Abstract
Lymphocyte microvilli mediate initial rolling-adhesion along endothelium but are lost during transmigration from circulation to tissue. However, the mechanism for resorption of lymphocyte microvilli remains unexplored. We show that chemokine stimulation of human peripheral blood T (PBT) cells is sufficient to induce rapid resorption of microvilli. Microvilli in other cells are regulated by ezrin/radixin/moesin (ERM) proteins, which link the plasma membrane to the cortical F-actin cytoskeleton; maintenance of these linkages requires ERM activation, reflected by phosphorylation at a specific carboxy-terminal threonine residue. Carboxyphosphorylated-ERM (cpERM) proteins in resting PBT cells show a punctate peripheral distribution consistent with localization to microvilli. cpERM dephosphorylation begins within seconds of stimulation by chemokines (stromal derived factor 1α [SDF-1α] or secondary lymphoid tissue cytokine), and ERM proteins lose their punctate distribution with kinetics paralleling the loss of microvilli. The cpERM proteins are preferentially associated with the cytoskeleton at rest and this association is lost with chemokine-induced dephosphorylation. Transfection studies show that a dominant-negative ERM construct destroys microvilli, whereas a construct mimicking cpERM facilitates formation of microvilli, retards chemokine-induced loss of microvilli, and markedly impairs chemokine-induced polarization. Thus, chemokine induces rapid dephosphorylation and inactivation of cpERM, which may in turn facilitate 2 aspects of cytoskeletal reorganization involved in lymphocyte recruitment: loss of microvilli and polarization.
Introduction
The microvilli of circulating lymphocytes play an important role in the specialized adhesion cascade1-6 that mediates emigration from circulation to tissue during normal immunosurveillance and in response to inflammation. The initial contact and rolling of lymphocytes along endothelial cells uses adhesion receptors that are selectively concentrated at the tips of microvilli, such as L-selectin and very late activation antigen 4 (VLA-4).7-10 Rolling lymphocytes can be triggered by chemokines to undergo an abrupt transition to strong, integrin-mediated adhesion,1-6,11 ultimately leading to transmigration across the endothelium. In contrast to L-selectin, the integrin leukocyte function-associated antigen 1 (LFA-1; αLβ2), which often mediates strong adhesion, is confined to nonmicrovillar regions of the membrane.7,9 Such localization of receptors excludes them from initial leukocyte-endothelial interactions.10 Consequently, it has been suggested that microvillus retraction might be important during strong adhesion to allow the cell body of the lymphocyte to contact the endothelium.12 Lymphocyte adhesion to and migration across endothelial cell surfaces in vivo has in fact been shown to involve a loss of microvilli.13,14 The elimination of microvilli is thus expected to increase the effectiveness of activated integrins in mediating lymphocyte adhesion and to facilitate the extended approximation of T-cell/endothelial cell membranes observed during transmigration.
Microvilli are regulated by the widely expressed proteins of the ezrin-radixin-moesin (ERM) family,15-18 of which moesin is particularly important in formation of microvilli in varied cell types, including hematopoietic cells.19 ERM proteins concentrate within peripheral processes and regulate their shape by forming reversible links between the plasma membrane and the cortical cytoskeleton (for reviews, see Gautreau et al,15 Bretscher et al,17 and Tsukita and Yonemura20 ). These links are mediated by the interaction of the ERM C-terminus with F-actin and of its N-terminus (called the FERM domain) with lipid- and membrane-associated proteins.21-26 The majority of cellular ERM proteins exist in a functionally dormant state in which intramolecular association between FERM and the C-terminus masks their binding sites for cytoskeleton and membrane.15,17,20 ERMs become activated by molecular events such as phosphoinositide binding and phosphorylation that decrease inhibitory self-interactions and thereby unmask actin- and membrane-binding sites.21-26 In vitro, phosphorylation of a carboxy-terminal threonine residue conserved among ezrin, radixin, and moesin disrupts intramolecular interactions and exposes the C-terminal actin-binding site.21,23,26 In vivo, carboxy-terminal phosphorylation is thought to maintain the active conformation of ERM proteins that have been “opened” by other factors, particularly by interactions of the lipid phosphatidylinositol 4,5-bisphosphate (PIP2) with the N-terminus.25,27 Accordingly, changes in the levels of carboxy-threonine phosphorylated ERM (cpERM) proteins have been shown to directly correlate with the remodeling of microvilli in many cell types.25,28,29
Because chemokines trigger conversion from rolling adhesion to strong adhesion, we examined whether chemokine stimulation also contributes to remodeling of the microvilli. Using freshly isolated normal human peripheral blood T (PBT) cells, we found that stromal-derived factor 1α (SDF-1α) induces a rapid loss of microvilli even in the absence of adhesion receptor engagement. Moreover, with similar rapid kinetics, SDF-1α induces the dephosphorylation and cytoskeletal dissociation of cpERM proteins. Our transfection studies demonstrate not only that cpERM proteins participate in microvilli, but also that inhibition of their dephosphorylation impedes lymphocyte polarization. We propose that chemokines at the endothelial surface, in addition to activating integrin-mediated adhesion,5,6 facilitate microvillus resorption and polarization during lymphocyte endothelial binding by a mechanism involving ERM dephosphorylation.
Materials and methods
Cells and reagents
PBT cells were isolated by leukapheresis of blood from healthy human volunteers, centrifugation of cells on a lymphocyte separation medium (ICN Biochemical, Aurora, OH) gradient, and immunomagnetic negative selection as previously described.30 The resulting PBT cells (> 95% purity) were suspended in Hanks balanced salt solution without phenol red containing 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) and 0.2% bovine serum albumin (BSA). Cells were either used immediately or held in suspension by rotation at 4°C for up to 24 hours. Cells were placed in polypropylene microcentrifuge tubes at 1 to 3 × 107/mL and warmed to 37°C on a rocking platform for at least 1 hour before use. The following antibody reagents were used for Western blots and immunofluorescence: monoclonal antibody (mAb) 297s (generously provided by Sa Tsukita, Kyoto University, Kyoto, Japan) whose reactivity includes moesin pT558, ezrin pT564, and radixin pT56724 ; phosphoezrin/radixin/moesin (ERM) antibody (Cell Signaling Technology, Beverly, MA); moesin-specific mAb 38/87 (Lab Vision, Fremont, CA); goat polyclonal antiezrin (C-19) and antimoesin (C-15), which react/cross-react with ezrin, moesin, and radixin (Santa Cruz Biotechnology, Santa Cruz, CA); the ezrin-specific mAb clone 18 and the antimoesin mAb clone 38, which recognizes ezrin and moesin, respectively (Transduction Labs, San Jose, CA); antihemagglutinin (HA)–fluorescein isothiocyanate (FITC) “high affinity” (Roche, Indianapolis, IN); and rabbit antiphospho-p44/42 mitogen-activated protein (MAP) kinase (Cell Signaling Technology). Preadsorbed secondary antibodies conjugated with FITC, rhodamine, Cy5, and horseradish peroxidase were obtained from Jackson ImmunoResearch (West Grove, PA). Other reagents included poly-l-lysine (PLL), rhodamine-conjugated phalloidin (Sigma-Aldrich, St Louis, MO); calyculin A, staurosporine, and pertussis toxin (Calbiochem, San Diego, CA); and recombinant human SDF-1α and secondary lymphoid tissue chemokine (SLC; PeproTech, Rocky Hill, NJ).
Cell lysates and Western blots
PBT whole cell lysates were generated by brief centrifugation of cell suspensions, aspiration of supernatants, and direct lysis of pellets in 2 × reducing sample buffer. Samples were vortexed to disrupt cell pellets, boiled for 3 minutes, and homogenized by sonication. Equal sample volumes were resolved by 8% or 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and analyzed by Western blot using enhanced chemiluminescence (ECL; Amersham, Piscataway, NJ). PBT cells were fractionated into soluble and insoluble/cytoskeletal pools using a modification of a previously described extraction procedure using the cationic detergent dodecyl-trimethyl ammonium chloride (DOTMAC; ICN Biochemical), which preserves actin association of cpERM proteins.22 Following treatment with SDF-1α or other agents, cells were pelleted, supernatants discarded, and pellets lysed with 0.5% DOTMAC in a cytoskeleton-stabilizing extraction buffer (0.1 M sodium-piperazine-N, N′-bis(ethanesulfonic acid) [PIPES], 2 M glycerol, 1 mM EGTA [ethylene glycol tetraacetic acid], 1 mM MgSO4, 2.5 mM sodium pyrophosphate, 1 mM sodium orthovanadate, 5 μM phalloidin, 50 nM calyculin A, 500 nM staurosporine, and Complete [Roche] protease inhibitor tablets, pH 6.9). After 5 minutes on ice, lysates were centrifuged at 15 000g for 10 minutes. Supernatants were mixed 1:1 with 2 × reducing sample buffer and boiled. Pellets were resuspended in 2 × reducing sample buffer, sonicated, boiled, and diluted 1:1 with lysis buffer. Soluble fractions were diluted 5-fold relative to insoluble fractions, equal volumes were resolved by SDS-PAGE, and Western blots were performed.
Immunofluorescence and microscopy
Immunofluorescent staining of lymphocytes with mAb 297s was performed using the trichloroacetic acid (TCA; ICN) fixation method described previously.31 PBT cell suspensions at 37°C were aliquoted at 5 × 106 cells/well into prewarmed 24-well tissue-culture plates containing 12-mm round, no. 1 glass coverslips coated with 10 μg/mL PLL. Cells were allowed to settle for 1 minute at 37°C and were then stimulated by adding SDF-1α to a final concentration of 100 ng/mL. Plates were drained by inversion and cells fixed by the addition of ice-cold TCA solution (10% TCA in distilled water). After 20 minutes on ice, samples were washed with phosphate-buffered saline (PBS), permeabilized with 0.2% Triton X-100, and blocked for 1 hour at room temperature in PBS with 10% normal donkey serum. Primary antibodies were added for 1 hour at room temperature in PBS with 10% donkey serum. After washing, fluorophore-conjugated secondary antibodies in PBS with 10% donkey serum were added for 1 hour at room temperature. Coverslips were washed, dried, and mounted using Prolong mounting medium (Molecular Probes, Thornwood, NY). Some samples (Figure 6) were examined using a Zeiss LSM410 laser scanning confocal microscope (Carl Zeiss) with a 100 × 1.4 numerical aperture (NA), planapochromat objective. Digital images were acquired as single approximately 1-μm thick optical sections at the approximate mid-plane of cells. Identical brightness and contrast settings were used for the comparison of 297s/ERM fluorescent intensity before and after SDF-1α stimulation. Other samples (Figures 8, 9) were examined with a Zeiss LSM 510 META equipped with a × 100 planapochromat (NA 1.4) objective lens, using 100 nm/pixel xy-sampling and optical slice thickness of 1.0 μm. Single-plane images were collected below the mid-plane of the cell.
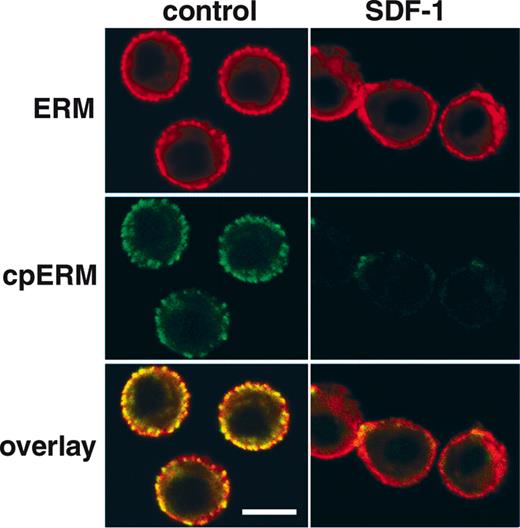
Dephosphorylation and redistribution of PBT cell ERM in response to SDF-1α Confocal micrographs showing the distribution of cpERM (green, mAb 297s) versus total ERM (red, polyclonal antiezrin) in human PBT cells detected by indirect immunofluorescence before and 1 minute after stimulation with 100 ng/mL SDF-1α. Resting cells exhibit a peripheral, punctate distribution of cpERM, colocalized with punctate staining of total ERM protein. Following SDF-1α stimulation, the cpERM signal is lost, as are punctate spots of total ERM staining, indicating ERM T558 dephosphorylation and redistribution. Images are single optical sections 1 μm in thickness acquired at the approximate mid-plane of cells (bar represents 5 μm).
Dephosphorylation and redistribution of PBT cell ERM in response to SDF-1α Confocal micrographs showing the distribution of cpERM (green, mAb 297s) versus total ERM (red, polyclonal antiezrin) in human PBT cells detected by indirect immunofluorescence before and 1 minute after stimulation with 100 ng/mL SDF-1α. Resting cells exhibit a peripheral, punctate distribution of cpERM, colocalized with punctate staining of total ERM protein. Following SDF-1α stimulation, the cpERM signal is lost, as are punctate spots of total ERM staining, indicating ERM T558 dephosphorylation and redistribution. Images are single optical sections 1 μm in thickness acquired at the approximate mid-plane of cells (bar represents 5 μm).
Transfection of dominant-negative ezrin construct into PBT cells ablates surface structures. PBT cells were “nucleofected” with either plasmid encoding ezrin FERM-GFP (A-C) or encoding GFP alone (D-F). After 24 hours of culture, portions of each preparation of cells were prepared for confocal fluorescence microscopy by fixation, permeabilization, and staining with Alexa Fluor 568 phalloidin. The remaining portions were prepared for conventional SEM using chemical drying with tetramethylsilane. FERM-transfected cells showed localization of the FERM-GFP protein (A) in submembranous regions, whereas GFP-transfected cells showed diffuse cytoplasmic and nuclear staining (D). GFP-transfected cells had a fine punctate distribution of actin (arrowheads), which is characteristic of normal PBT (E). In contrast, FERM-GFP–transfected cells generally lacked this fine punctate distribution of actin (B). SEM of PBT cells transfected with FERM-GFP revealed a distinctive “bald” phenotype (C). This “bald” phenotype was not observed in GFP-transfected cells (F). Bar indicates 5 μm.
Transfection of dominant-negative ezrin construct into PBT cells ablates surface structures. PBT cells were “nucleofected” with either plasmid encoding ezrin FERM-GFP (A-C) or encoding GFP alone (D-F). After 24 hours of culture, portions of each preparation of cells were prepared for confocal fluorescence microscopy by fixation, permeabilization, and staining with Alexa Fluor 568 phalloidin. The remaining portions were prepared for conventional SEM using chemical drying with tetramethylsilane. FERM-transfected cells showed localization of the FERM-GFP protein (A) in submembranous regions, whereas GFP-transfected cells showed diffuse cytoplasmic and nuclear staining (D). GFP-transfected cells had a fine punctate distribution of actin (arrowheads), which is characteristic of normal PBT (E). In contrast, FERM-GFP–transfected cells generally lacked this fine punctate distribution of actin (B). SEM of PBT cells transfected with FERM-GFP revealed a distinctive “bald” phenotype (C). This “bald” phenotype was not observed in GFP-transfected cells (F). Bar indicates 5 μm.
Increased prominence of peripheral processes in PBT cells transfected with a moesin construct that mimics the phosphorylated form. PBT cells were “nucleofected” with a moesin construct that mimics the unphosphorylated form (moesin-T558A-HA; A-C), or a construct that mimics the phosphorylated form (moesin-T558D-HA; D-F). After 24 hours of culture, the cells were fixed, permeabilized, and stained with Alexa Fluor 568 phalloidin, and anti-HA–FITC. Panels show the confocal images on distribution of actin (B,E; red) and that of the HA-tagged moesin (A,D; green) and corresponding conventional SEM images prepared using chemical drying with tetramethylsilane (C,F). Arrowheads in panels B and E refer to peripheral processes on the cell body representing microvilli. Bar represents 5 μm.
Increased prominence of peripheral processes in PBT cells transfected with a moesin construct that mimics the phosphorylated form. PBT cells were “nucleofected” with a moesin construct that mimics the unphosphorylated form (moesin-T558A-HA; A-C), or a construct that mimics the phosphorylated form (moesin-T558D-HA; D-F). After 24 hours of culture, the cells were fixed, permeabilized, and stained with Alexa Fluor 568 phalloidin, and anti-HA–FITC. Panels show the confocal images on distribution of actin (B,E; red) and that of the HA-tagged moesin (A,D; green) and corresponding conventional SEM images prepared using chemical drying with tetramethylsilane (C,F). Arrowheads in panels B and E refer to peripheral processes on the cell body representing microvilli. Bar represents 5 μm.
Flow cytometry
PBT cells were stimulated in suspension with 100 ng/mL SDF-1α at 37°C and fixed by transfer to a 10-fold volume of ice-cold 10% TCA in water. Cells were left on ice for 30 minutes and then washed 3 times with PBS. Cells were permeabilized with 0.1% Triton X-100 followed by 2 washes in PBS. Pellets were resuspended in mAb 297s (culture supernatant) for 1 hour at 37°C, washed 3 times with PBS with 0.5% BSA, and then stained with FITC-conjugated donkey antirat IgG in PBS with 10% normal donkey serum. Samples were washed 3 times and fluorescence intensity analyzed on a Becton Dickinson FACSscan (San Jose, CA).
Scanning electron microscopy
PBT cells were analyzed either by field emission scanning electron microscopy (SEM; Figure 1) or conventional SEM (Figures 7, 8). For field emission SEM, PBT cells were stimulated with SDF-1α in suspension at 37°C and fixed by transferring aliquots to a 20-fold volume of 37°C fixative (3% glutaraldehyde, 0.1 M cacodylate, 7.5% sucrose, 0.05% CaCl2, pH 7.4). Cells were fixed for 1 hour, washed in PBS, and then allowed to adhere to PLL-treated glass chips for 1 hour. Glass chips were transferred to and stored in fixative until subsequent processing as previously described.32 For conventional SEM, a solution of 8% formaldehyde and 4% glutaraldehyde in 2 × PBS was used to fix the cell suspensions. Equal volumes of the cell suspensions and fixative were mixed and incubated overnight at 4°C. Samples were filtered through a 0.45-μm nylon filter and washed twice with 0.1 M cacodylate buffer. The filter and attached cells were postfixed with 1% OsO4 for 1 hour at room temperature, washed 3 times with the 0.1 M cacodylate buffer, dehydrated in graded ethanol,33 and then immersed 3 times in tetramethylsilane and air-dried at room tempature.34 The filter was cut into smaller pieces, mounted on SEM stubs with double-sided carbon tape, and coated with gold-palladium in a vacuum evaporator. The cells were observed and photographed with the Hitachi S-570 scanning electron microscope operated at 10 kV (Hitachi, Tokyo, Japan).
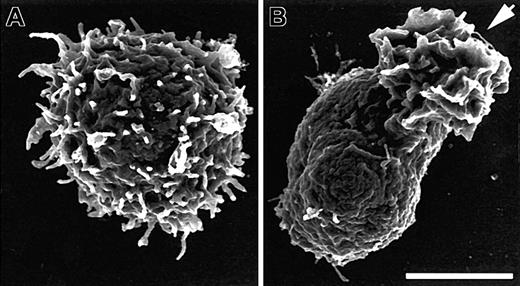
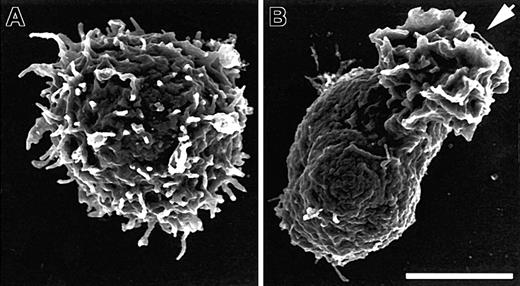
SDF-1α induces loss of microvilli concurrent with polarization. Scanning electron micrographs from field emission SEM of human PBT cells before (A) and after (B) 1 minute of stimulation in suspension with 100 ng/mL SDF-1α. Cells were stimulated, fixed, and processed for SEM as described in “Materials and methods.” The arrow indicates the ruffled leading edge of the polarized lymphocyte. Bar represents 2.5 μm.
SDF-1α induces loss of microvilli concurrent with polarization. Scanning electron micrographs from field emission SEM of human PBT cells before (A) and after (B) 1 minute of stimulation in suspension with 100 ng/mL SDF-1α. Cells were stimulated, fixed, and processed for SEM as described in “Materials and methods.” The arrow indicates the ruffled leading edge of the polarized lymphocyte. Bar represents 2.5 μm.
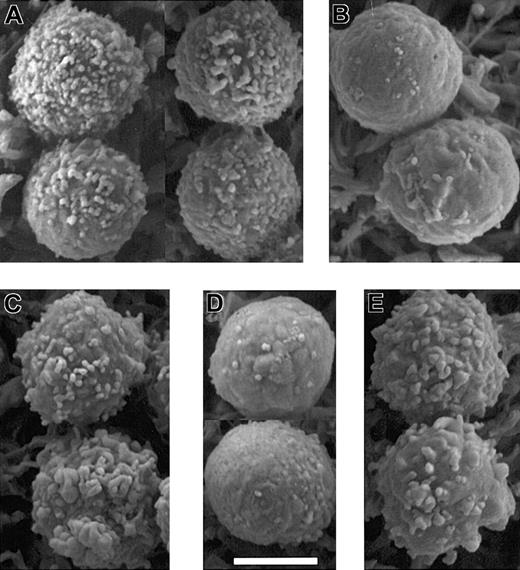
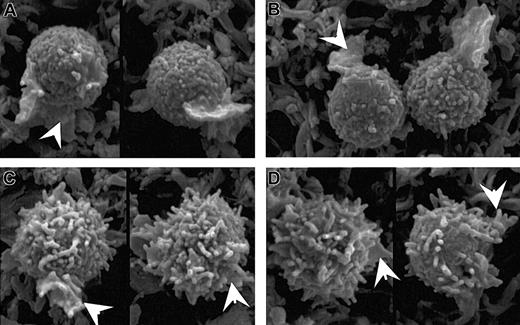
Kinase and phosphatase inhibitors alter microvilli in a manner predicted by their effects on ERM phosphorylation. Scanning electron micrographs of PBT cells that were: (A) untreated; (B) treated with 500 nM staurosporine A for 30 seconds; (C) treated with 50 nM calyculin A for 5 minutes; (D) treated with SDF-1α for 30 seconds; and (E) pretreated with 50 nM calyculin A for 5 minutes before treatment with SDF-1α for 30 seconds. Bar represents 5 μm. Microvilli appear shorter in these images acquired by conventional SEM using samples dehydrated by chemical drying with tetramethylsilane rather than field emission SEM of samples dehydrated by critical point drying (Figure 1).
Kinase and phosphatase inhibitors alter microvilli in a manner predicted by their effects on ERM phosphorylation. Scanning electron micrographs of PBT cells that were: (A) untreated; (B) treated with 500 nM staurosporine A for 30 seconds; (C) treated with 50 nM calyculin A for 5 minutes; (D) treated with SDF-1α for 30 seconds; and (E) pretreated with 50 nM calyculin A for 5 minutes before treatment with SDF-1α for 30 seconds. Bar represents 5 μm. Microvilli appear shorter in these images acquired by conventional SEM using samples dehydrated by chemical drying with tetramethylsilane rather than field emission SEM of samples dehydrated by critical point drying (Figure 1).
Transfection with moesin expression vectors
Expression vectors (pEF-BOS-HA) containing cDNAs encoding N-terminally truncated wild-type and mutant mouse moesins (moesin ΔN) were generously provided by Dr Kozo Kaibuchi (Nagoya University Graduate School of Medicine, Showa, Nagoya, Aichi, Japan). The substitution of alanine or aspartic acid for T558 by site-directed mutagenesis, producing moesin ΔN-T558A and moesin ΔN-T558D, has been described.35 Each of these was modified using polymerase chain reaction (PCR) to replace the truncated 11 N-terminal amino acids and to place the HA-coding sequence at the 3′ end of the original coding region. The resultant full-length moesin inserts (moesin wild type [WT], T558A, and T558D) obtained by PCR were then subcloned into the pcDNA3.1+ vector (Invitrogen, Carlsbad, CA). The ERM dominant-negative construct (FERM-green fluorescent protein [GFP]) was a kind gift from Dr Janis K. Burkhardt (University of Chicago, IL).36 The ezrin fragment 1-320 was amplified from a full-length human ezrin clone37 and the PCR product was inserted into pN1-eGFP (Clontech, Palo Alto, CA). PBT cells were transfected by Amaxa nucleoporator using a human T-cell nucleofector kit (Amaxa Biosystems, Gaithersburg, MD). Maximal expression was observed 16 to 24 hours after transfection. Cells were then fixed in suspension with 3% paraformaldehyde, permeabilized, stained with anti-HA FITC antibody, plated onto PLL-coated coverslips, and processed for indirect immunofluorescence as described previously.30 For studies in which the polarization response to SDF-1 was scored, cells were stained with phalloidin and anti-HA FITC. Transfected cells in the moesin-T558A– and moesin-T558D–nucleoporated populations were selected based only on the criteria of detectable green fluorescence; 250 such cells were scored as either round or polarized (ie, asymmetric actin distribution) and percent polarization calculated as (the number scored as polarized)/(total transfected cells counted).
Results
SDF-1α induces rapid resorption of PBT cell microvilli
Resting PBT cells exhibit heterogeneity of shape and surface features, but the majority of cells are spherical and have abundant microvilli of varying lengths and densities (Figure 1A). Within 1 to 2 minutes of SDF-1α stimulation in suspension the majority of PBT cells adopt a polarized morphology38 ; fully polarized PBT cells show a ruffled leading edge and a tail-like uropod visible by light microscopy. Scanning electron micrographs show that cells polarized by 1 minute of SDF-1α stimulation have undergone dramatic changes in surface features; the surface is devoid of microvilli and other distinctive features, with the exception of the ruffled leading edge (Figure 1B). At earlier time points, cells are observed that remain nearly spherical but show a marked reduction in microvilli relative to resting cells.
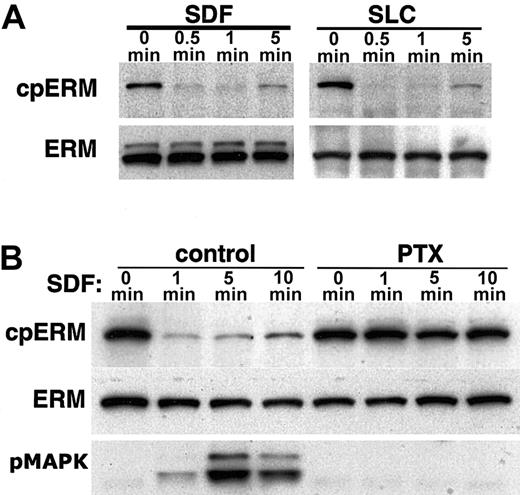
Because the breakdown of microvilli in several cell types correlates with the inactivation of ERM cross-linking activity,25,28,29 we examined whether the loss of PBT cell microvilli in response to SDF-1α could be attributed to changes in ERM activity. In agreement with previous studies,39,40 we found that moesin and to a lesser extent ezrin are expressed in human PBT cells, whereas radixin is not detected. As previously described (see “Introduction”), the phosphorylation state of ERM proteins is indicative of their activation not only in vitro but also in intact cells of different lineages. We therefore examined the effects of chemokine stimulation on ERM phosphorylation in human PBT cells. Western blots of whole cell lysates using antibodies that specifically recognize ERM proteins phosphorylated near their C-terminus (T567, T564, and T558 on ezrin, radixin, and moesin, respectively) show a dominant cpERM band in resting PBT cells at about 78 kDa, consistent with moesin. Treatment with SDF-1α results in a rapid and extensive dephosphorylation of cpERM (Figure 2A). To determine whether induction of ERM dephosphorylation is unique to SDF-1α, we also analyzed the effect of stimulation with another chemokine, SLC. Like SDF-1α, SLC (Figure 2A) induces strong and rapid dephosphorylation of ERM. Thus, ERM dephosphorylation is efficiently induced by 2 chemokines relevant to T-lymphocyte trafficking in vivo.
SDF-1α and SLC induce dephosphorylation of moesin. Lysates compared are either from untreated cells (0 min) or cells treated for the indicated time with the chemokines SDF-1α or SLC (100 ng/mL). Data shown are Western blots of whole cell lysates using mAb 297s (A; cpERM) which specifically recognizes moesin phosphorylated at T558 (as well as phospho-ezrin), compared to total ERM protein (B) detected with polyclonal antiezrin on a duplicate membrane. (A) Time course of dephosphorylation induced by chemokines; (B) inhibition of dephosphorylation by pertussis toxin (PTX). Samples on the right were preincubated for 2 hours with 100 ng/mL PTX. For comparison, Western blot was also performed with antibody specific for phosphorylated MAPK (B; pMAPK).
SDF-1α and SLC induce dephosphorylation of moesin. Lysates compared are either from untreated cells (0 min) or cells treated for the indicated time with the chemokines SDF-1α or SLC (100 ng/mL). Data shown are Western blots of whole cell lysates using mAb 297s (A; cpERM) which specifically recognizes moesin phosphorylated at T558 (as well as phospho-ezrin), compared to total ERM protein (B) detected with polyclonal antiezrin on a duplicate membrane. (A) Time course of dephosphorylation induced by chemokines; (B) inhibition of dephosphorylation by pertussis toxin (PTX). Samples on the right were preincubated for 2 hours with 100 ng/mL PTX. For comparison, Western blot was also performed with antibody specific for phosphorylated MAPK (B; pMAPK).
Many intracellular events triggered by SDF-1α are inhibited by pertussis toxin, including activation of the p44/42 MAP kinase pathway (MAPK, ERK1/2).41 The ERM dephosphorylation induced by SDF-1α is inhibited by pretreatment with pertussis toxin (Figure 2B), indicating that the mechanism involves pertussis toxin–sensitive heterotrimeric G-proteins coupled to the CXCR4 receptor. The rapidity of the ERM dephosphorylation is highlighted by the fact that ERM dephosphorylation is virtually complete at a time when MAPK phosphorylation is only weakly detectable.
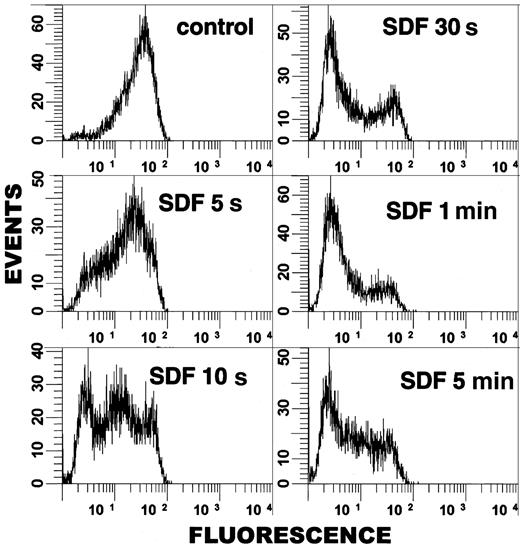
To examine the kinetics of ERM dephosphorylation in more detail, we adapted cpERM staining methods to allow analysis by flow cytometry. Single-cell analysis shows that the dephosphorylation of cpERM is evident in a significant proportion of cells as early as 5 seconds after SDF-1α stimulation (Figure 3). The cell population shows a progressive dephosphorylation response, with the majority of PBT cells exhibiting low cpERM levels within the first minute of stimulation. There is heterogeneity in the response, because some cells undergo dephosphorylation more rapidly than others, and a donor-variable subset (10%-15%) of cells remains nonresponsive. Our finding that the initiation of cpERM dephosphorylation in PBT cells occurs within seconds of chemokine stimulation suggests that ERM inactivation could contribute to the early phases of lymphocyte cytoskeletal reorganization involved in chemokine-induced arrest and subsequent transmigration (see “Discussion”).
Flow cytometric analysis demonstrates rapidity of cpERM dephosphorylation. PBT cells were stimulated in suspension with SDF-1α (100 ng/mL) for the indicated time and fixed, and cpERM proteins were labeled using mAb 297s and FITC-conjugated donkey antirat IgG. The fluorescence intensity of 10 000 cells/sample was measured by flow cytometry. Data were gated for single, intact cells and plotted as events versus log fluorescence intensity.
Flow cytometric analysis demonstrates rapidity of cpERM dephosphorylation. PBT cells were stimulated in suspension with SDF-1α (100 ng/mL) for the indicated time and fixed, and cpERM proteins were labeled using mAb 297s and FITC-conjugated donkey antirat IgG. The fluorescence intensity of 10 000 cells/sample was measured by flow cytometry. Data were gated for single, intact cells and plotted as events versus log fluorescence intensity.
Calyculin A inhibits SDF-1α–induced dephosphorylation
Investigations in other cell types have identified kinase and phosphatase inhibitors that perturb ERM phosphorylation.21,42 Our findings confirm that such agents are also effective and informative in PBT cells (Figure 4). Brief treatment with the kinase inhibitor staurosporine reduces cpERM to undetectable levels within 30 seconds (Figure 4A). Conversely, treatment with the serine/threonine phosphatase inhibitor calyculin A increases carboxy-threonine phosphorylation of moesin and ezrin; at the concentrations used in these studies, calyculin A effects are slower than staurosporine effects, becoming evident within 1 to 5 minutes. Thus, basal cpERM phosphorylation in lymphocytes reflects a dynamic kinase/phosphatase balance.
Calyculin A increases basal cpERM phosphorylation and inhibits SDF-1α–induced dephosphorylation. (A) Pharmacologic perturbation of basal kinase/phosphatase balance. Western blotting was performed for cpERM and total ERM on PBT cells treated with 50 nM calyculin A or 500 nM staurosporine for the indicated times. (B) ERM dephosphorylation following 1 minute of SDF-1α stimulation can be inhibited by costimulation (50 nM) or by brief pretreatment (10 nM, 2 minutes) with calyculin A. Western blots of duplicate membranes detecting cpERM versus total ERM versus phospho-p44/42 MAPK.
Calyculin A increases basal cpERM phosphorylation and inhibits SDF-1α–induced dephosphorylation. (A) Pharmacologic perturbation of basal kinase/phosphatase balance. Western blotting was performed for cpERM and total ERM on PBT cells treated with 50 nM calyculin A or 500 nM staurosporine for the indicated times. (B) ERM dephosphorylation following 1 minute of SDF-1α stimulation can be inhibited by costimulation (50 nM) or by brief pretreatment (10 nM, 2 minutes) with calyculin A. Western blots of duplicate membranes detecting cpERM versus total ERM versus phospho-p44/42 MAPK.
We extended the analysis of phosphatase inhibitor by investigating whether calyculin A is also able to inhibit SDF-1α–induced ERM dephosphorylation. The results demonstrate that it does so efficiently (Figure 4B). To facilitate interpretation, the experimental conditions were designed so that calyculin A would not grossly alter the basal phosphorylation; this can be achieved either by brief pretreatment with calyculin A before the SDF-1α or treatment with a somewhat higher concentration of calyculin A concurrent with exposure to SDF-1α. Examples of both are shown. With each approach, calyculin blocks SDF-1α–induced ERM dephosphorylation. Western blots for MAPK phosphorylation serve as an internal control identifying an SDF-1α–induced signaling pathway that is not influenced by calyculin A within the same time frame. Thus, a calyculin A–sensitive phosphatase is required for SDF-1α–induced dephosphorylation of cpERM.
SDF-1α triggers cytoskeletal dissociation and redistribution of ERM proteins
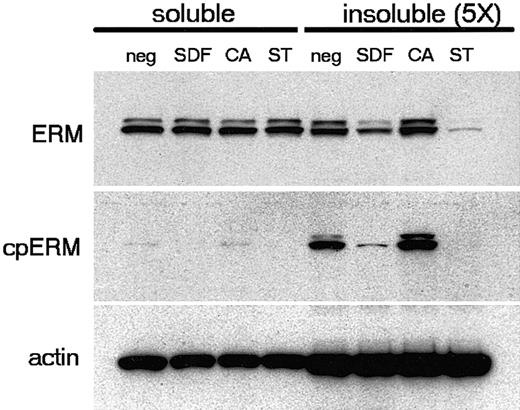
Recent studies22,25,42 suggest that the activation of ERM proteins is primarily driven by N-terminal interactions with polyphosphoinositides such as PIP2. Carboxy-threonine phosphorylation may not be necessary for ERM activation, but instead may serve to stabilize ERM proteins in their active conformation. Carboxy-threonine phosphorylation is, however, indicative of active ERM proteins, and dephosphorylation correlates with loss of ERM cross-linking function and the breakdown of ERM-stabilized surface features such as microvilli.28,29 We therefore examined the cytoskeletal association of lymphocyte ERM before and after stimulation with SDF-1α. Our results (Figure 5) demonstrate that a limited fraction (< 20%) of total ERM protein is retained in the detergent-insoluble cytoskeleton. In contrast to total ERM, cpERM proteins are enriched in the insoluble fraction, indicating a preferential association of cpERM with the cytoskeleton of resting PBT cells. Stimulation with SDF-1α results in the dephosphorylation of cpERM and a concomitant decrease in the total ERM retained in the cytoskeletal fraction. Relative to SDF-1α, staurosporine treatment causes more complete ERM dephosphorylation and a correspondingly greater loss of cytoskeletally associated ERM. Conversely, brief treatment with calyculin A increases ERM phosphorylation as well as the amount of total ERM retained in the cytoskeletal fraction. Thus, a direct relationship exists between ERM phosphorylation and cytoskeletal association in PBT cells. Chemokine-stimulated ERM dephosphorylation correlates with cytoskeletal dissociation and therefore with inactivation of ERM-mediated cross-linking between membrane and cytoskeleton.
Selective association of cpERM with the insoluble cytoskeleton in resting PBT cells. PBT cells were either untreated or treated with SDF-1α (100 ng/mL, 1 minute), calyculin A (CA; 25 nM, 5 minutes), or staurosporine (ST; 500 nM, 5 minutes). Data shown are Western blots of cell fractions detecting carboxy-threonine phosphorylated ERM (mAb 297s), total ERM (polyclonal anti-ezrin), and β-actin (mAb AC-15) on duplicate membranes. Note that the soluble fraction is diluted 5-fold relative to the insoluble fraction.
Selective association of cpERM with the insoluble cytoskeleton in resting PBT cells. PBT cells were either untreated or treated with SDF-1α (100 ng/mL, 1 minute), calyculin A (CA; 25 nM, 5 minutes), or staurosporine (ST; 500 nM, 5 minutes). Data shown are Western blots of cell fractions detecting carboxy-threonine phosphorylated ERM (mAb 297s), total ERM (polyclonal anti-ezrin), and β-actin (mAb AC-15) on duplicate membranes. Note that the soluble fraction is diluted 5-fold relative to the insoluble fraction.
The spatial distribution of ERM was examined in PBT cells before and after chemokine stimulation using confocal microscopy. In resting PBT cells, cpERM proteins exhibit a fine punctate cortical distribution (Figure 6). Such punctate cortical staining is characteristic of proteins found in microvilli. In contrast, staining with conventional anti-ERM antibodies in resting cells shows a combination of diffuse cortical staining, punctate cortical staining, and cytoplasmic localization. There is negligible signal in the nucleus, which occupies the majority of intracellular space in resting PBT cells. The cpERM staining is rapidly and dramatically decreased following SDF-1α stimulation for 30 seconds. Simultaneously, total ERM adopts a more diffuse staining pattern, suggesting ERM redistribution as a consequence of dephosphorylation. Thus, the SDF-1α–induced dephosphorylation of ERM (Figure 2) and its dissociation from cytoskeleton (Figure 5) is associated with a change in the cortical organization of ERM proteins (Figure 6) and with the loss of microvilli (Figure 1).
Kinase and phosphatase inhibitors influence microvilli in a manner predicted by their effects on ERM phosphorylation
The foregoing findings are consistent with a model in which SDF-1α–induced dephosphorylation of ERM causes resorption of microvilli. From that model arise 3 testable predictions regarding the effects of kinase and phosphatase inhibitors. First, because staurosporine causes ERM dephosphorylation, it should cause loss of microvilli. Our results confirm that staurosporine causes loss of most PBT surface features within 30 seconds (Figure 7B), consistent with the kinetics with which it causes cpERM dephosphorylation (Figure 4). Second, because the phosphatase inhibitor calyculin A increases ERM phosphorylation, and blocks SDF-1α–induced dephosphorylation, it should prevent SDF-1α–induced loss of microvilli. Five minutes of treatment with calyculin A causes no dramatic change in microvilli (Figure 7C); however, closer inspection reveals that the peripheral processes are somewhat coarser than typical microvilli. Because their spacing and length are grossly similar to those of microvilli present on such cells before treatment, we favor the interpretation that these are “broadened” microvilli. By 30 seconds, SDF-1α induces a reduction in microvilli without full polarization (Figure 7D; also Figure 6). In contrast, pretreatment of PBT cells with calyculin A inhibits the loss of peripheral processes in response to SDF-1α (Figure 7E). These results support the hypothesis that lymphocyte microvilli depend on cpERM, and that ERM dephosphorylation is causally related to the loss of microvilli in response to chemokine stimulation.
Transfection studies demonstrate the importance of ERM for PBT microvilli
PBT cells were transfected with a construct containing the N-terminal domain of ezrin (FERM) but lacking the C-terminal actin-binding domain. This FERM construct has been used as a dominant-negative in various model systems, and it most likely blocks ERM functions by occupying binding sites on transmembrane proteins without coupling them to actin16 and efficiently competing with endogenous active ERM. The absence of the C-terminus eliminates auto-inhibition, and therefore bypasses the need for C-terminal phosphorylation to form stable membrane interactions. Expression of the FERM construct markedly changes both the surface features and the F-actin distribution of PBT cells (Figure 8). The punctate F-actin staining characteristic of cells with fine peripheral processes such as microvilli and filopodia is consistently observed in untransfected PBT cells as well as in PBT cells transfected with control constructs (Figure 8E), but is absent in FERM-transfected cells (Figure 8B), suggesting that expression of FERM domain disrupts peripheral processes in PBT cells. SEM analysis of FERM-transfected PBT cells reveals a unique cellular phenotype—intact spherical cells devoid of peripheral processes (Figure 8C). This phenotype is observed in 15% to 30% of cells “nucleoporated” in the presence of FERM DNA but has not been observed in cells “nucleoporated” with control constructs (Figure 8F). These results are consistent with previous studies in which suppression of ERM expression in thymoma cells through the introduction of antisense probes resulted in the disruption of microvilli.19 Thus, suppression of peripheral processes is the distinctive outcome of transfection with FERM, consistent with the involvement of ERM proteins in lymphocyte peripheral processes including microvilli.
Role of cpERM phosphorylation in formation and dephosphorylation in chemokine-induced loss of PBT microvilli
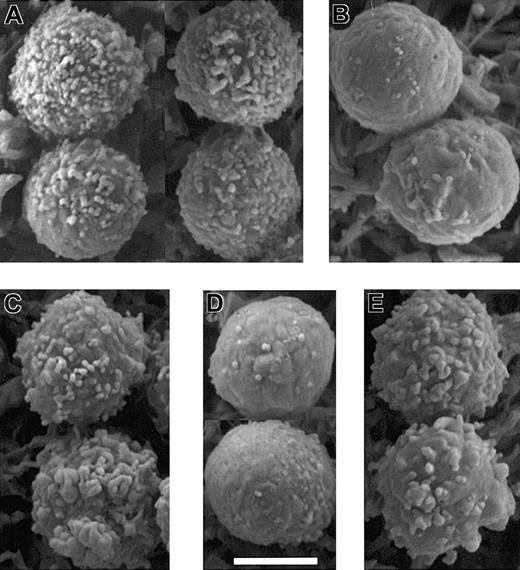
To directly address the importance of ERM carboxy-terminal threonine phosphorylation to PBT microvilli, cells were transfected with point-substitution mutant constructs of moesin in which residue T558 was replaced by either aspartic acid (T558D) to mimic phosphorylated moesin or alanine (T558A) to mimic dephosphorylated moesin. By fluorescence microscopy, a distinctive phenotype is seen in cells transfected with the T558D construct; of those T558D-transfected cells, 20% to 30% (depending on the experiment) have long peripheral processes rich in actin and moesin-T558D (Figure 9D-E). SEM images confirm the presence of long, coarse microvilli on such cells (Figure 9F). Such processes were not observed in moesin-T558A–transfected PBT cells (Figure 9A-C). The ability of T558D but not T558A to augment microvilli indicates the critical role of phosphorylation at T558 in their establishment/maintenance in resting PBT cells.
The microvilli of moesin-T558D–transfected PBT cells were also analyzed by SEM following SDF-1 stimulation. Cells fully polarized after 1 to 2 minutes of chemokine stimulation do not have the coarse microvilli characteristic of unstimulated moesin-T558D–transfected cells (data not shown). Thus, moesin-T558D does not prevent polarization or microvillus breakdown in response to chemokine stimulation. However, evidence for delayed loss of microvilli is observed in round moesin-T558D–transfected cells 30 to 60 seconds after chemokine stimulation. At those times, PBT cells are often not distinctly polarized, but the initiation of a lamellipodium is evident as the formation of one or more large ruffles on the cell surface. During this early phase of polarization, the moesin-T558A–transfected cells have largely lost their normal microvilli (Figure 10). In contrast, among moesin-T558D transfectants, many cells responding to SDF-1α, as demonstrated by the presence of ruffles, retained coarse microvilli.
Delayed loss of microvilli in moesin-T558D–transfected cells in early stages of polarization. PBT cells were “nucleofected” with a moesin construct that mimics the unphosphorylated form (moesin-T558A-HA; A-B), or a construct that mimics the phosphorylated form (moesin-T558D-HA; C-D). Samples were prepared for conventional SEM using chemical drying with tetramethylsilane. After 30 seconds (A,C) and 60 seconds (B,D) cells were observed in early stages of polarization, identifiable by the presence of ruffles (arrowheads), but absence of a well-formed lamellipodium. Such cells in T558D-transfected cells generally retained coarse microvilli but in T558A-transfected cells had generally lost microvilli. All images were acquired at × 6000 and relative size was maintained during figure composition.
Delayed loss of microvilli in moesin-T558D–transfected cells in early stages of polarization. PBT cells were “nucleofected” with a moesin construct that mimics the unphosphorylated form (moesin-T558A-HA; A-B), or a construct that mimics the phosphorylated form (moesin-T558D-HA; C-D). Samples were prepared for conventional SEM using chemical drying with tetramethylsilane. After 30 seconds (A,C) and 60 seconds (B,D) cells were observed in early stages of polarization, identifiable by the presence of ruffles (arrowheads), but absence of a well-formed lamellipodium. Such cells in T558D-transfected cells generally retained coarse microvilli but in T558A-transfected cells had generally lost microvilli. All images were acquired at × 6000 and relative size was maintained during figure composition.
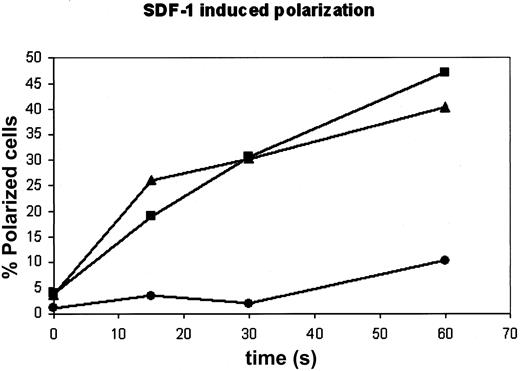
Analysis of the samples by fluorescence microscopy demonstrates a substantial inhibition of polarization by T558D transfection. In samples transfected with moesin-T558D, a 2- to 4-fold decrease in the fraction of cells fully polarized by 60 seconds of SDF-1α stimulation is observed (Figure 11). A decreased frequency of polarized cells was also evident in the SEM analysis of moesin-T558D–transfected cells (data not shown). Previous studies indicate that ERM phosphorylation may not be an absolute requirement for the formation and maintenance of microvilli.42 Our results are in agreement with these studies in that moesin-T558D expression cannot ultimately prevent the loss of microvilli and cell polarization. The finding, however, that overexpression of moesin-T558D impairs/delays polarization and the loss of microvilli indicates a significant role for chemokine-induced dephosphorylation of ERM in the facilitation of these processes, which occur rapidly in vivo.
Transfection with moesin-T558D inhibits polarization of PBT cells. PBT cells were “nucleofected” without any DNA (“mock,” ▴), or with moesin constructs that mimic the unphosphorylated form (moesin-T558A-HA; ▪), or the phosphorylated form (moesin-T558D-HA, •). Cells were fixed before stimulation or at varying intervals after stimulation with SDF-1α, permeabilized, and stained for HA plus actin and visualized by fluorescence microscopy. Determination of percentage polarization was performed single blind for polarized cells (percentage of all cells in “mock” and percentage of transfected cells in moesin-transfected populations).
Transfection with moesin-T558D inhibits polarization of PBT cells. PBT cells were “nucleofected” without any DNA (“mock,” ▴), or with moesin constructs that mimic the unphosphorylated form (moesin-T558A-HA; ▪), or the phosphorylated form (moesin-T558D-HA, •). Cells were fixed before stimulation or at varying intervals after stimulation with SDF-1α, permeabilized, and stained for HA plus actin and visualized by fluorescence microscopy. Determination of percentage polarization was performed single blind for polarized cells (percentage of all cells in “mock” and percentage of transfected cells in moesin-transfected populations).
Discussion
Lymphocyte adhesion to endothelium is a multistep process in which chemokines play the critical role of inducing strong adhesion by activating integrins. Although disappearance of microvilli is a predictable element in the adhesion cascade,13,14 the molecular basis is not known. Using freshly purified human PBT cells, we demonstrate that stimulation with a physiologically relevant chemokine alone is sufficient to induce the rapid loss of lymphocyte microvilli. This is a novel function of chemokines that is distinct from their role as integrin activators; we propose that these 2 chemokine functions cooperate in the adhesion cascade. Furthermore, our studies identify a key molecular process contributing to this loss of microvilli, the dephosphorylation of ERM proteins. Moreover, our studies indicate that chemokine-induced dephosphorylation of moesin plays another important role in the cascade, facilitation of lymphocyte polarization.
It has recently been shown that stimulation of resting neutrophils with 2 chemotactic and chemokinetic cytokines induces ERM dephosphorylation.43 Although the central focus of that report was on the role of Rho kinase in regulating ERM phosphorylation (and uropod retraction), Yoshinaga-Ohara et al's finding that formyl-methionine-leucine-phenylalanine (FMLP) or granulocyte-macrophage colony-stimulating factor (GM-CSF) rapidly induces ERM dephosphorylation in neutrophils parallels our findings that chemokines induce rapid dephosphorylation of lymphocyte ERM protein. The combination of our findings and those of Yoshinaga-Ohara et al suggests that ERM dephosphorylation is a molecular pathway that is commonly used during hematopoietic cell polarization in response to diverse chemotactic/chemokinetic agents.
Discussion hereafter will focus on 3 issues: (1) the evidence that dephosphorylation of ERM per se is a contributor to the loss of microvilli and polarization in response to chemokine, rather than simply being associated with it; (2) the molecular mechanisms regulating ERM dephosphorylation; and (3) the potential functional relevance of the loss of microvilli.
Dephosphorylation of cpERM per se is a contributor to loss of microvilli
Our findings indicate that ERM proteins are involved in formation of lymphocyte microvilli and specifically that dephosphorylation of cpERM proteins is causally related to their loss in PBT cells. The general importance of ERM to lymphocyte microvilli is clearly demonstrated by transfection studies with the dominant-negative FERM construct of ezrin, which results in ablation of lymphocyte surface processes (Figure 8). Because of the strong sequence conservation between the FERM domains of ezrin and moesin (94% amino acid similarity) and the overlap in the molecules to which they bind, the FERM domain from ezrin is believed to preempt binding sites normally used by both moesin and ezrin proteins, thereby preventing ERM proteins from establishing normal linkage between cytoskeleton and membrane proteins including L-selectin, CD43, and CD50. Such membrane anchorage for the cortical cytoskeleton is understood to be critical for organization of peripheral processes. Our finding is consistent with results of transfection of FERM in kidney epithelial cells16 and with an extensive literature documenting the importance of ERM in formation of microvilli in other cell types,15,17,20 including the loss of microvilli in mouse thymoma cells following exposure to ERM-antisense oligonucleotides.19
Moreover, it is the presence of carboxy-terminal phosphorylation of ERM that is critical to peripheral processes in lymphocytes. Multiple lines of evidence indicate a causal relationship between ERM phosphorylation and peripheral processes. First, direct demonstration is provided by the transfection studies with mutant ERM constructs that either mimic the phosphorylated form (eg, moesin-T558D) or the unphosphorylated form (eg, moesin-T558A). We find that moesin-T558D promotes long peripheral processes, but moesin-T558A does not (Figure 9); thus phosphorylated and nonphosphorylated forms differ markedly in their contribution to peripheral processes. These findings in lymphocytes are concordant with findings in epithelial cells and fibroblasts.15,35,44 Second, studies with pharmacologic agents provide supporting evidence. Staurosporine rapidly induces dephosphorylation of ERM proteins and associated loss of microvilli in lymphocytes (Figure 7); this confirms and extends analogous findings in L-cell fibroblasts.42 Conversely, calyculin A inhibits the dephosphorylation of cpERM (Figure 4) and prevents loss of peripheral processes in response to chemokine (Figure 7). Third, there is good correspondence between the rapid kinetics of ERM dephosphorylation and the disappearance of microvilli. Fourth, we demonstrate that chemokine stimulation induces both ERM dephosphorylation (Figure 2) and loss of ERM cytoskeletal association (Figure 5), which is central to their participation in microvilli.21,23,26 Fifth, overexpression of a moesin construct that mimics cpERM (but not a construct that mimics nonphospho ERM) retards loss of microvilli (Figure 10). In short, direct and indirect evidence converge to establish a causal relationship between ERM dephosphorylation and loss of microvilli in lymphocytes.
Although the presence of cpERM favors microvilli, current models suggest that cpERM alone is not sufficient for retention of microvilli.42 This is also evident in our results that microvillus loss, although delayed, does still occur in moesin-T558D–transfected PBT cells. A requirement for elements other than cpERM for the maintenance of microvilli is plausible, given the combinatorial requirements for complex molecular processes. One element likely to cooperate with cpERM in control of lymphocyte microvilli is phosphoinositide turnover; phosphoinositides interacting with the N-terminal FERM domain of ERM proteins are thought to collaborate with C-terminal phosphorylation in ERM activation.42 Moreover, chemokine responses in T lymphocytes involves acute regulation of phosphoinositides.45 Thus, in chemokine-stimulated PBT cells, phosphoinositide hydrolysis may be a key regulator of ERM inactivation and the loss of microvilli, but the concurrent dephosphorylation of ERM proteins may serve to increase the efficiency and rapidity of this important morphologic transition.
Molecular mechanisms involved in regulation of cpERM phosphorylation
Our studies demonstrate that the steady-state level of phosphorylated ERM in resting lymphocytes is subject to a dynamic kinase/phosphatase balance (Figure 4), as has been shown previously in fibroblasts and platelets.21,42 Analysis of the effects of kinase and phosphatase inhibitors suggests that, among the cell types studied, there are fundamental similarities in the regulators of ERM phosphorylation. Calyculin A, an inhibitor of type I serine/threonine phosphatases, augments cpERM phosphorylation in both platelets and epithelial cells. Staurosporine, an inhibitor of certain serine/threonine kinase of the AGC subfamily, tips the balance in the opposite direction, resulting in decreased cpERM phosphorylation in those cell types. Cellular responses to certain physiologic stimuli induce rapid changes in cpERM phosphorylation reminiscent of the effects of staurosporine or calyculin A. Rapid phosphorylation of cpERM is induced in platelets by treatment with thrombin21 and in serum-starved fibroblasts by treatment with epidermal growth factor (EGF).44 Rapid dephosphorylation has been observed in T lymphocytes in response to stimulation via the antigen-specific receptor,46 in neutrophils stimulated with FMLP,43 and in platelets following an initial wave of thrombin-induced phosphorylation.21 To our knowledge, the dephosphorylation response to chemokine demonstrated here is one of the fastest ERM-regulatory responses yet described. The identities of the kinases and phosphatases involved in regulating this balance are not yet definitively established.
Functional importance of rapid ERM dephosphorylation
The results present herein suggest 3 distinct functional roles for chemokine-induced ERM dephosphorylation: (1) facilitating collapse/resorption of microvilli; (2) facilitating polarization; and (3) modulating signal transduction. The resorption of microvilli during lymphocyte adhesion to endothelium is likely to be important in several ways. First, we propose that resorption of microvilli removes a physical obstacle to transmigration between endothelial cells. The transition during this time from a spherical circulating cell to a polarized cell involves a radical reorganization of lymphocyte cytoskeleton from an architecture optimized for function in blood to an architecture optimized for migration in tissue.30,38 Specifically, transmigration involves extreme deformation of the lymphocyte to move between endothelial cells. Stiffness is understood to be essential for microvilli to initiate adhesion; it allows them to penetrate glycocalyx and to establish selectin binding.47 However, persistence of microvilli on the lateral surfaces would be expected to pose a severe impediment to transendothelial migration. Additionally, if microvilli persist on lymphocytes as stiff “bristles,” they would limit access of LFA-1 on the cell body of the lymphocyte to ligands on endothelium such as intercellular adhesion molecule 1 (ICAM-1), ICAM-2, and ICAM-3. Because LFA-1 interactions are particularly important for transmigration,48 elimination of microvilli would eliminate barriers to LFA-1 interactions involved in transmigration. These proposed roles are consistent with observed resorption of microvilli from lymphocytes in vivo before transmigration.13,14
In addition to these roles, we raise the possibility that cpERM dephosphorylation might play a third role earlier in the adhesion cascade. Even partial ERM dephosphorylation would make the microvillus more likely to collapse during rolling, facilitating contact of the integrins on the lymphocyte cell body with ligands on the endothelium. As in the past, proposed contribution to the adhesion cascade of a chemokine-induced response may be anticipated49,50 long before assays are developed that validate the rapidity of the effect.11,51 This view fits with an emerging body of literature showing that microvilli play much more complex and interesting roles than originally imagined. Initial modeling considered lymphocytes as rigid spheres with homogeneous microvilli.4 It is now believed that heterogeneity in microvillus length is important52 and that longer microvilli can be especially important in penetrating through endothelial glycocalyx.47 Tension during rolling may initiate either of 2 outcomes—controlled lengthening of the microvillus or conversion into a long thin membrane tether, which can influence arrest.52,53 Thus, regulation of length and rigidity of microvilli can influence tethering, rolling, arrest, and thus the overall event of lymphocyte extravasation.
A role for ERM dephosphorylation in facilitating lymphocyte polarization is strongly suggested by the finding that transfected cpERM retards the process of lymphocyte polarization (Figure 11). Indeed, semiquantitative image analysis indicates that very high levels of cpERM overexpression may completely block polarization (data not shown). Although we had not anticipated this role, the finding fits well with the role of cpERM in stabilizing cortical cytoskeleton. Because ERM proteins are major stabilizers of cortical cytoskeleton and anchors for transmembrane proteins such as CD43 and ICAM-3, which redistribute during polarization, it is difficult to imagine the global reorganization involved in lymphocyte polarization occurring without at least a transient inactivation of ERM. The concept of ERM release facilitating cytoskeletal reorganization is supported by our previous findings that: (1) the VPF complex of vimentin-plectin-fodrin undergoes coordinated compaction into the uropod during lymphocyte polarization; and (2) that moesin dissociates from this complex during the first minute following SDF-1α stimulation.30 Indeed, studies of T-cell receptor–induced responses of T lymphocytes demonstrate that release from cpERM binding is important for reorganization of cytoskeleton at the immunologic synapse and movement of CD43 to the “distal pole complex.”54
Chemokine regulation of ERM activation may be important not only for the biophysical aspects of lymphocyte adhesion, polarization, and transmigration, but may also have biochemical implications. Recently, ERM proteins have been shown to be organizers of signal transduction complexes. For example, ERMs are connected to rhodamine signaling via their binding of RhoGDI, to protein kinase A, to phospholipase C, and to ion channels via ERM interactions with members of both the NHERF/EBP50 family and NHE1 families.17,55 Because many of these interactions depend on the activation state of ERM proteins, regulation of ERM phosphorylation may constitute an important node in chemokine-triggered signal transduction.
Prepublished online as Blood First Edition Paper, August 7, 2003; DOI 10.1182/blood-2002-12-3807.
M.J.B. and R.N. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the National Institutes of Health Department of Transfusion Medicine for blood products; Dr Sachiko Tsukita for generously providing reagents and for scientific discussion; Dr Kozo Kaibuchi for generously providing reagents; and Dr Ronald Germain for scientific discussion.