Abstract
Activated platelets release their granule content in a concentrated fashion at sites of injury. We examined whether ectopically expressed factor VIII in developing megakaryocytes would be stored in α-granules and whether its release from circulating platelets would effectively ameliorate bleeding in a factor VIIInull mice model. Using the proximal glycoprotein 1bα promoter to drive expression of a human factor VIII cDNA construct, transgenic lines were established. One line had detectable human factor VIII that colocalizes with von Willebrand factor in platelets. These animals had platelet factor VIII levels equivalent to 3% to 9% plasma levels, although there was no concurrent plasma human factor VIII detectable. When crossed onto a factor VIIInull background, whole blood clotting time was partially corrected, equivalent to a 3% correction level. In a cuticular bleeding time study, these animals also had only a partial correction, but in an FeCl3 carotid artery, thrombosis assay correction was equivalent to a 50% to 100% level. These studies show that factor VIII can be expressed and stored in platelet α-granules. Our studies also suggest that platelet-released factor VIII is at least as potent as an equivalent plasma level and perhaps even more potent in an arterial thrombosis model. (Blood. 2003;102:4006-4013)
Introduction
Hemophilia A is an X chromosome-linked bleeding disorder caused by defects in the factor VIII (FVIII) gene and affecting approximately 1:5000 male individuals.1 Treatment consists of factor replacement by using pooled FVIII concentrate or recombinant product. The limitations of these products include their expense2 and the limited ability of these products to prevent long-term sequelae unless used in a rigorous prophylaxis regimen.3 About 10% of the population with hemophilia A develops inhibitors to the infusion product, requiring alternative, even more expensive and less effective, forms of therapy.4 Concerns about infectious complications from blood-derived replacement products have continued to be an issue even with new preparative techniques.5 The high costs of treatment, the infectious and immune complications of therapy, and the limitations in preventing the long-term complications of hemophilia A make a gene-based strategy for the treatment of hemophilia A an attractive alternate form of therapy.
FVIII is a plasma protein necessary to accelerate FX activation by activated FIX.6 Activated FX then forms a similar complex with FVa, which activates prothrombin to form thrombin, which in turn cleaves fibrinogen to form a fibrin clot. Thrombin also cleaves FVIII into a more active form, whereas further cleavage by thrombin (and activation of the protein C complex) eventually inactivates FVIII. FVIII is translated as a 2351-amino acid (aa) protein, which includes a 908 aa B-domain,7 whose removal enhances FVIII secretion from cell lines without altering FVIII activity or its circulating half-life.8,9
Where FVIII is synthesized has not been definitively identified. Transplantation data in dogs and humans support the reticuloendothelial cells in the liver and spleen as the main sites of FVIII synthesis.10,11 FVIII is synthesized in the rough endoplasmic reticulum and then transported to the Golgi apparatus by way of an ERGIC-53 (endoplasmic reticulum-Golgi intermediate compartment 53)-dependent pathway, a pathway it shares with FV processing.12 In the Golgi apparatus, FVIII is cleaved at 2 sites surrounding the B-domain, releasing a heavy and light chain. The FVIII is then presumably either stored in granules (as FVIII levels increase with desmopressin acetate [DDAVP] treatment13 ) or is directly secreted from the cell where it circulates with its carrier protein von Willebrand factor (VWF).14 In Chinese hamster ovary (CHO) cells and AtT-20 pituitary cells, FVIII granule storage depends on the coexpression of VWF.15,16
We were interested in testing whether, in a transgenic mice model, FVIII could be ectopically expressed during megakaryopoiesis and stored in α-granules. There is no evidence that FVIII is present in either human or mouse platelets. FVIII from VWF/FVIII complex will bind to platelets when platelets are activated, but no FVIII has been detected in platelets. The literature is confusing because the old name for VWF was either FVIII:Ag or FVIII R:Ag.17-20 The first term persists in the pathologic literature but is incorrect because it refers to VWF (a protein that is synthesized in megakaryocytes) unlike FVIII clotting protein. Reports on FVIII mRNA did not demonstrate FVIII mRNA in peripheral blood or bone marrow.21 Extensive studies by one of us (R.R.M.) on human, canine, and mouse platelets failed to demonstrate FVIII protein (or message), and FVIII staining is only positive when platelet/megakaryocyte expression is driven by transfection or transduction.22 Although FVIII can be found in blood outgrowth endothelial cells after in vitro manipulation,23 mesenchymal cells have only demonstrated FVIII after transduction or transfection. Bone marrow transplantation has failed to correct the hemophilia A phenotype.24 Thus, we feel confident that the FVIII expressed in these platelets is unique to platelet expression by the transgene.
The impetus for these studies was the possible use of ectopic FVIII expression in developing megakaryocytes in gene therapy for FVIII hemophilia. The rationale for this approach included the knowledge that VWF is expressed and stored in α-granules in developing megakaryocytes25 and may help target ectopically expressed FVIII to these granules. At the time these studies were begun, FV was thought to be synthesized and stored in developing megakaryocytes,26 further supporting the suggestion that FVIII would be normally processed during megakaryopoiesis and be targeted to the α-granules.
There are a number of potential advantages to try to correct hemophilia A with FVIII ectopically expressed in platelets. It is possible that platelet FVIII would have a half-life 30 times longer than circulating FVIII (10 days versus 8 hours, respectively27,28 ). Platelet FVIII may be particularly useful in patients with hemophilia A with inhibitors, as the stored FVIII may be protected until needed at a site of injury and may represent a smaller antigenic stimulus than circulating FVIII. Finally, the release of small amounts of FVIII in a concentrated fashion at sites of injury may be an effective mechanism for maximizing clot formation. However, a recent report suggests that α-granule release begins deep within a developing thrombus29 so that it is possible that the released FVIII may occur too late during thrombus development and be physically removed from its intended site of action.
In this paper, we describe our studies with transgenic animals ectopically expressing human B-domainless (hB-) FVIII. This form of FVIII appears to be biologically as active as full-length FVIII and to be stored in the same fashion.15,16 A number of the generated transgenic lines had detectable hB-FVIII message in their platelets by reverse transcription-polymerase chain reaction (RT-PCR), but only line no. 38 had detectable protein antigen and FVIII activity in its platelets. No line had clearly detectable hB-FVIII in its plasma. Confocal microscopy studies showed that the hB-FVIII colocalized with VWF, presumably in the platelet α-granules. In in vitro and in vivo bleeding models, multiple transgenic lines had partial improvement. Line no. 38 had the greatest correction, and in an arterial thrombosis model had complete correction. The clinical implications of these studies are discussed.
Materials and methods
Vector construct and characterization of transgenic mice
The megakaryocyte-specific murine glycoprotein Ibα (GPIbα) (2.6 kb) proximal promoter region plus a 5′-untranslated region (UTR) 1st exon and part of its 1st intron were PCR amplified with an XhoI/artificial restriction sites at both ends.30 This promoter was subcloned into a previously described hB-FVIII vector,31 replacing the involucrin dermal-specific promoter. A 7.4-kb partial XhoI/SalI fragment containing the construct was used to create transgenic mice by pronuclear injections following standard methods at the University of Pennsylvania Transgenic Mice Core Facility. Genomic DNA was isolated from mouse tails using a QIAamp DNA Mini Kit (Qiagen, Valencia, CA). Positive founder lines were detected by Southern blots as previously described.32 BglII-digested genomic DNA was separated on a 1% (wt/vol) agarose gel. The probe used was the mouse GPIbα proximal promoter from -440 to +70 relative to the GPIbα transcriptional start site,30 and the final probed membrane was exposed on a PhosphorImaging screen (Amersham Biosciences, Sunnyvale, CA). The intensity of bands on film was analyzed using Imagequant Phosphor-Imager software (Amersham Biosciences). Copy number was determined by comparing the intensity of the 2.3-kb BglII transgenic GPIbα/hB-FVIII band to the native genomic 3.7-kb BglII native GPIbα band. Founder animals and their offspring were also characterized by genomic PCR analysis using a mouse GPIbα promoter region (5′-CTAGAGATAAAGACTTAAGT-3′) sense primer and an SV40 region (5′-CCCAGTTAACTTTCTGGTTTTTC-3′) antisense primer, which generates a 0.9-kb transgenic genomic band. The hypoxanthine phosphoribosyl-transferase (HPRT) coding region was RT-PCR amplified using previously described oligonucleotides as a widely expressed housekeeping gene.33
All biologic studies described here were done with transgenic animals that had been backcrossed at least 6 generations onto a C57BL/6J background. Wild-type littermates served as controls. Portal vein blood was drawn from mice into 1/10th volume of 3.8% sodium citrate. Studies were approved by the Animal Care and Use Committee of the Children's Hospital of Philadelphia.
Transgenic hB-FVIII message detection
Murine platelet RNA was isolated with the use of RNA STAT-60 (Tel-Test, Friendswood, TX) as previously described.33 Tissues (approximately 100 mg each) from these animals were collected, rinsed vigorously with saline to remove blood, and disaggregated in 500 μL RNA STAT-60, followed by RNA isolation.33 Reverse transcription was performed by using the SuperScript II Reverse Transcriptase Kit (Life Technologies, Bethesda, MD) as per the manufacturer's instructions. RT-PCR amplification of the transgenic message was done by using a mouse GPIbα exon 1 (5′-TCTGTTCCTCCAAAGGACTG-3′) sense primer and an hB-FVIII coding region (5′-CACAGGCAGCTCACCGAGATC-3′) antisense primer, yielding a 299-bp cDNA fragment. Platelet-specific control RT-PCR primers for mouse PF4 message were sense 5′-GTAGAACTTTATCTTTGGGT-3′ and antisense 5′-AATTTTCCTCCCATTCTTCA-3, with an expected cDNA product of 300 bp. The platelet murine transgenic hB-DNA platelet RT-PCR-amplified cDNA band was isolated from an agarose gel using a QIAkwik Gel Extraction Kit (Qiagen) and directly sequenced using an ABI 373A automated sequencer (ABI Instruments, Foster City, CA).
Mice were also studied on an FVIIInull background. The genetic defect in these animals involved deletion of exon 16 of their FVIII gene.34 These animals had been backcrossed onto a C57BL6 background more than 10 times and were kindly provided by Dr Haig Kazazian at the University of Pennsylvania. Genotype was determined as described by that group.34
Immunohistochemical staining for hFVIII
Femurs from wild-type and transgenic mice littermates were stained for hB-FVIII expression by using a sheep polyclonal α-human-specific FVIII antibody (Fitzgerald Industries International, Concord, MA). Specifically, formalin-fixed, paraffin-embedded 5-μm sections were deparaffinized in xylene and rehydrated. Endogenous peroxidase activity was quenched with 0.9% peroxide in methanol for a total of 20 minutes. Sections were incubated with anti-hFVIII (1:200 dilution) or normal sheep serum for 2 hours at room temperature. Slides were washed and incubated with biotinylated rabbit antisheep antibody (1:200 dilution; Vector Laboratories, West Grove, PA) for 30 minutes at 37°C. Slides were washed and incubated with streptavidin-horseradish peroxidase (HRP; DAKO, Glostrup, Denmark) for 15 minutes at room temperature. Slides were again washed and incubated with diaminobenzidine (DAB) reagent (DAKO) was applied for 5 minutes at room temperature and then counterstained with dilute (1:10) hematoxylin for 30 seconds.
Immunofluorescent studies for hFVIII
Platelets were fixed using 3.7% (vol/vol) buffered formalin, permeabilized in 1% Triton X-100 (in 20 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 300 mM sucrose, 50 mM NaCl, 3 mM MgCl2 · 6H2O, pH 7.0) for 30 minutes and blocked in 2% normal goat serum in Hanks balanced salt solution (HBSS; GibcoBRL, Gaithersburg, MD). Cells were incubated for several hours with primary antibodies diluted in HBSS/1% bovine serum albumin (BSA). Primary antibodies included a rabbit polyclonal antibody raised against hFVIII directly conjugated to AlexaFluor 568 (Alexa568*Conan, 5 μg/mL), a rabbit polyclonal raised against human VWF conjugated to HRP used at 1:500 dilution (Dako Corporation, Carpinteria, CA), or isotype controls (normal rabbit immunoglobulin G [IgG] and AlexaFluor568-conjugated normal rabbit IgG). After washing extensively with HBBS, cells were incubated in secondary antibody (fluorescein isothiocyanate [FITC]-goat-anti-HRP; Vector Laboratories) for 30 minutes followed by stringent washes with HBSS. Cells were mounted with Vectashield (Vector Laboratories). Immunostained cells were analyzed for the intracellular location of VWF and FVIII by confocal laser scanning microscopy in the Imaging Core of the Medical College of Wisconsin by using a Leica TCS SP2 confocal laser imaging system.
Determination of hFVIII antigenic and functional levels
Levels of hB-FVIII antigen in the plasma and platelets of transgenic animals were determined using an antihuman-specific FVIII enzyme-linked immunosorbent assay (ELISA) kit (Affinity Biological, Hamilton, ON, Canada) and compared with hB-FVIII recombinant protein (ReFacto Laboratory Standard; Genetics Institute, Cambridge, MA). For only these studies and for the functional studies described later, mice were kept on water containing 1 mg/mL aspirin for 24 hours prior to drawing portal blood samples. Platelet-rich plasma was prepared as previously described,35 pelleted at 600g for 10 minutes, and resuspended in 300 μL Tyrode buffer (139 mM NaCl, 2.9 mM KCl, 0.34 mM NaHPO4, 12 mM NaHCO3, 20 mM HEPES, 1 mM MgCl2, pH 7.28) and frozen at -20°C. Before assaying for hB-FVIII activity, the platelets were thawed and phorbol myristyl acetate (final concentration, 1 μM) and collagen (final concentration, 5 mg/mL) were added. After spinning down the platelet membranes at 600g for 15 minutes, hB-FVIII level was determined in the platelet releasate. To obtain platelet-poor plasma (PPP), prostaglandin E1 (final concentration 1 μM) was added to 1 mL platelet-rich plasma (PRP) prior to pelleting the platelets. The PPP was frozen at -20°C until assayed for hB-FVIII.
FVIII activity level was determined by a Coatest using a Coamatic Factor VIII kit (DiaPharma Group, West Chester, OH) as described by the manufacturer. Transgenic/FVIIInull mice and their wild-type (WT) and FVIIInull littermates were simultaneously measured. PPP and platelet releasate from PRP were prepared as described earlier for ELISA. Recombinant hB-FVIII protein was used to generate a standard curve.
Whole blood clotting time
Transgenic/FVIIInull mice and their FVIIInull littermates were studied simultaneously. The whole blood clotting time was done as previously described.36 Briefly, nonanticoagulated portal vein blood (600 μL) was divided equally among 3 glass tubes and placed in a 37°C water bath. One tube was tilted every 30 seconds, whereas the other tubes were left undisturbed. When a solid clot was observed in the first tube, the second tube was tilted every 30 seconds. This process was continued with the third tube, and the time to clot of the third tube was recorded as the whole blood clotting time.
Bleeding times
Bleeding times were determined by 2 methods, a standard tail bleeding time as previously described37 without cauterization, and a new cuticular bleeding time done on the middle hind digit. In the cuticular assay, the entire cuticle was removed with a sharp scissor and the animal was placed back in a cage lined with a sheet of 3M Whatman filter (Whatman International, Maidstone, England). Hourly, the filter was replaced, and the time to the first dry sheet was recorded. At the end of 7 hours, the animals had a complete blood count (CBC) drawn prior to being killed.
Carotid artery thrombi
Ferric chloride-induced arterial injury was performed according to published procedures38 in 6- to 8-week-old animals. Briefly, the right common carotid artery was exposed by blunt dissection, and a miniature Doppler flow probe (Model 0.5VB; Transonic Systems, Ithaca, NY) was positioned around the artery. A 1 × 2mm2 strip of 1M Whatman filter paper (Whatman International) soaked in 10% ferric chloride was then applied to the adventitial surface of the artery for 2 minutes. The field was flushed with saline, and blood flow was continuously monitored for 30 minutes. The time to the initial complete occlusion and the presence or absence of arterial occlusion at 30 minutes were recorded. In some experiments, the animals received an infusion of plasma or platelets into the right jugular vein immediately prior to the application of the FeCl3 injury to the adjacent artery.
Results
Construction of transgenic mice
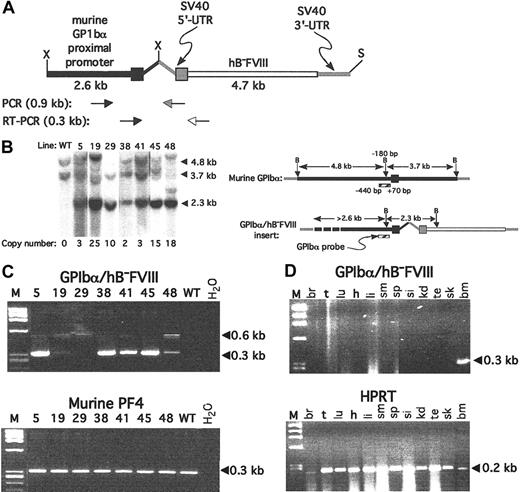
The 7.4-kb partial XhoI/SalI insert of the mouse GPIbα proximal promoter-driven hB-FVIII construct was used to create transgenic mice (Figure 1A). Seven founder animals were obtained that went germ line, and the copy number per haploid genome was determined and varied from 2 to 25 per haploid genome (Figure 1B). RT-PCR of platelet total RNA extracted from many of these lineages were positive for the transgenic message (Figure 1C), with the most intense RT-PCR band seen for lines nos. 5, 38, 41, and 45. This RT-PCR product was isolated from the gel and sequenced for one of these lines (line no. 38) for which further studies described hereafter showed the greatest hB-FVIII level and activity. The expected cDNA sequence with appropriate excision of the GPIbα intron30 was confirmed. Except for RNA extracted from whole marrow, RT-PCR analysis of multiple organs from line no. 38 was negative for the transgenic message (Figure 1D). These data are consistent with the transgenic message being transcribed in a megakaryocyte-specific fashion.
Transgenic line construction and initial genetic analysis. (A) Expression construct used with the murine GPIbα proximal promoter shown in black and the hB-FVIII in white. The SV40 5′-UTR and 3′-UTR are shown in gray. Primer pairs for the genomic analysis and for RT-PCR analysis are shown below. X indicates XhoI; S, SalI. (B) Genomic Southern blot after Bgl II digestion and probed for the murine GPIbα promoter region is shown on the left and the schematic representation of the probe and expected bands on the right. Calculated copy number for the transgene per haploid genome determine by phosphorimaging analysis is shown below the Southern blot. The position of the Bgl II site immediately upstream of the GPIbα transcriptional start site, and the ends of the probe are noted. B indicates Bgl II. (C) Platelet RT-PCR from various founder lines for the transgene is positive for multiple lines as shown in the top gel. The expected 0.3-kb cDNA band is indicated. In some lanes, a 0.6-kb genomic band is seen. The bottom gel is an RT-PCR amplification of PF4 message, confirming the platelet nature of the RNA. M indicates ϕX HaeIII marker; WT, wild-type mice platelet RNA; and H2O, lane with no RNA added. (D) In the top gel, RT-PCR on total RNA extracts from various tissues were studied for line no. 38, demonstrating that the only tissue that was positive for recombinant hB-FVIII message amplification of the transgene was the bone marrow. At the bottom, virtually all the tissues tested were positive for the widely expressed message of murine HPRT. M indicates ϕX HaeIII marker; br, brain; t, tongue; lu, lungs; h, heart; li, liver; sm, stomach; sp, spleen; si, small intestines; kd, kidney; te, testicle; sk, skeletal muscle; and bm, bone marrow.
Transgenic line construction and initial genetic analysis. (A) Expression construct used with the murine GPIbα proximal promoter shown in black and the hB-FVIII in white. The SV40 5′-UTR and 3′-UTR are shown in gray. Primer pairs for the genomic analysis and for RT-PCR analysis are shown below. X indicates XhoI; S, SalI. (B) Genomic Southern blot after Bgl II digestion and probed for the murine GPIbα promoter region is shown on the left and the schematic representation of the probe and expected bands on the right. Calculated copy number for the transgene per haploid genome determine by phosphorimaging analysis is shown below the Southern blot. The position of the Bgl II site immediately upstream of the GPIbα transcriptional start site, and the ends of the probe are noted. B indicates Bgl II. (C) Platelet RT-PCR from various founder lines for the transgene is positive for multiple lines as shown in the top gel. The expected 0.3-kb cDNA band is indicated. In some lanes, a 0.6-kb genomic band is seen. The bottom gel is an RT-PCR amplification of PF4 message, confirming the platelet nature of the RNA. M indicates ϕX HaeIII marker; WT, wild-type mice platelet RNA; and H2O, lane with no RNA added. (D) In the top gel, RT-PCR on total RNA extracts from various tissues were studied for line no. 38, demonstrating that the only tissue that was positive for recombinant hB-FVIII message amplification of the transgene was the bone marrow. At the bottom, virtually all the tissues tested were positive for the widely expressed message of murine HPRT. M indicates ϕX HaeIII marker; br, brain; t, tongue; lu, lungs; h, heart; li, liver; sm, stomach; sp, spleen; si, small intestines; kd, kidney; te, testicle; sk, skeletal muscle; and bm, bone marrow.
Immunohistochemistry studies for hFVIII
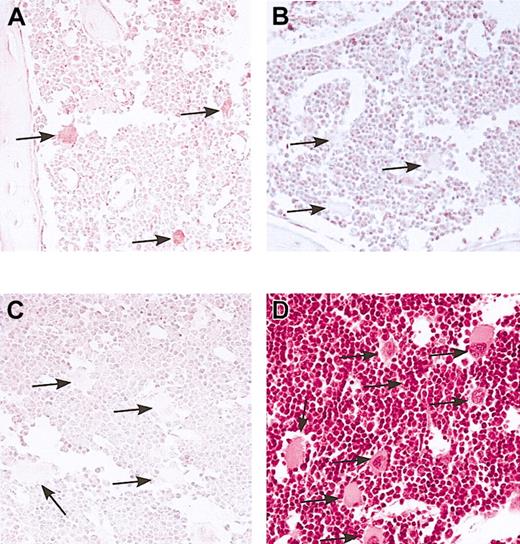
The studies shown in Figure 1 suggest that in line no. 38, hB-FVIII was expressed in developing megakaryocytes. To demonstrate specific megakaryocytic expression of hFVIII in these mice we examined bone marrow for hFVIII expression by immunohistochemistry. Staining of bone with anti-hFVIII antibody demonstrated the presence of VIII only in morphologically recognizable megakaryocytes in the transgenic hB-FVIII/FVIIInull animals examined (Figure 2A). No staining was observed using nontransgenic FVIIInull animals (Figure 2B) or when using a normal serum control (Figure 2C). These data demonstrate specific megakaryocyte expression of hFVIII in the bone marrow of our transgenic animals.
Marrow immunostain for transgenic hB-FVIII. Femoral marrow from line no. 38 hB-FVIII/FVIIInull animals (A,C,D) and from FVIIInull animals (B) was immunostained for hFVIII (A-B) or with a sheep serum control (C). Hematoxylin and eosin stain was done on the marrow from the hB-FVIII/FVIIInull mice to demonstrate the clear morphology of the megakaryocytes present (D). Only megakaryocytes in the line no. 38 marrow were positive for hFVIIIs. Arrows point to representative mature megakaryocytes in each figure. Original magnification, × 100.
Marrow immunostain for transgenic hB-FVIII. Femoral marrow from line no. 38 hB-FVIII/FVIIInull animals (A,C,D) and from FVIIInull animals (B) was immunostained for hFVIII (A-B) or with a sheep serum control (C). Hematoxylin and eosin stain was done on the marrow from the hB-FVIII/FVIIInull mice to demonstrate the clear morphology of the megakaryocytes present (D). Only megakaryocytes in the line no. 38 marrow were positive for hFVIIIs. Arrows point to representative mature megakaryocytes in each figure. Original magnification, × 100.
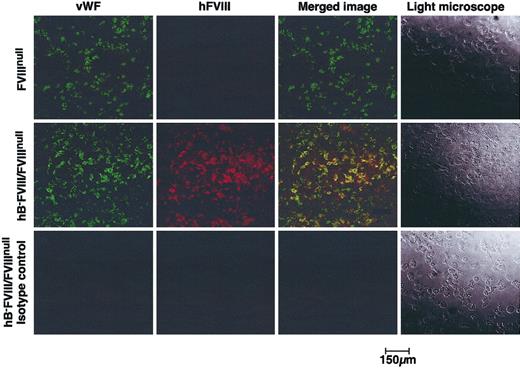
We next addressed whether the hB-FVIII was trafficking to α-granules as does VWF by confocal microscopy for hB-FVIII and murine VWF. As shown in Figure 3, both hB-FVIII and VWF are detected within the platelets of line no. 38 hB-FVIII/FVIIInull mice, but only VWF was detected in control FVIIInull platelets. In line no. 38, the distribution of the hB-FVIII and VWF strongly overlap, supporting a model in which the expressed hB-FVIII and VWF target together to the same granules as suggested by cell line studies with recombinantly expressed FVIII and VWF.13,14
Confocal fluorescent microscopic studies for transgenic hB-FVIII and murine VWF. Isolated platelets from FVIIInull animals (top row) and line no. 38 hB-FVIII/FVIIInull animals (middle and bottom rows) were immunostained for either mouse VWF (left column) or hFVIII (second column from left). The 2 images were overlapped in the third column, showing that in the line no. 38 platelets there was overlap of VWF and hFVIII distributions. Right column shows light microscopy of the platelets.
Confocal fluorescent microscopic studies for transgenic hB-FVIII and murine VWF. Isolated platelets from FVIIInull animals (top row) and line no. 38 hB-FVIII/FVIIInull animals (middle and bottom rows) were immunostained for either mouse VWF (left column) or hFVIII (second column from left). The 2 images were overlapped in the third column, showing that in the line no. 38 platelets there was overlap of VWF and hFVIII distributions. Right column shows light microscopy of the platelets.
Antigen and activity measurements
hB-FVIII antigen was measured in platelet-releasate derived from PRP and in parallel plasma samples using an anti-hFVIII-specific primary antibody-based ELISA and comparing outcome with a recombinant hB-FVIII standard. As expected, both plasma and platelet releasate from WT and FVIIInull mice had comparably low levels of hFVIII (Table 1 and data not shown). Line no. 38 plasma had low levels as well, but the platelet releasate as a measure of platelet-stored hB-FVIII had a level of approximately 0.086 U/mL above background. These levels were seen for line no. 38 whether it was on a wild-type (Table 1) or FVIIInull background (data not shown). None of the other transgenic lines tested had detectable hB-FVIII antigen (data not shown). These antigen studies suggest that within the sensitivity of the system only transgenic line no. 38 had detectable platelet hB-FVIII, and that there was little detectable hB-FVIII antigen circulating in the plasma.
FVIII activity level was determined in the transgenic animals on the FVIIInull background by using a Coatest on platelet releasate or plasma (Table 1). These studies detected FVIII activity only in line no. 38 transgenics, in which an approximate 0.03 U/mL correction was noted in the platelet releasate above background. Plasma levels were comparable to those seen in the FVIIInull animals, consistent with there being little free-circulating FVIII in the line no. 38/FVIIInull mice. None of the other transgenic lines had detectable FVIII activity (data not shown).
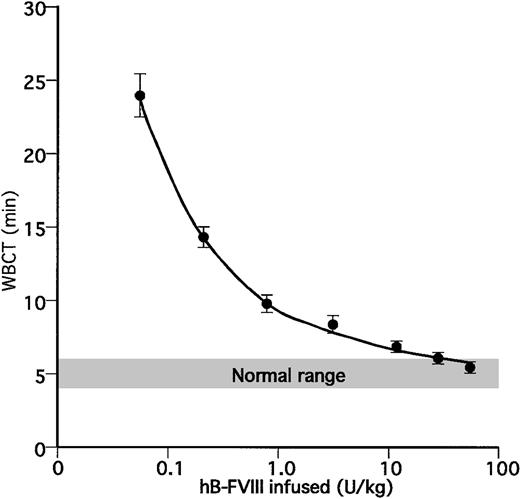
A more sensitive in vitro assay for FVIII activity is the whole blood clotting time (WBCT),36 which was determined in WT animals, transgenic animals on an FVIIInull background, and FVIIInull animals which were or were not infused with recombinant hB-FVIII 20 minutes before the study blood was withdrawn. Previous Coatest studies after hB-FVIII infusions showed that in the 30 to 40 minutes of the thrombotic study, the hB-FVIII levels decrease to 80% of the infused correction (data not shown). FVIIInull animals did not clot in this assay except after infusion of recombinant hB-FVIII when the degree of normalization of the whole blood clotting time was directly related to the dose infused (Figure 4). FVIIInull mice that were also transgenic for line no. 38 had the greatest degree of correction (Table 2). This correction to 8.7 ± 0.7 minutes was equivalent to infusing an FVIIInull animal with recombinant hB-FVIII at approximately 1.5 U/kg or a calculated 0.03 U/mL (assuming that 1 U infused hB-FVIII increases plasma levels by 2%). Interestingly, in this assay, several other transgenic lines on the FVIIInull background showed partial correction of the WBCT with mice transgenic for line no. 48 having the second greatest correction equivalent in this assay. These results suggest that the WBCT is more sensitive than assaying for hB-FVIII antigen or activity.
Whole blood clotting time studies. (A) Determination of whole blood clotting times in FVIIInull mice with infusions of various amounts of recombinant hB-FVIII. Each time point shows the mean ± 1SD(n = 4 or 5).
Whole blood clotting time studies. (A) Determination of whole blood clotting times in FVIIInull mice with infusions of various amounts of recombinant hB-FVIII. Each time point shows the mean ± 1SD(n = 4 or 5).
We took advantage of the sensitivity of the WBCT studies to further address the issue of whether hB-FVIII was localized to the platelets or also present circulating in the plasma. Plasma or platelets derived from 1 mL blood from line no. 38/FVIIInull mice was isolated and injected into recipient FVIIInull mice immediately prior to drawing blood for the WBCT. Infusion of 100 μL plasma did not improve the WBCT in FVIIInull mice as no clot was detected (Table 3). Infusion of 300 μL plasma did minimally correct the WBCT to a level equivalent to infusing less than 0.1 U/kg hB-FVIII level (Figure 4; Table 3). However, infusion of washed platelets in a total volume of 200 μL resulted in a rise of approximately 20% in platelet count (data not shown) and corrected the WBCT to a level equivalent to infusing approximately 0.3 U/kg hB-FVIII. Thus, these transfusion studies further confirm that little if any of the hB-FVIII is circulating in line no. 38 animals, but rather it is localized to the platelets. Control platelets from wild-type littermates do not correct the WBCT (Table 3), consistent with platelets not routinely containing FVIII.
In vivo studies
The in vitro studies described earlier demonstrate that line no. 38 and additional transgenic lines express hB-FVIII during megakaryopoiesis and store this protein in platelets, but they do not address whether the platelet-expressed hB-FVIII is effective in correcting the in vivo bleeding diathesis of FVIIInull mice. Murine FVIIInull animals do not develop the same clinical symptomatology, such as joint bleeds, commonly seen in humans with severe FVIII deficiency.34 The reference standard of a bleeding diathesis in the FVIIInull mice has been exsanguination on tail bleeding times.34 However, initial studies of transgenic animals with undetectable hB-FVIII message, antigen, and activity suggested that this assay was too sensitive for hB-FVIII expression. Even in transgenic lines with extremely low levels of hB-FVIII expression, there was sufficient FVIII present to prevent exsanguination on an FVIIInull background (data not shown). We designed a cuticular bleeding time that involved the time to stoppage of bleeding after exposing the nail bed by scissor of the middle digit of the right posterior extremity. Studies of wild-type animals and FVIIInull and the hB-FVIII/FVIIInull animals with this in vivo assay were performed (Table 4). The time to stoppage of bleeding as well as the clinical well-being of the animals at the end of the 8-hour study and the final hemoglobin levels were consistent with the in vitro whole blood clotting studies (Table 2). Line no. 38/FVIIInull mice had clearly an improved outcome compared with FVIIInull mice, but they clearly bleed more than wild-type animals and had final hemoglobin levels that were lower. The second best outcome again was seen with line no. 48/FVIIInull mice.
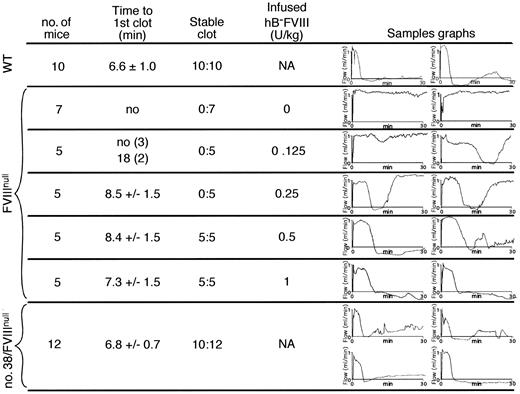
The cuticular bleeding study does not permit quantitative measurements of the degree of correction of the bleeding diathesis. However, another in vivo clotting model involving FeCl3 injury to the carotid artery allowed us to address this issue. The time to first complete occlusion and the percentage of animals with complete occlusion at the end of the 30-minute study were measured. The various hB-FVIII transgenic lines on an FVIIInull background were compared with wild-type littermates and with FVIIInull littermates. Additionally, FVIIInull animals were given infusion of varying amounts of hB-FVIII to achieve different levels of calculated correction. The correction in line no. 38 animals was equivalent to a 50% to 100% correction (25-50 U/kg infusion) (Figure 5). None of the other tested lines showed a detectable correction (data not shown).
FeCl3 carotid artery thrombosis study. Analysis of FeCl3 injury times to initial occlusion and whether stable occlusion occurred are shown for various study conditions. At the right for each study condition are 2 representative thrombosis curves. At the top of the figure are WT mice that were littermate controls, demonstrating that under the conditions of injury, all of the WT animals rapidly developed stable occlusive thrombi. Below are FVIIInull mice treated with various doses of hB-FVIII immediately prior to an FeCl3 injury to their carotid artery. At the bottom, similar studies were done with line no. 38 transgenic animals on an FVIIInull background, with 4 representative curves shown. NA indicates not applicable.
FeCl3 carotid artery thrombosis study. Analysis of FeCl3 injury times to initial occlusion and whether stable occlusion occurred are shown for various study conditions. At the right for each study condition are 2 representative thrombosis curves. At the top of the figure are WT mice that were littermate controls, demonstrating that under the conditions of injury, all of the WT animals rapidly developed stable occlusive thrombi. Below are FVIIInull mice treated with various doses of hB-FVIII immediately prior to an FeCl3 injury to their carotid artery. At the bottom, similar studies were done with line no. 38 transgenic animals on an FVIIInull background, with 4 representative curves shown. NA indicates not applicable.
We also performed FeCl3 carotid artery injury studies with infusions of either 200 μL platelets or 300 μL plasma from either no. 38 hB-FVIII/FVIIInull animals or littermate wild-type controls into FVIIInull recipients. Four of 6 FVIIInull recipients receiving platelets from the line no. 38 hB-FVIII/FVIIInull animals developed complete occlusions (time to first occlusion = 10.2 ± 0.7 minutes), whereas none of the 3 recipient animals receiving platelets from wild-type animals developed occlusions. On infusion of plasma, none of the 6 FVIIInull recipients receiving plasma from the no. 38 hB-FVIII/FVIIInull animals developed complete occlusions. All of the 3 recipient animals receiving platelets from WT animals never occluded, but the 3 animals receiving WT plasma developed stable occlusions (time to first occlusion = 11.3 ± 2.5 minutes). These studies further support the specific ability of hB-FVIII platelets in correcting the bleeding diathesis in an arterial injury model. Our estimate is that the correction is equivalent to a 12.5% hB-FVIII infusion correction (Figure 5; 6.3 U/kg), again suggesting that these platelets are particularly effective in correcting thrombosis in this model.
Discussion
Platelets release their granule content at sites of injury, and our group has been interested in testing whether platelets may serve as a delivery vehicle for prothrombotic and antithrombotic agents to a site of injury. We have recently shown that the ectopic expression of urokinase-type plasminogen activator (uPA) in platelets in a transgenic animal results in the expression of uPA during megakaryopoiesis, its storage within circulating platelets, and its release at sites of injury.39 No detectable uPA leaked out of the megakaryocytes or platelets. The platelet uPA not only resulted in a mild bleeding diathesis in the adult transgenic mice, but transfusion of these platelets into wild-type animals blocked untoward thrombosis. Thus, it appears that platelets can specifically store an antithrombolytic protein and release the protein of interest at sites of developing thrombi, interfering with their development.
The studies presented in this paper show that in a transgenic mouse model that FVIII message could also be ectopically expressed during megakaryopoiesis, and the resultant prothrombotic protein is not only expressed in the developing megakaryocytes but also is stored in circulating platelets. Confocal microscopy suggests that the platelet FVIII is stored along with VWF in the platelet α-granules. Whether the VWF is necessary for α-granule storage needs to be tested in the future by crossing these animals onto a vWFnull background.40 We had postulated that the reason megakaryocytes stored ectopically expressed uPA was because these cells express a uPA receptor.39 Thus, it may be fortuitous that the first 2 ectopically expressed proteins in megakaryocytes are stored in the platelets as both have a carrier protein that is naturally targeted to the α-granules.
However, there is no experimental evidence that megakaryocytes secrete any of their expressed proteins. Unlike endothelial cells, the VWF in megakaryocytes may only be stored into α-granules and not secreted into the medium. Whether megakaryocytes are exceptionally well suited for ectopic protein storage and delivery to sites of vascular injury will require further inquiry. The lack of an obvious pool of secreted proteins from megakaryocytes may explain why little to no plasma FVIII antigen and activity was detected in these transgenic mice when placed on an FVIIInull background.
One of the reasons we had initially pursued a platelet approach for gene therapy of hemophilia A was the suggestion in the literature that the related coagulation protein FV is expressed and stored in platelets26 and that platelet FV was important for normal hemostasis on the basis of observations in the disorder factor VQuebec.41 However, it has become clear that, although FV may be expressed during murine megakaryopoiesis and that FV mRNA can be detected in human platelets,26 the majority of human platelet FV is absorbed from the plasma.42 Furthermore, the abnormality in factor VQuebec appears to be unrelated to platelet FV stores and that the bleeding diathesis provides no insights into the biologic importance of FV stores.43 Indeed, the disease has been renamed Quebec platelet disorder. Our data support the idea that FVIII can be expressed and stored in murine platelet α-granules much like murine megakaryocyte FV.44 This stored FVIII can be released and correct the bleeding defect in FVIIInull animals as shown by our platelet transfusion studies from line no. 38 hB-FVIII/FVIIInull mice to FVIIInull recipient mice. Whether human megakaryocytes, which do not appear to express much human FV, would behave in a similar manner as the mouse is unclear. Preliminary data following retroviral transduction of human CD34+ cells and transduction into nonobese diabetic-severe combined immunodeficient (NOD-SCID) mice suggest that FVIII would also be ectopically expressed and stored during human megakaryopoiesis as well.45
Line no. 38 mice had approximately 5 U or approximately 10 ng hB-FVIII/mL blood, all stored within the platelets. Within the approximate 109/mL platelets in a mouse, there is approximately 100 μg total platelet granule-stored proteins.46 Thus, the expressed hB-FVIII would represent roughly 0.01% of all the stored proteins inside the platelet granules and is roughly equal to the molar level of platelet-derived growth factor in human platelets47 and 10-2 to 10-3 the level of FV in human platelets48 and would occupy 1% to 10% of the VWF monomers present in platelets.49
Although expressed and stored in platelet α-granules, would platelet FVIII be effective in ameliorating the bleeding diathesis in hemophilia A mice? Two types of in vivo studies were done: bleeding times and a carotid artery thrombosis model. Initial tail bleeding studies were too sensitive in that all transgenic lines on an FVIIInull background, including those lines in which little hB-FVIII message could be demonstrated by RT-PCR, had corrected exsanguination tail bleeding times. On a modified cuticular bleeding time, it was apparent that only those transgenic lines with higher levels of platelet hB-FVIII protein as measured the WBCT (Table 4) had noticeable improvement. However, even for the highest expressing line, line no. 38, the improvement in the cuticular bleeding time when crossed onto an FVIIInull background was partial and consistent with the partial replacement of FVIII in this transgenic line.
An alternative model, the FeCl3 carotid artery thrombosis model, allowed us to do short-term hB-FVIII infusions to various levels and compare these results with the transgenic lines on an FVIIInull background. In this model, line no. 38 on an FVIIInull background had a near complete correction; this finding suggests that platelet FVIII may be especially effective for arterial clotting. Arterial thrombi are especially platelet rich, and perhaps one would expect an exaggerated response to local release of FVIII in this setting. Thus, whether the ectopic expression of FVIII in platelets would be especially effective in preventing joint bleeds and intracranial bleeds in a large animal model is difficult to predict, as our data suggest that the level of platelet FVIII may be directly correlative with the degree of correction of venous bleeds but may underestimate its effectiveness in preventing arterial bleeds.
In conclusion, we show that platelet-specific expression of FVIII can be achieved in a transgenic setting with the resultant FVIII predominantly or exclusively stored in the platelet granules rather than being released in the plasma. Only low to moderate levels of platelet FVIII were achieved, but these levels were effective in in vivo studies to a varying degree. Large animal models in which the animals with hemophilia A incur bleeding complications similar to those seen in humans must now be tested to see how effective platelet FVIII is compared with plasma correction. Certainly, platelet FVIII expression may then be a viable strategy for gene therapy for hemophilia A, especially in the setting of inhibitors. Additionally, the model may have application for the delivery of other prothrombotic agents to sites of injury beyond the setting of inherited coagulation disorders.
Prepublished online as Blood First Edition Paper, July 31, 2003; DOI 10.1182/blood-2003-05-1519.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
H.V.Y. and D.K. contributed equally to this study.