Abstract
The oncofetal antigen immature laminin receptor protein (OFA-iLRP) is a highly conserved protein that is preferentially expressed in fetal tissues and in many types of cancer, including hematopoietic malignancies, whereas OFA-iLRP is not detectable on healthy differentiated adult cells. To investigate whether OFA-iLRP-specific cytotoxic T lymphocytes (CTLs) are capable of killing OFA-iLRP-expressing hematologic targets, CTLs were generated from healthy HLA-A*0201-positive volunteers by incubating T cells with autologous dendritic cells (DCs) transfected with OFA-iLRP RNA. OFA-iLRP-specific CTLs lysed HLA-A2+ OFA-iLRP+ tumor cells, including several lymphoma and leukemia cell lines, as well as fresh leukemic targets from patients with acute myeloid leukemia (AML) and chronic lymphatic leukemia (CLL), indicating that OFA-iLRP-derived peptides are naturally processed and presented by hematologic tumors. Healthy OFA-iLRP-negative target cells (CD14+ monocytes, activated B cells, DCs, bone marrow cells) were not attacked by OFA-iLRP-specific CTLs. Furthermore, in an established murine B-cell lymphoma model (A20), treatment with syngeneic DCs transfected with OFA-iLRP-coding RNA resulted in powerful antitumor effects in a significant portion of mice. For the first time, these data show that OFA-iLRP can be used as a target for T-cell-based immunotherapeutic strategies against hematologic malignancies. (Blood. 2003;102:4416-4423)
Introduction
The 37-kDa oncofetal antigen immature laminin receptor protein (OFA-iLRP) is an evolutionary conserved protein that has been detected in humans and rodents.1,2 OFA-iLRP appears to dimerize after acylation to form the high-affinity mature (m) 67-kDa mLRP that might serve as a cofactor to stabilize the binding of laminin to cell surface integrins.1,3 Experimental studies indicate that OFA-iLRP acts as a receptor for the uptake of prion protein into eukaryotic cells.4,5 Although the mature 67-kDa form is present on many healthy cells, 37-kDa OFA-iLRP is abundantly expressed in many types of human tumors, including breast, lung, ovary, prostate, renal cancer, and lymphoma.1,2,6,7 In embryos/early fetuses but not in term fetus, neonate, or adult differentiated tissues OFA-iLRP expression was detected.1,6,8 It has been documented in murine and, more recently, in human studies that OFA-iLRP is an immunogenic protein that can specifically activate both T and B lymphocytes.9-15 Therefore, OFA-iLRP might represent an ideal antigen for immunotherapeutic strategies directed against all human and rodent cancers.1,2,6-15
In the current study, we tested the hypothesis that OFA-iLRP can be used as a target structure for T-cell-based immunotherapeutic approaches against hematologic malignancies. We screened the expression of OFA-iLRP on healthy hematopoietic cells as well as on various hematologic malignancies by using a monoclonal antibody specific for OFA-iLRP. Using autologous OFA-iLRP RNA-transfected dendritic cells as antigen-presenting cells we generated polyclonal cytotoxic T lymphocytes (CTLs) from several healthy HLA-A*0201-positive donors capable of specifically recognizing and killing target cells endogenously expressing OFA-iLRP, including hematologic tumor lines and leukemic cells from patients with acute myeloid leukemia (AML) and chronic lymphatic leukemia (CLL). Tumor challenge experiments in a murine B-cell leukemia model reveal that treatment with OFA-iLRP RNA-transfected dendritic cells induces a potent antitumor immune response.
Patients, materials, and methods
Patients and healthy donors
Peripheral blood, bone marrow cells, and tumor samples were collected from healthy donors and from patients. Tumor samples used for cytotoxicity assay were obtained from patients with AML and CLL. All specimens were obtained after informed consent and approval by the institutional review board of the Medical Association of Hamburg.
Human tumor cell lines and nonmalignant cell subsets
K562 (erythroleukemia), IM-9 (plasmocytoma), Karpas-422, Balm-3, Ramos (B lymphoblastic lymphoma), and T2 cells were obtained from American Type Culture Collection (Manassas, VA). MEC-1, a chronic lymphatic leukemia was kindly provided by Dr F. Caligaris (Milano, Italy). Leukemic blasts (purity, > 80%) from patients with AML were obtained by Ficoll density gradient centrifugation. CD5+CD19+ CLL cells were obtained from peripheral blood of patients with CLL. After Ficoll density gradient centrifugation, more than 95% of resulting cells coexpressed CD19 and CD5. Isolation of CD8+ T lymphocytes, CD19+ B cells, CD14+ monocytes, and CD34+ peripheral blood progenitor cells was conducted using magnetically activated cell sorter (MACS) technology (Miltenyi, Bergisch-Gladbach, Germany) following the manufacturer's instructions. Fluorescence activated cell sorting (FACS) analysis revealed a cell purity of more than 90%.
Murine tumor cell lines
The OFA-iLRP+ A20 cell line, a nonimmunogenic B-cell leukemia/lymphoma that occurred spontaneously in a 15-month-old Balb/c mouse was used for in vivo dendritic cell (DC) vaccination experiments. MOPC-315, an OFA-iLRP-negative immunoglobulin A (IgA)-secreting myeloma (Balb/c, H-2d) and the OFA-iLRP-positive EL-4 thymoma cell line derived from C57Bl/6 mice (H-2b) were grown in RPMI 1640 medium (Gibco, Karlsruhe, Germany) containing 5% fetal calf serum (FCS; Biochrom, Cambridge, United Kingdom) and were used as control targets. All cell lines were obtained from American Type Culture Collection.
HLA-binding predictions
The sequence of iLRP (accession no. AAD26866) was screened for high-scoring peptides containing the HLA-A*0201 peptide motif using software by Rammensee (University of Tuebingen, Germany). The peptides iLR58-66 (LLLAARAIV; Rammensee score, 26; iLRP1), iLR60-68 (LAARAIVAI; Rammensee score, 23; iLRP2), iLR146-154 (ALCNTDSPL; Rammensee score, 22; iLRP3), iLR7-15 (VLQMKEEDV; Rammensee score, 22; iLRP4), FluM158-66 (GILGFVFTL; control peptide), and HIV-pol476-484 (ILKEPVHGV; control peptide) were obtained from Biosyntan (Berlin, Germany) and were provided at more than 90% purity, as verified by high-performance liquid chromatography (HPLC) and mass spectrometry (MS) analysis.
Peptides and loading of T2 cells
The HLA-A2-positive mutant cell line T2 was separately incubated with peptides for 2 hours at various concentrations, washed 3 times, and used as a target in a 51Chromium release assay.
Isolation of human OFA-iLRP-coding RNA
The coding sequence for human OFA-iLRP was obtained by extraction of total RNA from the OFA-iLRP-positive Karpas-422 cell line by using the RNeasy Kit (Qiagen, Hilden, Germany) following the manufacturer's instruction. Cells (1 × 107/mL) were used for the isolation. Conversion of RNA to cDNA was performed by using the First-Strand cDNA Synthesis Kit (Amersham, Buckinghamshire, United Kingdom). Synthesis was catalyzed by Molony murine leukemia virus reverse transcriptase. Resulting cDNA was used as template for DNA amplification by polymerase chain reaction (PCR) with specific primer for OFA-iLRP (OFA-iLRP forward primer, 5′-AgCggATCCATgTCCggAgCCCTTg-3′ with specific restriction cut site for BamHI, and reverse primer, 5′-AAggCCTCTCgAgTTAAgACCAgTCAgTgg-3′ with specific restriction cut site for XhoI). Coding sequence was then ligated into BamHI/XhoI site of the pCITE-2a(+) vector (Novagen, Madison, WI). The vector was transformed into Escherichia coli JM83 by heat-shock transformation. After ampicillin selection, large-scale plasmid isolation (Plasmid Maxi Kit; Qiagen) was performed. The correct size of the plasmid was checked on an 1% agarose gel, and the OFA-iLRP sequence was verified by automatic sequencing (ABI 310; Perkin Elmer, Shelton, CT). Resulting plasmid DNA was linearized, and in vitro transcription was performed by using the T7 RiboMAX Large Scale Transcription Kit (Promega, Mannheim, Germany). Linearized and purified plasmid DNA, enzyme mix, transcription buffer, and ribonucleoside triphosphates (rNTPs) were mixed and incubated at 37°C for at least 4 hours. DNA template was degraded by adding DNaseI. RNA was purified with RNeasy columns (RNeasy Kit; Qiagen) following the manufacturer's advice. Quantity and purity were determined by UV spectrophotometry. OFA-ILRP RNA was used for transfection of human and murine dendritic cells because of its 99% homologous protein sequence.
Transfection of EBV blasts with iLRP-RNA
Epstein-Barr virus (EBV)-transformed lymphoblasts were centrifuged, washed twice in phosphate-buffered saline (PBS), and resuspended at 2.5 × 107 cells/mL in Opti-MEM (Gibco). Cell suspension (200 μL) and 20 μg OFA-iLRP RNA were mixed in a 4-mm Cuvette (Bio-Rad, Munich, Germany). Transfection was achieved by electroporation (300 V, 150 μF; Gene Pulser II; Bio-Rad), and transfected cells were incubated at 37°C for 24 hours in complete RPMI 1640 medium (Gibco) supplemented with 10% heat-inactivated fetal calf serum (FCS; Biochrom, Cambridge, United Kingdom).
Isolation of total A20 RNA
A20 total RNA was obtained by using RNeasy Kit (Qiagen) following the manufacturer's instructions. Isolated RNA was visualized on an agarose gel. Quantity and purity of RNA were determined by UV spectrophotometry. RNA samples were stored at -80°C.
Cell isolation and generation of human immature DCs
Immature DCs (iDCs) were generated from peripheral blood mononuclear cells (PBMCs) from healthy HLA-A2pos volunteers following the protocol of Feuerstein et al.16 Briefly, a concentrated leukocyte fraction was generated through a 2-hour restricted peripheral blood leukapheresis processing 6 to 8 L blood with each collection. The leukapheresis product was further separated by density gradient centrifugation over polysucrose/sodium diatrizoate (Histopaque; Sigma, St Louis, MO), and cells were resuspended in serum-free AIM-V medium (Life Technologies, Silver Spring, MD). PBMCs were incubated at 2 × 108 cells/30 mL in T-150 culture flasks in a humidified incubator for 2 hours at 37°C to allow plastic adherence. The adherent cell fraction was used for DC culture by incubation in serum-free AIM-V medium supplemented with recombinant human interleukin 4 (IL-4; 500 U/mL) and recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF; 800 U/mL) (CellConcepts, Umkirch, Germany).
RNA transfection and maturation of human DCs
Transfection of iDCs was performed by a modified protocol from Su et al17 by using the liposomal transfection reagent DOTAP ((N-[1-(2,3-Dioleoyloxy)]-N,N,N-trimethylammonium propane methylsulfate in sterile water; Roche, Basel, Switzerland). DOTAP (20 μL) and OFA-iLRP RNA (10 μg) were mixed in 500 μL Opti-MEM (minimum essential medium) and incubated for 20 minutes at room temperature (RT). The RNA-Lipid complex was added to 1 × 106 iDC/mL (in Opti-MEM) and incubated at 37°C for 3 hours. Maturation of the transfected iDCs was performed by culturing for 2 days in serum-free medium supplemented with GM-CSF, IL-4, IL-1β, tumor necrosis factor α (TNF-α; 10 ng/mL; CellConcepts), IL-6 (1000 U/mL; CellConcepts), and prostaglandin E2 (PGE2; 1 μg/mL; Sigma-Aldrich, Deisenhofen, Germany). Flow cytometric analysis revealed that OFA-iLRP RNA-transfected and maturated DCs expressed more than 80% of OFA-iLRP and high levels of CD80, CD83, CD86, major histocompatibility complex (MHC) class I and II molecules.
Generation of human CTLs
Cytotoxic T cells (CTLs) were generated, as previously described.18,19 In brief, the T-cell-enriched nonadherent fraction of PBMCs obtained following the DC plastic adherence step was used for CTL generation. Nonadherent PBMCs were cultured in serum-free medium supplemented with 20 U/mL human IL-2 and 10 ng/mL human IL-7 (CellConcepts). Cells were weekly stimulated with autologous iLRP RNA-transfected DCs. As determined by flow cytometric analysis, 83% ± 5.3% of these effector cells were CD3+, 58% ± 4.2% were CD3+/CD8+, 7% ± 2.2% were CD3+/CD4+, 2% ± 1.2% were CD56+, and 2.2% ± 0.5% were CD3+/CD56+.
CTL assay
Target cells were labeled with 200 μCi (7.4 MBq) NaCrO4 (Amersham-Buchler, Braunschweig, Germany) in 0.5 mL complete medium for 1 hour. They were washed 3 times with complete medium and added at a concentration of 5 × 103 cells/well in round-bottomed microtiter plates (Nunc, Roskilde, Denmark). Effector cells were added at various effector-target ratios in a final volume of 200 μL/well. The plates were incubated for 4 hours at 37°C in a humid atmosphere with 5% CO2. Maximum chromium release was determined by the addition of 10% Triton-X, and spontaneous release was assessed by adding complete medium (RPMI 1640 + 10% FCS) to the target cells. The culture supernatant was harvested semiautomatically with a Scatron Titertek System (Scatron, Suffolk, United Kingdom) and counted in a gamma counter (Beckmann, Heidelberg, Germany). The percentage of specific lysis was calculated as (experimental cpm - spontaneous cpm)/(maximum cpm - spontaneous cpm) × 100. All determinations were made in triplicate.
Cold target inhibition assay
Specificity of tumor cell lysis was determined in a cold target inhibition assay by analyzing the capacity of unlabeled HLA-A2+ OFA-iLRP-negative K562 cells, HLA-A2+ OFA-iLRP-positive Karpas-422 cells, and HLA-A2+ OFA-iLRP-positive leukemic blasts from a patient with AML (AML-1) to block lysis of tumor cells at a ratio of 20:1 (inhibitor-to-target ratio).
Antibody-blocking inhibition assay
For antibody-blocking experiments, human CTLs were generated, as described earlier. Tumor cells were incubated with 10 μg/mL anti-HLA-A2 monoclonal antibody (BB7.2; BD Biosciences, Heidelberg, Germany), CTLs were incubated with anti-CD8 (T8); anti-Pan-TCR (T-cell receptor; BMA 031); anti-CD4 (T4); isotype-matched controls MAM-6 and HMFG-1 (Beckman Coulter, Krefeld, Germany) for 30 minutes. Cell suspensions were washed 2 times with complete medium and tested in a chromium release assay.
Enzyme-linked immunospot analysis
To determine the frequency of iLR peptide-specific T cells in polyclonal iLRP-specific CTL cultures of HLA-A2+ healthy individuals, enzyme-linked immunospot (ELISPOT) assays were performed by using the interferon-γ and IL-10 ELISPOT Kits (Becton Dickinson, Heidelberg, Germany) according to the manufacturer's instruction.
Generation of murine iLRP-RNA-transfected dendritic cells
For in vitro restimulation, bone marrow-derived dendritic cells were used as professional antigen-presenting cells and were generated as previously described.18 Transfection of iDCs was performed by using 50 μg DOTAP (Roche) and 25 μg OFA-iLRP RNA or total RNA mixed in 500 μL Opti-MEM (Gibco). The complex was incubated for 20 minutes at RT and then added to 5 × 106 immature DCs. DC maturation was achieved by adding lipopolysaccharide (LPS; 2 μg/mL for 5 × 106 iDC/mL; Sigma-Aldrich) for 4 hours at 37°C. Afterward, matured DCs were washed 3 times and applied for vaccination studies. Flow cytometric analysis demonstrated that these matured DCs expressed more than 80% of OFA-iLRP and high levels of CD45 (94% ± 2.0%), CD11c (92% ± 2.9%), CD18 (95% ± 1.8%), CD80 (89% ± 2.2%), CD86 (83% ± 2.9%), and class I (98% ± 0.3%) and class II MHC (92% ± 1.0%) antigens.
Vaccination protocol
Healthy Balb/c mice (8-12 weeks old) received 3 subcutaneous injections of 5 × 105 syngeneic DCs transfected with OFA-iLRP RNA at weekly intervals. Control groups were treated with nontransfected DCs or PBS (tumor control group). One week after the last vaccination, animals were inoculated subcutaneously with 1 × 105 A20 lymphoma cells. In another set of experiments, animals were treated with the identical protocol 5 days after tumor inoculation of 1 × 105 A20 cells. Animals were examined daily for palpable tumors and autopsied after death. Animals dying of lymphoma exhibited bulky subcutaneous tumors. Animals surviving the observation period of 140 days after tumor inoculation were killed under ether anesthesia and macroscopically, in some cases microscopically, inspected for signs of tumor manifestation.
Murine CTL assay
Balb/c mice were treated as described in “Vaccination protocol.” On day 100, DC-treated animals were killed under ether anesthesia. The spleens of 2 mice per group were removed, pooled, and pressed through wire mesh screens to obtain single cell suspensions. Mononuclear cells were isolated by Lympholite-M (Cedarlane, Hornby, British Columbia Canada) gradient centrifugation. Splenocytes were restimulated at a concentration of 2 × 106 cells/mL with 5 × 105/mL dendritic cells transfected with OFA-iLRP RNA or total A20 RNA in 24-well plates. After 5 days, viable cells were harvested and tested in a 51Cr-release assay for their ability to lyse A20, MOPC-315, and EL-4 cells.
Immunophenotyping
For marker analysis, mononuclear cells (MNCs) were incubated with appropriate primary and secondary antibodies and analyzed using a flow cytometer (FACScan; Becton Dickinson, Mountain View, CA). The following antibodies for murine MNCs were used: anti-Thy1.2 (30-H12), anti-NK (DX5), anti-CD19 (1D3), anti-CD11c (HL3), anti-CD45 (30-F11), anti-CD18 (GAME-46), anti-CD80 (1G10), anti-CD86 (GL1), anti-MHC class I (34-2-12), and anti-MHC class II (AMS-32.1). All monoclonal antibodies were obtained from Pharmingen (San Diego, CA). For flow cytometric analysis of human MNCs, fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated mouse monoclonal antibodies against CD4, CD3, CD8, CD56, CD14, CD40, CD80, CD86 (Becton Dickinson), HLADR, and CD83 (Coulter-Immunotech, Hamburg, Germany) were used.
Statistical analysis
Survival of animals was calculated according to the method of Kaplan and Meier. Group comparisons were made by the Wilcoxon test. The calculations were done on a personal computer with Statistica data analysis software (StatSoft, Tulsa, OK).
Results
OFA-iLRP is strongly expressed in malignant hematopoietic cells
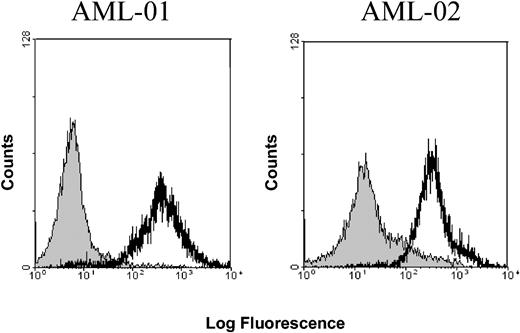
In a first set of experiments, the expression of OFA-iLRP on healthy and malignant hematopoietic cells by using the previously described7,14 monoclonal OFA-iLRP-specific antibody was determined. As depicted in Table 1, OFA-iLRP expression could be detected on all hematologic tumor cell lines tested. Furthermore, all blast samples of patients with AML and, interestingly, all samples of leukemic cells obtained from patients with B-CLL (Binet C) exhibited significant OFA-iLRP expression, whereas iLRP was not present on malignant B-CLL cells at an early stage of disease (Binet A). Figure 1 gives an example of OFA-iLRP expression on AML blasts from 2 patients (both AML-FAB-M5 [French-American-British classification M5]). We could not detect any expression of OFA-iLR protein on cell subpopulations purified from peripheral blood of patients with AML and of healthy donors like CD19+ (B lymphocytes), CD3+CD8+ (T lymphocytes), CD14+ (monocytes) cells, CD34+ peripheral blood progenitor cells, and immature and matured dendritic cells (Table 2).
OFA-iLRP expression in human acute myeloid leukemias. FACS analysis of OFA-iLRP expression on AML blasts obtained from 2 different patients with AML. AML-01: OFA-iLRP expression (black) versus isotype control (gray). P < .001. AML-02: OFA-iLRP (black) versus isotype control (gray). P < .005.
OFA-iLRP expression in human acute myeloid leukemias. FACS analysis of OFA-iLRP expression on AML blasts obtained from 2 different patients with AML. AML-01: OFA-iLRP expression (black) versus isotype control (gray). P < .001. AML-02: OFA-iLRP (black) versus isotype control (gray). P < .005.
Generation of polyclonal OFA-iLRP-specific CTLs using OFA-iLRP RNA-transfected DCs
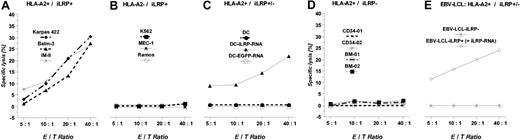
Polyclonal OFA-ILRP-specific CTLs were generated in vitro by priming T cells from healthy HLA-A2-positive individuals with autologous OFA-iLRP RNA-transfected DCs. CTL lines obtained after 4 weekly restimulations elicited strong T-cell responses recognizing and lysing endogenously OFA-iLRP-expressing tumor cell lines. As depicted in Figure 2A, the OFA-iLRP-positive, HLA-A2-expressing cell lines Karpas-422, Balm-3, and IM-9 were efficiently lysed. There was no lysis of the hematologic cell lines K562, Ramos, and Mec-1 (iLRP+/HLA-A2-). HLA-A2-matched but OFA-iLRP-negative target cells (dendritic cells, EBV-transformed lymphoblasts) resistant to lysis through OFA-iLRP-specific CTLs could be rendered susceptible after transfecting target cells with OFA-iLRP-coding RNA (Figure 2C,E).
Induction of OFA-iLRP-specific CTLs by using autologous DCs transfected with OFA-iLRP RNA. DCs from 2 healthy HLA-A2+ individuals were transfected with OFA-iLRP RNA and used to stimulate CTL responses. Cytotoxic reactivity was determined in a 4-hour 51Chromium release assay against HLA-A2+/OFA-iLRP+ hematologic tumor cell lines (A), HLA-A2-/OFA-iLRP+ targets (B), OFA-iLRP-transfected DCs (C), HLA-A2+ CD34+ PBPCs, (D) bone marrow cells from healthy individuals, and (E) HLA-A2+ EBV-transformed lymphoblasts transfected with OFA-iLRP RNA. One of 2 experiments is shown.
Induction of OFA-iLRP-specific CTLs by using autologous DCs transfected with OFA-iLRP RNA. DCs from 2 healthy HLA-A2+ individuals were transfected with OFA-iLRP RNA and used to stimulate CTL responses. Cytotoxic reactivity was determined in a 4-hour 51Chromium release assay against HLA-A2+/OFA-iLRP+ hematologic tumor cell lines (A), HLA-A2-/OFA-iLRP+ targets (B), OFA-iLRP-transfected DCs (C), HLA-A2+ CD34+ PBPCs, (D) bone marrow cells from healthy individuals, and (E) HLA-A2+ EBV-transformed lymphoblasts transfected with OFA-iLRP RNA. One of 2 experiments is shown.
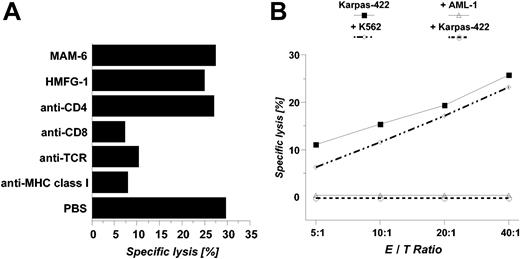
In addition, OFA-iLRP-negative CD34+ peripheral blood progenitor cells (PBPCs) as well as bone marrow cells obtained from healthy HLA-A2-positive individuals were not attacked by OFA-iLRP-reactive CTLs (Figure 2D). Antibody-blocking experiments (Figure 3A) and cold target inhibition assays (Figure 3B) revealed an MHC class I-restricted killing induced by specific CD8+ T lymphocytes.
Antibody-blocking experiments and cold target inhibition assaysusing OFA-iLRP-specific CTLs. To investigate MHC-dependent cytotoxicity, anti body blocking experiments (A) were performed in a 4-hour 51Chromium release assay against the Karpas-422 target. Cold target inhibitions assays (B) were conducted in a 4-hour 51Chromium release assay using the Karpas-422 lymphoma cell line (HLA-A2+/OFA-iLRP-). The specificity of the CTL lines was tested in the presence of unlabeled cold targets, ie, Karpas-422 cells, K562 blasts, and primary leukemic blasts from the patient with AML-1 (HLA-A2+/OFA-iLRP+). Cold targets were added at an inhibitor-to-target ratio of 20:1.
Antibody-blocking experiments and cold target inhibition assaysusing OFA-iLRP-specific CTLs. To investigate MHC-dependent cytotoxicity, anti body blocking experiments (A) were performed in a 4-hour 51Chromium release assay against the Karpas-422 target. Cold target inhibitions assays (B) were conducted in a 4-hour 51Chromium release assay using the Karpas-422 lymphoma cell line (HLA-A2+/OFA-iLRP-). The specificity of the CTL lines was tested in the presence of unlabeled cold targets, ie, Karpas-422 cells, K562 blasts, and primary leukemic blasts from the patient with AML-1 (HLA-A2+/OFA-iLRP+). Cold targets were added at an inhibitor-to-target ratio of 20:1.
OFA-ILRP-specific CTLs efficiently kill primary AML blasts and malignant B-CLL cells
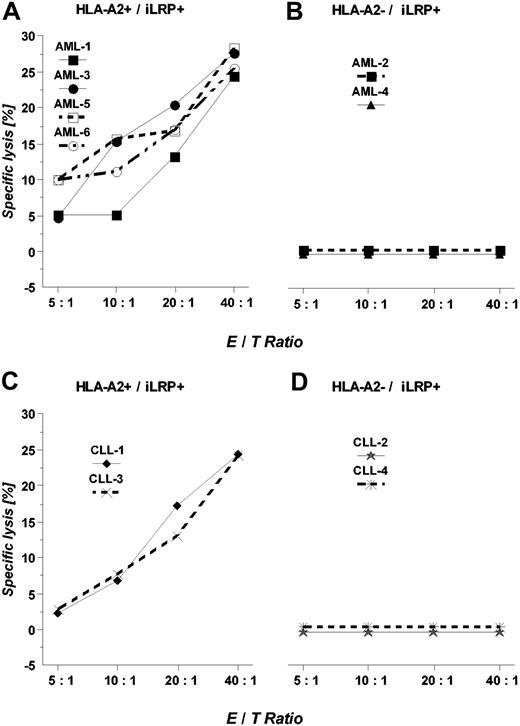
We then determined the cytotoxic potential of OFA-iLRP-specific CTLs against several primary leukemic targets obtained from patients with AML and CLL in a chromium release assay. As depicted in Figure 4A, in 4 cases of AML blasts T-cell lines were capable of efficiently lysing HLA-A2+/OFA-iLRP-expressing AML blasts, whereas HLA-A2-negative targets (Figure 4B) were not killed. Moreover, OFA-iLRP-specific CTLs elicited efficient killing of HLA-A2-matched OFA-iLRP+ CLL cells but did not react against HLA-A2-negative target cells (Figure 4C-D).
Lysis of primary AML blasts and malignant B-CLL cells endogenouslyexpressing OFA-iLRP. HLA-A2+/OFA-iLRP+ leukemic blasts (A; AML 1, 3, 5, 6), HLA-A2-/OFA-iLRP+ blasts (B; AML 2, 4), HLA-A2+/OFA-iLRP+ B-CLL cells (C; CLL1, CLL3), and HLA-A2-/OFA-iLRP+ B-CLL cells (D; CLL2, CLL4) were used as targets in a 4-hour 51Chromium release assay. One representative experiment is shown.
Lysis of primary AML blasts and malignant B-CLL cells endogenouslyexpressing OFA-iLRP. HLA-A2+/OFA-iLRP+ leukemic blasts (A; AML 1, 3, 5, 6), HLA-A2-/OFA-iLRP+ blasts (B; AML 2, 4), HLA-A2+/OFA-iLRP+ B-CLL cells (C; CLL1, CLL3), and HLA-A2-/OFA-iLRP+ B-CLL cells (D; CLL2, CLL4) were used as targets in a 4-hour 51Chromium release assay. One representative experiment is shown.
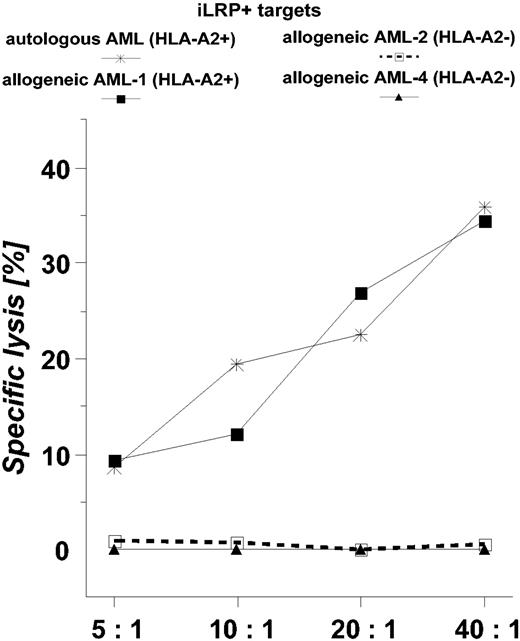
In a next step, iLRP-specific CTLs were generated from PBMCs of an HLA-A*0201-positive patient with AML being in a complete hematologic remission 6 months after the last chemotherapy. Figure 5 documents the potent cytotoxic reactivity of these effector cells against both autologous AML blasts (cryopreserved before cytostatic therapy) and allogeneic HLA-A*0201-matched targets.
Killing activity of OFA-iLRP-specific CTLs obtained from a patientwith AML. Autologous and allogeneic (AML-1, AML-2, AML-4) OFA-iLRP+ AML blasts were used as targets in a 4-hour 51Chromium release assay.
Killing activity of OFA-iLRP-specific CTLs obtained from a patientwith AML. Autologous and allogeneic (AML-1, AML-2, AML-4) OFA-iLRP+ AML blasts were used as targets in a 4-hour 51Chromium release assay.
Lysis of OFA-iLR peptide-loaded T cells by polyclonal OFA-iLRP-specific CTLs
To investigate whether polyclonal OFA-iLRP-specific CTL contain T lymphocytes capable of recognizing HLA-A2-binding OFA-ilRP-derived peptides, T2 cells were separately loaded with 4 different peptides (iLRP1-4) and used as target cells. As illustrated in Figure 6A, CTLs specific for OFA-iLRP lysed T2 cells coated with OFA-iLRP1 or OFA-iLRP2, but they did not kill T2 cells pulsed with iLRP3, iLRP4, or an irrelevant control peptide (FluM158-66). The findings are in line with the T-cell frequencies detected in an ELISPOT assay (Figure 6C).
Functional analysis of peptide-specific T cells contained in polyclonal OFA-iLRP-specific CTL cultures. T2 cells were separately pulsed with different peptides deduced from the OFA-iLRP protein (iLRP1-4) or with a control peptide (FluM158-66) and used as target cells in a 4-hour 51Chromium release assay (A). Killing activity of polyclonal OFA-iLRP-specific CTLs against T2 cells pulsed with different amounts of peptide iLRP1 and iLRP2 (B). Detection of iLR-peptide-specific T cells in polyclonal T-cell cultures using the IFN-γ and IL-10 ELISPOT assay (C). iLRP1 versus no peptide, P < .001; iLRP2 versus no peptide, P < .001; iLRP2 versus iLRP1, P < .005.
Functional analysis of peptide-specific T cells contained in polyclonal OFA-iLRP-specific CTL cultures. T2 cells were separately pulsed with different peptides deduced from the OFA-iLRP protein (iLRP1-4) or with a control peptide (FluM158-66) and used as target cells in a 4-hour 51Chromium release assay (A). Killing activity of polyclonal OFA-iLRP-specific CTLs against T2 cells pulsed with different amounts of peptide iLRP1 and iLRP2 (B). Detection of iLR-peptide-specific T cells in polyclonal T-cell cultures using the IFN-γ and IL-10 ELISPOT assay (C). iLRP1 versus no peptide, P < .001; iLRP2 versus no peptide, P < .001; iLRP2 versus iLRP1, P < .005.
Induction of tumor immunity using OFA-iLRP RNA-transfected DCs against a B-cell leukemia
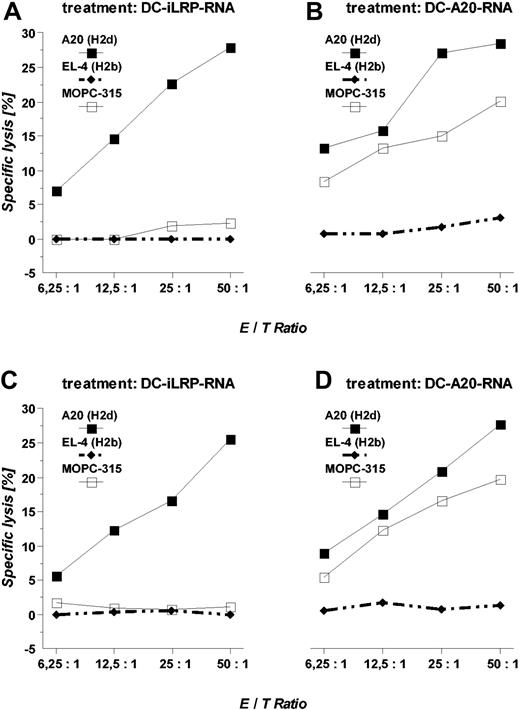
To evaluate the antitumoral potency of dendritic cells transfected with OFA-iLRP RNA, healthy Balb/c mice were treated with 3 subcutaneous injections of 5 × 105 OFA-iLRP RNA-transfected DCs, spaced 1 week apart. One week after the last DC vaccination, animals received an otherwise lethal tumor inoculation (subcutaneously) of 1 × 105 A20 leukemia cells. Vaccination of animals with unpulsed DCs did not significantly improve survival if compared with the tumor control group (Table 3). In contrast, animals treated with DCs transfected with the OFA-iLRP-coding RNA or with total RNA derived from the A20 leukemia completely rejected the tumor in 8 of 12 (67%) and 9 of 12 (75%) experiments. In an established tumor, long-term tumor-free survival could be documented in 50% (DC + iLRP RNA) and 67% (DC + total A20 RNA), respectively (Table 3). Furthermore, in animals surviving the observation period of 140 days, CTLs could be generated capable of efficiently killing the tumor lines A20 and MOPC-315. CTL analysis from mice treated with OFA-iLRP RNA-transfected DCs revealed an efficient tumor-specific killing of the OFA-iLRP+ A20 leukemia, whereas the syngeneic OFA-iLRP-negative tumor line MOPC-315 and the fully MHC-mismatched control target EL-4 were not lysed (Figure 7).
Induction of cytotoxic responses in mice treated with iLRP RNA- ortumor RNA-transfected DCs. Balb/c mice were treated with 3 subcutaneous injections of 1 × 105 OFA-iLRP RNA- or total A20 RNA-transfected syngeneic DCs and subsequently were challenged with 1 × 105 A20 leukemia cells inoculated subcutaneously 7 days after the last vaccination. At day 50, after tumor injection, splenocytes from vaccinated animals were harvested and restimulated in vitro in the presence of syngeneic OFA-iLRP RNA- or total-A20 RNA-transfected DCs for 5 days. Afterward, viable cells were separated and their cytotoxic potential (OFA-iLRP-CTL, A; A20-CTL, B) was determined against A20 (H-2d), MOPC-315 (H-2d), and EL-4 (H-2b) in a 4-hour 51Chromium release assay. Killing activity of polyclonal CTLs from animals receiving DC therapy 5 days after tumor inoculation was also measured (C + D).
Induction of cytotoxic responses in mice treated with iLRP RNA- ortumor RNA-transfected DCs. Balb/c mice were treated with 3 subcutaneous injections of 1 × 105 OFA-iLRP RNA- or total A20 RNA-transfected syngeneic DCs and subsequently were challenged with 1 × 105 A20 leukemia cells inoculated subcutaneously 7 days after the last vaccination. At day 50, after tumor injection, splenocytes from vaccinated animals were harvested and restimulated in vitro in the presence of syngeneic OFA-iLRP RNA- or total-A20 RNA-transfected DCs for 5 days. Afterward, viable cells were separated and their cytotoxic potential (OFA-iLRP-CTL, A; A20-CTL, B) was determined against A20 (H-2d), MOPC-315 (H-2d), and EL-4 (H-2b) in a 4-hour 51Chromium release assay. Killing activity of polyclonal CTLs from animals receiving DC therapy 5 days after tumor inoculation was also measured (C + D).
CTLs obtained from animals treated with total A20 RNA-transfected DCs were capable of killing both A20 and MOPC-315, suggesting that shared antigens might exist between MOPC-315 and A20. Determination of white blood cell counts at different time points demonstrate that administration of RNA-transfected DCs had no influence on marrow function (data not shown).
Discussion
In the present study, we show that OFA-iLRP-specific cytotoxic T-cell lines generated in the presence of OFA-iLRP RNA-transfected autologous dendritic cells are capable of efficiently lysing (1) OFA-iLRP-expressing hematologic tumor cell lines, (2) malignant blasts from patients with AML, and (3) leukemic cells obtained from patients with CLL. Furthermore, vaccination experiments in mice demonstrate that OFA-iLRP RNA-transfected DCs can serve as a potent cell vaccine to induce powerful antitumor effects against a hematologic tumor cell line.
OFA-iLRP has been recognized as a tumor-associated immunogen in both mice and humans and is detectable as an immunogen or antigen in carcinomas, sarcomas, and lymphomas, but not in a vast survey of healthy tissues from humans and rodents.1,6-8 In the current report, we extend these studies and demonstrate that OFA-iLR protein is abundantly expressed on various hematologic tumor cell lines but also on malignant blasts of patients with AML. To the best of our knowledge for the first time, we demonstrate that all samples of CD5+CD19+ leukemic cells obtained from patients with CLL with progressive disease (Binet C) constitutively express OFA-iLRP as a tumor-associated immunogen, whereas B-CLL cells from an early stage (Binet A) were OFA-iLRP-negative, as well as the nonmalignant counterpart and other cell subsets, such as monocytes, DCs, and T cells obtained from healthy individuals (Table 2).
On the basis of several in vitro and in vivo immunogenicity studies, OFA-iLRP has been shown to be a T- and B-lymphocyte-stimulating tumor-associated rejection immunogen.9-15 For example, in patients with metastatic renal cell carcinoma vaccinated with tumor lysate-loaded autologous DCs, Zelle-Rieser et al14 and Holtl et al15 demonstrated that those patients who clinically responded to DC therapy developed significant OFA-iLRP-specific T-cell reactivity, suggesting that OFA-iLRP might contain immunodominant epitopes. We here investigated whether OFA-iLRP can be used as a target structure for T-cell-based immunotherapeutic approaches against hematologic malignancies, especially for AML and CLL. For this purpose, tumor antigen-specific CTLs were generated by stimulating naive T cells from HLA-A2-positive healthy volunteers in the presence of autologous DCs prior transfected with tumor antigen-coding RNA, an efficient technique to stably produce polyclonal CTLs, as extensively reported.20-22 We observed that both hematologic tumor cell lines and malignant cells from patients with AML and CLL endogenously expressing OFA-iLRP could be recognized and killed by OFA-iLRP-specific CTLs, whereas nonmalignant cell subpopulations derived from the hematopoietic system (CD34+ progenitor cells, bone marrow cells, B lymphoblasts, dendritic cells) were not affected. Using peripheral blood from a patient with HLA-A2-positive AML who was in a hematologic remission 6 months after the last cytostatic chemotherapy, we were able to generate OFA-iLRP-specific CTLs capable of efficiently lysing both the autologous and allogeneic HLA-A2-matched OFA-iLRP-expressing AML blasts. It is important to note that OFA-iLRP-specific CTLs do not react against HLA-A2-matched CD34-purified peripheral blood progenitor cells and bone marrow cells obtained from healthy donors, suggesting that nonmalignant hematopoietic progenitor cells might not be the target for T-cell-based immunotherapeutic strategies using OFA-iLRP as a target structure.
In the present data, polyclonal OFA-iLRP-specific CTLs generated with autologous OFA-iLRP RNA-transfected dendritic cells contain T lymphocytes capable of recognizing 2 peptides (OFA-iLRP58-66 and OFA-iLRP60-67) as demonstrated by their potential to kill T2 cells loaded with these peptides. This recognition suggests that at least 2 clonotypes had been generated by using autologous OFA-iLRP RNA-transfected DCs as stimulator cells.
In an established murine B-cell leukemia model, a significant proportion of animals treated with OFA-iLRP RNA-transfected DCs was capable of completely rejecting an otherwise lethal tumor dose of A20 leukemia cells most probably mediated through OFA-iLRP-specific CTLs. Treated animals surviving the observation period of 140 days did not exhibit any signs of autoimmune manifestations. In earlier studies, it could be demonstrated that immunization of mice with syngeneic tumor cells expressing OFA-iLRP even results in cross-protective immunity against a spectrum of syngeneic tumors.1,13,23 Interestingly, treatment of animals with purified recombinant OFA-iLRP can induce T- and B-cell responses critically dependent on the dose of iLRP-OFA administered for immunization.1 The low dose induces cytotoxic T cells capable of lysing a syngeneic OFA-iLRP-expressing tumor, whereas a higher dose of OFA-iLRP generates a humoral anti-OFA-iLRP immune response. In the present data, vaccination of animals with dendritic cells transfected with OFA-iLRP-coding RNA resulted in a powerful CTL reactivity against syngeneic OFA-iLRP-expressing targets. Further studies are required to analyze whether vaccination with OFA-iLRP RNA-transfected DCs can generate anti-iLRP antibodies in vivo as well.
In summary, our data show that OFA-iLRP-specific CTLs efficiently lysed hematologic tumor cell lines as well as malignant cells from leukemia patients. These findings provide strong evidences for the use of OFA-iLRP as a good candidate for the design of T-cell-based immunotherapeutic strategies of OFA-iLRP-expressing tumors such as AML and CLL. Future studies will focus on the detection of OFA-iLRP-specific T-cell responses in the peripheral blood of patients with AML and CLL.
Prepublished online as Blood First Edition Paper, July 17, 2003; DOI 10.1182/blood-2003-01-0198.
Supported by the National Institutes of Health National Cancer Institute (grant RO1 CA 82603) (J.C. Jr, A.B., and J.R.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.