhibition of ConA-induced ALT and TNFα by inosine. C57BL/6 (A-B), A2aR-/- (C-D), A3R-/- (E-F), and A2aA3R-/- (G-H) mice were injected with ConA, inosine (100 mg/kg), or both, and the level of TNFα and ALT in the serum at 1 hour and 24 hours, respectively, after challenge was determined. Because excessive inflammation is observed in the absence of the A2aR,3 A2aR-/- and A2aA3R-/- mice were injected with 15 mg/kg, whereas the C57BL/6 and A3R-/- mice were injected with 20 mg/kg ConA. This figure shows that inosine inhibited ConA-induced hepatitis only in mice expressing the A3R. Representative results from one of 3 separate experiments.
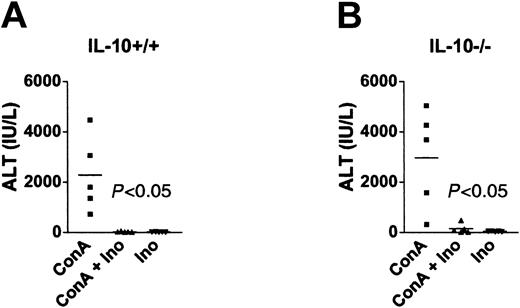
Since inosine had been shown to induce production of the anti-inflammatory cytokine IL-10,19 which was reported to have protective effects over ConA-induced hepatitis,40,41 we compared IL-10+/+ and IL-10-/- mice for susceptibility to inhibition of liver injury by inosine. Figure 2 shows that ALT accumulation was inhibited by inosine in both IL-10+/+ and IL-10-/- mice, indicating that inhibition of liver injury by inosine in vivo was independent of IL-10. Thus, inosine inhibited ConA-induced fulminant hepatitis through an IL-10-independent but A3R-dependent mechanism.
Inosine inhibits ConA-induced liver injury by an IL-10-independent mechanism. IL-10+/+ (A) and IL-10-/- (B) mice were injected with ConA (20 mg/kg), inosine (100 mg/kg), or both, and serum ALT was determined 24 hours after injection. This figure shows that inosine inhibited ConA-induced ALT regardless of the lack of IL-10 production in IL-10-/- mice. Representative results from one of 3 separate experiments.
Inosine inhibits ConA-induced liver injury by an IL-10-independent mechanism. IL-10+/+ (A) and IL-10-/- (B) mice were injected with ConA (20 mg/kg), inosine (100 mg/kg), or both, and serum ALT was determined 24 hours after injection. This figure shows that inosine inhibited ConA-induced ALT regardless of the lack of IL-10 production in IL-10-/- mice. Representative results from one of 3 separate experiments.
Inosine protects liver tissue by inhibiting ConA-induced hepatocyte apoptosis
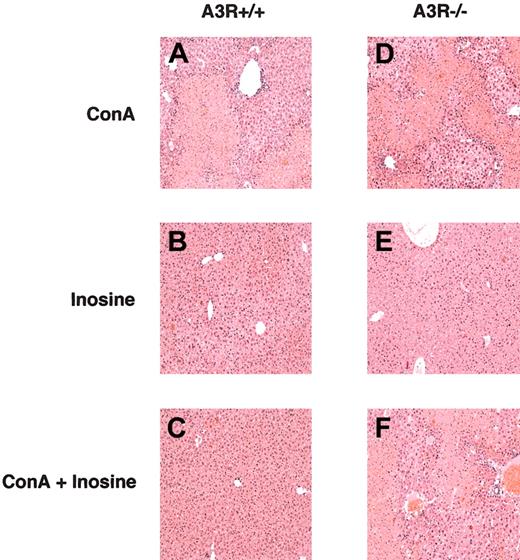
The A3R-dependent anti-inflammatory effect of inosine in the ConA model of hepatitis was further demonstrated by histopathologic examination of tissue sections of liver taken from A3R+/+ and A3R-/- mice. Figure 3 shows ConA induced severe liver damage in both A3R+/+ and A3R-/- mice, but that only A3R+/+ mice were rescued from liver damage by inosine as assessed by H & E staining. It is shown that livers from A3R+/+ and A3R-/- mice sustained severe damage from ConA (Figure 3A,D) but no gross damage from inosine alone (Figure 3B,E). Moreover, Figure 3C,F shows that liver tissue from A3R+/+, but not A3R-/-, mice was protected from ConA-induced damage in vivo by inosine.
Inosine protects A3R+/+ liver tissue from ConA-induced damage in vivo. Tissue sections of liver taken from A3R+/+ (A-C) and A3R-/- (D-F) mice 24 hours after injection with ConA (A,D), inosine (B,E), and ConA plus inosine (C,F) were stained with haemotoxylin and eosin (H&E). This figure shows that inosine protected A3R+/+, but not A3R-/-, liver from ConA-induced damage. Original magnification, × 10.
Inosine protects A3R+/+ liver tissue from ConA-induced damage in vivo. Tissue sections of liver taken from A3R+/+ (A-C) and A3R-/- (D-F) mice 24 hours after injection with ConA (A,D), inosine (B,E), and ConA plus inosine (C,F) were stained with haemotoxylin and eosin (H&E). This figure shows that inosine protected A3R+/+, but not A3R-/-, liver from ConA-induced damage. Original magnification, × 10.
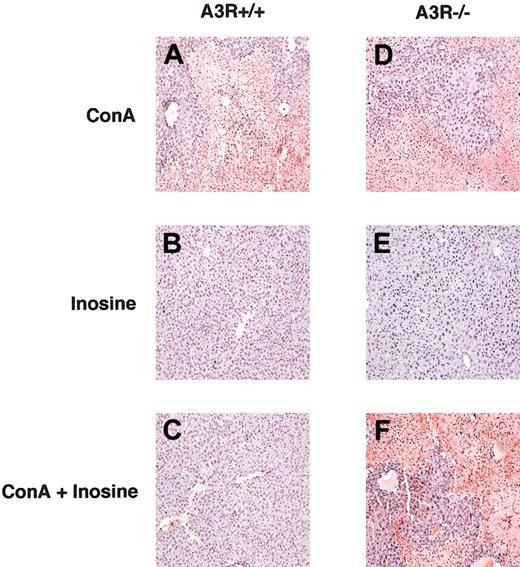
Since the apoptosis of hepatocytes had been suggested to be involved in ConA-induced liver injury,33 we analyzed the effect of inosine on hepatocyte apoptosis in livers from ConA-challenged A3R+/+ and A3R-/- mice by in situ staining of single-strand breaks in nuclear DNA (TdT assay). Figure 4 demonstrates that livers of both A3R+/+ and A3R-/- mice had large numbers of apoptotic cells 24 hours after ConA challenge (Figure 4A,D), but not with inosine treatment alone. In addition, it shows that hepatocyte apoptosis was inhibited in A3R+/+ mice that had been coinjected with inosine as shown in Figure 4C. In contrast, ConA-induced hepatocyte apoptosis was not inhibited by inosine in A3R-/- mice (Figure 4F).
Inosine inhibits ConA-induced hepatocyte apoptosis in vivo. Tissue sections of liver taken from A3R+/+ (A-C) and A3R-/- (D-F) mice 24 hours after injection with ConA (A,D), inosine (B,E), and ConA plus inosine (C,F) were assessed for apoptosis by staining single-strand breaks in nuclear DNA to detect apoptotic cells (TdT assay). This figure shows that inosine protected A3R+/+, but not A3R-/-, liver from ConA-induced damage by inhibiting hepatocyte apoptosis. Original magnification, × 10.
Inosine inhibits ConA-induced hepatocyte apoptosis in vivo. Tissue sections of liver taken from A3R+/+ (A-C) and A3R-/- (D-F) mice 24 hours after injection with ConA (A,D), inosine (B,E), and ConA plus inosine (C,F) were assessed for apoptosis by staining single-strand breaks in nuclear DNA to detect apoptotic cells (TdT assay). This figure shows that inosine protected A3R+/+, but not A3R-/-, liver from ConA-induced damage by inhibiting hepatocyte apoptosis. Original magnification, × 10.
Taken together, these experiments show that inosine inhibited T-cell-triggered inflammatory processes in liver and protected mice from tissue damage by signaling through A3 receptors to inhibit hepatocyte apoptosis. Importantly, they also show that inosine did not utilize A2a receptors in the inhibition of the pathogenesis of acute liver inflammation studied here.
Inosine acts through both A2a and A3 adenosine receptors to inhibit endotoxin-induced inflammation in vivo
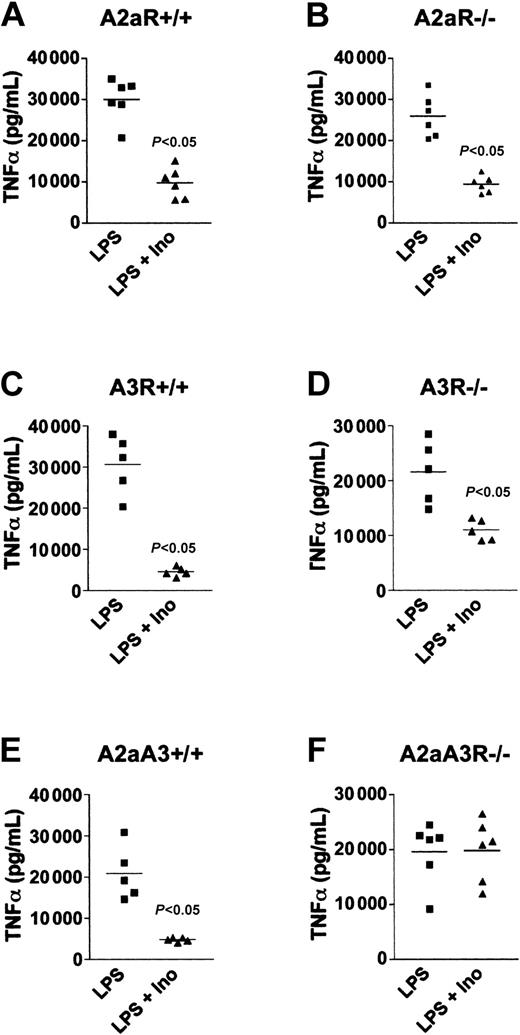
To determine if the mechanism by which inosine inhibited LPS-induced inflammation was also dependent only on A3R, we compared the effect of inosine on wild-type, A2aR-null, A3R-null, and A2aA3R-double-null mice in an in vivo model of LPS-induced endotoxemia. In these experiments, mice were pretreated with inosine (100 mg/kg) followed by an injection of LPS (10 mg/kg). The mice were bled 1 hour after LPS injection and the concentration of TNFα in the serum was measured. As expected, LPS-stimulated TNFα was inhibited in wild-type mice as shown in Figure 5A,C. In addition, we found that wild-type mice when injected with 60 mg/kg of inosine alone had 85% mortality by 24 hours whereas 15% of mice injected with LPS plus inosine died within the same time frame (data not shown). Thus, inosine protected mice over a broad range of inflammatory insult. Importantly, the accumulation of TNFα was inhibited even in the absence of A2aR or A3R in single-gene knockouts (Figure 5B,D), suggesting that inosine used both A2a and A3 receptors subtypes to inhibit endotoxin-stimulated TNFα production in vivo.
Inosine utilizes both A2aR and A3R to inhibit LPS-induced TNFα in vivo. Mice were injected intraperitoneally with 100 mg/kg inosine followed by 10 mg/kg LPS and the level of TNFα in the serum collected 1 hour after LPS injection was assessed. This figure shows that inosine inhibited LPS-induced TNFα production in wild-type (A,C,E), A2aR-/- (B), and A3R-/- (D) mice, but did not inhibit TNFα in A2aA3R-/- (F) mice, which lacked both receptors. Representative results from 1 of 3 separate experiments.
Inosine utilizes both A2aR and A3R to inhibit LPS-induced TNFα in vivo. Mice were injected intraperitoneally with 100 mg/kg inosine followed by 10 mg/kg LPS and the level of TNFα in the serum collected 1 hour after LPS injection was assessed. This figure shows that inosine inhibited LPS-induced TNFα production in wild-type (A,C,E), A2aR-/- (B), and A3R-/- (D) mice, but did not inhibit TNFα in A2aA3R-/- (F) mice, which lacked both receptors. Representative results from 1 of 3 separate experiments.
To test this, we developed and studied A2aA3R-/- double-knockout mice. As predicted from the hypothesis that both A2a and A3 receptors are involved in mediating the anti-inflammatory signaling by inosine, the A2aA3R-/- mice were unresponsive to the inhibitory effect of inosine and maintained high levels of LPS-induced TNFα production in vivo as compared with wild-type littermates (Figure 5E-F). These results thus prove that inosine utilized both A2a and A3 receptor subtypes to inhibit endotoxin-induced inflammation in vivo in this model of LPS-induced endotoxemia.
Pharmacologic activation of A2a or A3 adenosine receptors inhibits endotoxin-induced TNFα production in vivo
The data shown in Figures 1, 2, 3, 4, 5 firmly implicate A2a and A3 receptors in the anti-inflammatory effects of inosine. These receptors have been suggested to be anti-inflammatory in earlier studies of the inhibitory effects of adenosine and pharmacologic agonists of both A2aR and A3R on proinflammatory cytokine production from monocytes/macrophages and mast cells.4,5,42-47 It was important to confirm these pharmacologic observations by genetic in vivo experiments to allow the conclusive implication of individual subtypes of adenosine receptors in the anti-inflammatory effect of inosine.
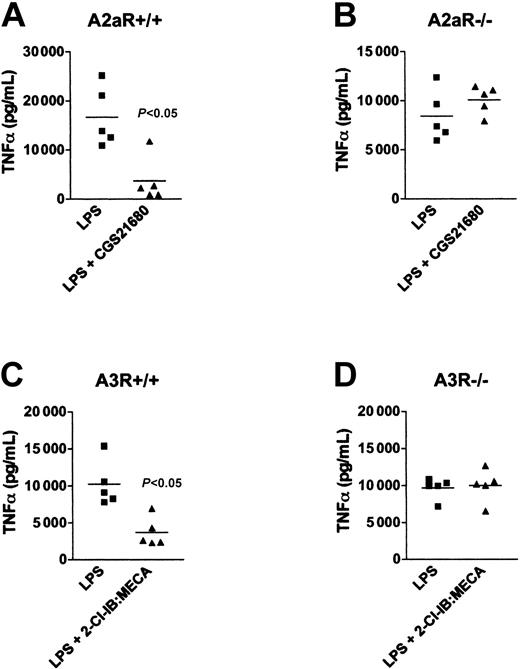
Therefore, to verify that it was the activation of A2a or A3 receptors that caused the inhibition of LPS-induced TNFα production in vivo, we treated wild-type and A2aR-deficient or A3R-deficient mice with selective pharmacologic agonists to A2aR or A3R followed by challenge with LPS. It is shown in Figure 6 that injection of the A2aR agonist CGS21680 or A3R and A1R agonist 2-Cl-IB:MECA into wild-type mice resulted in decreased TNFα levels in blood serum following lethal injection of LPS (Figure 6A,C). In addition, Figure 6 shows that CGS21680 had no effect in A2aR-/- mice (Figure 6D) and, likewise, 2-Cl-IB:MECA had no effect in A3R-/- mice (Figure 6C), confirming earlier studies by Salvatore et al.39 Thus, the data presented here provide the genetic proof that both A2a and A3 receptor subtypes play a role in the negative regulation of LPS-induced TNFα, and support our observation that inosine utilized both receptor subtypes to negatively regulate endotoxin-induced inflammation in vivo. These data also confirm the selectivity of tested pharmacologic agonists in the assays of acute inflammation used in these experiments.
Pharmacologic activation of A2aR or A3R inhibits LPS-induced TNFα production in vivo. A2aR+/+ (A) and A2aR-/- (B) mice were injected with 0.5 mg/kg of CGS21680, and A3R+/+ (C) and A3R-/- (D) mice were injected intraperitoneally with the 0.5 mg/kg of 2-Cl-IB:MECA. Ten minutes later the mice were injected with 10 mg/kg LPS. The mice were bled 1 hour after LPS injection and the level of TNFα in the serum was measured. This figure shows that LPS-induced TNFα was inhibited by CGS21680 or 2-Cl-IB:MECA in A2aR+/+ (A) and A3R-/- (C) mice, respectively. However, these adenosine receptor-specific agonists did not affect A2aR-/- and A3R-/- mice (B and D, respectively). Representative results from 1 of 3 separate experiments.
Pharmacologic activation of A2aR or A3R inhibits LPS-induced TNFα production in vivo. A2aR+/+ (A) and A2aR-/- (B) mice were injected with 0.5 mg/kg of CGS21680, and A3R+/+ (C) and A3R-/- (D) mice were injected intraperitoneally with the 0.5 mg/kg of 2-Cl-IB:MECA. Ten minutes later the mice were injected with 10 mg/kg LPS. The mice were bled 1 hour after LPS injection and the level of TNFα in the serum was measured. This figure shows that LPS-induced TNFα was inhibited by CGS21680 or 2-Cl-IB:MECA in A2aR+/+ (A) and A3R-/- (C) mice, respectively. However, these adenosine receptor-specific agonists did not affect A2aR-/- and A3R-/- mice (B and D, respectively). Representative results from 1 of 3 separate experiments.
Discussion
In this report, we addressed the issue of which adenosine receptors were essential for the in vivo protective anti-inflammatory effect of the endogenous purine inosine. This was important since the biochemical and pharmacologic evidence to support or refute the use of adenosine receptors other than the A3R by inosine was conflicting. Therefore, genetic in vivo testing in assays of inflammatory tissue damage was required to resolve the issue. The question as to which adenosine receptor(s) is/are utilized functionally by inosine was intriguing given that inosine was reported to induce mast cell degranulation (via A3R binding),26,27,39 a proinflammatory event, yet inosine inhibited endotoxin-induced proinflammatory cytokine production from macrophages, an anti-inflammatory event, and protected mice from endotoxemia.19 This ability of inosine to trigger both pro- and anti-inflammatory actions suggested to us that inosine utilizes more than one type of adenosine receptor.
Our study specifically addressed the requirement for A2a and/or A3 adenosine receptors for the protective effect of inosine in 2 experimental in vivo murine models of disease. Our experimental data clearly show that A3R was required for the inosine-mediated inhibition of ConA-induced liver damage since wild-type and A2aR-/-, but not A3R-/- or A2aA3R double-knockout mice, were rescued from inflammatory damage. The data also show that the requirement for adenosine receptors in the LPS model studied was different than that in the ConA model. In this case, the data show that both A2aR and A3R were employed in protecting mice from LPS-induced endotoxemia since both A2aR and A3R single-knockout mice, but not A2aA3R double-knockout mice, were protected by inosine in this model. Based on these data, we concluded that both A2a and A3 adenosine receptors were utilized for inosine to protect tissue from excessive inflammatory damage in vivo. Furthermore, we concluded that the type of inflammatory stimuli determines the overall requirement for one or both receptors.
The possibility that the observed protective effect was a consequence of an indirect action of inosine should be considered. It was possible that the anti-inflammatory effect of inosine was a consequence of indirect activation of A3 receptors leading to the release of mast cell mediators including adenosine triphosphate, which might be converted to adenosine and activate other adenosine receptors, and histamine, which has been shown to modulate cytokine production.48 Indeed, our data showing that only A3R-expressing mice were protected from ConA-induced liver damage might be suggestive of such an indirect effect of inosine rather than a direct activation of A2a receptors. However, if this were the case then one would expect similar results in the model of LPS-induced endotoxemia as in the ConA experiments such that only mice expressing A3R would be rescued from inflammatory damage by inosine. That is, if the observed effect of inosine is due to the release of anti-inflammatory mediators from mast cells following activation of A3 receptors by inosine then only mice expressing A3 receptors should be protected from inflammation following injection of inosine. In fact, this was not the case since A3R-/- mice were rescued from inflammation in our in vivo model of LPS-induced endotoxemia. Moreover, it was only when both A2a and A3 receptors were absent that mice were unprotected from inflammatory damage caused by LPS in this model.
It was also possible that inosine had systemic effects that influenced the observed experimental results. Inosine had been shown to have positive effects on the cardiovascular system and on neuronal growth.49-51 Perhaps most interesting is the possibility that alterations in cellular metabolic processes or of inosine itself were responsible for our observations. In a very recent article, Scott et al52 reported that administration of inosine or inosinic acid suppressed clinical signs of experimental allergic encephalomyelitis (EAE) and promoted recovery from the disease in their model. Importantly, they showed that intraperitoneal administration of inosine or inosinic acid resulted in a transient, minor increase in serum levels of inosine but a strong increase in uric acid. In addition, they showed that uric acid but not inosine accumulated in the spinal cord tissue of mice with EAE fed inosine or inosinic acid. The authors concluded that the mode of action of inosine and inosinic acid on EAE was due to its metabolism to uric acid. These data suggest that metabolism of inosine might be responsible for the observations reported here. However, if the protective effect of inosine in our studies was due to an indirect consequence of its effect on a nonimmune system rather than a direct activation of adenosine receptors then one might expect the same or no requirement for adenosine receptors under different inflammatory conditions. In our study, there was clearly a differential requirement for both A2a and A3 receptors in the models studied. Nevertheless, it remains to be determined if direct administration of uric acid exerts the same protective anti-inflammatory effect as inosine. If so, does uric acid have the same requirement for A2a and A3 adenosine receptors as reported here for inosine?
Although the possibility exists that activation of A2a receptors was an indirect consequence of the effect of inosine on A3 receptors, the data from the LPS model of endotoxemia clearly show the independent participation of both A2aR and A3R in response to inosine in vivo.
The differences in the effect of inosine on the different inflammatory models have important mechanistic implications. The obtained results are consistent with the hypothesis that different inflammatory stimuli will result in recruitment of different types of immune cells with a different repertoire of purinergic receptors. Accordingly, it is the type of immune cells and the expression of different subtypes of adenosine receptors that will determine the final outcome of regulation by extracellular purines, including inosine. For example, ConA-induced liver injury is triggered by activation of T-cell receptors by ConA and the resulting hepatocyte death is mediated by the complex interplay of T cells, NK T cells, neutrophils, Kupffer cells, and cytokines such as IFNγ, TNFα, IL-4, IL-12, and IL-18, as well as nitric oxide and other molecules.28-34 On the other hand, LPS-induced endotoxemic processes are triggered by activation of Toll-like receptors mostly on macrophages and other cells, but not T cells.35-37 It remains to be determined in detailed future studies as to why the activation of either A2a or A3 receptors was sufficient for inhibition of LPS-induced inflammation, whereas only A3 receptors were required for the inhibition of ConA-induced liver damage. At least one explanation may be that there are differences in the time course of up-regulation of A2aR and/or A3R on T cells versus macrophages and neutrophils during the course of ConA-induced or LPS-induced immune cell activation in the models of acute inflammation tested. It seems likely that excessive inflammatory tissue damage causes accumulation of extracellular inosine and signaling through adenosine receptors on immune cells in local tissue environment. Since the expression of purinergic receptors on immune cells is dependent on activation and differentiation, only those immune cells that express sufficient numbers of adenosine receptors will be susceptible to the protective effect of inosine. In addition, it is possible, but has to be experimentally tested, that the time course of up-regulation of A2a receptors will differ from that of A3 receptors and, as a result, different immune cells will express different combinations of A2a versus A3 receptors at different time points in vivo.
Taken together, the data reported here indicate that inosine has properties that are consistent with it being an endogenous metabolic “switch” that serves to modulate inflammation in vivo. The ability of inosine to functionally activate A2a receptors as reported here extends the anti-inflammatory effect of inosine to include negative regulation of immune cells that do not express A3R, but do express a sufficient number of A2a receptors as well as enhanced inhibition of immune cells that express both A2aR and A3R. Thus, we propose that both A2a and A3 purinergic receptors are functionally utilized, directly or indirectly, by endogenous inosine with potential to protect tissue from excessive inflammatory damage, and that down-regulation of tissue damage in different inflammatory conditions is under control of the complex interplay of different types of inflammatory cells with different repertoire of adenosine receptors. Although the models used in this study are experimental and might have limited similarities with actual disease states in humans, these findings may prove important in the understanding of molecular mechanisms of the pathogenesis of acute inflammation-associated diseases and in the search for pharmacologic anti-inflammatory agents for the treatment of inflammatory/autoimmune disease such as hepatitis, arthritis, and sepsis.
Prepublished online as Blood First Edition Paper, August 28, 2003; DOI 10.1182/blood-2002-11-3624.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Marlene Jacobson for the A3R-/- mice; Jiang Fan Chan for the A2aR-/- mice; and Ricardo Orellana, Charles Caldwell, Manfred Thiel, and Akio Ohta for technical support and helpful discussions. We also thank Mrs Leticia Gomez for help in preparation of the manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal