Abstract
Using separate adeno-associated viral 2 (AAV2) vectors to deliver the heavy and light chains of factor VIII (FVIII) we have overcome the packaging limitations of AAV, achieving phenotypic correction of hemophilia A in mice. AAV vectors were constructed that use a liver-specific promoter and the cDNA sequences of either the human or canine heavy and light chains of FVIII. After intraportal vein injection of these vectors in hemophilia-A mice, therapeutic to superphysiologic levels of active FVIII were achieved in plasma in a dose-dependent manner. Phenotypic correction of the bleeding diathesis was demonstrated by survival of all treated mice after tail clipping. Biochemical analysis demonstrated lower levels of heavy-chain (25- to 100-fold) compared with light-chain protein in the plasma of treated animals. Differences in gene transfer and transcription did not account for the differences in protein expression. We hypothesize that improvements in FVIII activity could be achieved by improvements in FVIII heavy-chain expression. This work demonstrates that cotransduction of liver with AAV vectors expressing the heavy and light chains of FVIII corrects hemophilia A in vivo, providing an alternative approach to the use of a single vector. This strategy may potentially be useful for other large therapeutic proteins that contain functionally distinct domains.
Introduction
Hemophilia A (Hem-A) is a common bleeding disorder characterized by a deficiency in blood coagulation factor VIII (FVIII). Intravenous administration of plasma-derived or recombinant FVIII protein is currently the most widely accepted treatment for bleeding episodes in this patient population. However, in severe hemophilic patients (< 1% FVIII), spontaneous bleeds into muscles and joints occur frequently causing progressive debilitation. Studies have shown that prophylactic infusion of FVIII protein, sufficient to maintain levels higher than 1%, prevents chronic arthropathy in patients.1 However, high costs and difficulties associated with accessing peripheral veins have prevented the widespread implementation of primary prophylactic treatments in the United States. Protein replacement therapy is also complicated by the fact that approximately 20% of severe hemophilic patients develop inhibitory antibodies to FVIII.2
Gene therapy could provide an attractive alternative to current therapies by maintaining continuous expression of FVIII at levels above 1% of normal. Encouraging data have been generated in normal and hemophilic mice using retroviral and adenoviral vectors.3-5 Using a third generation E1/E2A/E3 deleted adenoviral vector, sustained expression of canine FVIII (cFVIII) was achieved in Hem-A mice6 ; however, subsequent studies in dogs resulted in short-term expression, inhibitor development, and liver toxicity.7 Balague et al,5 using a gutted adenovirus, demonstrated sustained FVIII expression and phenotypic correction in Hem-A mice. However, in the follow-up clinical trial, liver toxicity was observed in the single subject that was treated at a low dose. Only one clinical trial using a nonviral approach has reported significant levels of FVIII expression.8 In this trial, autologous fibroblasts were transplanted into patients after transfection with plasmid DNA encoding B-domain–deleted human FVIII (hFVIII). FVIII levels of up to 4% were seen in one patient; however, expression was transient and disappeared by one year after implantation.
Adeno-associated viral (AAV) vectors have been demonstrated to provide sustained transgene expression without toxicity in mice, rats, dogs, and nonhuman primates.9,10 In hemophilia-B genetherapy studies, intrahepatic injections of AAV–factor IX (FIX) vectors in dogs resulted in sustained expression of FIX above 1% of normal.11,12 No toxicity or inhibitor development was seen in a phase 1 clinical trial when an AAV-hFIX vector was delivered intramuscularly to hemophilia B patients.13,14 However, in another phase 1 clinical trial, intrahepatic artery delivery of an AAV-FIX vector resulted in transient elevations in transaminases in one subject.15 Therefore, the toxicity of this vector in humans awaits further clinical evaluation.
Delivery of the FVIII gene using AAV vectors has been limited by its small packaging capacity (4.7 kb). One strategy, currently under investigation, involves using minimal regulatory sequences to drive expression of a B-domain–deleted form of FVIII16 (H. J. et al, manuscript in preparation). An alternative approach, which permits the use of larger regulatory elements, uses 2 AAV vectors to deliver the heavy chain (HC) and light chain (LC) of FVIII separately. This approach exploits the fact that FVIII is normally expressed as a large single-chain polypeptide of 280 kDa with a domain structure as follows: A1-A2-B-A3-C1-C2.2 Upon secretion, the B-domain is proteolytically cleaved to generate the HC and LC polypeptides. A number of groups have demonstrated that cotransfection of Chinese hamster ovary (CHO) cells with plasmids encoding the FVIII-HC and FVIII-LC reconstitutes FVIII activity in vitro.17,18 We previously demonstrated that codelivery of AAV-hFVIII-HC and -LC vectors into the livers of normal C57 Bl/6 mice produces superphysiologic levels of active FVIII.19 In the current study, we have extended our initial work to the Hem-A mouse model, and confirm that superphysiologic levels of biologically active FVIII are produced, following intrahepatic delivery of 2 AAV vectors expressing the heavy and light chains of FVIII under the control of liver-specific regulatory sequences. In addition, we have demonstrated complete correction of the bleeding diathesis in this model. Furthermore, biochemical characterization indicated that both the heavy and light chains of FVIII were processed correctly, although the stoichiometry of the secreted chains was altered, resulting in a chain imbalance.
Materials and methods
Vector construction, production, and purification
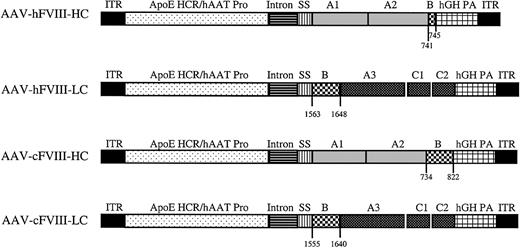
The AAV-hFVIII-HC, AAV-hFVIII-LC, AAV-cFVIII-HC, and AAV-cFVIII-LC constructs have identical regulatory sequences, which include the apolipoprotein E hepatic control region, the human alpha one antitrypsin promoter, a cytomegalovirus (CMV)/β-globin chimeric intron, and a human growth hormone polyadenylation sequence.20 Each construct also contains the first 57 bp of either the human (h) or canine (c) FVIII-HC, which encodes the 19–amino acid (aa) signal sequence. The AAV-hFVIII-HC construct encodes the A1 and A2 domain and 5 aa (residues 741-745) from the amino terminus of the B-domain. The AAV-cFVIII-HC construct contains the corresponding canine sequences, except the A1-A2 domain is followed by the sequence encoding 89 aa (residues 734-822) from the amino terminus of the canine B-domain. The human and canine FVIII-LC constructs contain the sequence corresponding to 86 aa (human residues, 1563-1648; canine residues, 1555-1640) of the carboxy terminal B-domain plus the entire A3-C1-C2 domains of the LC. The hFVIII-HC and hFVIII-LC sequences were derived from AAV-EF1α-hFVIII-HC and AAV-EF1α-hFVIII-LC, respectively.19 The canine HC and LC cDNA sequences were derived by reverse transcription (RT) of canine RNA purified from normal canine liver using the method of Chomczynski and Sacchi.21 Cloning of the canine sequences is described by R. S. et al (manuscript in preparation).
AAV vectors were produced using a triple-transfection method22 and purified by column chromatography.23 Vector genomes (vg) were quantified using quantitative real-time polymerase chain reaction (Q-PCR) with primers and probes specific for human or canine FVIII-HC and -LC and using linearized plasmid DNA as a standard.
In vitro transduction assay
HepG2 cells were plated in 6-well tissue-culture plates at a density of 1 × 106 cells per well and cultured for 24 hours. The cells were then infected at a dose of 1 × 104 vector genomes (vg)/cell, and 48 hours later the media were replaced with media containing 10% heat-inactivated fetal bovine serum (FBS), and grown for a further 24 hours. Media were analyzed for activity using the Coatest (Dipharma, West Chester, OH) or by enzyme-linked immunosorbent assay (ELISA), for antigen determination.
In vivo analysis: animal care and manipulation
Hem-A mice,24 homozygous for the exon 16 FVIII knock-out (KO) allele, were licensed from Johns Hopkins University. Hem-A/Rag1 mice were generated by breeding male B6/129-Rag1 knock-out mice (Jackson Laboratories, Bar Harbor, ME) with Hem-A females to generate F1 Rag1 heterozygotes, which were crossed to produce mice homozygous for both the Hem-A and Rag1 phenotype. All animal procedures were performed according to “Guide for the Care and Use of Laboratory Animals”41 and involved injection of AAV vectors into the portal vein of mice. Hem-A mice were injected with the AAV vectors encoding canine FVIII-HC and FVIII-LC at increasing doses of a 1:1 ratio (3 × 1010, 3 × 1011, or 9 × 1011 vg/mouse). Hem-A/Rag1 mice were injected with the vectors encoding the human FVIII heavy and light chains. In these experiments mice were injected with 3 × 1011 vg/mouse of each vector alone or a combination of the 2 vectors. Blood samples were collected in sodium citrate from the retro-orbital plexus at biweekly or monthly intervals. Plasma was harvested and stored at -80°C. To assess phenotypic correction, mice were subjected to a tail-clip survival assay.
FVIII assays
The concentration of hFVIII-HC and -LC antigen was determined using chain-specific ELISAs. For the hFVIII-HC ELISA, Nunc maxisorp (Nalge Nunc International, Rochester, NY) plates were coated with 5 μg/mL GMA012 (Green Mountain Antibodies, Burlington, VT). Samples and standards were diluted in phosphate-buffered saline (PBS)/0.05% Tween-20/10% horse serum. Depending on the test sample, standards were spiked with either media or naive mouse plasma at the same concentration as the diluted samples. Samples and standards (100 μL/well) were incubated at room temperature for 2 hours. After washing, a horseradish peroxidase (HRP)–conjugated antibody ESH5-HRP (100 μL/well, 3 μg/mL; American Diagnostica, Greenwich, CT) was added, and the plates were incubated for 2 hours at room temperature. The HRP enzyme was conjugated using EZ-link Plus Activated labeling kit (Pierce, Rockford, IL). After a final wash the antigen was detected using o-phenylenediamine dihydrochloride peroxidase substrate (Sigma Chemical, St Louis, MO) and the absorbance read at 490 nm. The hFVIII-LC ELISA was used as described previously.19 For both ELISAs, the standard used was Refacto, recombinant B-domain–deleted FVIII.
To measure cFVIII antigen, a commercially available hFVIII-LC ELISA (Asserachrom VIIIC: Ag; Diagnostico Stago, Parsippany, NJ), which crossreacts with cFVIII-LC, was used. Normal canine plasma was used as the standard (0.625-20 ng/mL). Otherwise, the ELISA was performed according to the manufacturer's instructions.
Biologically active FVIII in media and plasma was measured using the Coatest assay (Dipharma) as previously described.19 Refacto (Genetics Institute, Cambridge, MA) was used as the standard. The specific activity of the expressed FVIII was calculated by dividing the activity value derived from the Coatest by the antigen value derived from either the FVIII-LC or -HC ELISA. Anti-FVIII inhibitory antibodies were measured using the Bethesda assay.25
Immunoprecipitation and Western analysis of human HC and LC polypeptides
For the immunoprecipitation of FVIII from plasma, the volume (10 μL) was adjusted to 500 μL with PBS/2% protease inhibitor cocktail (Sigma Chemical)/0.1 mg/mL phenylmethanesulfonyl fluoride (PMSF). Samples were precleared with 50 μL protein-G beads (Life-Tech, Stafford, TX) for 2 hours at 4°C. Selective immunoprecipitation of LC or HC polypeptides was accomplished by dissociating the chains using 20 mM EDTA (ethylenediaminetetraacetic acid) and immunoprecipitation with chain-specific antibodies. Then, 10 μg of each LC antibody (ESH2 and ESH8) and 20 μg of the HC antibody (ESH5) (American Diagnostica) were prebound to 100 μL protein-G beads, crosslinked as described,26 and resuspended in 0.5 mL PBS/0.02% NaN3. Precleared samples were incubated with crosslinked beads (50 μL) and immunoprecipitated overnight at 4°C. The beads were washed sequentially in PBS containing 1%, 0.1%, and 0.05% Triton X-100. Immunoprecipitated FVIII was eluted in 1 × sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer and analyzed by 8% SDS-PAGE and Western blotting. After blocking the membrane overnight in PBS/5% skimmed milk powder/0.3% Tween-20, a sheep anti-hFVIII antibody, SAF8C-AP (1:1000 dilution; Affinity Biologicals, Ancaster, ON, Canada), was added and incubated for one hour at room temperature. The membrane was washed, incubated with a rabbit antisheep antibody (1:2000 dilution; Zymed, South San Francisco, CA), and developed using an enhanced chemiluminescent substrate (ECL; Amersham-Pharmacia Biotech, Piscataway, NJ). For analysis of intracellular protein, liver tissue (50 mg) was homogenized in lysis buffer (150 mM NaCl, 50 mM Tris [tris(hydroxymethyl)aminomethane, pH 7.5], 0.05% SDS, 1% nonidet P-40 [NP40], 0.1 mM PMSF, and 2% Sigma Chemical's protease inhibitor cocktail), incubated on ice for 10 minutes, and then centrifuged at 10 000g for 5 minutes. The supernatant was retained and immunoprecipitated as described for plasma.
DNA and RNA analysis of human and canine FVIII transgenes
DNA was extracted from liver tissue and analyzed by Southern blotting27 to determine copy number. For the hFVIII-LC copy number determination, 10 μg genomic DNA was digested with PstI, separated on a 1% agarose gel, Southern blotted, and hybridized with a 32P-labeled 499-bp PCR fragment encoding the A3/C1 junction of the mature hFVIII protein. For the determination of the hFVIII-HC copy number, the DNA was digested with HindIII, Southern blotted, and hybridized with a 642-bp HindIII probe encoding a portion of the A1 domain. Copy number controls were generated by spiking naive mouse liver DNA, digested with either PstI or HindIII, with HC or LC plasmid DNA digested with the same enzymes, at ratios of 10, 5, 1.0, 0.1, and 0.01 copies per diploid genome. The hybridized membranes were analyzed using a Storm 860 PhosphoImager (Molecular Devices, Sunnyvale, CA) and the copy numbers determined using IMAGE-QUANT SOFTWARE (Molecular Devices).
For the cFVIII-HC or -LC copy number determinations, 10 μg genomic DNA was digested with EcoRI, separated on a 1% gel, and hybridized with a 32P-labeled 1707-bp EcoRI fragment from cFVIII-HC or a 32P-labeled 1214-bp BglII/EcoRI cFVIII-LC fragment.
RNA copies were determined by RT-QPCR using 10 ng total RNA. The standards used were in vitro–transcribed single-chain human and canine FVIII RNAs. Primers and probes to both the human and canine HC and LC sequences were used. The human primer and probe sets are as follows: human FVIII-HC forward primer, 5′ TCATTGGCCCTCTCCTCATCT 3′ and hFVIII-HC reverse primer, 5′ TCTGTGAGGTACCAGCTTCGG 3′; hFVIII-HC probe, 5′ 6-FAM-TCTGTAGATCAAAGAGGAAACCAGATAATGTCAGACA-TAMRA 3′; hFVIII-LC forward primer, 5′ CAATGGCTACATAATGGATACACTACCT 3′ and hFVIII-LC reverse primer, TGTCCACTGAAATGAATAGAATGGAT; and hFVIII-LC probe, 5′ 6-FAM-CTGCCCATGCTGAGCAGATACCATCG-TAMRA 3′.
RNA fluorescence in situ hybridization (FISH) analysis of canine FVIII
RNA in situ hybridization was performed as described.28 In brief, liver tissue was harvested and mounted in Optimal Cutting Temperature Compound (Sakura Finetek USA, Torrance, CA). Sections (12 μm) were generated using a cryostat and fixed in 4% formaldehyde/PBS. After washing with PBS and 2 × SSC (0.5 M sodium chloride, 0.05 M sodium citrate, pH 7.0), the sections were treated with 3% H2O2 at room temperature for 20 minutes. Sections were then washed in nuclease-free H2O and dehydrated sequentially in 70%, 95%, and 100% ethanol. The following HRP-conjugated canine chain-specific antisense probes were designed: cFVIII-HC probe (5′GAGCTGTCGTCATCAAAGCTAACT ACGTCC 3′); cFVIII-LC probe cocktail (probe 1: 5′GAGGCCCTGATTTTCATAGTC GCCGTAGAT3′; probe 2: 5′ATTGCAGGGAGCTCTACA GTTCCTTTCCAG3′) (Epoch Biosciences, Bothell, WA). Sections were first hybridized with the cFVIII-HC probe, and the hybrids were detected as a green signal with tyramide signal amplification (TSA)–fluorescein isothiocyanate (FITC) (Perkin Elmer, Boston, MA). A consecutive hybridization was performed on the same sections with the cFVIII-LC probe, and the hybridization signal was detected as a red signal using TSA-Alexa 554 (Molecular Probes, Eugene, OR). Sections were mounted in Prolong antifade (Molecular Probes) and examined using a Zeiss Axioskop fluorescence microscope (Carl Zeiss, Thornwood, NY). Images were recorded using a Quips imaging system and software (Applied Imaging, Santa Clara, CA). The transduction efficiency was determined after counting at least 2000 nuclei per section.
Results
In vitro production of biologically active FVIII
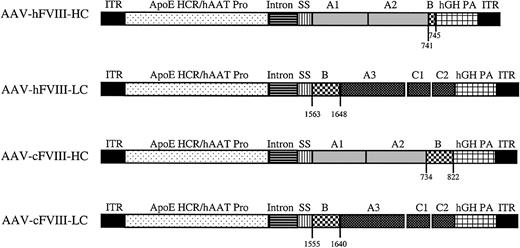
Previously we demonstrated that biologically active hFVIII can be produced following coinjection of normal mouse liver with hFVIII-HC and -LC vectors using a ubiquitous promoter.19 In the present study, we further optimized this approach by designing AAV human and canine FVIII-HC and -LC vectors that use liver-specific regulatory elements (the apolipoprotein E hepatic control region [HCR] and the human alpha one antitrypsin [hATT] promoter) (Figure 1) and evaluated these vectors in the Hem-A mouse model.
Map of AAV human and canine FVIII heavy- and light-chain vectors (AAV-hFVIII-HC, AAV-hFVIII-LC, AAV-cFVIII-HC, and AAV-cFVIII-LC). The regulatory regions in each vector are as follows: ITR indicates AAV inverted terminal repeat; ApoE-HCR, apolipoprotein E hepatic control region, hAAT, human alpha one antitrypsin promoter; intron, CMV-β-globin chimeric intron; hGH PA, human growth hormone poly A. The human heavy chain is composed of the A1 and A2 domains preceded by the FVIII signal sequence, SS. The hFVIII-HC is followed by 5 aa (741-745) of the B-domain. The cFVIII-HC construct is identical except in addition to the regulatory and FVIII elements in the human construct, the cFVIII-HC construct is followed by 89 aa of the upstream B-domain (734-822). The human and canine light chain constructs are preceded by 86 aa of the B-domain, then the A3, C1, and C2 regions.
Map of AAV human and canine FVIII heavy- and light-chain vectors (AAV-hFVIII-HC, AAV-hFVIII-LC, AAV-cFVIII-HC, and AAV-cFVIII-LC). The regulatory regions in each vector are as follows: ITR indicates AAV inverted terminal repeat; ApoE-HCR, apolipoprotein E hepatic control region, hAAT, human alpha one antitrypsin promoter; intron, CMV-β-globin chimeric intron; hGH PA, human growth hormone poly A. The human heavy chain is composed of the A1 and A2 domains preceded by the FVIII signal sequence, SS. The hFVIII-HC is followed by 5 aa (741-745) of the B-domain. The cFVIII-HC construct is identical except in addition to the regulatory and FVIII elements in the human construct, the cFVIII-HC construct is followed by 89 aa of the upstream B-domain (734-822). The human and canine light chain constructs are preceded by 86 aa of the B-domain, then the A3, C1, and C2 regions.
These liver-specific elements allowed high levels of FIX expression in vivo.20 The FVIII constructs were evaluated for FVIII activity following transduction of HepG2 cells. When the HC or LC vectors were used alone, no FVIII activity was detected (Table 1); however, cotransduction with HC and LC vectors resulted in 220 ± 8 mU/106 cells/24 hours for the hFVIII vectors and 1966 ± 37 mU/106 cells/24 hours for the cFVIII vectors. This difference in activity between the human and canine vectors is consistent with the 7-fold difference in specific activity that we have observed between human FVIII (5000 U/mg) and canine FVIII (35 000 U/mg). Another significant observation was that higher levels of hFVIII-LC (92 ± 8 ng/106 cells/24 hours) were observed compared with hFVIII-HC (28 ± 4 ng/106 cells/24 hours) antigen in the media of the cotransduced cells.
In vivo production of biologically active canine FVIII
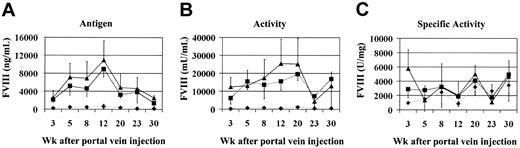
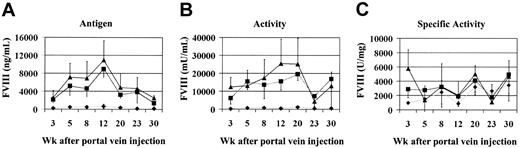
To test the efficacy of this approach in vivo we coinjected vectors encoding the canine heavy and light chains of FVIII into the portal veins of Hem-A mice. There were 3 groups of 6 animals injected with increasing doses of AAV-cFVIII-HC and AAV-cFVIII-LC vectors at a 1:1 ratio (3 × 1010, 3 × 1011, or 9 × 1011 vg/mouse). FVIII activity and FVIII-LC antigen levels were measured at various time points. Prior to these experiments, we determined, using the Coatest and hFVIII as a standard, that the normal activity of cFVIII is 7000 mU/mL. We also determined, using an hFVIII-LC ELISA that crossreacts with cFVIII and hFVIII as a standard, that the normal FVIII-LC antigen level in canine plasma is 200 ng/mL. Therefore, unlike hFVIII, which has a specific activity of 5000 U/mg, the specific activity of cFVIII is 35 000 U/mg relative to a hFVIII standard. Superphysiologic levels of cFVIII-LC antigen and FVIII activity were generated in animals injected with the 2 highest vector doses (Figure 2A-B).
Efficacy of AAV-cFVIII vectors in vivo. Hem-A mice were injected via the portal vein with either 3 × 1010 (♦), 3 × 1011 (▪), or 9 × 1011 (▴) vg/mouse of both AAV-cFVIII-HC and AAV-cFVIII-LC. Mice were bled periodically via the retro-orbital plexus; plasma was harvested and analyzed by ELISA for cFVIII-LC antigen expression (A) and by Coatest for FVIII activity (B). The specific activity was calculated based on the determined activity and LC antigen levels (C). In normal cFVIII plasma, the antigen and activity levels are 200 ng/mL and 7000 mU/mL, respectively. Error bars indicate standard deviation.
Efficacy of AAV-cFVIII vectors in vivo. Hem-A mice were injected via the portal vein with either 3 × 1010 (♦), 3 × 1011 (▪), or 9 × 1011 (▴) vg/mouse of both AAV-cFVIII-HC and AAV-cFVIII-LC. Mice were bled periodically via the retro-orbital plexus; plasma was harvested and analyzed by ELISA for cFVIII-LC antigen expression (A) and by Coatest for FVIII activity (B). The specific activity was calculated based on the determined activity and LC antigen levels (C). In normal cFVIII plasma, the antigen and activity levels are 200 ng/mL and 7000 mU/mL, respectively. Error bars indicate standard deviation.
For example, at the 12-week time point animals injected with 3 × 1011 vg secreted an average of 8952 ng/mL cFVIII-LC antigen, resulting in FVIII activity levels of 15 854 mU/mL. At this same time point, the 3 × 1010 vg group had considerably lower levels of both antigen and activity (622 ng/mL, 454 mU/mL), but these activity levels (6.5%) were still in the therapeutic range, that is, more than 1% of normal cFVIII levels. At all doses, more FVIII-LC antigen was observed in the plasma than would have been expected based on the FVIII activity, suggesting that the specific activity of the protein is lower than normal. However, given the absence of a cFVIII-HC ELISA, the specific activity for the expressed cFVIII was based only on the circulating LC levels. Following administration of 3 × 1011 vg/mouse, FVIII-specific activity measurements ranged from 900 to 6000 U/mg (Figure 2C), which is lower than that of normal canine plasma (35 000 U/mg).
High levels of both FVIII antigen and activity were sustained for more than 30 weeks after vector injection, although a decrease from peak levels was observed. This decrease in expression was associated with the development of inhibitory antibodies (1-5 Bethesda units [BUs]) in some animals (6/8 mice) (data not shown). To determine whether these levels of cFVIII could correct the bleeding phenotype in the animals, we performed a tail-clip survival assay 12 weeks after injection. All the mice in the treated groups survived the tail clipping, whereas no mice in the control group survived (Table 2). These data demonstrate that biologically active FVIII can be produced in vivo following delivery of 2 AAV vectors encoding the heavy and light chains of cFVIII, resulting in correction of the bleeding phenotype.
Biochemical analysis of secreted human FVIII in vivo
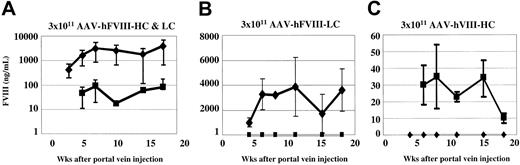
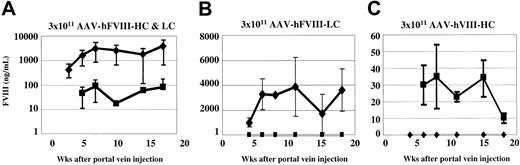
Our in vitro data demonstrated that approximately 5-fold more LC antigen was secreted compared with the HC antigen. To determine whether there was a similar chain imbalance between the secretion of the 2 chains in vivo, we performed ELISA and immunoprecipitation analysis on plasma from Hem-A/Rag1 mice that were injected with AAV-hFVIII-HC and -LC vectors. Human FVIII vectors were chosen for this analysis as the techniques for qualitative and quantitative analysis of the hFVIII proteins, not the cFVIII proteins, were developed. In this experiment, 3 groups of animals were injected with 3 × 1011 vg/mouse of both vectors or with the individual vectors alone. Among the animals injected with both vectors, FVIII activity ranged between less than 5% (50 mU/mL) and 55.9% (559 mU/mL) of normal, with 4 of 6 animals having levels higher than 50 mU/mL, the sensitivity of the assay (Table 3). These levels are lower than those seen when Hem-A animals were injected with the cFVIII constructs. We believe the reason for this is 2-fold. The first is that the specific activity of cFVIII is 7-fold higher than hFVIII, and second, we have previously observed up to 5-fold lower levels of expression in animals with the Rag1 phenotype compared with immunocompetent animals (C.D.S., unpublished data, January 2001).
ELISA analysis of plasma from coinjected animals demonstrated considerably higher levels (average of 25- to 100-fold) of hFVIII-LC antigen compared with hFVIII-HC antigen (Figure 3A). This discrepancy between FVIII-HC and -LC expression was also observed by comparing the plasma levels achieved in animals injected with a single vector (Figure 3B-C). Using the hFVIII-LC antigen to determine the specific activity we calculate values up to 348 U/mg (7% of normal). In contrast, when the hFVIII-HC antigen was used to calculate the specific activity, values up to 6969 U/mg (139% of normal) were determined.
Comparison of human heavy- and light-chain antigen expression in vivo. Hem-A/Rag1 mice were injected with 3 × 1011 vg/mouse of both AAV-hFVIII-HC and AAV-hFVIII-LC (A), AAV-hFVIII-LC (B), and AAV-hFVIII-HC (C). Mice were bled periodically, plasma was harvested, and FVIII heavy-chain (▪) and light-chain (♦) antigen levels were measured by chain-specific ELISAs. Error bars indicate standard deviation.
Comparison of human heavy- and light-chain antigen expression in vivo. Hem-A/Rag1 mice were injected with 3 × 1011 vg/mouse of both AAV-hFVIII-HC and AAV-hFVIII-LC (A), AAV-hFVIII-LC (B), and AAV-hFVIII-HC (C). Mice were bled periodically, plasma was harvested, and FVIII heavy-chain (▪) and light-chain (♦) antigen levels were measured by chain-specific ELISAs. Error bars indicate standard deviation.
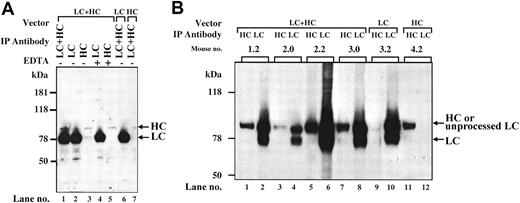
Immunoprecipitation analysis of pooled plasma from coinjected Hem-A/Rag1 mice with monoclonal antibodies to the hFVIII-HC and hFVIII-LC revealed a large excess of the LC peptide (78 kDa) compared with the HC peptide (90 kDa) (Figure 4A, lane 1), confirming the ELISA results. The magnitude of the difference cannot be accounted for by the minor difference (2- to 3-fold) in the immunoprecipitation efficiency between the HC and LC antibodies (data not shown). The LC band is of the expected size indicating that the cleavage site at amino acid 1648 is recognized and cleaved efficiently. When the same plasma was immunoprecipitated with only an anti–hFVIII-LC antibody, an intense 78-kDa band and a faint 90-kDa (HC) band were observed. However, when an anti–hFVIII-HC antibody was used, faint bands at the positions of the HC (90 kDa) and LC (78 kDa) were observed. These data suggest that the majority of the LC in the plasma is not associated with HC. However, the HC that is secreted appears to be complexed with the LC. When EDTA was included in the immunoprecipitations, the dissociated HC and LC peptides were precipitated at levels similar to that seen in the absence of EDTA (Figure 4A, lanes 4-5). These data demonstrate that following coinjection of AAV-hFVIII-HC and AAV-hFVIII-LC vectors, the HC and LC peptides are properly processed and associated, however, a large excess of inactive, noncomplexed LC antigen is produced.
Characterization of hFVIII expressed in vivo. (A) Immunoprecipitation from plasma. At 6 weeks after vector injection, plasma from Hem-A/Rag1 mice was harvested and pooled. Of each plasma pool, 10 μL was brought up to 500 μL with PBS. Immunoprecipitation was performed as described in “Materials and methods.” Lanes 1-5: plasma from LC- and HC-treated animals. Lane 6: plasma from AAV-hFVIII-LC-alone–treated animals. Lane 7: plasma from AAV-hFVIII-HC-alone–treated animals. The immunoprecipitating antibodies used were as follows: lane 1, LC and HC antibodies; lane 2, LC antibody; lane 3, HC antibody; lane 4, LC antibody and 20 mM EDTA; lane 5, HC antibody and 20 mM EDTA; lane 6-7, HC and LC antibodies. (B) Immunoprecipitation from liver lysates. At 18 weeks after vector infusion, liver lysates were prepared from 50 mg tissue harvested from Hem-A/Rag1 mice and FVIII was immunoprecipitated as described in “Materials and methods.” Mice 1.2, 2.0, 2.2, and 3.0 were coinjected with 3 × 1011 vg/mouse of AAV-hFVIII-HC and AAV-hFVIII-LC; mice 3.2 and 4.2 were injected with 3 × 1011 vg/mouse of AAV-hFVIII-LC and AAV-hFVIII-HC, respectively. Samples in lanes 1, 3, 5, 7, 9, and 11 were immunoprecipitated with an anti–hFVIII-HC antibody, while lanes 2, 4, 6, 8, 10, and 12 were immunoprecipitated with an anti–hFVIII-LC antibody.
Characterization of hFVIII expressed in vivo. (A) Immunoprecipitation from plasma. At 6 weeks after vector injection, plasma from Hem-A/Rag1 mice was harvested and pooled. Of each plasma pool, 10 μL was brought up to 500 μL with PBS. Immunoprecipitation was performed as described in “Materials and methods.” Lanes 1-5: plasma from LC- and HC-treated animals. Lane 6: plasma from AAV-hFVIII-LC-alone–treated animals. Lane 7: plasma from AAV-hFVIII-HC-alone–treated animals. The immunoprecipitating antibodies used were as follows: lane 1, LC and HC antibodies; lane 2, LC antibody; lane 3, HC antibody; lane 4, LC antibody and 20 mM EDTA; lane 5, HC antibody and 20 mM EDTA; lane 6-7, HC and LC antibodies. (B) Immunoprecipitation from liver lysates. At 18 weeks after vector infusion, liver lysates were prepared from 50 mg tissue harvested from Hem-A/Rag1 mice and FVIII was immunoprecipitated as described in “Materials and methods.” Mice 1.2, 2.0, 2.2, and 3.0 were coinjected with 3 × 1011 vg/mouse of AAV-hFVIII-HC and AAV-hFVIII-LC; mice 3.2 and 4.2 were injected with 3 × 1011 vg/mouse of AAV-hFVIII-LC and AAV-hFVIII-HC, respectively. Samples in lanes 1, 3, 5, 7, 9, and 11 were immunoprecipitated with an anti–hFVIII-HC antibody, while lanes 2, 4, 6, 8, 10, and 12 were immunoprecipitated with an anti–hFVIII-LC antibody.
Biochemical analysis of intracellular hFVIII in vivo
To further evaluate the low levels of hFVIII-HC, we analyzed liver lysates from animals injected with either vector alone or both the AAV-hFVIII-HC and AAV-hFVIII-LC vectors together. EDTA was included in all the immunoprecipitation reactions to dissociate the heavy and light chains. This was necessary to distinguish the heavy and light chains in the coinjected animals, as the primary FVIII-LC translation product, which contains 86 aa of the carboxy terminal of the B-domain, migrates on an SDS-PAGE gel at 90 kDa, which is similar to the size of the FVIII-HC. Upon intracellular cleavage of the primary translation product at amino acid 1648, a FVIII-LC peptide of 78 kDa is produced.2 By including EDTA in the reaction, the HC antibody precipitates only the 90-kDa FVIII-HC peptide and the LC antibody precipitates both, the 90- and 78-kDa FVIII-LC peptides. This could be seen when FVIII from the liver of 4 coinjected animals was immunoprecipitated with either the HC or LC antibody (Figure 4B, lanes 1-8). In addition, it is clear that the LC is more abundant in liver lysates of coinjected animals, compared with the HC. Animals injected with the LC vector alone produced both forms of the LC (Figure 4B, lanes 9-10), while animals injected with the HC vector alone produced a 90-kDa HC polypeptide in the liver (Figure 4B, lanes 11-12). The combined data demonstrate that the nonstoichiometric levels of FVIII-HC and -LC observed in the plasma of coinjected animals correlate with lower intracellular levels of the FVIII-HC.
Molecular analysis of vector DNA and RNA in vivo
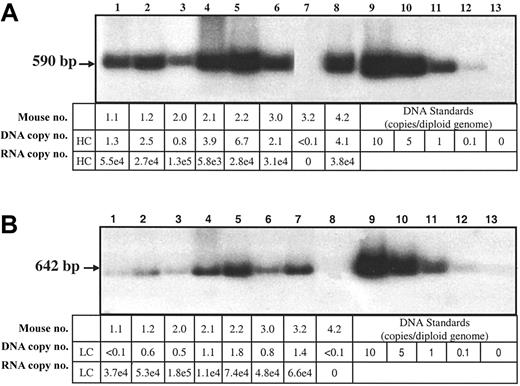
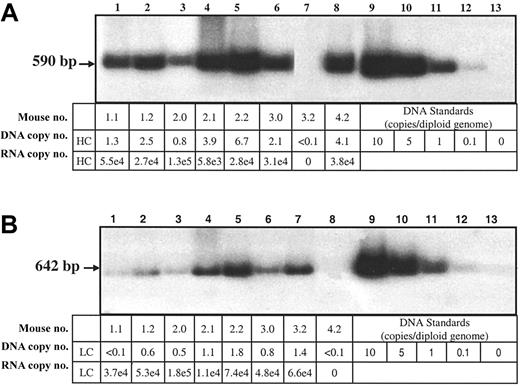
In order to determine if differential gene transfer could account for the differences in HC and LC protein in liver lysates, the copy numbers of the hFVIII-HC and hFVIII-LC transgenes in the coinjected Hem-A/Rag1 mice were determined by Southern blot analysis. Mice injected at a dose of 3 × 1011 vg of each vector were killed 18 weeks after injection, and DNA was extracted from the liver. The copy number of the hFVIII-HC transgene in 4 coinjected animals was greater than that of the hFVIII-LC transgene by 1.6- to 4-fold (Figure 5A-B).
Southern and RT-QPCR analysis of transduced livers. Liver DNA from Hem-A/Rag1 mice was extracted 18 weeks after portal vein injection with 3 × 1011 vg AAV-hFVIII-HC and AAV-hFVIII-LC (samples 1.1, 1.2, 2.0, 2.1, 2.2, 3.0), AAV-hFVIII-LC (sample 3.2), or AAV-hFVIII-HC (sample 4.2). For the hFVIII-HC copy number determination, the DNA (10 μg) was digested with HindIII (A), while for the light chain copy number the DNA was digested with PstI (B). 32P-random primer labeled heavy- and light-chain probes were used for the respective blots. Heavy- and light-chain RNA levels were determined by RT-QPCR using heavy- and light-chain–specific primers and probes. The DNA copy numbers are expressed as copies per diploid genome. The RNA levels are expressed as copies/10 ng RNA and have been normalized to 1 DNA copy/cell by dividing by the DNA copy number that was determined by Southern blot analysis.
Southern and RT-QPCR analysis of transduced livers. Liver DNA from Hem-A/Rag1 mice was extracted 18 weeks after portal vein injection with 3 × 1011 vg AAV-hFVIII-HC and AAV-hFVIII-LC (samples 1.1, 1.2, 2.0, 2.1, 2.2, 3.0), AAV-hFVIII-LC (sample 3.2), or AAV-hFVIII-HC (sample 4.2). For the hFVIII-HC copy number determination, the DNA (10 μg) was digested with HindIII (A), while for the light chain copy number the DNA was digested with PstI (B). 32P-random primer labeled heavy- and light-chain probes were used for the respective blots. Heavy- and light-chain RNA levels were determined by RT-QPCR using heavy- and light-chain–specific primers and probes. The DNA copy numbers are expressed as copies per diploid genome. The RNA levels are expressed as copies/10 ng RNA and have been normalized to 1 DNA copy/cell by dividing by the DNA copy number that was determined by Southern blot analysis.
RNA levels were also evaluated using quantitative RT-PCR analysis. When normalized for gene copy, similar levels of hFVIII-HC and hFVIII-LC RNA were observed (Figure 5A-B). Thus, the lower levels of hFVIII-HC protein are not due to less efficient gene transfer or lower steady-state levels of RNA transcripts.
Gene transfer and RNA expression were also analyzed in Hem-A mice that were injected with the canine vectors at a dose of 3 × 1011 vg each. Similar levels of gene transfer and RNA expression were seen for both the AAV-cFVIII-HC and -LC vectors (data not shown), again ruling out gene transfer or inefficient transcription of FVIII-HC as contributing to the lower levels of FVIII-HC protein.
Histologic analysis of cFVIII in vivo
FISH analysis was used to evaluate the level of gene transfer and the frequency of cotransduction in the livers of Hem-A mice injected with 3 × 1011 vg of the AAV-cFVIII-HC and AAV-cFVIII-LC vectors. Liver tissue was harvested 18 weeks after vector administration and analyzed by in situ hybridization for RNA synthesis (Figure 6A-D).
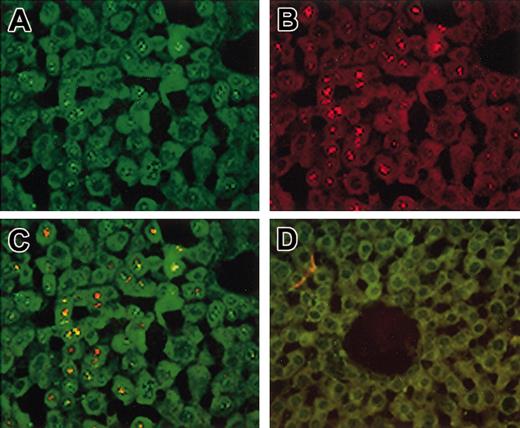
Cotransduction of hepatocytes in vivo. Hem-A mice were injected with 3 × 1011 vg/mouse of both AAV-cFVIII-HC and AAV-cFVIII-LC. Treated and naive livers were harvested 15 weeks after injection and analyzed by FISH for RNA expression (A-D). Sections were probed sequentially with a heavy-chain–specific probe (green; A) and then with a cocktail of 2 light chain–specific probes (red; B). Colocalization of both transcripts is indicated by the yellow staining (yellow; C). No signal was seen in sections from untreated mice (D). Original magnification, ×40.
Cotransduction of hepatocytes in vivo. Hem-A mice were injected with 3 × 1011 vg/mouse of both AAV-cFVIII-HC and AAV-cFVIII-LC. Treated and naive livers were harvested 15 weeks after injection and analyzed by FISH for RNA expression (A-D). Sections were probed sequentially with a heavy-chain–specific probe (green; A) and then with a cocktail of 2 light chain–specific probes (red; B). Colocalization of both transcripts is indicated by the yellow staining (yellow; C). No signal was seen in sections from untreated mice (D). Original magnification, ×40.
The quantitative data presented here were obtained by analysis of multiple sections from a single animal, which had similar copy numbers of the 2 transgenes. Using an antisense probe specific for the HC, we observed approximately 42% positive hepatocytes (Figure 6A). When the antisense LC-specific oligonucleotide was used, 18% of cells were positive (Figure 6B). This difference in staining between the HC and LC signal was demonstrated to be due to differences in sensitivity between the 2 probes (data not shown). When liver sections were hybridized with both probes, 47% of the cells stained positive for HC and/or LC RNA (Figure 6C), and cotransduction was determined in 12% of total hepatocytes, or 26% of all transduced hepatocytes. No signal was seen in sections from naive mice hybridized with the antisense probes (Figure 6D). Similarly, no signal was seen in livers from injected mice hybridized with the sense probes, or pretreated with RNAse and hybridized with the antisense probes (data not shown), confirming that the signal was due to RNA.
Discussion
Previously we demonstrated in normal mice that coinjection of AAV vectors encoding the HC and LC of FVIII resulted in the secretion of biologically active FVIII.19 In this study, we made use of the Hem-A mouse model to confirm and extend our earlier observations. Following coinjection of Hem-A mice with liver-specific AAV-cFVIII-HC and -LC vectors at a dose of 3 × 1011 vg/mouse, peak plasma LC antigen levels up to 10 560 ng/mL were observed 12 weeks after injection, and activity levels in excess of 200% of normal were seen. Using the cFVIII-LC antigen levels detected using an LC ELISA and the activity levels determined using the Coatest assay to calculate the specific activity, we found that the specific activity of cFVIII in the plasma of coinjected animals was considerably lower than normal, which we have calculated to be 35 000 U/mg. In this study, the specific activity of cFVIII ranged from 870 to 5779 U/mg (2.4%-16.5% normal), implying that a large excess of inactive protein is secreted. When AAV-hFVIII-HC and -LC vectors were coinjected into Hem-A/Rag1 mice, therapeutic levels of FVIII were achieved (up to 60% of normal at 6 weeks). However, chain-specific ELISAs demonstrated that 25- to 100-fold more FVIII-LC was produced compared with FVIII-HC. When the LC antigen was used to calculate the specific activity, the expressed hFVIII had a lower-than-normal value (up to 7% of normal). In contrast, when the hFVIII-HC was used to calculate the specific activity, near-normal levels (up to 139% of normal) were determined. These data suggest that the active FVIII complex formed using this approach has a specific activity similar to that of normal hFVIII. Presumably, this chain imbalance also exists for the cFVIII.
There are a number of reasons that could account for this chain imbalance, including differences in vector gene transfer, transgene transcription, and/or protein translation and secretion. Using biochemical techniques designed for hFVIII, we demonstrated that posttranscriptional events contribute to the chain imbalance observed. A sensitive immunoprecipitation technique demonstrated that hFVIII heavy and light chains of the expected size are secreted into the bloodstream of Hem-A mice and that these chains associate in a metal ion-dependent manner, as has been noted for wild-type FVIII. However, unlike wild-type FVIII, the chains are found in a nonstoichiometric ratio, with an excess of LC when compared with HC as determined by chain-specific ELISAs. This chain imbalance also appears to exist intracellularly, suggesting that the difference in plasma levels is not simply due to enhanced secretion or plasma stability of the LC. Molecular analysis demonstrated that the lower levels of hFVIII-HC protein were not the result of less efficient gene transfer of the AAV-hFVIII-HC vector or lower steady-state hFVIII-HC mRNA levels. Thus, the observed chain imbalance is likely due to inefficient translational or posttranslational processing, and/or intracellular trafficking of the HC polypeptide. Problems in the production of wild-type FVIII in CHO cells have also been observed and have been correlated with binding of FVIII to the immunologic binding protein (BiP).29 Mutagenesis of the BiP binding site resulted in improved FVIII secretion in vitro.30 Whether this mutation could lead to improved FVIII secretion in vivo remains to be determined. Our ability to express the FVIII heavy and light chains separately in the livers of mice has allowed us to confirm in vivo the problems with FVIII expression that have been elucidated in tissue culture2 and have identified the expression of the HC as a problem. This will undoubtedly provide valuable insights toward improving the expression of single-chain FVIII as well.
One strategy that we used to address the chain imbalance was to reduce the amount of LC vector while keeping the HC vector constant. We demonstrated in vitro that the input LC vector could be reduced up to 5-fold without decreasing FVIII activity (data not shown). However, this finding was not observed when a similar approach was attempted in vivo. A more promising strategy may be to maintain a 1:1 ratio of vectors to ensure high levels of cotransduction and modify the strength of the promoters to yield stoichiometric secretion of the chains.
Another interesting observation from these studies was the high levels of transduction and cotransduction in vivo. RNA FISH analysis on livers of mice injected with the cFVIII vectors demonstrated that 18% to 42% of all hepatocytes were transduced and that 12% of all hepatocytes (or 26% of transduced hepatocytes) were cotransduced. These levels are significantly higher than those previously published, where the percentage of transduced hepatocytes did not exceed 10% using similar vector doses.31,32 The reason for this difference is not clear.
Production of biologically active FVIII and correction of the bleeding disorder required coinjection of AAV vectors encoding the FVIII-HC and FVIII-LC, and was not seen in animals that were injected with only one vector (data not shown). It has been suggested that the HC of FVIII is expressed in the livers of Hem-A mice33 that have an insertion in exon 16, which encodes part of the LC. Thus, it was theoretically possible that injection of these animals with the LC vector alone might complement the FVIII deficiency. We have shown in vitro that human, canine, and murine heavy and light chains can cross-complement and produce active FVIII (data not shown). Injection of AAV-FVIII-LC vector alone was unable to correct the Hem-A phenotype in these mice. Had this approach been successful, its use for the subset of human Hem-A patients that contains an exon 22 inversion may have been considered. The mouse data suggest that expression of only the LC in this patient population is unlikely to correct the hemophilia A phenotype.
Recombinant AAV (rAAV) vectors have a number of advantages over other gene therapy vectors. However, a major limitation is their packaging capacity, which is approximately 5 kb.34 Several studies have attempted to increase the packaging capacity of rAAV in order to express large genes.35-40 In this study we demonstrate that this limitation can be overcome by using 2 AAV vectors to express discrete functional domains of this protein. Using this strategy, we demonstrated the generation of functional FVIII protein at superphysiologic levels. Furthermore, we showed that this approach could lead to phenotypic correction of the bleeding diathesis in the hemophilic animals. No deleterious effects associated with the nonstochiometric expression of the FVIII heavy and light chains have been observed in Hem-A and normal mice. Thus, AAV vectors can now be considered for the delivery of other large therapeutic proteins using strategies that involve codelivery of 2 AAV vectors.
Prepublished online as Blood First Edition Paper, July 31, 2003; DOI 10.1182/blood-2003-01-0222.
Several of the authors (C.D.S., T.L., A.E.P., S.L.P-W., H.C., H.J., J.V., D.N., S.K.P., J.F.W., A.M., L.B.C.) are employed by Avigen Inc., whose potential product was studied in the present work
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Robin Scheer for help in the preparation of the figures, Gibrail Haniff for the small animal surgery, and Dr Alejandra Arbetman for reviewing the manuscript.