Abstract
Interaction of very late antigen-4 (VLA-4) with its ligand vascular cell adhesion molecule-1 (VCAM-1) is required for central nervous system (CNS) migration of encephalitogenic T cells in relapsing experimental autoimmune encephalomyelitis (R-EAE). Anti-VLA-4 monoclonal antibody (mAb) treatment prior to EAE onset inhibits disease induction; however, treatment initiated after the appearance of clinical symptoms increases relapse rates, augments Th1 responses, and enhances epitope spreading perhaps due to the activation of costimulatory signals. To negate the potential costimulatory activity of intact anti-VLA-4, we examined the ability of BIO 5192, a small-molecule VLA-4 antagonist, to regulate active proteolipid protein 139-151 (PLP139-151)-induced R-EAE. BIO 5192 administered one week after peptide priming (ie, before clinical disease onset) delayed the clinical disease onset but led to severe disease exacerbation upon treatment removal. BIO 5192 treatment initiated during disease remission moderately enhanced clinical disease while mice were on treatment and also resulted in posttreatment exacerbation. Interestingly, BIO 5192 treatment begun at the peak of acute disease accelerated entrance into disease remission and inhibited relapses, but treatment removal again exacerbated disease. Enhanced disease was caused by the release of encephalitogenic cells from the periphery and the rapid accumulation of T cells in the CNS. Collectively, these results further demonstrate the complexity of VLA-4/VCAM interactions, particularly in a relapsing-remitting autoimmune disease. (Blood. 2003;102:4464-4471)
Introduction
Migration of encephalitogenic CD4+ T cells to the central nervous system (CNS) induces relapsing experimental autoimmune encephalomyelitis (R-EAE), a disease model of multiple sclerosis (MS) characterized by inflammation and demyelination of the CNS.1 Active immunization of Swiss Jackson Laboratory (SJL) mice with the immunodominant epitope of proteolipid protein, proteolipid protein 139-151 (PLP139-151), induces a moderate to severe acute paralytic phase of R-EAE followed by remission and subsequent relapses.2 Relapses are mediated by epitope spreading, a process in which T cells respond against endogenous myelin peptides recruited secondary to acute CNS damage.3,4 Tolerization with the PLP178-191 peptide during remission from acute PLP139-151-induced R-EAE inhibits development of clinical relapses, confirming the predominant pathologic role of PLP178-191-specific T cells in disease progression.3,5,6
Elucidation of the mechanisms by which activated T cells cross the blood-brain barrier and gain entry to the CNS is of significant importance to the pathogenesis of R-EAE and MS. The integrin α4β1, also known as the very late activation antigen-4 (VLA-4), has been shown to play an integral part in the CNS homing of cells that induce disease.7-10 Interaction of VLA-4 with its ligand, vascular cell adhesion molecule-1 (VCAM-1), which is expressed on CNS endothelium, allows entry of encephalitogenic T cells into the CNS.7 Interfering with this interaction is postulated to have a potential beneficial therapeutic effect for many autoimmune diseases, including MS.
In addition to its role in T-cell entry into the CNS, VLA-4 likely plays an important role in other immune functions. For example, VLA-4 may act as a costimulatory molecule on T cells and could therefore influence T-cell activation and differentiation.11-14 Potential costimulatory activities could explain the results observed in our previous study in which treatment with an anti-VLA-4 monoclonal antibody (mAb) initiated during ongoing disease increased disease relapses and accumulation of CD4+ T cells in the CNS.15 Most significantly, treatment with anti-VLA-4 either prior to or during ongoing R-EAE enhanced Th1 responses to both the priming peptide and to endogenous myelin epitopes released secondary to acute tissue damage (ie, epitope spreading). These results suggest that treatment with anti-VLA-4 mAb has multiple effects on the immune system. Despite the enhanced clinical EAE observed when mice with pre-existing disease were treated with anti-VLA-4, small-molecule VLA-4 antagonists that block homing of activated T cells without triggering costimulatory or other potential effector activities remain as promising therapeutics for treatment of autoimmune diseases.
Considering the complex role of VLA-4 in immune responses and to obviate the potential delivery of agonistic T-cell costimulatory signals by bivalent anti-VLA-4 mAbs, we examined the effects of long-term treatment with the small-molecule VLA-4 antagonist, BIO 5192, begun either prior to or after the appearance of clinical symptoms in active R-EAE. In this study, 21 daily treatments of BIO 5192 were preclinically administered at the peak of acute disease or during the first disease remission. The subsequent effect on disease course was determined. Although preclinical administration of anti-VLA-4 delayed R-EAE induction, 50% of animals began to exhibit clinical signs of EAE while on treatment and 80% experienced a striking posttreatment disease exacerbation 5 days after termination of treatment. Treatment initiated during disease remission moderately enhanced disease of mice during treatment and posttreatment disease exacerbation was again observed. In contrast, the relapse rate was significantly lowered in mice in which treatment was initiated at the peak of acute disease while the animals remained on BIO 5192 despite the fact that the mice exhibited augmented peripheral myelin peptide-specific Th1 responses. However, following cessation of treatment, encephalitogenic cells were released from peripheral lymphoid compartments again resulting in a striking posttreatment disease exacerbation characterized by increased infiltration of CD4+ T cells into the CNS.
Materials and methods
Animals
Six- to 7-week-old female SJL mice were obtained from Harlan Laboratories (Indianapolis, IN). Mice were housed under barrier conditions according to a protocol approved by the Northwestern University Animal Care and Use Committee. Paralyzed mice were afforded easier access to food and water.
Peptides
PLP139-151 (HSLGKWLGHPDKF), PLP178-191 (NTWTTCQSIAFPSK), and myelin basic protein (MBP84-104) (VHFFKNIVTPRTPPPSQGKGR) were synthesized by the peptide facility at the University of North Carolina (Chapel Hill, NC).Amino acid composition of these peptides was verified by mass spectrometry, and purity (> 97%) was confirmed by mass spectroscopy at the Michigan State University Biotechnology Center.
Active induction of R-EAE
Mice were subcutaneously immunized with 100 μL complete Freund adjuvant (CFA) emulsion containing 200 μg Mycobacterium tuberculosis H37Ra (Difco, Detroit, MI) and 50 μg PLP139-151 distributed over 3 spots across the flank.
In vivo small-molecule antagonist treatment
BIO 5192 [2(S)-{[1-3,5-dichloro-benzenesulfonyl)-pyrrolidine-2(S)-carbonyl]-amino}-4-[4-methyl-2(S)-(methyl-{2-[4-(3-o-tolyl-ureido)-phenyl]acetyl}-amino)-pentanoylamino]-butyric acid] has been shown to be a potent (Kd of < 10 pM) and highly selective inhibitor of both the unactivated and activated forms human, mouse, and rat α4β1 integrin.16 BIO 5192 as well as the tris lactose vehicle were supplied by Biogen (Cambridge, MA). In initial experiments, mice were treated daily for 21 days by the intraperitoneal route with vehicle or 600 μg BIO 5192. Treatments were initiated prior to disease onset (day 7), at the peak of acute disease (approximately day 15), or during the first remission (approximately day 21). For acute and remission treatments, treatment was individualized in order to begin treatment at the appropriate stage of disease. In a subsequent experiment, treatment was initiated at the peak of acute disease and animals received daily treatment for either 22 or 44 days with vehicle or 600 μg BIO 5192. Treatment was again individualized and administered subcutaneously in a total volume of 100 μL.
Clinical evaluation
Clinical severity was assessed on a 0 to 5 scale as follows: grade 0, no abnormality; grade 1, limp tail or hind limb weakness; grade 2, limp tail and hind limb weakness (waddling gait); grade 3, partial hind limb paralysis; grade 4, complete hind limb paralysis; and grade 5, moribund. A relapse was defined as a sustained increase (> 2 days) of at least one full grade in clinical score after the animal had previously improved at least a full clinical score and had stabilized for at least 2 days. The data are plotted as the mean clinical score for all animals in a particular treatment group or as the relapse rate (total number of relapses in a group during the observation period divided by the total number of mice in that group). Mean peak clinical score is defined as the greatest clinical score reached during the specified phase of disease/treatment. The severity index was calculated by adding the clinical scores for each mouse over a specified time period and dividing this number by the number of scores examined.
Preparation of tissue for immunohistochemistry
At days 39 and 44 after priming (for mice in which treatment was initiated at the peak of acute disease phase and treated for 22 days), 2 mice per time point were perfused with phosphate-buffered saline (PBS) and fresh 4% paraformaldehyde (Sigma, St Louis, MO). Spinal cords and brains were dissected out and blocks of tissue were flash frozen in octopamine (OCT) compound (Miles Laboratories, Elkart, IN) with liquid nitrogen. Tissue from the lower thoracic region of the spinal cord was sectioned at 6 μmona Reichert-Jung 1800 cryotome (Leica Instruments, Deerfield, IL) and mounted on Superfrost Plus electrostatically charged slides (Fisher Scientific, Pittsburgh, PA).
Immunohistochemistry
Slides were stained using tyramide signal amplification (TSA) Direct Kit (NEN Life Science Products, Boston, MA) according to the manufacturer's protocol. Tissue was thawed, air dried, and rehydrated in PBS. After catalyzing endogenous peroxidases in 0.3% hydrogen peroxide, nonspecific staining was blocked using an avidin/biotin blocking kit (Vector Labs, Burlingame, CA) in addition to the blocking reagent provided by the TSA kit. For CD4, CD8, B220, and VCAM-1 staining, slides were incubated with biotinylated anti-L3T4, anti-Ly-2, RA3-6B2, and 429 (MVCAM.A; PharMingen, San Diego, CA), respectively, for 1 hour at room temperature. Sections were counterstained with 0.1 μg/mL DAPI (4,6 diamidino-2-phenylindole; Sigma) and coverslipped with Vectashield mounting medium (Vector). Tissue was examined by epifluorescence using a chroma triple-band filter (Chroma Technology, Brattleboro, VT).
Isolation of mRNA, first-strand cDNA synthesis, and reverse transcriptase-polymerase chain reaction (RT-PCR)
At day 39 and day 44 after priming (for mice treated for 22 days beginning at the acute phase), 5 mice per group were anesthetized and perfused through the left ventricle with 30 mL PBS. Spinal cords were extruded by flushing the vertebral canal with PBS and then rinsed in PBS. Tissues were forced through a 100-mesh stainless steel screen to give a single cell suspension and pelleted by centrifugation (500g) for 5 minutes at 4°C. The pellets were resuspended vigorously in 16 mL 4 M guanidinium isothiocyanate/50 mM Tris-Cl (pH 7.5)/25 mM EDTA (ethylenediaminetetraacetic acid; Life Technologies, Gaithersburg, MD), and 1% 2-mercapto-ethanol (2-ME) and 0.5% N-lauroylsarcosine (Sigma-Aldrich, St Louis, MO). Shearing of DNA was facilitated by repeatedly forcing the resulting suspension through a 23-gauge needle. Total RNA was isolated by high-speed gradient centrifugation (27 000 rpm) of 8-mL lysate through a 3-mL 5.7 M CsCl pad using a SW41 swinging bucket rotor (Beckman Coulter, Palo Alto, CA) for 20 hours at 4°C. The resulting RNA pellet was resuspended to a final concentration of 1 μg/μL with diethylpyrocarbonate-treated water and stored in aliquots at -70°C. First-strand cDNA was generated from 2 μg total RNA using Advantage-RT Kit (Clontech, Palo Alto, CA) using 20 pmol oligo(dT) primer, per the manufacturer's provided protocol, in a total volume of 20 μL. Following first-strand synthesis, each cDNA sample was brought to a final volume of 100 μL with distilled water. Final PCR conditions included 50 mM KCl, 10 mM Tris-Cl (pH 8.3), 2.5 to 5 mM MgCl2, 2 mM deoxynucleoside triphosphates (dNTPs), 100 pmol each of 5′ and 3′ gene-specific primer, 1 U Taq polymerase (Qiagen, Chatsworth, CA), and 5 to 10 μL diluted cDNA. The primers were synthesized by Life Technologies and amplify both the competitor plasmid and wild-type cDNA. Expression of interleukin 4 (IL-4; 5′CAT CGG CAT TTT GAA CGA GGT CA, 3′ CTT ATC GAT GAA TCC AGG CAT CG), IL-5 (5′GAA AGA GAC CTT GAC ACA GCT G, 3′GAA CTC TTG CAG GTAATC CAG G), IL-10 (5′CCA GTT TTA CCT GGT AGAAGT GAT G, 3′TGT CTA GGT CCT GGA GTC CAG CAG ACT CAA), interferon γ (IFN-γ; 5′CTT GGA TAT CTG GAG GAA CTG GC, 3′GCG CTG GACCTGTGGGTTGTTGA), macrophage inflammatory protein-1α (MIP-1α; 5′ ATG AAG GTC TCC ACC ACT GCC CTT G, 3′ GGC ATT CAG TC CAG GTC AGT GAT), and tumor necrosis factor α (TNF-α; 5′GTT CTA TGG CCC AGA CCC TCA CA, 3′TAC CAGGGTTTGAGCTCAGC) genes were examined. Cycling conditions were 94°C, 40 seconds; 60°C, 20 seconds; and 72°C, 40 seconds for a total of 35 cycles, linked to a final 72°C extension program for 3 minutes and then to a final 4°C soak program. PCR products were run on an ethidium bromide-containing 2% agarose gel and illuminated using a UV light source, then photographed using Polaroid type 667 film.
In vitro T-cell proliferation and cytokine assays
Spleen and draining lymph node cells (axillary and inguinal) were obtained from the same 5 animals used for RT-PCR analyses. Cells were dissociated and cultured in 96-well microtiter plates (Costar, Cambridge, MA) at a density of 5 × 105 viable cells/well in a total volume of 200 μL peptide Dulbecco modified eagle medium (DMEM) supplemented with 10% fetal calf serum (FCS; Sigma), 100 U/mL penicillin (Life Technologies), 100 μg/mL streptomycin (Life Technologies) and 2 mM L-glutamine (Life Technologies). Cells were cultured at 37°C in 100% humidity and 5% CO2 in the presence or absence (background) of varying concentrations of either PLP139-151 or PLP178-191. As a control, concanavalin A (Sigma) was used at a concentration of 5 μg/mL. Cultures were pulsed with 1 μCi (0.037 MBq) of 3H-TdR ([3H]thymidine incorporation; ICN Pharmaceuticals, Irvine, CA) after 72 hours, harvested at 96 hours, and 3H-TdR uptake was detected using a Packard Topcount microplate scintillation counter (Packard Instruments, Meriden, CA). Results are expressed as mean counts per minute (CPM) of triplicate cultures ± SEM. For cytokine analysis, 2.5 × 106 spleen and lymph node cells were cultured in a total volume of 1 mL supplemented DMEM and 25 μM PLP139-151, PLP178-191, or DMEM alone. Supernatants were harvested at 48 and 72 hours and analyzed for IL-2, IL-4, and IFN-γ by capture enzyme-linked immunosorbent assay (ELISA). ELISA reagents were purchased from Endogen (Woburn, MA) and were used according to the manufacturer's protocol. Briefly, 96-well Nunc Maxisorb plates were coated overnight with anticytokine capture antibody followed by washing. Cytokine standards and samples were added to coated wells and were incubated at 20°C overnight and washed. Biotinylated anticytokine antibody was added followed by washing and the addition of streptavidin-horseradish peroxidase (HRP) conjugate. DAKO (Carpinteria, CA) tetramethyl benzidine (TMB) liquid substrate system was used. The plates were read at 450 nM.
Statistical analysis
Differences in clinical scores between control and treatment groups were analyzed by Student t test. Differences in relapse rates between control and treatment groups were analyzed by Chi-square. P values less than .05 were considered significant.
Results
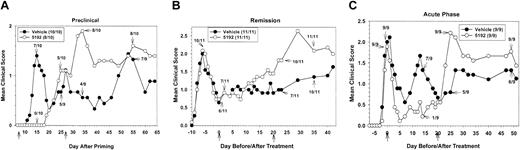
Treatment with small-molecule VLA-4 antagonist initiated prior to disease onset delays clinical signs of R-EAE but results in a posttreatment exacerbation
Previous studies suggest that VLA-4 (CD49d) interaction with its ligand VCAM-1 (CD106) is important for the induction of EAE7 and that surface expression of the α4 integrin on CD4+ T cells is required for their entry into the CNS parenchyma.9,17 Treatment with mAb to CD49d, the α4 integrin, initiated at time of priming effectively inhibited clinical signs of EAE.7,9 We thus asked whether treatment with BIO 5192, a monovalent small-molecule VLA-4 antagonist, initiated at day 7 after priming, after the initial myelin-specific T-cell activation in the periphery but prior to clinical disease onset, would also inhibit initiation of R-EAE. BIO 5192 works by blockading α4β1/ligand interactions without down-modulating the receptor.16 By day 15 after priming (after 9 treatments), 7 of 10 of vehicle-treated mice showed signs of clinical R-EAE, whereas 0 of 10 in the BIO 5192-treated group were affected (Figure 1A). However, clinical signs of R-EAE began to develop in the BIO 5192 group at day 19 after priming. The average mean day of disease onset for the BIO 5192-treated group was 25.1, which was significantly greater than the mean day of onset of 15.9 for the vehicle controls (Table 1). By day 25 (after the final treatment), 5 of 10 BIO 5192-treated animals exhibited clinical signs of R-EAE (acute phase) compared with 5 of 9 (relapsing phase) in the vehicle-treated controls. A synchronous, striking disease exacerbation was observed 5 days after treatment with BIO 5192 was withdrawn. In fact, one week after treatment was stopped, animals that had been treated with BIO 5192 reached a mean clinical score of 1.90 compared with a mean clinical score of 0.56 in the vehicle-treated group (P < .05). Furthermore, the 15-day posttreatment severity index of 1.40 was significantly greater (P < .05) in BIO 5192-treated animals than the index of 0.57 in vehicle-treated animals (Table 1). Following the posttreatment exacerbation, 5 of 10 of the animals that had received the small-molecule antagonist compared with 2 of 9 control animals entered into a chronic phase of R-EAE.
Differential effects of treatment with the monovalent anti-VLA-4 inhibitor BIO 5192 on R-EAE. The indicated numbers of SJL mice were treated intraperitoneally daily for 21 treatments with either tris lactose vehicle or 600 μg of the small-molecule VLA-4 antagonist, BIO 5192, beginning prior to disease onset (A), during disease remission (B), or at the peak of the acute phase of disease (C). In Panel A, data are represented as mean clinical score versus day after priming. In panels B and C, treatment was tailored to each mouse depending on disease state and therefore data are represented as mean clinical score versus days prior to the first treatment, the first day of treatment, and days after treatment onset.
Differential effects of treatment with the monovalent anti-VLA-4 inhibitor BIO 5192 on R-EAE. The indicated numbers of SJL mice were treated intraperitoneally daily for 21 treatments with either tris lactose vehicle or 600 μg of the small-molecule VLA-4 antagonist, BIO 5192, beginning prior to disease onset (A), during disease remission (B), or at the peak of the acute phase of disease (C). In Panel A, data are represented as mean clinical score versus day after priming. In panels B and C, treatment was tailored to each mouse depending on disease state and therefore data are represented as mean clinical score versus days prior to the first treatment, the first day of treatment, and days after treatment onset.
BIO 5192 treatment initiated during disease remission moderately enhances clinical signs of R-EAE while on treatment and also results in posttreatment exacerbation
When treatment was initiated during disease remission, animals in the vehicle- and BIO 5192-treated group had a similar mean day of disease onset and disease incidence (Figure 1B). While on treatment, clinical disease parameters were not affected compared with controls until day 15, when disease in BIO 5192-treated mice worsened somewhat. However, when treatment ended, the BIO 5192 group displayed a synchronous exacerbation of disease. As seen in Table 1, the 15-day posttreatment severity index of 2.23 for BIO 5192-treated animals was significantly greater than the 1.41 in vehicle-treated mice (P < .05). None of the BIO 5192-treated mice recovered from relapses and 11 of 11 animals in the BIO 5192 group remained chronic compared with only 4 of 11 in the vehicle-treated group (P < .01), accounting for similar relapse rates once treatment was removed (1.09 in the vehicle group vs 1.18 in the BIO 5192 group).
Treatment initiation at the peak of the acute phase accelerates entrance into disease remission, inhibits relapses while on treatment, but results in a posttreatment exacerbation
BIO 5192 was also administered at the peak of the acute phase and the resultant effects on R-EAE were monitored. It is important to note that animals in the vehicle-treated group had similar mean day of onset of R-EAE (12.6 in the vehicle group vs 13.0 in the BIO 5192 group), incidence (9/9), and severity compared with the BIO 5192-treated group before the start of treatment. In contrast to initiation of treatment in remission, disease relapses were inhibited when BIO 5192 was administered at the peak of the acute phase (Figure 1C). After 15 treatments, only 1 of 9 BIO 5192-treated animals displayed clinical signs of R-EAE compared with 7 of 9 in the vehicle group (P < .02). This inhibition is further demonstrated by the 15-day severity index of 0.36 for the BIO 5192 group compared with 1.03 in the vehicle control group during treatment (Table 1; P < .05). Interestingly, not only were relapses inhibited, but mice in the BIO 5192 group also entered remission faster. After 4 treatments, BIO 5192-treated animals had a mean clinical score of 0.44 compared with a mean clinical score of 1.22 for vehicle-treated mice (P < .02). Once treatment was stopped, however, a striking posttreatment clinical exacerbation was again observed. Five days after cessation of treatment, all 9 BIO 5192-treated animals displayed clinical signs of R-EAE with a mean clinical score of 2.22 compared with 5 of 9 animals in the vehicle control group with a mean clinical score of 0.80. The 15-day posttreatment severity index was 1.81 in BIO 5192-treated animals versus 1.20 in vehicle-treated animals. A summary of disease parameters for preclinical, remission, and acute initiation of treatment is shown in Table 1.
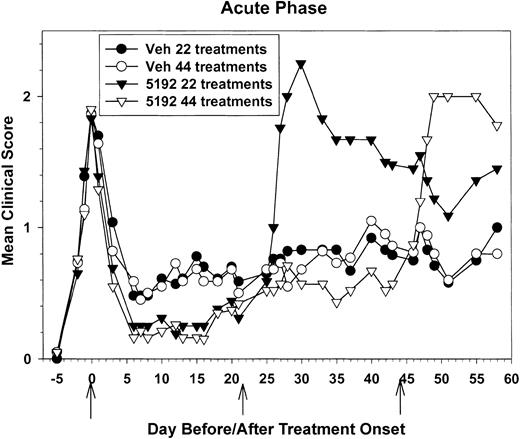
Due to the severe exacerbation observed after removal of BIO 5192, we compared the ability of short-term treatment (22 daily treatments) versus long-term drug treatment (44 daily treatments) beginning at the peak of acute phase to maintain disease regulation. Once again, the onset of disease remission was accelerated in the BIO 5192-treated animals (Figure 2). After 4 treatments, the BIO 5192-treated groups had a mean clinical score of 0.56 compared with a mean clinical score of 0.93 for the vehicle control group (P < .05). However, beginning around day-15 posttreatment initiation (PTI), BIO 5192-treated groups started to exhibit slightly increased disease severity. This trend continued to a point around day 20 PTI where no inhibition of disease was seen and the mean clinical scores of treated and control groups were equal (Figure 2). This confirms the results seen in Figure 1. However, as shown in Table 2, while the mice were on treatment, both the relapse rate and the severity index were significantly decreased in mice treated with BIO 5192 for 22 or 44 days compared with the vehicle control group.
Treatment initiation at the peak of the acute phase accelerates entrance into disease remission, inhibits relapses while on treatment, but results in posttreatment disease exacerbation. Twenty-two recipient mice per group were treated with control tris lactose vehicle or 600 μg of the small-molecule VLA-4 antagonist BIO 5192 for 22 consecutive days beginning at the peak of acute disease. Also, twenty-one recipient mice per group were treated with control tris lactose vehicle or BIO 5192 for 44 consecutive days beginning at the peak of acute disease. Treatment was individualized to each mouse depending on disease state and therefore data are represented as mean clinical score versus days prior to the first treatment, the first day of treatment, and days after treatment onset.
Treatment initiation at the peak of the acute phase accelerates entrance into disease remission, inhibits relapses while on treatment, but results in posttreatment disease exacerbation. Twenty-two recipient mice per group were treated with control tris lactose vehicle or 600 μg of the small-molecule VLA-4 antagonist BIO 5192 for 22 consecutive days beginning at the peak of acute disease. Also, twenty-one recipient mice per group were treated with control tris lactose vehicle or BIO 5192 for 44 consecutive days beginning at the peak of acute disease. Treatment was individualized to each mouse depending on disease state and therefore data are represented as mean clinical score versus days prior to the first treatment, the first day of treatment, and days after treatment onset.
Mice removed from the 22-day BIO 5192 treatment experienced a severe disease exacerbation 4 to 5 days after treatment was stopped and this peaked at a mean clinical score of 2.25 (vs 0.83 in sham-treated controls; P < .05) just 4 days later (Figure 2). The mean peak clinical scores (2.64 vs 1.08), the 20-day severity index (1.77 vs 0.81), and the relapse rate (1.0 vs 0.42) were significantly higher (P < .05) in the BIO 5192-treated groups after treatment was discontinued (Table 2). A similar pattern was observed when mice receiving the 44-day regimen were removed from BIO 5192 treatment. These animals exhibited posttreatment exacerbation that peaked at a mean clinical score of 2.0 six days after treatment ceased compared with the vehicle control group, which had a mean clinical score of only 0.80 (Figure 2). The mean peak clinical scores, the severity indices, and relapse rates were all significantly higher in the BIO 5192 versus vehicle control groups (P < .05) after treatment cessation, regardless of whether the animals had been treated for 22 or 44 days (Table 2). Thus, reduced relapses were observed as long as BIO 5192 treatment continued, but a severe relapse occurred shortly after treatment was discontinued.
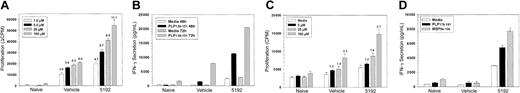
Acute phase initiation of treatment with BIO 5192 enhances proliferation and IFN-γ secretion in response to the priming epitope and augments epitope spreading
PLP139-151-specific proliferative responses (Figure 3A) and IFN-γ secretion (Figure 3B) of splenic T cells taken on day 22 PTI (day 39 after priming) were elevated upon stimulation with a range of doses of the priming peptide in mice that had begun BIO 5192 treatment at the peak of the acute disease (Figure 2). In addition, 48-hour IL-2 secretion was also substantially elevated and enhanced proliferative responses in the lymph nodes were also observed at this time (data not shown). Proliferation of splenic T cells to the primary relapse-associated PLP178-191 epitope5,6 was slightly higher in the BIO 5192-treated animals (Figure 3C). More significantly, secretion of IFN-γ at 72 hours to PLP178-191 and to MBP84-104, a second epitope often associated with disease relapse, was also substantially higher in BIO 5192-treated animals (Figure 3D). Interestingly, this was at a time when BIO 5192-treated mice displayed a slightly less severe clinical disease than vehicle controls (Figure 2). By day-27 PTI, when BIO 5192-treated mice were approximately at the peak of the posttreatment exacerbation (Figure 2), splenic PLP139-151-specific proliferation (Figure 4A), IFN-γ secretion (Figure 4B), and IL-2 secretion (Figure 4C) had returned to levels observed in the vehicle control group. Also, at this time point, proliferation, IFN-γ secretion, and IL-2 secretion of PLP139-151-specific lymph node T cells returned to vehicle control levels (data not shown). Proliferation of splenic T cells to the primary relapse-associated PLP178-191 epitope was similar in both the BIO 5192- and vehicle control-treated animals (data not shown).
Mice undergoing short-term treatment with BIO 5192 initiated at the peak of acute disease display enhanced proliferation and IFN-γ secretion in response to the priming PLP139-151 epitope and augmented epitope spreading. Spleen cells from 5 mice per group were harvested after 22 treatments (day 39 after priming) from the mice in Figure 2. (A) Viable spleen cells (5 × 105/well) were cultured with indicated concentrations of PLP139-151 for 4 days and proliferation was assessed by incorporation of 3H-TdR. Results are expressed as ΔCPM (media only backgrounds subtracted). Stimulation indices are shown above the relevant bars. (B) Supernatants from the above culture in panel A were harvested at 48 and 72 hours and analyzed for IFN-γ secretion in response to PLP139-151 by ELISA as described in “Materials and methods.” (C) Proliferative responses to the relapse-associated PLP178-191 epitope were also assessed from splenic cultures. (D) Supernatants from the above culture in panel C were harvested at 72 hours and analyzed for IFN-γ secretion in response to PLP178-191 and MBP 84-104 by ELISA as described in “Materials and methods.” Error bars indicate SEM.
Mice undergoing short-term treatment with BIO 5192 initiated at the peak of acute disease display enhanced proliferation and IFN-γ secretion in response to the priming PLP139-151 epitope and augmented epitope spreading. Spleen cells from 5 mice per group were harvested after 22 treatments (day 39 after priming) from the mice in Figure 2. (A) Viable spleen cells (5 × 105/well) were cultured with indicated concentrations of PLP139-151 for 4 days and proliferation was assessed by incorporation of 3H-TdR. Results are expressed as ΔCPM (media only backgrounds subtracted). Stimulation indices are shown above the relevant bars. (B) Supernatants from the above culture in panel A were harvested at 48 and 72 hours and analyzed for IFN-γ secretion in response to PLP139-151 by ELISA as described in “Materials and methods.” (C) Proliferative responses to the relapse-associated PLP178-191 epitope were also assessed from splenic cultures. (D) Supernatants from the above culture in panel C were harvested at 72 hours and analyzed for IFN-γ secretion in response to PLP178-191 and MBP 84-104 by ELISA as described in “Materials and methods.” Error bars indicate SEM.
Normal peripheral proliferative and cytokine responses are observed in BIO 5192-treated mice 5 days following cessation of short-term treatment. Spleen cells from 5 mice per group were harvested 5 days after the last of 22 treatments were administered (day 44 after priming at the peak of the posttreatment disease exacerbation) from the mice in Figure 2. (A) Viable spleen cells (5 × 105/well) were cultured with indicated concentrations of PLP139-151 for 4 days and proliferation was assessed by incorporation of 3H-TdR. Results are expressed as Δ CPM (media only backgrounds subtracted). Stimulation indices are shown above the relevant bars. Supernatants from the above culture in panel A were harvested at 48 and 72 hours and analyzed for IFN-γ secretion (B) and IL-2 secretion (C) in response to PLP139-151 by ELISA as described in “Materials and methods.”
Normal peripheral proliferative and cytokine responses are observed in BIO 5192-treated mice 5 days following cessation of short-term treatment. Spleen cells from 5 mice per group were harvested 5 days after the last of 22 treatments were administered (day 44 after priming at the peak of the posttreatment disease exacerbation) from the mice in Figure 2. (A) Viable spleen cells (5 × 105/well) were cultured with indicated concentrations of PLP139-151 for 4 days and proliferation was assessed by incorporation of 3H-TdR. Results are expressed as Δ CPM (media only backgrounds subtracted). Stimulation indices are shown above the relevant bars. Supernatants from the above culture in panel A were harvested at 48 and 72 hours and analyzed for IFN-γ secretion (B) and IL-2 secretion (C) in response to PLP139-151 by ELISA as described in “Materials and methods.”
Cessation of BIO 5192 treatment enhances lymphoid cell infiltration, expression of VCAM-1, and proinflammatory cytokine mRNA in the CNS
We wished to determine if a possible mechanism to explain the decrease in T-cell reactivity in the periphery in mice recently removed from BIO 5192 treatment was the release of T cells from peripheral lymphoid compartments followed by their migration into the CNS. Immunohistochemical analysis of spinal cords taken on day-22 PTI (day 39 after priming) from mice receiving BIO 5192 at the peak of acute disease (Figure 2) revealed significantly fewer CD4+ T cells in the CNS compared with controls (Figure 5A versus 5B). However, at day-27 PTI, when BIO 5192-treated mice undergo the posttreatment exacerbation (Figure 2), influx of CD4+ T cells into the spinal cords of the BIO 5192 group was markedly enhanced compared with vehicle-treated controls (Figure 5C versus 5D). This pattern was observed in multiple spinal cord sections from 3 to 4 representative mice. Interestingly, VCAM-1 expression, which was not found in either vehicle control or BIO 5192 groups at day-22 PTI (data not shown), was significantly increased 5 days after treatment cessation in BIO 5192-treated mice (Figure 5F) compared with that of control animals (Figure 5E). Slightly elevated CNS counts of CD8+ T cells were also observed in the BIO 5192 group during the posttreatment disease exacerbation. No CD4+ and CD8+ T cells and no VCAM-1 expression was seen in the CNS of naive animals (data not shown). RT-PCR analysis of cytokine mRNA levels at day-27 PTI in the CNS of mice treated with BIO 5192 beginning at the peak of acute disease revealed an increase in expression of IFN-γ, TNF-α, and IL-10 (data not shown). IL-4 and IL-5 were not detected in either group and MIP-1α expression was similar to that of controls (data not shown). These results are consistent with a posttreatment release of activated myelin-specific T cells from the periphery resulting in significantly enhanced CNS inflammation.
Enhancement of CNS infiltration of CD4+ T cells and expression of VCAM-1 following cessation of BIO 5192 treatment. Sections of the lower thoracic spinal cord from representative animals described in Figure 2 were stained for infiltration of CD4+ T cells and for VCAM-1 expression after 22 treatments (day 39 after priming—on treatment) and 5 days after treatment was terminated (day 44 after priming—off treatment). DAPI, in blue, stains all cells; CD4+ cells and VCAM-1 expression are marked in red. (A) Day-39 spinal cord CD4+ infiltrate in a vehicle-treated animal. (B) Day-39 spinal cord CD4+ infiltrate in a BIO 5192-treated animal. (C) Day-44 spinal cord CD4+ infiltrate in a vehicle-treated animal. (D) Day-44 spinal cord CD4+ infiltrate in a BIO 5192-treated animal. (E) Day-44 spinal cord VCAM-1 expression in a vehicle-treated animal. (D) Day-44 spinal cord VCAM-1 expression in a BIO-5192 animal. All sections are 6-μM thick with original magnification, × 100.
Enhancement of CNS infiltration of CD4+ T cells and expression of VCAM-1 following cessation of BIO 5192 treatment. Sections of the lower thoracic spinal cord from representative animals described in Figure 2 were stained for infiltration of CD4+ T cells and for VCAM-1 expression after 22 treatments (day 39 after priming—on treatment) and 5 days after treatment was terminated (day 44 after priming—off treatment). DAPI, in blue, stains all cells; CD4+ cells and VCAM-1 expression are marked in red. (A) Day-39 spinal cord CD4+ infiltrate in a vehicle-treated animal. (B) Day-39 spinal cord CD4+ infiltrate in a BIO 5192-treated animal. (C) Day-44 spinal cord CD4+ infiltrate in a vehicle-treated animal. (D) Day-44 spinal cord CD4+ infiltrate in a BIO 5192-treated animal. (E) Day-44 spinal cord VCAM-1 expression in a vehicle-treated animal. (D) Day-44 spinal cord VCAM-1 expression in a BIO-5192 animal. All sections are 6-μM thick with original magnification, × 100.
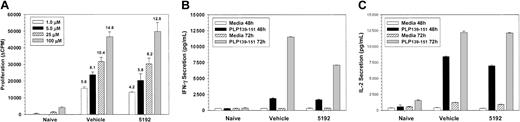
Long-term treatment with BIO 5192 results in accumulation of highly activated cells in the periphery
PLP139-151-specific proliferative responses (Figure 6A) and IFN-γ secretion (Figure 6B) of splenic T cells taken on day-44 PTI were also strikingly elevated in mice treated with BIO 5192 beginning at the peak of the acute disease (Figure 2). In addition, 48-hour IL-2 secretion was also substantially elevated in splenic T cells (data not shown). There was also enhanced proliferation and IFN-γ secretion in the lymph node at this time (data not shown). In contrast, delayed-type hypersensitivity (DTH) responses, an in vivo measure of Th1 trafficking, were significantly lower upon challenge with both the disease-inducing PLP139-151 epitope and the relapse-associated PLP178-191 epitope, indicating that the BIO 5192 was effective in inhibiting Th1 homing to a peripheral antigen challenge similar to other small-molecular VLA-4 inhibitors,18 despite enhanced peptide-specific responses in peripheral lymphoid organs. By day 63, when BIO 5192-treated mice were at the peak of the posttreatment exacerbation (Figure 2), splenic PLP139-151-specific IFN-γ secretion returned to levels similar to vehicle control (data not shown), again consistent with the migration of a significant number of activated peripheral T cells to the CNS.
Mice undergoing long-term treatment with BIO 5192 initiated at the peak of acute disease display enhanced proliferation and IFN-γ secretion in response to the priming PLP139-151 epitope. Spleen cells from 5 mice per group were harvested after 44 treatments (day 58 after priming) from the mice in Figure 2. (A) Viable spleen cells (5 × 105/well) were cultured with indicated concentrations of PLP139-151 for 4 days and proliferation was assessed by incorporation of 3H-TdR. Results are expressed as ΔCPM (media only backgrounds subtracted). Stimulation indices are shown above the relevant bars. (B) Supernatants from the above culture in panel A were harvested at 48 and 72 hours and analyzed for IFN-γ secretion in response to PLP139-151 by ELISA as described in “Materials and methods.” (C) Five mice per treatment group were ear challenged with 10 μg PLP139-151 (left ear) and PLP178-191 (right ear) at day 57 after priming and increase in ear thickness as a measure of DTH was determined 24 hours thereafter. Values shown are mean net ear thickness (background swelling in challenged naive recipients subtracted) in units of 10-4 inches ± SEM. *DTH responses significantly less than those of the vehicle-treated mice; P < .05.
Mice undergoing long-term treatment with BIO 5192 initiated at the peak of acute disease display enhanced proliferation and IFN-γ secretion in response to the priming PLP139-151 epitope. Spleen cells from 5 mice per group were harvested after 44 treatments (day 58 after priming) from the mice in Figure 2. (A) Viable spleen cells (5 × 105/well) were cultured with indicated concentrations of PLP139-151 for 4 days and proliferation was assessed by incorporation of 3H-TdR. Results are expressed as ΔCPM (media only backgrounds subtracted). Stimulation indices are shown above the relevant bars. (B) Supernatants from the above culture in panel A were harvested at 48 and 72 hours and analyzed for IFN-γ secretion in response to PLP139-151 by ELISA as described in “Materials and methods.” (C) Five mice per treatment group were ear challenged with 10 μg PLP139-151 (left ear) and PLP178-191 (right ear) at day 57 after priming and increase in ear thickness as a measure of DTH was determined 24 hours thereafter. Values shown are mean net ear thickness (background swelling in challenged naive recipients subtracted) in units of 10-4 inches ± SEM. *DTH responses significantly less than those of the vehicle-treated mice; P < .05.
Discussion
Small-molecule inhibitors of VLA-4 are currently being developed for treatment of a variety of immune inflammatory conditions including autoimmune diseases such as multiple sclerosis and inflammatory bowel disease, kidney diseases and disorders, asthma, as well as allograft rejection, graft-versus-host disease (GVHD), and other hematologic disorders. Targeting such a range of diseases represents the hope for an extremely versatile therapeutic based on regulating trafficking of inflammatory cells to sites of inflammation. The promise of these inhibitors has been shown in a recent report by Abraham et al19 that showed that administering BIO-1211, a small-molecule, tight-binding inhibitor of α4, blocked allergen-induced late airway responses (LAR) and post-antigen-induced airway hyper-responsiveness (AHR) in allergic sheep. This study supported previous work that found that pretreatment of allergic sheep with BIO-1211 inhibited early and late airway responses following antigen challenge and prevented development of nonspecific airway hyper-responsiveness to carbachol.20 In addition, small-molecule VLA-4 inhibitors have been shown to inhibit ovalbumin delayed-type hypersensitivity and oxazolone contact hypersensitivity in BALB/c mice, suggesting their potential efficacy as anti-inflammatory agents,18 and a recent report in rat MBP-induced EAE showed that BIO 5192 administered daily on days 5 to 13 inhibited disease onset by 3 days and reduced the mean peak clinical score from grade 3 to grade 2.16
In EAE, a mouse model for MS, previous studies have concentrated predominantly on VLA-4 mAbs as a method of blocking VLA-4/VCAM-1 and fibronectin interactions and thereby inhibiting entry of disease-inducing encephalitogenic T cells into the CNS. These reports have shown the efficacy of inhibiting induction of EAE with anti-VLA-4 mAb.7,9 Relevant to human autoimmune disease, a recent clinical trial reported significantly reduced numbers of new brain lesions and relapses in relapsing MS patients receiving monthly intravenous injections of large doses of natalizumab, an α4 integrin antagonist monoclonal antibody, when compared with patients receiving a placebo control.21 However, our recent study indicated that the dose and timing of treatments with anti-VLA-4 mAb may be critical to the clinical outcome in that SJL mice treated during ongoing R-EAE exhibited significantly exacerbated disease relapses and peripheral Th1 responses.15 Using a limited number of anti-VLA mAb injections in the guinea pig model of chronic EAE, Kent et al22 and Keszthelyi et al23 demonstrated a temporary reduction in disease severity after preclinical or postclinical treatment. Disease was followed long term and posttreatment disease exacerbation was observed but was slight compared with what we observed in the SJL relapsing-remitting EAE model. The differences in these results may be due to the relapsing nature of the SJL model, which involves epitope spreading,3,4 or to species differences in disease mechanism. One possible mechanism for the profound disease exacerbation we noted is a proposed role for VLA-4 in T-cell costimulation.11-14 In fact, we demonstrated that the addition of anti-CD49d to anti-CD3/anti-CD28-coated polystyrene beads induced significantly greater proliferation15 and proinflammatory cytokine production (B.E.T., unpublished results, March 2002) over cells stimulated with anti-CD3/anti-CD28 alone. Additionally, cyclic hexapeptide CWLDVC (TBC 772), an integrin-selective antagonist of α4 integrins and a potent inhibitor of lymphocyte interactions with fibronectin, VCAM-1, and mucosal addressin cell adhesion molecule 1 (MAdCAM-1), was shown to suppress T-cell costimulation induced by anti-CD49d coimmobilized with anti-CD3 mAb.24 Interestingly, these authors provided evidence that integrin coactivation systems are neutralized by a mechanism independent of competitive binding by showing that binding of the α4-specific mAb L25 and the β1-specific mAb 33B6 was not altered by TBC 772; however, binding of mAb 19H8, which is specific for a combinatorial epitope of α4β1, was dramatically inhibited. It is important to note that BIO 5192 is a competitive antagonist of VLA-4 and could potentially induce an in vivo costimulatory signal that could be negated through the development of antagonists such as TBC 772 that function independently of competitive binding. Our preliminary data suggest that a BIO 5192/bovine serum albumin (BSA) conjugate adhered to anti-CD3/anti-CD28-coated polystyrene beads can induce minimally enhanced proliferation over cells stimulated with anti-CD3/anti-CD28/BSA alone, and this is the subject of ongoing investigation in our laboratory.
Another level of complexity in the VLA-4 system is that Chen et al25 have detected multiple activation states of integrin α4β1 through their different affinities for a small-molecule ligand. This report showed that activation of VLA-4 is not defined by a single state but rather by several distinct states that give rise to a range of affinities and that the affinity differences are tightly coupled to dissociation rates of the integrin-ligand complex. In the previously mentioned study by Abraham et al, the small-molecule inhibitor BIO 1211 was shown to be 200-fold selective for the activated form of α4β1.19 In our study, differential effects of BIO 5192 on R-EAE were observed when animals were on treatment. Although disease was inhibited when treatment began at the peak of the acute phase, animals beginning treatment during remission exhibited no such protection. It is possible the acute and remission phases of R-EAE are defined by different activation states of α4β1 and that treatment with BIO 5192 during a particular activated state of VLA-4 results in a higher affinity, more efficacious therapeutic. It is important to note that activation state does not necessarily correlate with expression levels. Finally, we have recently reported that resting CD4+CD25+ T-regulatory cells express high constitutive levels of both VLA-4 and P-selectin.26 Thus, it is also possible that in situations where animals on BIO 5192 therapy exhibited disease exacerbations, the drug may have selectively blocked trafficking of T-regulatory cells as opposed to encephalitogenic T cells. This possibility is currently under investigation.
Treatment with BIO 5192 begun at the time of priming of acute disease and continued for 22 or 44 days resulted in temporary protection from clinical symptoms. However, it is important to note that while on treatment, clinical signs returned and, more strikingly, when treatment was terminated, clinical symptoms worsened dramatically. Most significantly, relapse rate and disease severity were significantly lowered in mice in which BIO 5192 treatment was initiated at the peak of acute disease while the animals were being treated with BIO5192 (Figures 1C and 2) despite the fact that the mice exhibited augmented peripheral myelin peptide-specific Th1 responses during this period and, at the same time, reduced DTH as an in vivo measure of Th1 trafficking (Figures 3 and 6). However, a striking posttreatment disease exacerbation was observed within 5 to 7 days following cessation of treatment. The posttreatment exacerbation of R-EAE in animals for all treatment regimens correlated with a release of activated CD4+ T cells from the periphery into the CNS (Figure 5) and a lessening of peripheral myelin-specific T-cell responses (Figure 4) and this timing is consistent with the t1/2 of BIO 5192, which is approximately 12 hours.16 The inhibition of relapses in animals beginning treatment at the peak of acute phase suggests continual long-term treatment begun early in disease may be efficacious in treating established autoimmune diseases such as MS. However, similar to treatment with intact mAb,15 autoreactive responses in the peripheral immune system appear to be enhanced, or at least maintained, during BIO 5192 treatment. The striking accumulation of highly activated encephalitogenic cells in the periphery not only to the priming peptide but also to spread epitopes associated with disease relapses raises a potential concern in treatment with VLA-4 inhibitors. Although no exacerbation was observed in a Lewis rat EAE model upon cessation of treatment with BIO 5192,16 this is a monophasic, self-limiting EAE model that does not involve epitope spreading and no disease relapse would be expected in either controls or treated animals. A recent report showed improvement in clinical disease progression in guinea pigs with chronic spinal cord homogenate-induced EAE upon daily long-term treatment with a different small-molecule VLA-4 inhibitor, CT301, initiated at 40 days after immunization.27 Similar to the present results, a small subset of animals in which drug treatment was withdrawn after 30 days progressed to control disease levels within 7 days, indicating that the rebound phenomena is observed in a different EAE model in a different species.
Collectively, these results further illustrate the complexity of VLA-4/VCAM-1 interactions in a chronic relapsing-remitting autoimmune disease model (ie, R-EAE) and illustrate potential benefits and drawbacks to targeting VLA-4 with small-molecule inhibitors to treat established autoimmune diseases such as MS, as well as GVHD and allograft rejection. Our results suggest that continual small-molecule treatment may be effective in such diseases by preventing cell trafficking; however, the treatment appears to facilitate activation and peripheral accumulation of Th1 cells potentially resulting in a significant rebound upon cessation of treatment. These cells may be beneficial or destructive depending on the effector role of Th1 and Th2 cells in the disease being treated. Continued examination of the multiple effects of anti-VLA-4 and VLA-4 inhibitors on the immune system will be important to determine the potential efficacy of small molecule VLA-4 inhibitors in a variety of diseases.
Prepublished online as Blood First Edition Paper, August 21, 2003; DOI 10.1182/blood-2003-03-0974.
Supported by United States Public Health Service (USPHS) National Institutes of Health (NIH) grants NS26543, NS30871, and NS34819, and a grant from Biogen, Inc.
Several of the authors (C.N.-N., M.C., D.M.S., S.J.P., and E.T.W.) are or were employed by Biogen Inc, whose potential product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.