Abstract
SOCS1-/- mice die prematurely of increased interferon-γ (IFNγ) signaling with severe thymic atrophy and accelerated maturation of T cells. However, it was unclear whether the thymic defects were caused by SOCS1 deficiency or by increased IFNγ signaling. Using SOCS1-/-IFNγ-/- mice, we show in this study that SOCS1 deficiency skews thymocyte development toward CD8 lineage independently of IFNγ. Fetal thymic organ cultures and intrathymic transfer of CD4-CD8- precursors into Rag1-/- mice show that the lineage skewing in SOCS1-/- mice is a T-cell autonomous defect. Interestingly, SOCS1 is not required for attenuating interleukin-7 (IL-7) signaling at the CD4-CD8- stage but is essential for regulating IL-15 and IL-2 signaling in CD8+ thymocytes. IL-15 selectively stimulates SOCS1-/- CD8+ thymocytes, inducing sustained signal transducer and activator of transcription 5 (STAT5) phosphorylation and massive proliferation. IL-15 also strongly up-regulates Bcl-xL and CD44 in CD8+ thymocytes lacking SOCS1. The SOCS1 gene is induced in CD4+ thymocytes by γc cytokines, whereas CD8+ thymocytes constitutively express SOCS1 mRNA even in the absence of cytokine stimulation. Because many different cell types express IL-15, our results strongly suggest that SOCS1 functions as an indispensable attenuator of IL-15 receptor signaling in developing CD8+ thymocytes. (Blood. 2003;102:4115-4122)
Introduction
Cytokines are critical regulators of the development and maturation of T lymphocytes within the thymus.1-3 T-cell development in the thymus occurs in distinct stages defined by the rearrangement of T-cell receptor (TCR) genes and the modulation of various surface markers.4 Early thymocyte survival and proliferation are critically dependent on c-Kit and interleukin-7 (IL-7), which provide partially overlapping antiapoptotic and mitogenic signals during CD4-CD8- (double-negative, DN) pre-T-cell stage 2. Disruption of normal c-Kit signaling profoundly reduces thymic cellularity, with a transitional block from CD44+CD25+ DN-2 to CD44-CD25+ DN-3 stage.5 Ablation of genes encoding IL-7, IL-7 receptor α (IL-7Rα), or the γc chain causes a similar developmental block with an accumulation of CD44+CD25+ DN-2 stage cells.6-9 A combined deficiency for both c-Kit and the γc chain totally abolishes T-cell development.10 Subsequent stages of thymocyte development, which are dependent on pre-TCR and TCR signals, can be modulated by cytokine receptor signaling; however, these cytokine-mediated signals are not critical for the development of TCR-αβ+ T cells.2,3,11
c-Kit is a receptor tyrosine kinase activated by autophosphorylation after interaction with its ligand. Activated c-Kit stimulates multiple signal transduction pathways, including phosphoinositide 3-kinase (PI3-K), Src kinase, Janus kinase-signal transducer and activator of transcription (JAK-STAT), and Ras-mitogen-activated protein kinase (Ras-MAPK) pathways.12 IL-7R, composed of IL-7Rα and the γc chain, signals through JAK-STAT and PI3-K pathways.13-15 The magnitude and the duration of cytokine receptor signals are controlled by negative regulatory mechanisms to prevent excessive signaling and abnormal cellular activation.16 Members of the SOCS family proteins—SOCS1, SOCS2, and SOCS3—function as feedback-negative regulators of cytokine receptor signaling by binding and inhibiting JAKs.17 Gene-deletion studies have shown that SOCS1, SOCS2, and SOCS3 play nonredundant roles in attenuating cytokine receptor signaling.18-23
SOCS1 is expressed during all thymic developmental stages.24 SOCS1 can inhibit c-Kit-induced cell proliferation signals without interfering with cell survival signals.25 Using retroviral-mediated transfer of SOCS1 cDNA into developing thymocytes in fetal thymic organ cell cultures (FTOCs), we have shown that SOCS1 can inhibit T-cell development at DN stage 2.4 Similarly, SOCS1 transgenic mice show severe impairment of T-cell maturation.26 However, these studies did not reveal whether SOCS1 plays a nonredundant role in regulating c-Kit or IL-7 signaling during thymopoiesis. SOCS1-/- mice, which die within 2 to 3 weeks of birth, failed to provide a conclusive answer to whether SOCS1 is dispensable in regulating cytokine signaling during T-cell development.18-20 Instead of showing increased proliferation because of augmented c-Kit or IL-7 signaling, SOCS1-/- mice showed severe thymic atrophy.18-20,27 Given that premature death in SOCS1-/- mice results from uncontrolled interferon-γ (IFNγ) signaling and that the injection of IFNγ into neonatal mice causes severe perturbation of thymocyte development,28 the thymic atrophy observed in SOCS1-/- mice was presumed to result from excessive IFNγ signaling.20 SOCS1-/- mice also showed accelerated maturation of CD4+CD8+ double-positive (DP) cells to CD4+ and CD8+ single-positive (SP) T cells, with an increased frequency of the latter.20 In this study, we have examined the development of SOCS1-/- T cells without the influence of systemic IFNγ. Our results show that SOCS1 plays a nonredundant role in regulating IL-15 and IL-2 signaling in CD8+ thymocytes.
Materials and methods
Mice
SOCS1+/- and SOCS1+/-IFNγ-/- mice were generous gifts from Dr J. Ihle. SOCS1-/- and SOCS1-/-IFNγ-/- mice were bred from SOCS1 heterozygous parents because SOCS1-/- mice die prematurely and SOCS1-/-IFNγ-/- mice breed poorly. SOCS1+/- and SOCS1+/-IFNγ-/- mice have been backcrossed to C57Bl/6 for 5 generations. Rag1-/- (C57Bl/6) mice were bred in our facility. SOCS1-/- mice were analyzed at 8 to 14 days, and SOCS1-/-IFNγ-/- mice were analyzed at 4 to 6 weeks. All animal work was performed according to the guidelines of the Ontario Cancer Institute.
Antibodies, cytokines, and reagents
Antibodies against mouse CD4, CD8, TCR-β (H57), CD44, and Bcl-2 conjugated to fluorescein isothiocyanate (FITC), phycoerythrin (PE) or Biotin, and Fc block (CD16/CD32) were purchased from BD PharMingen Biosciences (San Diego, CA). Bcl-xL-FITC and streptavidin-spectral red (ST-SPRD) were from Southern Biotechnology Associates (Birmingham, AL). J558/murine IL-7 (mIL-7) secreting mIL-7 was a kind gift from Dr Ana Cumano (Pasteur Institute, Paris, France). Rabbit polyclonal antibody against phospho-STAT5 was from Cell Signaling Technology. Goat antirabbit immunoglobulin G (IgG) conjugated to Oregon green 488, and 5-6carboxyfluorescein diacetate succinimidyl ester (CFSE) were from Molecular Probes (Eugene, OR). Culture supernatants (CS) from X630/mIL-2 and J558/mIL-7 cells were used as sources of IL-7 and IL-2. Recombinant human IL-15 (hIL-15) was purchased from R&D Systems (Minneapolis, MN). OPTI-MEM cell culture medium and fetal bovine serum (FBS) were from Gibco (Grand Island, NY).
Flow cytometry
Single-cell suspensions in phosphate-buffered saline (PBS) containing 5% FBS and 0.05% sodium azide were preincubated with Fc block for 10 minutes. Expression of various cell surface markers was estimated by standard 3-color staining using FITC, PE, and biotin-conjugated primary antibodies followed by ST-SPRD. For staining intracellular Bcl-2 and Bcl-xL, thymocytes stimulated with IL-15 (40 ng/mL) or IL-7 (at 1:20 dilution of the CS) for 36 hours at 2 × 106 cells/mL were stained for CD4 and CD8 and were fixed in 1% paraformaldehyde (PFA). After permeabilization with 0.5% saponin in PBS/5% FBS, cells were incubated with anti-Bcl-2-PE or anti-Bcl-xL-FITC, washed, and analyzed. Data acquisition and analysis were performed using FACSCalibur using CellQuest software (Becton Dickinson, Mountain View, CA).
Fetal thymic organ culture
Fetal thymi from day 14.5 embryos were cultured on Nucleopore membrane (Whatman, Newton, MA) placed on Gel Foam sponges (Pharmacia & Upjohn, Kalamazoo, MI). At each indicated time point, at least 3 thymi representing the SOCS1-/- or SOCS1+/+ genotype were analyzed for the distribution of different T-cell markers.
Cell proliferation assays
Total thymocytes (1 × 105) were stimulated with indicated concentrations of IL-7, IL-2, or IL-15 for 48 hours in 96-well culture plates. One microcurie (0.037 MBq) methyl-3H-thymidine (NEN, Boston, MA) was added per well during the last 8 hours. Cells were harvested onto glass fiber filter mats, and the incorporated radioactivity was measured using TopCount (Canberra/Packard, Mississauga, ON, Canada).
The CFSE dye dilution assay was used to estimate the number of cell division cycles within each T-cell subset after cytokine stimulation. Total thymocytes were labeled with CFSE by incubating cells at 2 × 107 cells/mL in PBS containing 5 μM CFSE for 10 minutes at room temperature. The reaction was quenched with an equal volume of FBS. Cells were washed twice and stimulated with IL-15 (40 ng/mL), IL-2, or IL-7 (at 1:20 dilution of the CS) for 72 hours and then labeled for surface markers. Sequential reduction in dye content, reflecting successive cell division, was followed within each gated thymocyte subset.
Flow cytometric detection of intracellular phospho-STAT5
Cytokine-induced STAT5 phosphorylation in different thymocyte subsets was examined by intracellular staining and flow cytometry. Briefly, freshly isolated cells in OPTI-MEM containing 1 mg/mL bovine serum albumin (BSA) and 0.5% FBS were suspended at 2 × 106 cells/mL and stimulated with IL-15 (100 ng/mL) or IL-7 (at 1:10 dilution of the CS) for 20 minutes to achieve maximal STAT5 phosphorylation. Immediately after stimulation, an aliquot of cells was fixed in 2% PFA at room temperature for 10 minutes. The rest of the cells were washed twice and returned to culture without cytokines. Aliquots were drawn at indicated time points and fixed. Fixed cells were washed in PBS and incubated in methanol/acetone (1:1 vol/vol) on ice for 30 minutes. Cells were rehydrated by washing in PBS/5% FBS. After incubation with anti-phospho-STAT5 antibody in PBS/FBS for 30 minutes, antirabbit IgG-Oregon green 488 was added for another 30 minutes. After blocking with normal rat IgG, the cells were stained for CD4 and CD8, and phospho-STAT5 in gated thymocyte subsets was analyzed by flow cytometry.
Reverse transcription-polymerase chain reaction analysis of SOCS1 gene expression in thymocyte subsets
Five to 10 × 104 flow cytometrically sorted DN, DP, CD4+, or CD8+ thymocytes from SOCS1-/-IFNγ-/- and SOCS1+/+IFNγ-/- mice were stimulated with IL-2, IL-7 (1:10 dilution of CS), or IL-15 (40 ng/mL) or were left untreated in medium alone. After 16 hours of incubation, total RNA was isolated using 0.5 mL Trizol (Gibco-BRL) according to the manufacturer's instructions. Equivalent amounts of RNA were primed with oligo(dT), and first-strand cDNA was synthesized using Thermoscript reverse transcriptase (Life Technologies). Polymerase chain reaction (PCR) amplification of SOCS1 cDNA was performed as described earlier.24
Results
SOCS1 deficiency skews intrathymic T-cell development toward CD8 lineage independently of IFNγ
SOCS1-/- mice show early thymic atrophy characterized by the depletion of CD4+CD8+ thymocytes and the accumulation of CD4+ and CD8+ SP cells, suggesting a rapid maturation from the DP to the SP stage18-20 (Figure 1A, upper panels). Perinatal lethality caused by SOCS1 deficiency hindered the characterization of the precise mechanism(s) that accelerates DP to SP transition in these mice. Given that SOCS1-/- mice survive in an IFNγ-/- background20 and IFNγ deficiency does not affect T-cell development,29 we investigated T-cell maturation in SOCS1-/-IFNγ-/- mice. Although SOCS1-/- thymi atrophied within a few days of birth, thymi from 4-week-old SOCS1-/-IFNγ-/- mice were of normal size and yielded thymocyte numbers comparable to those of SOCS1+/+IFNγ-/- thymi (99 ± 26 × 106 in SOCS1-deficient thymi vs 96 ± 17 × 106 in control thymi; n = 6).
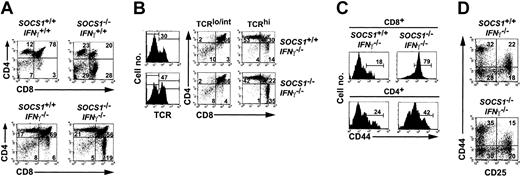
T-cell development in SOCS1-deficient mice is skewed toward CD8 lineage. (A) Thymocytes from 8-day-old SOCS1-/-IFNγ+/+ and 4-week-old SOCS1-/-IFNγ-/- mice and their respective controls were stained for CD4 and CD8. Numbers within each quadrant represent the frequency of thymocytes in DN, DP, CD4+, or CD8+ stages of development. Data shown are representative of several independent experiments. (B) CD8+ SP cells in SOCS1-deficient thymus are mature T cells. Thymocytes from 4-week-old SOCS1-/-IFNγ-/- mice and their littermate controls were stained for TCR-β, CD4, and CD8, and the distributions of DN, DP, CD4+, and CD8+ cells within TCRlo/int and TCRhi populations were estimated. Frequency of TCRhi cells is indicated within histograms, and numbers within the quadrants denote the percentages of each subset within TCRlo/int and TCRhi populations. Representative data from at least 4 animals per group are shown. (C) Increased CD44 expression on CD8+ SP cells in SOCS1-/-IFNγ-/- thymus. Expression of CD44 on CD8+ and CD4+ SP thymocytes from SOCS1-/-IFNγ-/- mice and their littermate controls is shown as histograms. Percentage of positive cells within the marker boundary is indicated. (D) SOCS1 is not a critical regulator of pre-T-cell development during the DN stage. Thymocytes from 4-week-old SOCS1-/-IFNγ-/- mice and their littermate controls were stained with biotinylated anti-CD4, anti-CD8, anti-CD25-PE, and anti-CD44-FITC, followed by ST-SPRD. Surface expression of CD25 and CD44 on gated CD4-CD8- DN cells is shown as dot plots. Percentages of cells within the 4 DN developmental stages are indicated. Results shown are representative of at least 3 animals per group from 2 experiments.
T-cell development in SOCS1-deficient mice is skewed toward CD8 lineage. (A) Thymocytes from 8-day-old SOCS1-/-IFNγ+/+ and 4-week-old SOCS1-/-IFNγ-/- mice and their respective controls were stained for CD4 and CD8. Numbers within each quadrant represent the frequency of thymocytes in DN, DP, CD4+, or CD8+ stages of development. Data shown are representative of several independent experiments. (B) CD8+ SP cells in SOCS1-deficient thymus are mature T cells. Thymocytes from 4-week-old SOCS1-/-IFNγ-/- mice and their littermate controls were stained for TCR-β, CD4, and CD8, and the distributions of DN, DP, CD4+, and CD8+ cells within TCRlo/int and TCRhi populations were estimated. Frequency of TCRhi cells is indicated within histograms, and numbers within the quadrants denote the percentages of each subset within TCRlo/int and TCRhi populations. Representative data from at least 4 animals per group are shown. (C) Increased CD44 expression on CD8+ SP cells in SOCS1-/-IFNγ-/- thymus. Expression of CD44 on CD8+ and CD4+ SP thymocytes from SOCS1-/-IFNγ-/- mice and their littermate controls is shown as histograms. Percentage of positive cells within the marker boundary is indicated. (D) SOCS1 is not a critical regulator of pre-T-cell development during the DN stage. Thymocytes from 4-week-old SOCS1-/-IFNγ-/- mice and their littermate controls were stained with biotinylated anti-CD4, anti-CD8, anti-CD25-PE, and anti-CD44-FITC, followed by ST-SPRD. Surface expression of CD25 and CD44 on gated CD4-CD8- DN cells is shown as dot plots. Percentages of cells within the 4 DN developmental stages are indicated. Results shown are representative of at least 3 animals per group from 2 experiments.
SOCS1-/-IFNγ+/+ and SOCS1-/-IFNγ-/- thymi showed an increase in the proportion of CD8+ cells compared with wild-type and IFNγ-deficient control mice (Figure 1A). Because the frequencies of the CD4+ subset were comparable between SOCS1-/-IFNγ-/- and SOCS1+/+IFNγ-/- thymi, the increased frequency of CD8+ SP cells in SOCS1-deficient thymi was caused by an absolute increase in the numbers of CD8+ SP cells. As a result, the CD4/CD8 ratio in SOCS1-/-IFNγ-/- thymi was decreased. We also observed that the severe diminution in the proportion of DP cells in the SOCS1-/-IFNγ+/+ thymi was almost completely reversed in SOCS1-/-IFNγ-/- mice, suggesting that this was primarily an IFNγ-mediated effect. These results show that SOCS1 deficiency causes T-cell developmental skewing toward the CD8 lineage, which occurs independently of IFNγ.
To investigate whether SOCS1-/- CD8+ thymocytes undergo normal developmental changes that accompany T-cell maturation, we examined TCR expression. The frequency of TCRhi cells was elevated in the SOCS1-/-IFNγ-/- thymi compared with the control thymi (47% vs 30%) (Figure 1B, left panels). Examination of the distribution of CD8+ cells within TCRhi and TCRlo/int thymocyte populations (Figure 1B, right panels) showed that almost all CD8+ SP cells in SOCS1-/-IFNγ-/- thymi expressed high levels of TCR, indicating that CD8+ SP cells in SOCS1-deficient thymi are mature T cells. Interestingly, more than 75% of SOCS1-/-IFNγ-/- CD8+ SP cells expressed high levels of CD44, an activation marker for antigen-experienced and memory T cells,30 compared with less than 20% in SOCS1+/+IFNγ-/- thymi (Figure 1C). CD4+ cells from SOCS1-/-IFNγ-/- mice also showed an increase in CD44 expression, but it was not as pronounced as in CD8+ SP cells.
Because c-Kit- and IL-7-mediated signals regulate the survival and proliferation of pre-T cells,2 we examined whether the absence of SOCS1 would perturb pre-T-cell development. The frequencies of the total DN subpopulations were comparable between SOCS1-/-IFNγ-/- and SOCS1+/+IFNγ-/- thymi (Figure 1A). To examine whether SOCS1 deficiency affects the transition through DN stages, we analyzed the DN population for CD25 and CD44 expression. SOCS1-/-IFNγ-/- and SOCS1+/+IFNγ-/- mice showed frequencies comparable to those of DN-1 (CD44+CD25-), DN-2 (CD44+CD25+), DN-3 (CD44-CD25+), and DN-4 (CD44-CD25-) cells (Figure 1D), suggesting that SOCS1 is not a critical negative regulator of IL-7 and c-Kit signaling during the DN stage of thymocyte development.
T-cell developmental skewing toward CD8 lineage is intrinsic to SOCS1 deficiency
To address whether the increased frequency of CD8+ cells in SOCS1-/- thymi arises from a systemic stress response or from the dysregulated elaboration of cytokines other than IFNγ, we analyzed T-cell development in SOCS1-/- fetal thymi in vitro. FTOCs from wild-type mice showed a CD8/CD4 ratio of almost 1:1, which decreased to 1:1.5 after 12 days (Figure 2A). Compared with this, SOCS1-/- FTOCs generated 4- to 5-fold more CD8+ cells than CD4+ cells throughout the culture period, indicating that the developmental skewing toward CD8 lineage in SOCS1-/- mice is not the result of an indirect effect or of any systemic cytokine or hormonal imbalance. Similar skewing toward CD8 lineage in SOCS1-/-IFNγ-/- FTOCs ruled out the possibility that locally produced IFNγ might be responsible for the developmental skewing (data not shown).
Skewed T-cell development toward CD8 lineage in SOCS1-deficient mice arises from an IFNγ-independent, T-cell intrinsic defect. (A) SOCS1-deficient FTOCs recapitulate T-cell developmental skewing toward the CD8 lineage. FTOCs were established from day 14.5 SOCS1-/- embryos and their littermate controls. Single-cell suspensions were prepared on indicated days and analyzed for the frequency of DN, DP, CD4+, and CD8+ cells. Results shown are representative of at least 6 thymi per group from 2 experiments. (B) Maturation of SOCS1-deficient DN precursors in Rag1-/- thymus is skewed toward the CD8 lineage. DN cells sorted from 4-week-old SOCS1-/- thymi and their littermate controls were injected into Rag1-/- thymi, and the frequencies of DN, DP, CD4+, and CD8+ cells were estimated after 14 days. Data shown are representative of 3 animals per group.
Skewed T-cell development toward CD8 lineage in SOCS1-deficient mice arises from an IFNγ-independent, T-cell intrinsic defect. (A) SOCS1-deficient FTOCs recapitulate T-cell developmental skewing toward the CD8 lineage. FTOCs were established from day 14.5 SOCS1-/- embryos and their littermate controls. Single-cell suspensions were prepared on indicated days and analyzed for the frequency of DN, DP, CD4+, and CD8+ cells. Results shown are representative of at least 6 thymi per group from 2 experiments. (B) Maturation of SOCS1-deficient DN precursors in Rag1-/- thymus is skewed toward the CD8 lineage. DN cells sorted from 4-week-old SOCS1-/- thymi and their littermate controls were injected into Rag1-/- thymi, and the frequencies of DN, DP, CD4+, and CD8+ cells were estimated after 14 days. Data shown are representative of 3 animals per group.
The thymic microenvironment plays important roles in T-cell development by elaborating cytokines and by providing cell-cell contact signaling to developing thymocytes.31 To investigate whether the T-cell developmental abnormalities seen in SOCS1-/- mice are intrinsic to T cells or are secondary to any perturbation within the thymic microenvironment, DN thymocytes from SOCS1-/- and SOCS1+/+ thymi were sorted and injected intrathymically into Rag1-/- mice. After 14 days, the reconstituted thymi were examined for DP and SP cells (Figure 2B). Although the proportion of CD4+ cells was comparable, the SOCS1-/- DN thymocytes yielded 3 times more CD8+ cells, demonstrating that an SOCS1-/- thymic epithelium is not a prerequisite for the preferential accumulation of CD8+ thymocytes. Therefore, the developmental skewing toward CD8 lineage in the SOCS1-/- thymus arises from a T-cell-intrinsic defect caused by SOCS1 deficiency.
Examining CD44 expression on CD8+ cells showed an increased accumulation of CD8+CD44hi SP cells over time in SOCS1-/- FTOCs (Table 1). This observation ruled out the possibility that the elevated CD44 expression on CD8+ cells in SOCS1-/- thymi could have been caused by recirculating mature T cells. In addition, this observation suggests that CD44 expression on thymocytes is modulated by a cytokine(s).
CD8+ thymocytes lacking SOCS1 proliferate vigorously and up-regulate CD44 after IL-15 stimulation
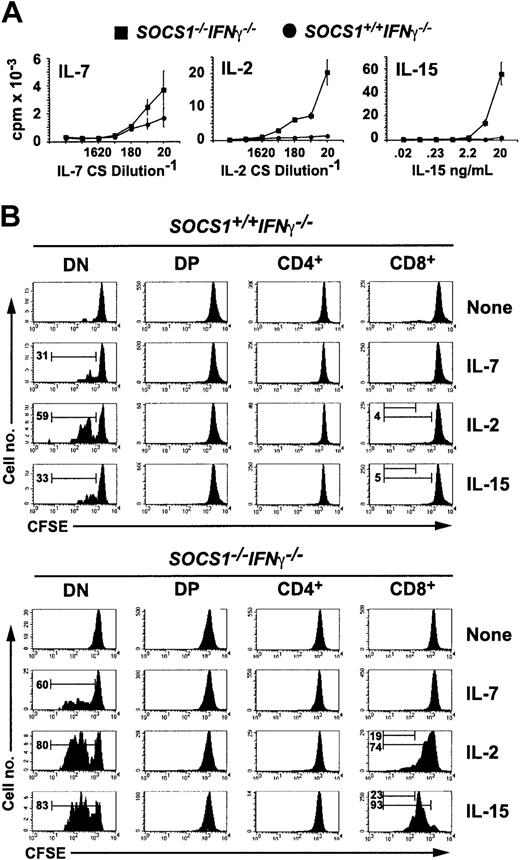
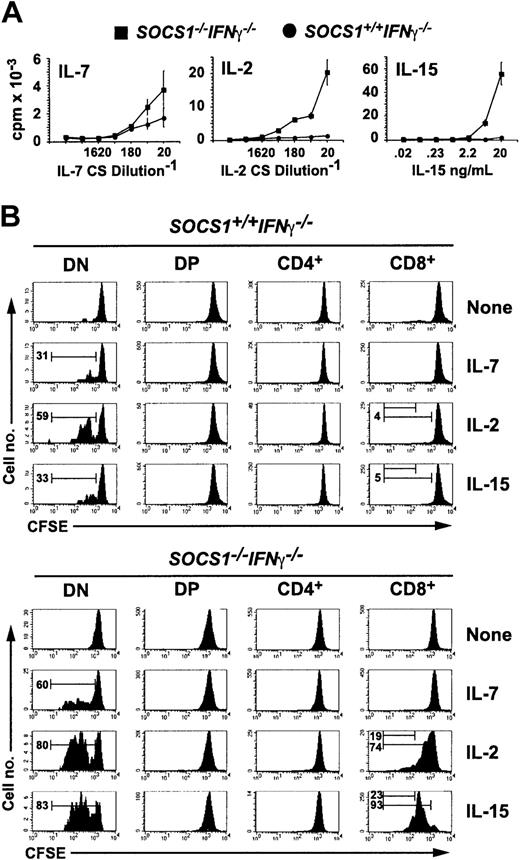
Because CD44 is an activation marker for CD8+ T lymphocytes,30 the elevated expression of CD44 on SOCS1-/- CD8+ thymocytes, ex vivo and in FTOCs, suggested that it could result from dysregulated cytokine stimulation in the absence of SOCS1. To examine this possibility, we first examined the proliferative response of SOCS1-/- thymocytes to γc cytokines IL-7, IL-2, and IL-15 (Figure 3A). Total thymocytes from SOCS1+/+IFNγ-/- mice responded marginally to IL-7 but not to IL-15 or IL-2. SOCS1-/-IFNγ-/- thymocytes showed increased responsiveness to IL-7; surprisingly, IL-2 caused a 5-fold higher response, but IL-15 stimulated more than a 15-fold increase in the proliferation of SOCS1-/-IFNγ-/- cells compared with SOCS1+/+IFNγ-/- thymocytes.
SOCS1-deficient CD8+ SP thymocytes hyperproliferate to IL-15 stimulation. (A) SOCS1-deficient thymocytes show increased proliferative responses to γc cytokines. Total thymocytes from 6-week-old SOCS1-/-IFNγ-/- mice and their littermate controls were stimulated with indicated concentrations of IL-7, IL-2, and IL-15 for 48 hours. 3H-thymidine incorporation during the last 8 hours is shown. Error bars indicate standard error. (B) SOCS1-deficient DN and CD8+ SP thymocytes selectively respond to IL-15 and IL-2. Total thymocytes from SOCS1-/-IFNγ-/- and control mice were loaded with CFSE and stimulated with a 1:20 dilution of IL-7- or IL-2-containing CS or with 40 ng mL-1 IL-15. After 72 hours, the cells were stained for CD4 and CD8 and were analyzed for diluted CFSE as a function of cell division. Representative data from at least 6 animals for each group from 3 experiments are shown. Numbers within histograms denote the frequency of cells that underwent 1 or 3 cell divisions.
SOCS1-deficient CD8+ SP thymocytes hyperproliferate to IL-15 stimulation. (A) SOCS1-deficient thymocytes show increased proliferative responses to γc cytokines. Total thymocytes from 6-week-old SOCS1-/-IFNγ-/- mice and their littermate controls were stimulated with indicated concentrations of IL-7, IL-2, and IL-15 for 48 hours. 3H-thymidine incorporation during the last 8 hours is shown. Error bars indicate standard error. (B) SOCS1-deficient DN and CD8+ SP thymocytes selectively respond to IL-15 and IL-2. Total thymocytes from SOCS1-/-IFNγ-/- and control mice were loaded with CFSE and stimulated with a 1:20 dilution of IL-7- or IL-2-containing CS or with 40 ng mL-1 IL-15. After 72 hours, the cells were stained for CD4 and CD8 and were analyzed for diluted CFSE as a function of cell division. Representative data from at least 6 animals for each group from 3 experiments are shown. Numbers within histograms denote the frequency of cells that underwent 1 or 3 cell divisions.
To identify the thymocyte developmental stage that showed increased sensitivity to IL-15 or IL-2 in the absence of SOCS1, we labeled total thymocytes with CFSE before cytokine stimulation and evaluated the proliferative response of various thymocyte subsets after 72 hours by diluting CFSE fluorescence. IL-7 stimulated DN cells to proliferate but had no effect on the other thymocyte subsets (Figure 3B). SOCS1 deficiency sensitized DN cells 2-fold to IL-7-mediated proliferation. IL-15 and IL-2 also stimulated greater DN cell proliferation in SOCS1-/- cells than wild-type controls. Whereas IL-15 had a negligible effect on the proliferation of SOCS1-sufficient CD8+ thymocytes, it caused a massive proliferation of CD8+ cells lacking SOCS1. By 72 hours virtually all SOCS1-deficient CD8+ cells had divided at least once in response to IL-15. This effect was unique to the CD8 lineage because IL-15 failed to stimulate CD4+ thymocytes even from SOCS1-/-IFNγ-/- mice. IL-2 showed a similar, but less intense effect, on SOCS1-deficient CD8+ thymocytes.
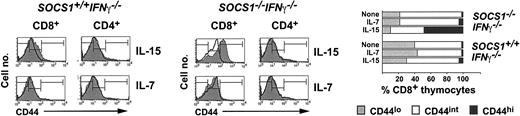
Examining CD44 expression after IL-15 stimulation showed that IL-15-induced proliferation of CD8+ thymocytes is accompanied by CD44 up-regulation. Although IL-15 induced a marginal increase in CD44 expression in SOCS1-sufficient cells, it potently up-regulated CD44 in SOCS1-deficient CD8+ thymocytes (Figure 4). Quantitating the level of CD44 expression as high, medium, or low showed that 50% of the CD8+ thymocytes from SOCS1-deficient mice expressed high levels of CD44 after IL-15 stimulation, whereas less than 10% of SOCS1-sufficient cells became CD44hi. This effect is specific to IL-15 because IL-7 did not up-regulate CD44 on SOCS1-deficient cells.
IL-15 up-regulates CD44 expression in SOCS1-deficient CD8+ SP thymocytes. Total thymocytes from SOCS1-/-IFNγ-/- and control mice were stimulated with 40 ng mL-1 IL-15 or a 1:20 dilution of IL-7-containing CS. After 48 hours, the cells were stained for CD4, CD8, and CD44. Expression of CD44 in gated CD8+ and CD4+ SP thymocytes from 1 of 4 representative animals per group is shown. Histograms of the stimulated cells are shaded gray, and unfilled line histograms represent the unstimulated cells. The bar graph shows the proportion of CD8+ cells showing high, intermediate, or low levels of CD44 expression, based on the marker boundaries shown in the histograms.
IL-15 up-regulates CD44 expression in SOCS1-deficient CD8+ SP thymocytes. Total thymocytes from SOCS1-/-IFNγ-/- and control mice were stimulated with 40 ng mL-1 IL-15 or a 1:20 dilution of IL-7-containing CS. After 48 hours, the cells were stained for CD4, CD8, and CD44. Expression of CD44 in gated CD8+ and CD4+ SP thymocytes from 1 of 4 representative animals per group is shown. Histograms of the stimulated cells are shaded gray, and unfilled line histograms represent the unstimulated cells. The bar graph shows the proportion of CD8+ cells showing high, intermediate, or low levels of CD44 expression, based on the marker boundaries shown in the histograms.
IL-15 induces Bcl-xL expression selectively in SOCS1-deficient CD8+ thymocytes
Cytokine-induced up-regulation of the antiapoptotic protein Bcl-2 plays an important role in lymphocyte survival. IL-7 favors the survival of DN thymocytes by up-regulating Bcl-2, and the transgenic expression of Bcl-2 can rescue thymopoiesis in IL-7Rα- or γc-chain-deficient mice.32-34 CD8+ thymocytes from IL-15Rα-deficient mice show decreased levels of Bcl-2 and increased sensitivity to corticosteroid-induced death.35 Therefore, we examined whether Bcl-2 induction by IL-15 or IL-7 in thymocytes derived from SOCS1-/-IFNγ-/- mice was dysregulated. IL-15 and IL-7 induced comparable levels of Bcl-2 in CD8+ thymocytes from SOCS1-deficient and SOCS1-sufficient thymi (Figure 5), indicating that SOCS1 is not a critical regulator of Bcl-2 induction by these cytokines in CD8+ thymocytes. Although IL-7 efficiently up-regulated Bcl-2 in CD4+ thymocytes, IL-15 caused only a marginal increase in Bcl-2 expression even in SOCS1-deficient CD4+ thymocytes. Compared with Bcl-2 expression, IL-15 up-regulated Bcl-xL only in SOCS1-deficient CD8+ thymocytes (Figure 5). IL-7 also up-regulated Bcl-xL in CD8+ thymocytes even though it did not induce proliferation or CD44 expression in these cells. Neither IL-15 nor IL-7 induced Bcl-xL in SOCS1-deficient CD4+ thymocytes. These results suggest that in addition to stimulating proliferation, IL-15 may provide a survival advantage to CD8+ thymocytes lacking SOCS1 by up-regulating Bcl-xL expression.
IL-15 and IL-7 up-regulate Bcl-xL expression selectively in SOCS1-deficient CD8+ SP thymocytes. Total thymocytes from SOCS1-/-IFNγ-/- and control mice were stimulated with 40 ng mL-1 IL-15 or a 1:20 dilution of IL-7-containing CS. After 36 hours, the cells were stained for CD4 and CD8, fixed, and stained for intracellular Bcl-2 or Bcl-xL. Expression of Bcl-2 or Bcl-xL in gated CD8+ and CD4+ SP thymocytes from a representative of several experiments is shown. Histograms of the stimulated cells are shaded gray, and unfilled line histograms represent the unstimulated cells.
IL-15 and IL-7 up-regulate Bcl-xL expression selectively in SOCS1-deficient CD8+ SP thymocytes. Total thymocytes from SOCS1-/-IFNγ-/- and control mice were stimulated with 40 ng mL-1 IL-15 or a 1:20 dilution of IL-7-containing CS. After 36 hours, the cells were stained for CD4 and CD8, fixed, and stained for intracellular Bcl-2 or Bcl-xL. Expression of Bcl-2 or Bcl-xL in gated CD8+ and CD4+ SP thymocytes from a representative of several experiments is shown. Histograms of the stimulated cells are shaded gray, and unfilled line histograms represent the unstimulated cells.
IL-15 stimulates sustained STAT5 phosphorylation in SOCS1-deficient CD8+ thymocytes
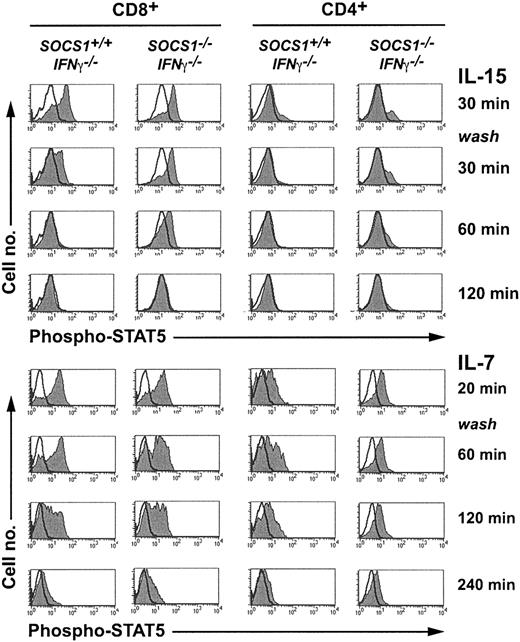
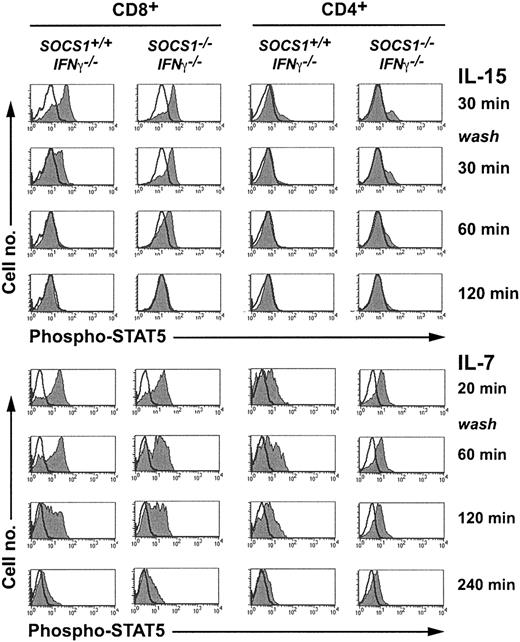
IL-2, IL-7, and IL-15 bind to the unique α-chains of each respective receptor complex and transduce signals through the γc chain using JAK3 and STAT5.13,36,37 To examine whether IL-15 signaling was potentiated in the absence of SOCS1, we evaluated STAT5 phosphorylation in CD8+ thymocytes using flow cytometry (Figure 6). Total thymocytes stimulated with IL-15 for 30 minutes were washed and incubated without cytokines. Aliquots of cells drawn before and after stimulation, and at various time points after cytokine withdrawal, were stained for intracellular phospho-STAT5, CD4, and CD8. IL-15 stimulated stronger STAT5 phosphorylation in CD8+ thymocytes than in CD4+ cells. The signal decayed completely after 30 minutes in SOCS1-sufficient CD8+ cells, whereas the signal persisted in SOCS1-deficient CD8+ cells for more than 60 minutes. IL-7 stimulated strong STAT5 phosphorylation in CD4+ and CD8+ thymocytes, and the signal persisted for more than 2 hours after cytokine withdrawal. However, neither the magnitude of the response nor the decay kinetics of phospho-STAT5 after IL-7 stimulation was different between SOCS1-sufficient and -deficient cells. These results indicate that SOCS1 is a critical regulator of IL-15-induced, but not IL-7-induced, STAT-5 phosphorylation in CD8+ thymocytes.
IL-15 induces sustained STAT5 phosphorylation in SOCS1-deficient CD8+ SP thymocytes. Total thymocytes from 6-week-old SOCS1-/-IFNγ-/- mice and their littermate controls were stimulated with 40 ng mL-1 IL-15 or a 1:20 dilution of IL-7-containing CS for 30 minutes. An aliquot of 2 × 106 cells was fixed immediately after stimulation. The remaining cells were washed twice and cultured without cytokines. At indicated time points, aliquots of cells were fixed, permeabilized, and stained for intracellular phospho-STAT5 followed by CD4 and CD8 surface staining. Phospho-STAT5 levels in gated CD8+ and CD4+ thymocytes are shown in histograms. Histograms of the stimulated cells are shaded gray, and unfilled line histograms represent the unstimulated cells. Results shown are representative of at least 4 animals per group from 2 independent experiments.
IL-15 induces sustained STAT5 phosphorylation in SOCS1-deficient CD8+ SP thymocytes. Total thymocytes from 6-week-old SOCS1-/-IFNγ-/- mice and their littermate controls were stimulated with 40 ng mL-1 IL-15 or a 1:20 dilution of IL-7-containing CS for 30 minutes. An aliquot of 2 × 106 cells was fixed immediately after stimulation. The remaining cells were washed twice and cultured without cytokines. At indicated time points, aliquots of cells were fixed, permeabilized, and stained for intracellular phospho-STAT5 followed by CD4 and CD8 surface staining. Phospho-STAT5 levels in gated CD8+ and CD4+ thymocytes are shown in histograms. Histograms of the stimulated cells are shaded gray, and unfilled line histograms represent the unstimulated cells. Results shown are representative of at least 4 animals per group from 2 independent experiments.
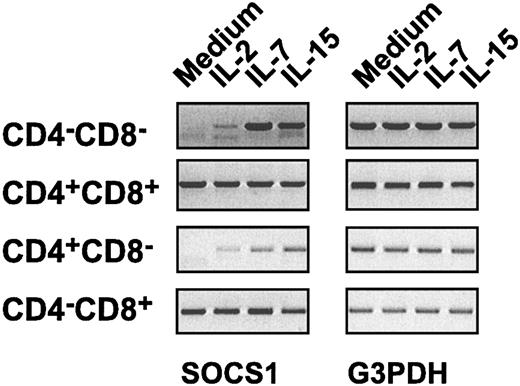
Differential regulation of SOCS1 gene expression in thymocyte subsets
The slow decay kinetics of phospho-STAT5 in SOCS1-deficient CD8+ thymocytes stimulated by IL-15, but not by IL-7, could be explained if SOCS1 is not induced by IL-7 in these cells. To address this issue, we examined SOCS1 gene expression in sorted thymocyte subsets after stimulation with IL-2, IL-7, or IL-15. We observed that the SOCS1 transcript was undetectable in DN and CD4+ thymocytes after overnight culture, whereas in DP and CD8+ thymocytes SOCS1 gene expression was stable even without cytokine stimulation (Figure 7). Moreover, IL-2, IL-7, and IL-15 did not significantly increase SOCS1 gene expression in CD8+ thymocytes above the basal level. These results suggest that SOCS1 is a negative feedback regulator of cytokine signaling in DN and CD4+ thymocytes, whereas SOCS1 functions as a constitutive attenuator of γc cytokine signaling in DP and CD8+ thymocytes.
Differential induction ofSOCS1gene expression in thymocyte subsets. Sorted DN, DP, CD4+, or CD8+ thymocyte subsets from SOCS1-/-IFNγ-/- mice and SOCS1+/+IFNγ-/- controls were stimulated with indicated cytokines or were left untreated in medium alone. After 16 hours of incubation, total RNA was isolated and first-strand cDNA was synthesized. cDNAs encoding SOCS1 and G3PDH genes were amplified by PCR, as described in “Materials and methods.” The SOCS1 transcript was absent in SOCS1-/-IFNγ-/- cells (not shown).
Differential induction ofSOCS1gene expression in thymocyte subsets. Sorted DN, DP, CD4+, or CD8+ thymocyte subsets from SOCS1-/-IFNγ-/- mice and SOCS1+/+IFNγ-/- controls were stimulated with indicated cytokines or were left untreated in medium alone. After 16 hours of incubation, total RNA was isolated and first-strand cDNA was synthesized. cDNAs encoding SOCS1 and G3PDH genes were amplified by PCR, as described in “Materials and methods.” The SOCS1 transcript was absent in SOCS1-/-IFNγ-/- cells (not shown).
Discussion
SOCS1 is abundantly expressed in the thymus at all stages of thymocyte development24 ; however, the precise role of SOCS1 in thymocyte development is unclear. Others and we24,26 have shown that forced expression of SOCS1 can inhibit stem cell factor (SCF)- and IL-7-mediated signaling during early thymocyte development. Examining SOCS1 gene expression in thymocyte subsets after cytokine stimulation suggests that the SOCS1 gene is critically regulated in DN cells and explains the hypersensitivity of SOCS1-deficient DN cells to γc cytokines. However, this did not result in a significant expansion of DN cells in vivo. The lack of DN thymocyte expansion in vivo may be a reflection of limiting IL-7 concentration in the microenvironment of developing DN cells within the thymic cortex because IL-7 is also a critical survival factor for differentiating DP cells and naive CD4+ T cells in the periphery.38,39 Under such minimal IL-7 stimulation, SHP-1, another negative regulator of γc cytokines, may be sufficient to control cytokine signaling in the absence of SOCS1.40,41 IL-2 is unlikely to be present in the thymic microenvironment because it is produced only by activated CD4+ T cells, whereas IL-15, though expressed by many cells types,42,43 might be avidly used by a larger pool of CD8+ thymocytes, making it unavailable to DN cells in SOCS1-/- mice.
The SOCS1 transcript is detectable in DP and CD8+ thymocytes even after 16 hours of culture without cytokine stimulation. The increased amount of SOCS1 mRNA in these cells could arise from either constitutive expression of SOCS1 or increased stability of the SOCS1 transcript. These results suggest that constitutive expression of SOCS1 may be crucial for preventing inadvertent cytokine signaling in DP and CD8+ thymocytes. Consistent with this prediction, the absence of SOCS1 caused increased proliferation of CD8+ thymocytes to IL-15. However, SOCS1-deficient DP thymocytes did not proliferate to IL-15, suggesting that SOCS1 is a crucial regulator of IL-15 signaling in CD8+ thymocytes, whereas additional negative regulatory mechanisms may be operating at the DP stage to prevent cytokine-induced cell proliferation.
DP cell differentiation to the CD4 or CD8 lineage is closely coupled to the interaction of the TCR on DP thymocytes with major histocompatibility complex (MHC) molecules.44 According to the signal strength model, both the amplitude and the duration of the TCR/coreceptor signal facilitates commitment toward the CD4 lineage, whereas weaker signals favor the CD8 lineage.44-46 Genetic and biochemical data support the signal strength model. Attenuation of TCR expression, as seen in mice deficient in TCF-1, results in increased levels of CD8+ thymocytes.47 Interference with Lck, Itk, or components of the MAPK pathway also influences a lineage decision toward the CD8 pathway.48-50 Finally, the expression of truncated Notch1 expands the CD8+ thymocyte subset,51,52 whereas the expression of full-length NotchIC inhibits the development of CD4+ and CD8+ thymocytes.53 These contradictory data may result from the partial inhibition of TCR signaling by truncated NotchIC, whereas full-length NotchIC might completely terminate TCR signaling.54 SOCS1 has not been implicated in regulating MAPK or Notch signaling pathways, but recent reports suggest that SOCS1 may modulate TCR signaling in thymocytes.55-58 TCR signaling has been shown to activate JAK3 and STAT5 and to induce many cytokine-inducible genes.55-57 SOCS1 has been reported to directly interact with immunoreceptor tyrosine activation motif (ITAMs) on the CD3ζ chain and with Syk in heterologous cells.58 If such an interaction occurs in developing thymocytes and if SOCS1 indeed attenuates TCR signaling, SOCS1 deficiency would be expected to potentiate TCR-mediated signals and to increase CD4+ thymocytes, whereas SOCS1-/- mice actually show an increase in CD8+ thymocytes.
Using a genetic model, we have shown that in the absence of SOCS1, γc cytokines, particularly IL-15, may influence thymocyte maturation. This may occur through increased cell survival by way of the up-regulation of Bcl-2 and Bcl-xL and through increased proliferation. Cytokine-dependent survival signals are vital for the maturation of CD8+ cells, but they are not absolutely required for CD4+ cells.44 In fact, mice transgenic for Bcl-2 or Bcl-xL show more CD8+ thymocytes.59,60 In the absence of SOCS1, we observed that IL-7 and IL-15 stimulated Bcl-xL in CD8+ cells but not in CD4+ cells. Because γc cytokine-induced Bcl-2 expression is comparable between SOCS1-deficient and SOCS1-sufficient thymocytes, the up-regulation of Bcl-xL by IL-15 and IL-2 in SOCS1-deficient CD8+ cells is likely to contribute to increased frequency of these cells in SOCS1-deficient thymi. Inhibiting cytokine signaling by the constitutive expression of SOCS1 decreased the number of CD8+ thymocytes,26 further supporting the notion that CD8+, but not CD4+, thymocytes are dependent on cytokine-induced survival signals.
We also observed that SOCS1-deficient CD8+, but not CD4+, thymocytes selectively proliferated in response to IL-15 and IL-2. However, SOCS1-deficient CD8+ thymocytes showed no additional proliferative advantage to IL-7 compared with wild-type cells, even though they responded to IL-7 with increased Bcl-xL expression. The IL-15-mediated proliferation of SOCS1-deficient CD8+ thymocytes was accompanied by elevated CD44 expression. The increased frequency of CD8+44hi cells in SOCS1-/-IFNγ-/- mice and in SOCS1-deficient FTOCs strongly suggested that SOCS1-deficient CD8+ thymocytes undergo proliferation in vivo. This proliferation is more likely to be mediated by IL-15 than by IL-2 because IL-15 is expressed by many cell types, whereas IL-2 is primarily secreted by activated T lymphocytes.42,43
The significance of SOCS1 governing the exquisite sensitivity of CD8+ thymocytes to IL-15 appears to be related to the role of SOCS1 in maintaining T-cell homeostasis. IL-15, induced by type 1 and type 2 interferons, stimulates memory CD8+ T cells.43,61,62 We have observed that peripheral CD8+ T cells from SOCS1-/-IFNγ-/- mice show the CD44hiLy6C+D122hiCD25lo memory phenotype and are hypersensitive to IL-15, resulting in a loss of T-cell homeostasis.63 The data presented here show that CD8+ T cells acquire the sensitivity to IL-15 even before emigrating from the thymus. Although the responsiveness of CD8+ memory T cells to IL-15 is beneficial to rapid recall responses to viral infections,64 our data strongly suggest that SOCS1 restrains the naive and developing CD8+ T cells from undergoing dysregulated proliferation to IL-15. Consistent with this notion, the SOCS1 gene is constitutively expressed in CD8+ thymocytes, but it is inducible in CD4+ thymocytes.
The γc-chain shared by the receptors for IL-2, IL-7, and IL-15 is associated with JAK3, which phosphorylates STAT5 on activation.13 However, the JAK3-STAT5 signaling pathway appears to be differentially used by γc cytokines in various thymocyte subsets. IL-15 stimulated efficient STAT5 phosphorylation only in CD8+ thymocytes, which persisted longer in SOCS1-/- cells and correlated with their increased proliferative response and Bcl-xL expression. IL-15 stimulated significantly higher proliferation of SOCS1-/- DN thymocytes (Figure 3B) without inducing STAT5 phosphorylation (data not shown), suggesting that the receptor complex, the signaling pathways, or both, are used differently in DN cells than in CD8+ thymocytes. In contrast to IL-15, IL-7 induced strong STAT5 phosphorylation in CD8+ and CD4+ thymocytes. Although IL-15-induced STAT5 phosphorylation persisted longer in SOCS1-/- cells, the comparable decay kinetics of IL-7-induced phospho-STAT5 between SOCS1-deficient and SOCS1-sufficient SP cells suggests that IL-15 and IL-7 may differentially stimulate other negative regulatory mechanisms. Despite inducing strong STAT5 phosphorylation, IL-7 did not stimulate the proliferation of SP thymocytes even in SOCS1-/- cells, indicating that additional signaling pathways stimulated by IL-15 in CD8+ thymocytes are required for stimulating cell proliferation.
In conclusion, T-cell developmental abnormalities observed in SOCS1-/- mice arise from IFNγ-dependent and IFNγ-independent mechanisms. Although depletion of the DP subset by increased maturation into SP cells is caused by excessive IFNγ signaling in the absence of SOCS1, eliminating IFNγ-mediated effects has revealed that SOCS1 deficiency alone can skew T-cell development. Although the IL-7-dependent DN stage is not regulated by SOCS1, maturation of CD8+ cells, not known to be regulated by cytokines, is dysregulated in the SOCS1-/- thymus. We have shown that IL-15 and IL-2 can modulate CD8+ thymocyte maturation through the induction of antiapoptotic proteins and cell proliferation. Even though several negative regulatory mechanisms can control signaling stimulated by γc cytokines, SOCS1 plays a nonredundant role in attenuating IL-15 signaling in CD8+ thymocytes to ensure normal T-cell development.
Prepublished online as Blood First Edition Paper, 2003-01-0175; DOI 10.1182/blood-August 7, 2003.
Supported by Terry Fox Program project of the National Cancer Institute of Canada (R.R.) and by Canadian Institutes of Health Research (CIHR) grants MOP-15706 and MOP-37882 (P.P.). S.I. was supported by the CIHR postdoctoral fellowship. R.R. is a CIHR Senior Research Scientist.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.