Abstract
Interleukin 21 (IL-21) is a newly described cytokine with homology to IL-4 and IL-15. They belong to a cytokine family that uses the common γ chain for signaling but also have their private high-affinity receptors. Since it is well known that IL-4 modulates differentiation and activation of dendritic cells (DCs), we analyzed effects of IL-21 compared with IL-15 on DC differentiation, maturation, and function. Here we show that DCs generated with granulocyte-macrophage colony-stimulating factor (GMCSF) in the presence of IL-21 (IL-21DCs) differentiated into phenotypically and functionally altered DCs characterized by reduced major histocompatibility complex class II (MHCII) expression, high antigen uptake, and low stimulatory capacity for T-cell activation in vitro. Additionally, IL-21DCs completely failed to induce antigen (Ag)-specific T-cell mediated contact hypersensitivity. Furthermore, IL-21 blocked lipopolysaccharide (LPS)-induced activation and maturation of DCs, which was not mediated by release of the anti-inflammatory cytokine IL-10. In contrast, when supplementing GMCSF with IL-15, DCs differentiated into mature antigen-presenting cells (APCs) with low antigen uptake and highly significant increased capacities to stimulate T cells in vitro and in vivo. Taken together, these results identify a dichotomous action of these structurally related cytokines on DCs, establishing IL-21 as inhibitory cytokine on DC activation and IL-15 as potent stimulator of DC function, making both cytokines interesting targets for therapeutic manipulation of DC-induced immune reactions. (Blood. 2003;102: 4090-4098)
Introduction
Dendritic cells (DCs) possess specialized features such as pathogen recognition, antigen (Ag) capturing and processing machinery, migratory capacity, and constitutively expressed costimulatory molecules that allow them to act as professional antigen-presenting cells (APCs). Thus, DCs display an extraordinary capacity to initiate T-cell-dependent immune responses.1 In mediating this, DCs pass through different functional activation states.2 Thereby DCs convert from a highly efficient antigen capture and uptake to an antigen-presenting state. The capacity of mature DCs to prime naive T cells and to promote their differentiation is critically attributed to their cytokine-secretion pattern. Vice versa, polarizing signals from the microenvironment directly shape the DC maturation. A network of cytokines modulates this sensitive system of cell communication, including interleukin 15 (IL-15), a proinflammatory cytokine that activates APCs such as DCs and macrophages.3
IL-15 was introduced as a member of the 4-helix-bundle cytokine family able to reproduce biologic effects of IL-2.4 This is related to the fact that the IL-15 receptor (IL-15R) complex contains the β and common γ chain of the IL-2R complex beside its private high-affinity α chain.5 However, IL-15 was also found to have distinct functions compared with those mediated by IL-2.6,7 In addition, IL-2 expression is nearly restricted to T cells, whereas IL-15 is hardly detectable in T cells but produced by a variety of tissues, monocytes, and DCs.8 Therefore, IL-15 may be an important candidate for modulating DC-mediated immune responses.9
It is established that combining granulocyte-macrophage colony-stimulating factor (GMCSF) and IL-4 promotes DC generation in vitro.10 Therefore, it was of interest to investigate other members of this cytokine family acting on DC development. It has been recently reported that IL-15 can skew monocyte differentiation into DCs with a surface phenotype of Langerhans cells11 and supports the maturation of monocytes to DCs ex vivo.12 Interestingly, although IL-4 and IL-15 both mobilize the common γ chain, IL-4 was unable to induce comparable effects. Moreover, IL-15 is produced by DCs itself after inflammatory stimuli,13,14 suggesting that IL-15 might be important to link innate response to infection with the initiation of an adaptive immune response by DCs.
IL-21, a novel cytokine, has close structural similarities with IL-15, IL-2, and IL-4.15 Like IL-4 and IL-15, IL-21 has its private high-affinity receptor chain and shares the common γ chain as functional subunit of its IL-21 receptor.16 The IL-21R is expressed in lymphoid tissues such as thymus, lymph nodes, and leukocytes as well as natural killer (NK) cells,15 suggesting that this receptor/ligand pair could play a role in innate and acquired immunity. Interestingly, IL-21R expression was also found in bone marrow (BM) cells, confirming the fact that IL-21 promotes the differentiation of lymphoid cells.15 Unlike its receptor, IL-21 appeared more restricted; production was found in activated peripheral T cells.15 These observations open up the possibility that IL-21 is involved in T-cell-dependent immune responses mediated by professional APCs such as DCs.
This led us to the following question: how do IL-15 and IL-21 influence differentiation, maturation, and function of bone marrow-derived DCs (BMDCs) as well as DC-T-cell interaction? The principle method to generate murine BMDCs in vitro was adapted many years ago and yields high amounts of pure myeloid CD11c+ DCs.17,18 Expansion of in vitro-generated BMDCs is promoted by IL-4.10 Given the structural similarity between IL-21 and the IL-15/IL-4 family of cytokines together with their common use of the γ chain it was reasonable to speculate that they influence DC development in similar ways.
We report here that despite structural similarities and shared receptor components, signals from IL-15 during DC generation induced highly immunogenic DCs, whereas IL-21 kept DCs in an immature state characterized by low T-cell stimulatory capacity.
Materials and methods
Mice and culture medium
C57BL/6 mice, 10 to 12 weeks of age, were from Charles River (Sulzfeld, Germany). OTIItg mice, transgenic for a CD4 T-cell-restricted T-cell receptor, recognizing ovalbumin (OVA(323-339)) were from IFFA Credo (Les Oncins, France). All in vivo experiments were performed in compliance with the national German guidelines and the guidelines of the Research Center Borstel.
Cells were cultured in RPMI 1640 medium plus 10% heat-inactivated fetal calf serum (FCS; Biochrom, Berlin, Germany), 2 mM l-glutamine (Lifetechnologies, Karlsruhe, Germany), 50 μM β-mercaptoethanol, 100 U/mL penicillin, and 100 μg/mL streptomycin (PAA, Linz, Austria), at 37°C in 5% CO2.
DC preparation
BMDCs were generated as described.18 In brief, BM cells from femurs were isolated and seeded (2 × 105/mL) in bacterial-grade Petri dishes (Falcon, Heidelberg, Germany) and differentiated for 8 days in 10 mL medium plus 20 ng/mL GMCSF (Tebu, Frankfurt, Germany) alone or plus 20 ng/mL IL-15 (RnD, Wiesbaden, Germany) or 20 ng/mL IL-21 (ZymoGenetics, Seattle, WA). At day 3, 10 mL medium containing 20 ng/mL GMCSF alone or plus IL-15 or IL-21 were added. At day 6 half of the cell-free supernatant was exchanged and fresh medium containing 10 ng/mL GMCSF alone or plus cytokines was added. At day 8 cells were harvested using Accutase (PAA, Pasching, Austria).
Histomorphometry
Harvested cells (5 × 104) were transferred in fresh medium onto chamber slides (Nunc, Wiesbaden, Germany), incubated for 48 hours to adhere, and stained with Pappenheim (Merck, Darmstadt, Germany).
Reverse transcriptase-polymerase chain reaction (RT-PCR)
RNA was extracted from 5 × 106 cells after 8 days of culture using RNAzol (Lifetechnologies). cDNA was synthesized using random hexanucleotide primers and Superscript preamplification system II (Lifetechnologies). cDNA was amplified using 1 U AmpliTaq DNA polymerase (Roche, Mannheim, Germany), 250 μM of each deoxynucleoside triphosphate (dNTP) and 2 μL 10-fold PCR buffer (Roche). The final primer concentration was 0.5 μM. Cycling conditions were 5 minutes at 94°C and an additional 30 seconds at 94°C. Annealing temperature was 60°C for 30 seconds followed by 30 seconds at 72°C. Thirty cycles were performed and ended with final extension at 72°C for 10 minutes. Primers (Table 1) were from TIB Molbiol (Berlin, Germany) or Metabion (Martinsried, Germany). To exclude contaminations all experiments were run with a mock PCR. β-actin was used to normalize cDNA amount. As positive controls for IL-21 and its receptor, cDNA from CD4+ T cells from C57BL/6 mice was isolated.15 For IL-15 and its receptor we used L929 fibroblasts19 and CTLL-2 cells for all IL-2R chains.20
Fluorescence-activated cell sorter (FACS) analysis
BMDCs were characterized using anti-CD11c (fluorescein isothiocyanate [FITC]), major histocompatibility complex (MHC) class II (IA/IE), CD80, CD86, and CD11b (phycoerythrin [PE]-conjugated, all from Pharmingen, Heidelberg, Germany). Propidium iodide (Sigma, Deisenhofen, Germany) was added to exclude dead cells. Anti-CD3, CD8α, CD45/B220, DX5, NK1.1, and F4/80 (Serotec, Eching, Germany) were used as control. Analysis was performed on CD11c+ gated DCs with a FACSCalibur CELLQuest (Becton Dickinson, Heidelberg, Germany).
Analysis of endocytosis
To quantify endocytic activity, FITC-dextran uptake (molecular weight [MW]: 70 000; Molecular Probes, Göttingen, Germany) was monitored by FACS as described by Stumbles et al.21
DC activation
All DC types were cultured for 24 hours with low-dose lipopolysaccharide (LPS; 10 ng/mL) to induce DC activation and maturation. In addition all DCs were labeled with FITC (12.5 μg/mL; Sigma) as described in “Assay for contact hypersensitivity (CHS) to FITC.” Concentrations were chosen out of several titration experiments. Medium was used as control. After 24 hours DCs were analyzed by FACS.
Proliferation assay
DCs were harvested after 8 days of culture and seeded into 96-well flat-bottom plates (Costar Corning, Cambridge, MA) at a density of 1 × 105/well to adhere for 12 hours. T cells were isolated from lymph nodes of OTIItg mice and used at 1 × 105 per well. OVA(323-339) peptide was supplemented at 0.3 μM and 1 μM to a final volume of 200 μL. Cells were incubated for 72 hours and labeled for an additional 12 hours with 0.2 μCi (0.0074 MBq) [3H]thymidine (Amersham, Freiburg, Germany). Proliferation of T cells was quantified by liquid scintillation counting (Wallac/PerkinElmer, Freiburg, Germany).
Assay for contact hypersensitivity (CHS) to FITC
To estimate the capacity of the differently generated DCs in inducing in vivo T-cell sensitization and an antigen-specific immune response, 1 × 106 cells/mL were labeled with 12.5 μg/mL FITC (Sigma) for 20 minutes at 37°C as described.22 Fifty microliters (5 × 105 cells) were injected in one footpad. After 5 days, mice were challenged by applying 50 μL FITC (3.5 mg/mL in acetone-dibutylphtalate, 1:1) on the right ear.22 As the control, the left ear was painted with diluent, and unsensitized mice were painted with FITC. The CHS response was determined by measuring ear swelling at 24, 48, and 72 hours after challenge using a micrometer (Mitutoyo, Elk Grove Village, IL).
In vivo DC migration
DCs were labeled with FITC as described in “Assay for contact hypersensitivity (CHS) to FITC” and 5 × 105 labeled DCs were injected subcutaneously in the hind footpad. To assess that FITC was not taken up by other cells, FITC-labeled DCs were fixed with 0.1% glutaraldehyde and injected subcutaneously in the hind footpad.23 After 24 hours local draining lymph nodes (DLNs) were removed and prepared as described.24 Cells were stained with anti-CD11c APCs and analyzed by FACS.
DC activation by LPS
DCs generated with GMCSF only were cultured for another 24 hours in medium or with low-dose LPS (10 ng/mL) to induce DC activation. To analyze the effects of IL-15 and IL-21 on this in vitro process, DCs were incubated with a combination of LPS and IL-15 or IL-21 (100 ng/mL). Cytokine and LPS concentrations were chosen out of several titration experiments. As control, the anti-inflammatory cytokine IL-10 (100 ng/mL) was used, which inhibits DC maturation.25 After 24 hours, the surface phenotype of the DCs was analyzed by FACS and the supernatant was analyzed for cytokine production using a Bio-Plex kit (BioRad, Munich, Germany). Fifty microliters per sample were analyzed on the Luminex 100 (BioRad) according to manufacturer's instruction.
Results
CD11c+ DCs differentiate in the presence of IL-15 or IL-21 in vitro
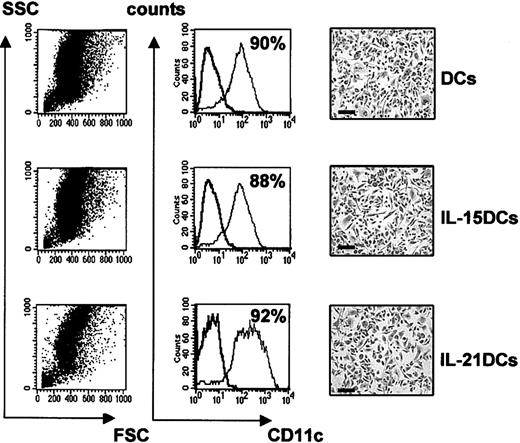
IL-21R expression was found in BM cells. Therefore, we first analyzed whether IL-21 might play a role in generation of myeloid DCs compared with IL-15. Freshly isolated BM cells were cultured following established protocols18 with GMCSF alone (subsequently designated DCs) or GMCSF plus 20 ng/mL IL-15 (subsequently designated IL-15DCs) or 20 ng/mL IL-21 (subsequently designated IL-21DCs). Cells aggregated within 48 hours and after 8 days of culture had a characteristic DC morphology (not shown). At day 8, DCs were transferred into microchamber slides. The differentially generated DCs displayed a typical DC phenotype such as cytoplasmic protrusions (Figure 1), indistinguishable between the 3 types. In FACS analysis after 8 days of culture, the IL-15DCs and IL-21DCs showed comparable size and granularity to DCs (Figure 1). Further, the percentage of CD11c+ cells (a specific DC marker in mice) was similarly high in all conditions (Figure 1). Moreover, we assessed by FACS that adding IL-15 or IL-21 to BM cells in combination with GMCSF did not lead to increased differentiation of other cell types such as B220+ B cells, NK1.1+ NK cells, CD3+ T cells, Gr1+ granulocytes, Mac3 or CD14+ macrophages, or CD8α+ lymphoid DCs (not shown). Thus, IL-15 and IL-21 altered neither the purity of the generated DCs nor the total yield compared with GMCSF alone (mean of 7.5 × 106 cells out of 2 × 106 seeded for all conditions).
CD11c+ DCs differentiate in the presence of IL-15 or IL-21. Murine BM cells were induced to differentiate to DCs by GMCSF alone (DCs; top row) or by addition of IL-15 (IL-15 DCs; middle row) or IL-21 (IL-21 DCs; bottom row) during the entire culture period of 8 days. Cells were analyzed by forward (FSC) and sideward (SSC) scatter (left column) and CD11c expression (middle column) using FACS analysis at day 8. The percentage of CD11c-expressing cells is shown in each panel in the middle column. One representative of 10 experiments is shown. Morphology was assessed by culturing the DCs for 48 hours on microchamber slides (right column) following Pappenheim staining. The scale bar is equal to 100 μm.
CD11c+ DCs differentiate in the presence of IL-15 or IL-21. Murine BM cells were induced to differentiate to DCs by GMCSF alone (DCs; top row) or by addition of IL-15 (IL-15 DCs; middle row) or IL-21 (IL-21 DCs; bottom row) during the entire culture period of 8 days. Cells were analyzed by forward (FSC) and sideward (SSC) scatter (left column) and CD11c expression (middle column) using FACS analysis at day 8. The percentage of CD11c-expressing cells is shown in each panel in the middle column. One representative of 10 experiments is shown. Morphology was assessed by culturing the DCs for 48 hours on microchamber slides (right column) following Pappenheim staining. The scale bar is equal to 100 μm.
IL-21 and IL-15 receptors are expressed in BM-derived DCs
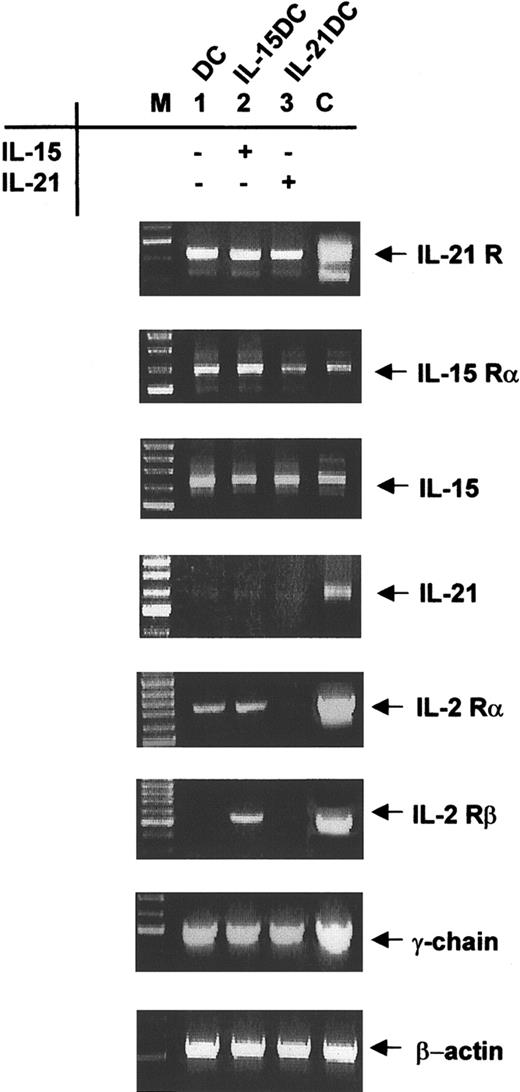
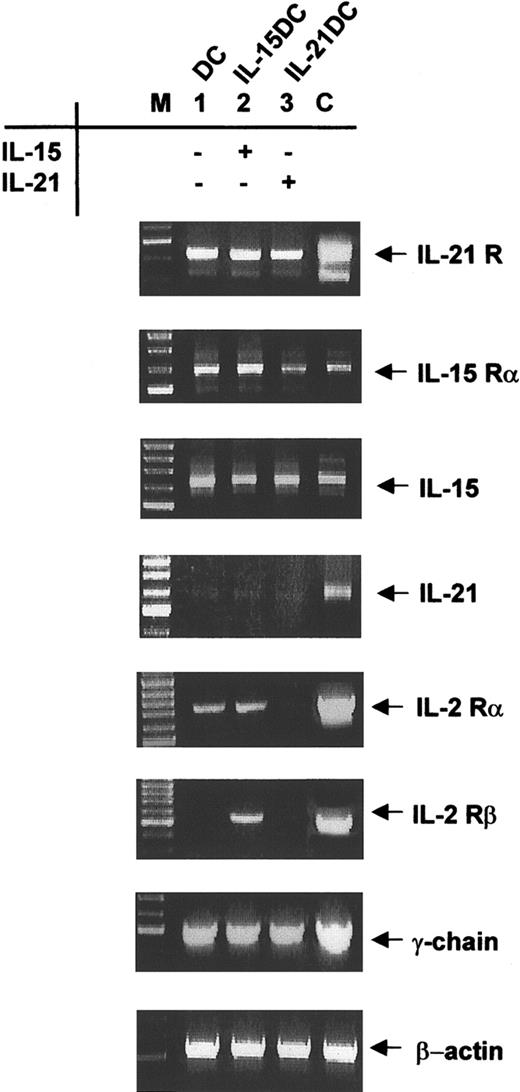
The IL-15 receptor complex (IL-15R), which is composed of a private α subunit, the IL-2/IL-15Rβ chain and the common γ chain, is expressed on DCs.13 Message expression for the private IL-21R subunit was previously found in lymphoid tissues.15 In order to elucidate whether myeloid DCs express the private receptor subunits and the common γ chain we performed RT-PCR analysis. We found both private receptor chains, IL-15Rα and IL-21R, expressed after 8 days in BMDCs (Figure 2). Adding IL-15 or IL-21 to the culture did not change expression levels of the IL-21R subunit. Analyzing the expression of IL-15Rα mRNA in the differently generated DC types revealed that all cells showed IL-15Rα transcripts (Figure 2).
Expression of IL-15/IL-21 receptor components in DCs. Eight-day-cultured DCs (lane 1), IL-15DCs (lane 2), and IL-21DCs (lane 3) were analyzed by RT-PCR for mRNA expression of IL-21R, IL-15Rα, IL-15, IL-21, and IL-2Rα and β chains as well as for the common γ-chain. β-actin message expression was used to normalize the cDNA amount. To exclude contaminations all experiments were run with a mock PCR and found negative (not shown). The correct size of PCR product was assessed with a marker (M). As positive controls (C) for IL-21 and its receptor, cDNA from CD4+ T cells from C57BL/6 mice was isolated. For IL-15 and its receptor we used cDNA from L929 fibroblasts, and CTLL-2 cells were the positive reference for all IL-2R chains. One representative experiment of 5 is shown.
Expression of IL-15/IL-21 receptor components in DCs. Eight-day-cultured DCs (lane 1), IL-15DCs (lane 2), and IL-21DCs (lane 3) were analyzed by RT-PCR for mRNA expression of IL-21R, IL-15Rα, IL-15, IL-21, and IL-2Rα and β chains as well as for the common γ-chain. β-actin message expression was used to normalize the cDNA amount. To exclude contaminations all experiments were run with a mock PCR and found negative (not shown). The correct size of PCR product was assessed with a marker (M). As positive controls (C) for IL-21 and its receptor, cDNA from CD4+ T cells from C57BL/6 mice was isolated. For IL-15 and its receptor we used cDNA from L929 fibroblasts, and CTLL-2 cells were the positive reference for all IL-2R chains. One representative experiment of 5 is shown.
We next determined whether endogenous expression of IL-15 and IL-21 was modulated by the different culture conditions since IL-15 mRNA up-regulation was identified during an increase of DC activation. Transcripts for IL-15 were expressed in all 3 differentiated DC types at comparable levels, however, IL-21 expression was not detected (Figure 2).
Besides its high-affinity receptor, IL-15Rα, IL-15 is also able to signal through the low-affinity IL-2Rβ chain. For both cytokines, the common γ chain is an indispensable subunit so we also examined expression of the β and γ subunit and, in addition, the IL-2Rα chain. IL-2Rα mRNA was expressed at low levels in IL-21DCs compared with DCs or IL-15DCs (Figure 2). Interestingly, the mRNA expression of the β subunit was restricted to IL-15DCs (Figure 2), whereas in DCs or IL-21DCs no expression was detectable, probably since only IL-15 uses and therefore up-regulates this receptor. All DC types had comparable mRNA levels for the common γ chain (Figure 2). Representative data of 5 experiments are shown. Positive controls exhibit the expected results.
Taken together, BM-derived myeloid DCs expressed the private receptor chains IL-15Rα and IL-21R plus the common γ chain and therefore may respond to IL-21 and IL-15, where the latter was expressed by DCs itself. IL-21 was not expressed in any condition, supporting the finding that IL-21 is mainly produced by activated T cells.15 Since no antibodies against the IL-21R are currently available, the protein expression remains to be determined.
IL-21 reduced MHCII and CCR7 expression
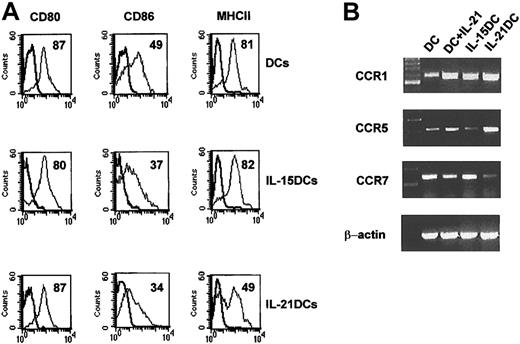
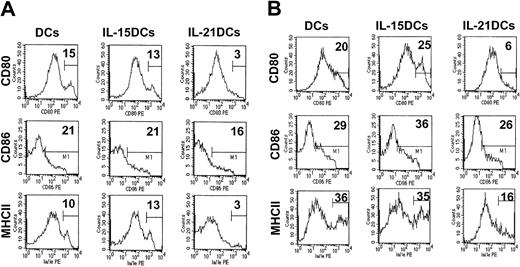
Antigen presentation to and costimulation of T cells by DCs in innate and acquired immunity are mediated by MHC molecules and a variety of costimulatory molecules on DCs. To assess the repertoire of surface molecules on DCs we did phenotyping by FACS after 8 days of culture. Interestingly, generation in the presence of IL-21 resulted in a significantly decreased expression of MHCII (Figure 3A) compared with DCs or IL-15DCs.
IL-21DCs express low MHC II and CCR7mRNA. (A) DCs, IL-15DCs, and IL-21DCs were analyzed by multicolor flow cytometry (gated on CD11c+ cells). Representative data of 10 experiments are shown. The percentage of positive cells is given in each histogram. Unstained cells or isotype control antibodies were used as negative controls. (B) IL-21 enhances CCR1 and CCR5 but suppresses CCR7 expression. Chemokine receptor expression was analyzed in DCs, IL-21-stimulated DCs (100 ng/mL for 24 h), IL-15DCs, or IL-21DCs by PCR.
IL-21DCs express low MHC II and CCR7mRNA. (A) DCs, IL-15DCs, and IL-21DCs were analyzed by multicolor flow cytometry (gated on CD11c+ cells). Representative data of 10 experiments are shown. The percentage of positive cells is given in each histogram. Unstained cells or isotype control antibodies were used as negative controls. (B) IL-21 enhances CCR1 and CCR5 but suppresses CCR7 expression. Chemokine receptor expression was analyzed in DCs, IL-21-stimulated DCs (100 ng/mL for 24 h), IL-15DCs, or IL-21DCs by PCR.
In contrast, all 3 differently generated DCs showed comparable levels of CD80 and slightly reduced CD86 expression (Figure 3A). Moreover, all DCs did not differ in the expression of OX40L, CD95, CD95L, CD11b, and CD54 (not shown). Although adding IL-21 or IL-15 during generation did not alter the quantity and morphology of DCs, the phenotype of the DCs shown by expression of the functionally relevant MHCII antigen-presenting molecule was modulated.
Chemokine receptors are regulated during DC maturation and modulate trafficking of DCs. Immature DCs express high CCR1 and CCR5, whereas mature DCs up-regulate CCR7, which mediates migration to the DLN. Incubating normal DCs with IL-21 for 24 hours increased CCR1 and CCR5 but decreased CCR7 expression (Figure 3B). IL-21DCs presented the same phenotype, supporting their immature state compared with IL-15DCs (Figure 3B).
High endocytosis in IL-21DCs versus low activity in IL-15DCs
The phenotypic changes of DCs generated in the presence of IL-21, particularly the significantly reduced expression of the MHCII molecule, point to different maturational stages and suggest that IL-21 might modulate APC functions. In vitro, immature DCs are characterized by an increased antigen-capture activity. Therefore, we studied the endocytic activity using a fluorescent model antigen. FITC-dextran uptake (at 37°C) was monitored after 8 days of DC culture as described.21 IL-21DCs showed a significantly increased FITC-dextran uptake (mean fluorescent intensity [MFI], 495) compared with DCs (Figure 4). Prolonged incubation did not increase the uptake (not shown). In contrast to IL-21DCs, FITC-dextran uptake was strongly reduced in IL-15DCs (MFI, 59) compared with normal DCs (MFI, 156). To control passive FITC diffusion, all experiments were additionally performed on ice, showing very low FITC-dextran uptake. Internalization was further confirmed by fluorescence microscopy (not shown). In addition, using the hapten FITC, IL-21DCs also showed the highest uptake in vitro (not shown). Thus, the presence of IL-21 in vitro leads to the differentiation of “functional immature” DCs, characterized by high unspecific antigen uptake, whereas IL-15 induces DCs with a mature phenotype accompanied by lower unspecific antigen uptake.
High versus low antigen uptake (endocytosis) by IL-21DCs or IL-15DCs. Cells were incubated 30 minutes at 37°C with FITC-labeled dextran, washed, and analyzed for FITC-dextran uptake by FACS. One representative experiment of 3 is shown.
High versus low antigen uptake (endocytosis) by IL-21DCs or IL-15DCs. Cells were incubated 30 minutes at 37°C with FITC-labeled dextran, washed, and analyzed for FITC-dextran uptake by FACS. One representative experiment of 3 is shown.
IL-21DCs keep their immature phenotype after antigen uptake and LPS stimulation
The high FITC-dextran uptake, shown in Figure 4, indicates that IL-21DCs have functional alterations. To study this in greater detail, we investigated the activation of DCs by different stimuli. It is known that contact sensitizers and bacterial products such as LPS trigger DC activation in vitro.26,27 DC activation and maturation is accompanied by up-regulation of MHC and costimulatory molecules.
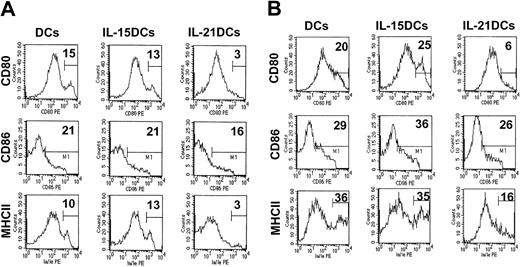
To examine whether IL-21DCs are blocked in maturation by keeping their immature phenotype even after strong stimulation by LPS or after antigen uptake, we incubated all DC types with the contact sensitizer FITC (Figure 5A) and with low-dose LPS (Figure 5B) for 24 hours. FACS analysis of CD80, CD86, and MHCII revealed that IL-21DCs showed a significantly inhibited expression of these representative molecules in all conditions (Figure 5A-B compared with unstimulated cells; Figure 3). This inability of IL-21DCs to up-regulate these molecules was not due to altered Toll-like receptor 2 (TLR2) and TLR4 LPS receptor expression (which was comparable to DCs and IL-15DC; not shown) or due to insufficient Ag uptake, which we have shown in Figure 4 to be rather enhanced. In contrast, DCs and IL-15DCs clearly showed an up-regulation of CD80 and MHCII, indicating that IL-21DCs were blocked in their expression, suggesting that they are unable to “mature” after these stimuli.
IL-21DCs are blocked in their maturation. All 3 generated DC types were stimulated with (A) FITC and (B) LPS for an additional 24 hours after 8 days of culture. The percentage of positive (CD86) or high positive (CD80, MHCII) cells (fluorescence channel > 102) with high surface density/number defined by the marker M1 is given. One representative of 2 experiments is shown.
IL-21DCs are blocked in their maturation. All 3 generated DC types were stimulated with (A) FITC and (B) LPS for an additional 24 hours after 8 days of culture. The percentage of positive (CD86) or high positive (CD80, MHCII) cells (fluorescence channel > 102) with high surface density/number defined by the marker M1 is given. One representative of 2 experiments is shown.
IL-21DCs inhibit antigen-specific T-cell proliferation
The capacity to induce specific T-cell activation and proliferation is a functional hallmark of mature DCs, whereas immature DCs fail to prime T-cell responses. To assess whether IL-21DCs are inhibited to induce T-cell response, we set up an antigen-specific T-cell proliferation assay. The different DCs after 8 days of culture were pulsed with OVA(323-339) peptide and cocultured for 72 hours with T cells from lymph nodes of syngenic OTIItg mice. We compared the T-cell stimulatory capacity of DCs that had been cultured with GMCSF alone or in combination with IL-15 or IL-21. As shown in Figure 6, IL-15DCs showed a highly significant increased ability to prime T-cell proliferation, compared with DCs, with a maximum at OVA(323-339) peptide concentration of 0.3 μM. In contrast, IL-21DCs induced significantly lower proliferation. This reduced ability of IL-21DCs to prime specific T cells could refer to the above-described (Figure 3) reduced MHCII expression supporting the deduction that DCs generated in the presence of IL-21 are less mature and, most importantly, do not acquire an immunogenic T-cell activating phenotype after Ag uptake.
High versus low antigen-specific T-cell stimulation in vitro by IL-15DCs or IL-21DCs. DCs (□), IL-15DCs (▪), and IL-21DCs (▦) were labeled with different OVA323-339 peptide concentrations and incubated with lymph node cells from OTII mice. After 72 hours of incubation, cells were labeled with 0.2 μCi (0.0074 MBq) [3H]thymidine. One representative of 3 experiments is shown. Significance compared with DCs was calculated using Student t test (**P ≤ .01). Error bars indicate standard deviation.
High versus low antigen-specific T-cell stimulation in vitro by IL-15DCs or IL-21DCs. DCs (□), IL-15DCs (▪), and IL-21DCs (▦) were labeled with different OVA323-339 peptide concentrations and incubated with lymph node cells from OTII mice. After 72 hours of incubation, cells were labeled with 0.2 μCi (0.0074 MBq) [3H]thymidine. One representative of 3 experiments is shown. Significance compared with DCs was calculated using Student t test (**P ≤ .01). Error bars indicate standard deviation.
IL-21DCs are unable to prime in vivo contact hypersensitivity
Small molecules, which act as antigens after protein binding, are designated as haptens and induce a contact hypersensitivity (CHS) in the skin. Following application to skin, epidermal DCs take up hapten-protein complexes, process them, and migrate toward the regional DLN to prime antigen-specific T cells. During this process, DCs convert from an immature into an activated functional state.28 In addition to the in vitro data, we examined whether IL-15DCs and IL-21DCs showed also a modulated capability for T-cell priming in vivo. Therefore, we labeled the different DCs in vitro with the fluorescent hapten FITC and injected DCs subcutaneously in the footpad of syngenic C57BL/6 mice. After 5 days mice were challenged at one ear with FITC, and the ability to initiate an antigen-specific T-cell-mediated immune response was examined by measuring the FITC-specific ear swelling 24, 48, and 72 hours after challenge. Unsensitized mice served as negative controls for unspecific ear swelling.
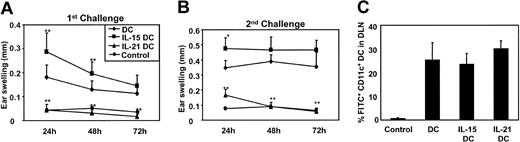
Mice that had been actively sensitized with IL-15DCs showed a highly significant increase in the CHS ear swelling response 24 hours after challenge compared with mice that had been injected with DCs or IL-21DCs (Figure 7A). In contrast, injection of IL-21DCs resulted in highly significant reduced CHS response at all 3 time points (ie, no swelling above unsensitized controls was observed).
Failed versus enhanced induction of T-cell-mediated contact hypersensitivity by IL-21DCs or IL-15DCs in vivo. DCs, IL-15DCs, and IL-21DCs were labeled in vitro with FITC and injected into the hind footpad. (A) First challenge: 5 days later, mice were challenged with FITC painting to the ear and after 24, 48, and 72 hours the ear swelling reaction was analyzed. Painting of unsensitized mice served as control. (B) Second challenge: 14 days after the first challenge, the mice were painted again on the other ear and swelling was analyzed as before. (C) Migration of DCs was analyzed by FACS of the draining lymph nodes 24 hours after injection of 5 × 105 FITC-labeled DCs. Cells were double stained for CD11c. The CD11c/FITC+ DCs as percent of total CD11c+ cells in the DLN are shown. Controls were injected with unlabeled DCs. The experiments were repeated at least twice; the mean ± SD of 12 mice is shown. Significance between IL-15DCs and IL-21DCs compared with DCs was calculated using Student t test (*P ≤ .05; **P ≤ .01).
Failed versus enhanced induction of T-cell-mediated contact hypersensitivity by IL-21DCs or IL-15DCs in vivo. DCs, IL-15DCs, and IL-21DCs were labeled in vitro with FITC and injected into the hind footpad. (A) First challenge: 5 days later, mice were challenged with FITC painting to the ear and after 24, 48, and 72 hours the ear swelling reaction was analyzed. Painting of unsensitized mice served as control. (B) Second challenge: 14 days after the first challenge, the mice were painted again on the other ear and swelling was analyzed as before. (C) Migration of DCs was analyzed by FACS of the draining lymph nodes 24 hours after injection of 5 × 105 FITC-labeled DCs. Cells were double stained for CD11c. The CD11c/FITC+ DCs as percent of total CD11c+ cells in the DLN are shown. Controls were injected with unlabeled DCs. The experiments were repeated at least twice; the mean ± SD of 12 mice is shown. Significance between IL-15DCs and IL-21DCs compared with DCs was calculated using Student t test (*P ≤ .05; **P ≤ .01).
To confirm that sensitization was based on active migration of the injected viable DCs from the footpad to DLN, additional control mice were injected with FITC-labeled glutaraldehyde-fixed (dead) DCs,23 which failed to induce any CHS response (not shown).
Two weeks after the first challenge, a second challenge was performed by painting FITC on the other ear and analyzing ear swelling as before (Figure 7B). Mice, sensitized by DCs and IL-15DCs, showed in repetition a strong ear swelling, even more pronounced as after the first challenge. However, mice sensitized with IL-21DCs again did not mount a significant response compared with negative controls.
These findings are completely in line with our previous in vitro data stressing the fact that combining GMCSF and IL-15 generates “high immunogenic” DCs, whereas IL-21 induces “functional immature” DCs, unable to mature to fully effective T-cell-priming APCs after antigen uptake neither in vitro nor in vivo.
Comparable migration of all DC types to DLN
To exclude that the differences in CHS responses are due to altered migratory DC migration, we investigated their abilities to enter DLN in vivo.29 For this purpose FITC-labeled DCs were injected in the hind footpad. After 24 hours cell suspensions from the DLNs were prepared as described,24 stained with anti-CD11c antibody, and analyzed by FACS. Double-positive cells (FITC+/CD11c+), which migrated in the DLN, are given in percent of total CD11c+ DCs (the total number of DCs in the DLN did not differ). As control, unlabeled DCs were injected. All DCs showed similar migratory capacities; 25% to 30% of the total lymph node CD11c+ DCs migrated within the 24 hours from the periphery into DLNs (Figure 7C). This exhibits that the reduction of CHS responses by IL-21DCs in vivo is not attributed to limited migration.
In contrast, footpad-injected glutaraldehyde-fixed (ie, “dead”) FITC-labeled DCs did not reach the DLN and no FITC uptake by surrounding DCs of recipient mice was observed (not shown), indicating that the observed migration into DLN is an active and specific process.
IL-21 inhibits LPS-induced activation and release of proinflammatory cytokines by normal DCs
When IL-21 is present during the entire differentiation it prevents DC maturation, so we subsequently elucidated whether IL-21 could act also in short time course on DC activation. To this end, we studied the effects of IL-21 and IL-15 when given in parallel to LPS. Therefore, DCs differentiated 8 days with GMCSF only were activated for an additional 24 hours with low-dose LPS (10 ng/mL) alone or in combination with 100 ng/mL of IL-15 or IL-21. As control we used the anti-inflammatory cytokine IL-10 that inhibits DC activation.25 To prove that IL-15 and IL-21 were taken up by DCs, we did confocal microscopy and found both cytokines internalized after 30 minutes (not shown).
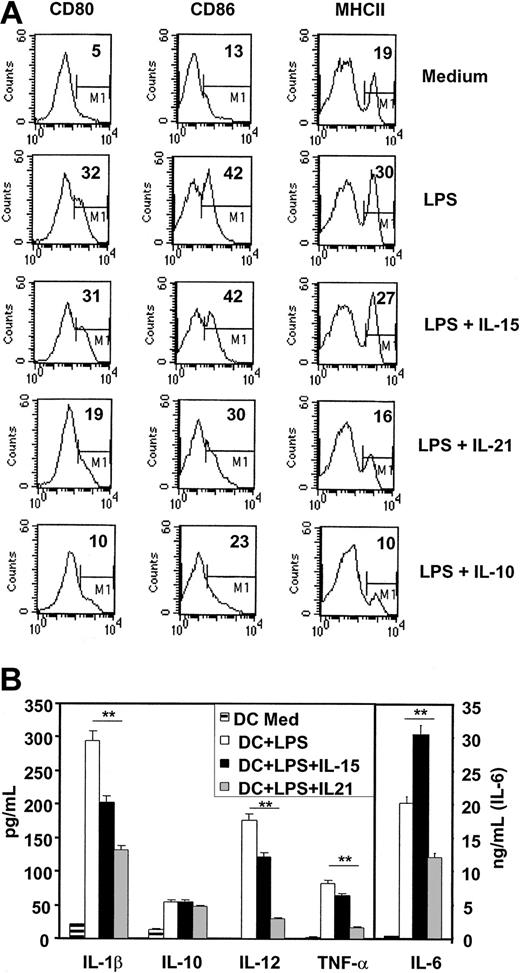
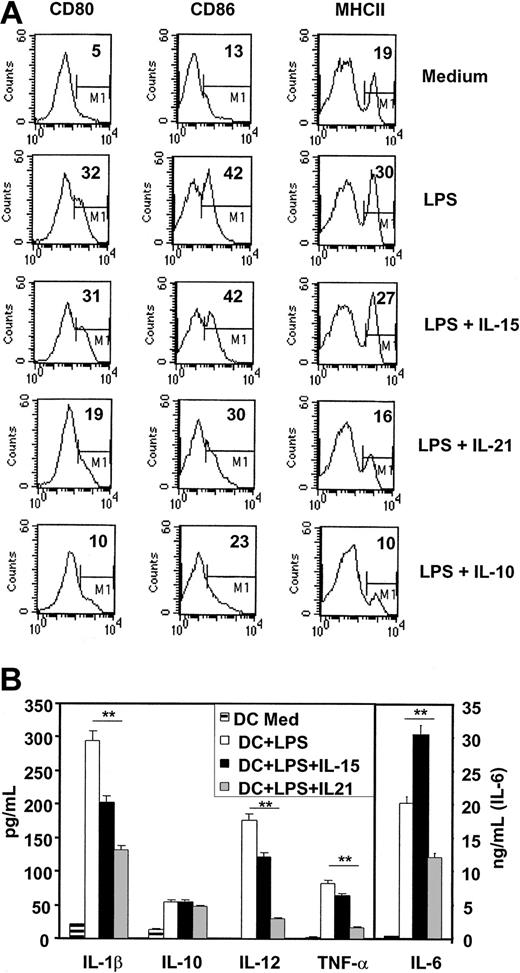
It was evident that adding IL-21 showed properties similar to IL-10 in blocking the LPS-induced up-regulation of CD80, CD86, and MHCII molecules, which were expressed 40%, 70%, and 90% higher, respectively, when LPS was used alone (Figure 8A). This inhibitory effect was already seen at IL-21 concentrations of 1 ng/mL (not shown). These observations strongly support the evidence that IL-21 prevents also in vitro-induced maturation by LPS. Supplementing LPS with IL-15 did not further enhance the effects mediated by LPS alone, which is likely due to maximal activation reached by LPS. Additionally, we analyzed the effects of the cytokines alone and stimulated DCs with the IL-15, IL-21, and IL-10 (100 ng/mL) for 24 hours and did subsequent FACS analysis. We found no pronounced up-regulation of the indicated markers due to the stimulation solely with cytokines and no induction of apoptosis by IL-21 at concentrations up to 200 ng/mL (not shown).
IL-21 inhibits short-time, LPS-induced DC activation and proinflammatory cytokine production in vitro. Immature DCs (after 8 days of culture with GMCSF only) were activated with low-dose LPS (10 ng/mL) or with LPS plus IL-15, IL-21, or IL-10 for 24 hours. (A) Surface expression of costimulatory and MHC II molecules were analyzed by FACS. Given is the percentage of high positive cells defined by the marker M1. (B) Cytokines were analyzed in the DC culture supernatant. Significance was calculated using Student t test (**P ≤ .01) and shown is the mean ± SD (note different scale for IL-6 concentration). One representative out of 4 experiments is shown.
IL-21 inhibits short-time, LPS-induced DC activation and proinflammatory cytokine production in vitro. Immature DCs (after 8 days of culture with GMCSF only) were activated with low-dose LPS (10 ng/mL) or with LPS plus IL-15, IL-21, or IL-10 for 24 hours. (A) Surface expression of costimulatory and MHC II molecules were analyzed by FACS. Given is the percentage of high positive cells defined by the marker M1. (B) Cytokines were analyzed in the DC culture supernatant. Significance was calculated using Student t test (**P ≤ .01) and shown is the mean ± SD (note different scale for IL-6 concentration). One representative out of 4 experiments is shown.
To check how IL-21 influences release of proinflammatory cytokines and whether the observed effects of IL-21 were mediated by the release of the inhibitory cytokine IL-10, we analyzed the supernatant of the stimulated DCs after 24 hours. As shown in Figure 8B, addition of IL-21 could highly significant suppress the proinflammatory cytokines IL-1β, IL-12, IL-6, and tumor necrosis factor α (TNF-α) but did not significantly enhance DC production of IL-10. Incubation of DCs with IL-21 alone (using increasing concentrations from 1-100 ng/mL) for 24 hours resulted in no significant increase of the analyzed cytokines compared with medium alone and had no toxic effect as cell survival was checked by Annexin V/propidium iodide (PI) staining (not shown).
In conclusion, IL-21 inhibits not only DC maturation when present during the generation but also the activation and maturation of DCs in the presence of highly potent stimuli such as LPS. These findings could also be verified for human DCs, suggesting that these effects of IL-21 are not species specific (S.B.-P., manuscript submitted).
Discussion
Our results show that the newly described cytokine IL-2115 and its structural relative IL-15 modulate DC differentiation, maturation, and function in vitro and in vivo. Giving IL-21 to BM cultures in addition to GMCSF provided phenotypic and functional “immature” DCs with reduced MHCII expression, elevated endocytic activity, and limited T-cell stimulatory ability. This is the first report showing that IL-21 in combination with GMCSF is able to modulate differentiation of myeloid BMDCs and exhibits a regulatory impact on APC function, therefore expanding results from literature demonstrating that IL-21 also influences lymphoid cell development and function.15 By contrast, IL-15, which is structurally related and shares the common γ chain with IL-21, mediated effects opposite from IL-21 by promoting maturation of DCs in vitro and significantly enhanced DC-mediated antigen-specific T-cell response in vitro and in vivo.
DCs are able to respond directly to pathogens like microbial cell wall components and indirectly by sensing infection through inflammatory cytokines.30 In response to these “danger” signals,30 DCs are activated to enter maturation. Our data imply that IL-21 is able to modulate maturation of DCs. Thus, we showed that LPS-induced activation of DCs, characterized by an elevated expression of functionally relevant molecules including MHCII, CD80, and CD86, and production of proinflammatory cytokines (IL-6, IL-12, IL-1β and TNF-α) was significantly reduced when IL-21 was given in parallel to LPS. Other cytokines in the extracellular environment, notably from T-cell-released IL-10, have been implicated in impeding DC maturation, which was associated with the retention of an “immature” phenotype.31 Concomitant incubation of DCs with LPS and IL-10 blocked maturation likewise and impaired expression of antigen-presenting and costimulatory molecules, suggesting that IL-21 has inhibitory effects comparable to IL-10. We further could show that the inhibition by IL-21 is not mediated by IL-10 release from DCs but rather seems to be a direct effect of IL-21. This is in line with recently published data32 showing that IL-21 did not induce IL-10 or transforming growth factor β (TGF-β) release from T cells. However, further attempts have to be undertaken to elucidate the exact actions of IL-21 in blocking DC activation.
Nevertheless, our data provide the first evidence that IL-21 is an important negative regulator of DC activation in response to microbial stimuli. In contrast, IL-15 did not enhance the LPS action, most probably due to the fact that IL-15 is already produced by LPS-stimulated DCs,13 thus exogenously added IL-15 could not further enhance the activation.
Because IL-21 was shown to support differentiation of myeloid NK cells from BM progenitor cells and act synergistically in this respect with IL-1515 we investigated whether IL-21 and its structural relative IL-15 have modulatory impact on DC differentiation. Moreover, IL-15 and IL-21 belong to a receptor family that shares a common γ chain in its receptor complex. With the knowledge that IL-4, another member of this receptor-sharing family, modulates DC differentiation we are searching for a comparable influence of IL-15 and IL-21. Our studies revealed completely contrary outcomes of DCs generated in the presence of either IL-15 or IL-21. On the one hand we observed highly immunogenic IL-15DCs, which represent mature DCs after several, by an exhaustive body of literature, defined criteria.33 On the other hand IL-21DCs were blocked in their activation and were unable to enter maturation after various stimuli.
It is well known that DC maturation is directly linked to T-cell stimulatory capacities.34 Indeed, our experiments revealed that antigen-specific T-cell response was significantly reduced with IL-21DCs. By contrast, IL-15DCs mediated highly significant increased T-cell proliferation. While IL-15 led to more mature DCs characterized by enhanced capability to prime T-cell response, IL-21 silenced DC functions in this respect. The immature phenotype of IL-21DCs was also stable after in vitro incubation of IL-21DCs with the contact sensitizer FITC, where normal DCs and IL-15DCs changed their phenotype by up-regulating B7 and MHC molecules, which has been expected from recent reports.26,35
The CHS28 model provides a unique approach to assess multiple functions of DCs in vivo. DCs transport antigens from the periphery to the DLN to prime T cells, which then become effectors of cell-mediated immunity. Indeed, IL-15DCs induced a significantly increased CHS response, whereas the same number of IL-21DCs failed to induce CHS. To exclude that the inability of IL-21DCs to induce a CHS response was due to reduced migration capacity, we examined the local draining lymph nodes and found that IL-21DCs, DCs, and IL-15DCs traffic to the local DLN to the same extent. Therefore, the lack of T-cell stimulation by IL-21DCs is most likely attributed to their “immature” phenotype with reduced MHCII expression and not due to a defect in homing to DLN. This is supported by the observation that the lack of IL-21 signaling in IL-21R-/- mice results in an increased delayed-type hypersensitivity (DTH) response.36 This correlated with a marked increase in interferon γ (IFN-γ) production by T cells. Wurster and coworkers36 also showed that IL-21 treatment dampened the responsiveness of T cells to IL-12 through a reduction of STAT-4 (signal transducers and activators of transcription 4). We reported that DCs developing under the aegis of IL-21 displayed immunosuppressive functions by abrogating Ag-specific CHS. In addition, mice that had been injected with IL-21DCs, even after a second application of FITC 2 weeks after DC injection, were unable to mount an ear swelling. This implicates that IL-21DCs not only failed to undergo maturation after antigen uptake and migration to DLNs but also failed to establish a T-cell response and hence might not be able to induce memory T cells. The possibility and the extent to which IL-21DCs may induce anergy and therefore may be used for tolerance induction must be clarified in future experiments. Interestingly, we further observed that normal DCs also act immunosuppressively in vivo when preincubated with IL-21 only for a short time (K.B., unpublished data, September 2003). Thus, we could state that IL-21 not only plays a role in a discrete developmental window but also is able to act in short time courses, making it an attractive therapeutic target.
Indeed, preliminary experiments showed that injection of IL-21 in mice significantly down-regulates expression of costimulatory molecules such as CD80, CD86, and MHCII up to 50% in splenic DCs (R.R., unpublished data, September 2003). Subsequently, antigen presentation by these immature DCs might induce differentiation of naive T cells toward suppressor/regulatory phenotype.37,38 Moreover, we also observed a suppressive effect of IL-21 on macrophage function, which further supports our data that IL-21 has silencing properties on APCs (K.B., unpublished data, September 2003).
Because IL-15 was found to induce DC activation after short-time incubation,3,13 it has been considered to be important in maturation processes. Here we present evidence that during generation of DCs permanently given IL-15 induced highly potent DCs with regard to T-cell stimulation. Since IL-15 is produced in vivo by BM cells and lymph node stromal cells,4 the presence of this cytokine in the extracellular matrix may shape DC phenotype during hematopoiesis and DC function during a variety of immune responses. Recently we showed that DC-derived IL-15 indeed is essential for induction of Th1 immune responses using IL-15- and IL-15Rα-deficient DCs (R.R., unpublished data, September 2003).
Despite the fact that IL-15 and IL-21 share structural motives and the common γ chain, they mediate completely contrasting effects on DCs. These effects are most likely due to their unique private receptor α chains that complete the IL-15Rαβγ and IL-21R complexes and thereby allow differential responsiveness, depending on the ligand and high-affinity receptor expressed. How the γ chain in the multipart receptors of these cytokines is involved in the DC differentiation has to be determined; however, it is known that the effects of IL-4 on DCs are independent from the γ chain and its associated kinase, Janus kinase 3 (Jak3).39 In this respect it will be necessary to unravel the modulation of the GMCSF signaling pathway by IL-15 and IL-21.
Alternatively, blocking of IL-21 and its private receptor could give more insight into the mechanisms that keep DCs controlled in an immature state. Moreover, it is reasonable to speculate that T cells release IL-21 during or after contact with DCs, providing a negative feedback signal keeping DCs in vivo in an immature state. In addition to suppressing DC maturation, IL-21 was also shown to prevent IL-15-mediated proliferation of murine CD44+CD8+ memory T cells and the up-regulation of receptors for IL-2, IL-15, and IFN-γ.40
In vivo, the tolerogenity of immature DCs could lead to therapeutic applications, for instance in prolonging allograft survival41 or treating autoimmune diseases. Vice versa, IL-15DCs might serve as potent immune stimulating DCs and possibly provide new or expanded properties for immunotherapy based on the injection of antigen-pulsed DCs.42,43
In conclusion, we found that despite structural similarities, IL-15 and IL-21 have completely opposite functional consequences for DC biology including maturation and antigen presentation in vitro and in vivo. Our results display that IL-15 induces high-immunogenic DCs and IL-21 release may lead to inhibition of DC activation and maturation.
Prepublished online as Blood First Edition Paper, July 31, 2003; DOI 10.1182/blood-2003-03-0669.
Supported by a grant from the University Lübeck (R.R., STIF 110/MUL12).
D.C.F. is employed by Zymogenetics, a company whose product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank A. Jenckel, J. Schenck, and M. V. Odenwald for excellent technical assistance.


![Figure 6. High versus low antigen-specific T-cell stimulation in vitro by IL-15DCs or IL-21DCs. DCs (□), IL-15DCs (▪), and IL-21DCs (▦) were labeled with different OVA323-339 peptide concentrations and incubated with lymph node cells from OTII mice. After 72 hours of incubation, cells were labeled with 0.2 μCi (0.0074 MBq) [3H]thymidine. One representative of 3 experiments is shown. Significance compared with DCs was calculated using Student t test (**P ≤ .01). Error bars indicate standard deviation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/12/10.1182_blood-2003-03-0669/6/m_h82335301006.jpeg?Expires=1763524604&Signature=O9kW5XCvpdWA0QGc~1sXV3kGz0rCrvHLA3sKndWhv8fPeAuMpFaomFtuoxCcD15pIGP6uU~G8-4xEnKIA3xShcMo03eCBkRcZIw9TYuSmDCilcKkkzL0vthb7zpqKSVcTw7XpkmQ67ZzsLmMDVek1pRvK4kRyAcpFsKhsm04kC7pFpJqgIgo5C8duuUmG9F10MnMfm6YrQETdZigN58aknHqGfEm0udbinapOUb~kLKY846XIZE9hVI-nhyyM-9OVOURH91TdvJSfw1V00qiyAE8hfv3X8QO49FTQDJ~WFHf0y5VRItU19V1C~JbTvNVBHhl-kqqIsjIhvaTDAZdKQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)





![Figure 6. High versus low antigen-specific T-cell stimulation in vitro by IL-15DCs or IL-21DCs. DCs (□), IL-15DCs (▪), and IL-21DCs (▦) were labeled with different OVA323-339 peptide concentrations and incubated with lymph node cells from OTII mice. After 72 hours of incubation, cells were labeled with 0.2 μCi (0.0074 MBq) [3H]thymidine. One representative of 3 experiments is shown. Significance compared with DCs was calculated using Student t test (**P ≤ .01). Error bars indicate standard deviation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/12/10.1182_blood-2003-03-0669/6/m_h82335301006.jpeg?Expires=1763524605&Signature=ED4APZxoraZMZN7cfQ-zAGpWoGWvHfaWz4kqthngy~pP5abV--208MB7eXa-~40eBKMBozbo4BzXXUsIGHYGbzIOhCnPqhA6rLWPu0drkELxd23G415ieOtXpN1uZMZoNOZzKNQnAII-g-ZCXcXifyAvvnqpyZ1ayRt2zaYsnT~A8Z-ZQV4RTSiWs4mdnmH96Gb8yC6DxT8MhTj1drUxTd0tBvwwhqK-0XpGTrpGQ3HXvbPMcM-o6YSk1vRLejZ9qkMgCA~c-a2iehibkbGEkRNAlZcTl09p9rJjnWNPafdzg5ik6MGh3uYQu5m0F4EPgEoFRa70fppRH3XNEdE22Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)