Abstract
Farnesyltransferase inhibitors (FTIs) are small-molecule inhibitors that selectively inhibit farnesylation of a number of intracellular substrate proteins such as Ras. Preclinical work has revealed their ability to effectively inhibit tumor growth across a wide range of malignant phenotypes. Many hematologic malignancies appear to be reasonable disease targets, in that they express relevant biologic targets, such as Ras, mitogen-activated protein kinase (MAPK), AKT, and others that may depend on farnesyl protein transferase (FTase) activity to promote proliferation and survival. A host of phase 1 trials have been recently launched to assess the applicability of FTIs in hematologic malignancies, many of which demonstrate effective enzyme target inhibition, low toxicity, and some clinical responses. As a result, phase 2 trials have been initiated in a variety of hematologic malignancies and disease settings to further validate clinical activity and to identify downstream signal transduction targets that may be modified by these agents. It is anticipated that these studies will serve to define the optimal roles of FTIs in patients with hematologic malignancies and provide insight into effective methods by which to combine FTIs with other agents.
Introduction
Research into the pathophysiology of hematologic malignancies has unveiled an abundance of intracellular signaling events that governs proliferation and survival of the malignant cell. Such discoveries have subsequently promoted recognition of new molecular targets, to which therapeutic development may be aimed, and on which malignant cells may rely disproportionately for their own maintenance. Indeed, chronic myelogenous leukemia (CML) has been the prototype disease for which identification of a unique oncogenic molecular target (BCR-ABL) has led to the development of a relatively specific pharmacologic inhibitor to that target, subsequently inducing therapeutic success.1-3 CML not withstanding, however, few other malignant hematologic diseases are driven primarily by a single aberrant molecular signaling event. As such, large-scale therapeutic success is unlikely to occur with a single agent in diseases such as acute myelogenous leukemia, in which multiple and unknown molecular events may trigger expansion of the malignant clone. Nonetheless, progress is being made into the dissection of specific signaling pathways that may be important, although not absolutely necessary, in the propagation of the cancerous process.
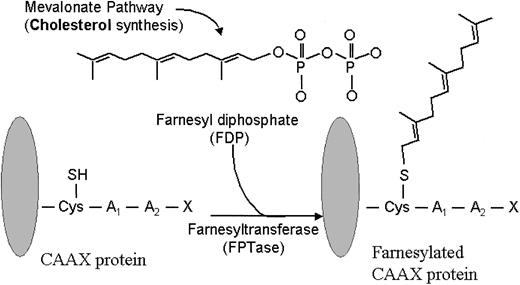
Farnesyl transferase inhibitors (FTIs) represent a new class of signaling inhibitors that is emerging in the clinical arena of hematologic malignancies and that may inhibit critical growth and survival signals. These agents are potent and selective competitive inhibitors of intracellular farnesyl protein transferase (FTase), an enzyme that catalyzes the transfer of a farnesyl moiety to the cysteine terminal residue of a substrate protein (Figure 1).4 A host of intracellular proteins are substrates for prenylation via FTase, including Ras, RhoB, Rac, and Lamin proteins.5 As such, interrupting the normal prenylation process of these substrate proteins may prove useful for inhibiting cellular events that are governed by them.
Farnesylation reaction. FPTase transfers a farnesyl group onto the cysteine terminal portion of a substrate protein, such as Ras. (Adapted from Rowinsky et al.5 J Clin Oncol 1999;17:3635. Used with permission from the author.)
Farnesylation reaction. FPTase transfers a farnesyl group onto the cysteine terminal portion of a substrate protein, such as Ras. (Adapted from Rowinsky et al.5 J Clin Oncol 1999;17:3635. Used with permission from the author.)
Expanded knowledge about FTase and its substrates has culminated in the rational design of several classes of FTIs.6 One approach, through screening of libraries and natural products, has identified farnesyl diphosphate (FDP) analogues as a class of FTIs. A second class of FTIs, known as peptidomimetics, competes with the terminal cysteine portion (C-A-A-X) of Ras and related proteins for FTase. A third class of FTIs is the bisubstrate analogues, which incorporate the structural motifs of both FTase and the C-A-A-X tetrapeptide. Nonpeptidomimetic inhibitors of FTase, identified from random screening of compound libraries, comprise a fourth class of FTIs. Collectively, the FTI subtypes share an ability to potently and selectively inhibit farnesylation; however, it appears likely that individual classes of FTIs, and perhaps the individual compounds within each class, ultimately display dissimilar functional activity within specific tumor repertoires.
Molecular targets in hematologic malignancies
Knowledge of molecular events that govern intracellular signaling events is critical to the appropriate application and development of therapeutic compounds that may affect a disease process. In the arena of hematologic malignancies, many molecular signaling pathways have been identified as important contributors to disease pathophysiology, including pathways that may be susceptible to effects of FTIs.
Ras
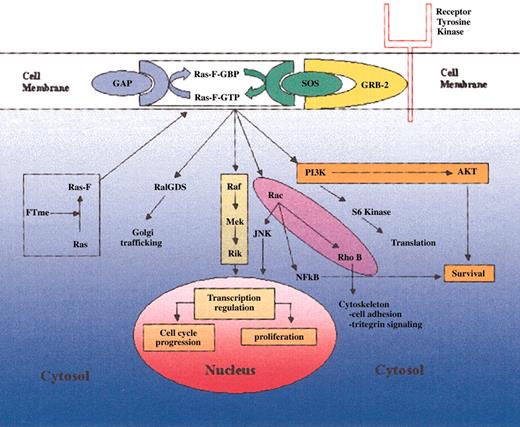
The Ras family of oncoproteins is a critical network of signal transduction pathways that affects cellular proliferation, survival, and differentiation (Figure 2).5,7,8 Following isoprenylation in the cytosol, the Ras protein migrates to the cell membrane, where it is capable of activating downstream signaling events.4,9 The transfer of a farnesyl group is mediated by the enzyme farnesyltransferase (FT), whereas transfer of a geranyl group is mediated by geranylgeranyl transferase.4,9,10 The Ras protein exists in specific isoforms (N-Ras, K-Ras, H-Ras) that differ in their affinity for specific isoprenyl groups. For example, K-Ras is able to be farnesylated or geranylgeranylated, whereas H-Ras is preferentially farnesylated.11-13 While membrane bound, Ras fluctuates between an active deoxyguanosine triphosphate (GTP)-bound form and an inactive guanosine diphosphate (GDP)-bound form, an equilibrium that is regulated by surface proteins such as SOS (a guanine nucleotide exchange factor), and GTPase-activating protein (GAP).7 When mutated, Ras may fail to interact appropriately with its negative regulators, thereby leading to its constitutive activation in the GTP-bound form.8 It is the high frequency of ras mutations in human malignancies that has spurred the development of pharmacologic inhibitors such as FTIs.
Ras-mediated signaling. Posttranslationally farnesylated Ras localizes to the cell membrane, where it interacts with its regulatory factors GAP and SOS. In its activated GTP-bound form, Ras is capable of mediating a variety of kinase pathways that ultimately govern cellular proliferation and survival.
Ras-mediated signaling. Posttranslationally farnesylated Ras localizes to the cell membrane, where it interacts with its regulatory factors GAP and SOS. In its activated GTP-bound form, Ras is capable of mediating a variety of kinase pathways that ultimately govern cellular proliferation and survival.
Central to the development of FTIs has been their potential to inhibit Ras-driven malignant cell growth and propagation. A large body of preclinical data now exists that confirms this hypothesis. One point of importance, which has been reproducibly demonstrated, is that FTIs are able to induce morphologic reversion of ras-transformed cell lines.4,14-16 Furthermore, evidence shows that they block both anchorage-independent growth in cell lines and also inhibit farnesylation of newly synthesized Ras protein.14,15,17,18 As a next step in development, FTIs have been studied for in vivo antitumor efficacy as well; in mouse models, a variety of orally administered FTI compounds clearly cause regression in ras-transformed tumors.19-22 Although these agents have well-established preclinical activity in ras-transformed tumors and cell lines, their activity is not necessarily limited to ras-mutated tumors nor is the effect equal among tumors bearing different mutated ras isoforms. In addition, there are likely differences in the degree of activity against specific tumor phenotypes among the various FTIs. For example, one group demonstrated potent activity of the nonpeptidomimetic R115777, both in vitro and in vivo, against tumors bearing wild-type ras and H-ras mutations, whereas K-ras-mutated tumors were much less sensitive.19 Similarly, the peptidomimetic FTI, B956, was most active against H-ras-mutated cells, whereas K-ras tumors were significantly more resistant.23 Alternatively, one study demonstrated the peptidomimetic FTI-276 to be inactive against tumors expressing wild-type ras.24 These collective findings suggest that Ras itself may be a rational target for FTIs, but that Ras-driven tumors likely exhibit significant variability in their susceptibility to FTIs. This susceptibility likely depends both on the pharmacologic properties of the specific FTI being tested as well as the Ras isoform driving a specific tumor's growth.
Certain molecular events pathognomonic to hematologic malignancies may depend on Ras signaling to facilitate the ultimate transformation or leukemogenic process, thereby making Ras itself a rational target for new therapeutic agents in these disease settings. The critical role of Ras in leukemogenesis can be demonstrated, for example, in a chronic myelogenous leukemia (CML) model, in which leukemic transformation depends on the presence of a specific oncoprotein, BCR-ABL, resulting from a translocation between chromosomes 9 and 22. In this model, introduction of dominant negative (inactive) ras into BCR-ABL-transfected hematopoietic or fibroblast cells results in a complete blockage of malignant transformation.25 In a similar light, disrupting the Grb-2/SOS complex, a key guanine nucleotide exchange factor that positively regulates Ras, greatly inhibits Ras-mediated cell proliferation in both BCR-ABL cell lines and freshly isolated CML blasts.26 Other key molecular signaling events in hematologic malignancies may also depend on Ras. FLT-3 is a receptor that is known to play a central role in the proliferation, survival, and differentiation of early murine and human hematopoietic precursor cells.27 Internal tandem duplication (ITD) of the FLT3 gene occurs in up to 20% to 35% of acute myelogenous leukemia (AML) cases.28-30 Although this mutated receptor can induce transformation as well as growth factor-independent activation of various signaling pathways, it has also been demonstrated that transfection with a dominant-negative form of Ras inhibits colony formation in cells containing the FLT-3 ITD mutation.31 Ras may also play a central role in the pathogenesis of myelodysplasia. In vitro work indicates that mutated ras can impair erythroid differentiation of both erythroleukemia and normal human CD34+ cells.32,33 Further evidence pointing to the importance of ras in myeloid leukemogenesis emanates from an in vivo model, in which a majority of irradiated mice, reconstituted with bone marrow transfected with activated N-ras, developed severe hematopoietic defects pathologically similar to myeloproliferative disorders, AML, and myelodysplastic syndrome (MDS).34
Data extracted from clinical samples also lend evidence to the relevance of Ras in hematologic malignancies and to its potential as a direct therapeutic target. In AML and MDS, ras (particularly the N-ras isoform), may be mutated in up to 48% of cases, although there is considerable variation in these rates among published series.35-40 Similarly, in multiple myeloma, activating ras mutations may be present in a high percentage of pathologic specimens at baseline, likely involving both K-ras and N-ras.41,42 Mutation of ras in other hematologic malignancies is less common. For example, in acute lymphoblastic leukemia, the rate of N-ras mutations is on the order of 2% to 10%.43-45 Low-grade lymphoproliferative disorders and Hodgkin disease are rarely associated with ras mutations.46,47
Mitogen-associated protein kinase (MAPK)
Downstream from Ras, several effector molecules and pathways influence a variety of cellular processes thought to be important in both normal and malignant hematopoiesis (Figure 2). One key pathway is the Raf-MEK-MAPK cascade. The Raf protein is a serine-threonine protein kinase that binds Ras to become active.48-50 At this point Raf is able to initiate a downstream cascade of phosphorylation, ultimately resulting in the phosphorylation of mitogen-activated protein kinase (also known as extracellular signal-regulated kinase, or ERK).51-53 MAPK, in turn, localizes to the nucleus where it activates transcription factors that help to govern cellular proliferation and apoptosis.54-56 Transcription-independent mechanisms may also account for the pro-survival effect of this pathway, perhaps via phosphorylation and subsequent inactivation of the proapoptotic BAD (Bcl-2-associated death promoter) protein.56 Because of its connection with Ras, MAPK itself may be a viable indirect target of FTIs. Indeed, in vitro studies have demonstrated that FTIs can inhibit the phosphorylation and, hence, activation of MAPK in NIH3T3 cells, although this effect appears to be directly related to the ability of the FTI to inhibit Ras farnesylation.57,58 Similarly, in mice receiving transplants of neurofibromatosis type 1 (Nf1)-deficient hematopoietic cells, FTI treatment failed to induce an antitumor effect or MAPK inhibition. It was speculated that the failure to induce tumor or MAPK inhibition resulted from the inability of FTI L-744832 to effectively block farnesylation of Ras isoforms (eg, K-Ras and N-Ras) other than H-Ras.59
The Raf-MEK-MAPK signaling pathway likely has significant implications in the development and propagation of malignant hematopoiesis. For example, mutant Raf (in a constitutively activated form) can abrogate growth factor dependency in hematopoietic cells.60 Further evidence in support of a major role for this pathway in malignant hematopoiesis is demonstrated by the finding that pharmacologic inhibitors of MEK function and antisense RNA to MEK can block Ras- or Raf-mediated transformation and cytokine-stimulated growth.61 In its activated form, Raf may also induce autocrine growth factor synthesis and subsequently inhibit apoptosis.62 In multiple myeloma models, activation of the MAPK pathway appears to promote cellular proliferation, whereas pharmacologic inhibition of MEK can effectively reverse the proliferation, particularly when this process is driven by interleukin 6 (IL-6) or angiogenic factors.63,64
Similar to Ras, MAPK itself may be overexpressed or constitutively activated in hematopoietic malignancies. For example, constitutive activation of MAPK in clinical AML marrow samples, as determined by immunoblot and in vitro kinase analyses, has been demonstrated in a high proportion of AML cases at baseline.65-67 Interestingly, it has been suggested that the presence of strong phospho-MAPK expression at baseline may adversely affect survival.66
Phosphatidyl inositol-3 kinase (PI3K)/AKT
The PI3K/AKT pathway occupies a critical position in the transduction of signals that begin with growth-stimulating cytokines and end with cell proliferation and survival.68,69 At least one trigger for this pathway is activated Ras, which interacts directly with the PI3K catalytic subunit as a critical first step in the phosphorylation and activation of the pivotal “second messenger” serine-threonine kinase AKT.70-72 AKT phosphorylates a panoply of substrates involved in cell proliferation and survival following DNA damage or other cellular stresses.
The PI3K/AKT pathway is germane to hematopoiesis and leukemogenesis. For example, an in vitro model has shown that intact PI3K/AKT signaling is essential for BCR-ABL-induced transformation and colony growth.73 Further evidence to support the critical role of the PI3K/AKT pathway in malignant hematopoiesis can be found in a study whereby Ras mutants incapable of activating the Raf-MEK-ERK pathway were still able to inhibit apoptosis in IL-3-dependent cell lines. This effect was blocked by wortmannin (an inhibitor of PI3K), emphasizing the importance of the PI3K/AKT pathway in hematopoiesis.60 Constitutive AKT activity may also be present in primary tumor cells from patients with hematologic malignancies. In AML, for example, constitutively activated (phosphorylated) AKT was detected in 17 of 17 leukemic marrow samples but in none of the 6 normal control marrows.74 Similarly, in primary non-Hodgkin lymphoma tissue samples, constitutively activated AKT was frequently detected, further highlighting the relevance of this signaling network as a potential target in hematologic cancers.75
Another case in point is the Ras-dependent Akt-driven production of vascular endothelial growth factor (VEGF), a pivotal growth and survival factor for diverse leukemias, which occurs in response to hypoxia.76 VEGF is thought to drive leukemic cell proliferation via both an internal autocrine loop77-79 and an external paracrine process, where it induces marrow stromal cell cytokine production that can stimulate AML blasts.80 Given that VEGF likely plays a pivotal role in sustaining and expanding the leukemic cell population, it is not surprising that VEGF is produced in response to certain cellular stresses and that there are multiple signaling pathways leading to VEGF gene expression. In this regard, the hypoxia-triggered, Ras-mediated activation of PI3K/Akt-2 that culminates in VEGF gene transcription76 may explain the link between Ras-related oncogenesis and VEGF expression that has been demonstrated in certain epithelial cell lines and in vivo animal tumor models.81,82 Taken together, these findings raise the possibility that FTIs could promote apoptosis by suppressing Ras-PI3K/AKT-induced VEGF activity.
Other substrates, including those involved in cell cycle regulation and DNA repair, are inhibited as a result of AKT phosphorylation. For example, AKT leads to serine phosphorylation and inactivation of the proapoptotic molecule BAD and the oxidative stress-induced apoptosis-regulating kinase-1 (ASK-1)83-85 Similarly, AKT can counteract DNA damage-induced G1 and G2 checkpoint arrests86,87 perhaps because of threonine phosphorylation and inactivation of the cyclin-dependent kinase inhibitors p21 and p27.88-91 AKT can also abrogate cell cycle arrest at both the G1/S and G2M checkpoints by inactivating forkhead transcription factors such as FOXO3a, which protect cells from oxidative stress.92-94 The ultimate effect of these activities may confer a net resistance to cytotoxic drugs, and the ability to inhibit PI3K/AKT signaling may reinstate drug sensitivity by permitting those drugs to induce apoptosis. Indeed, the reinstatement of drug-induced cytotoxicity has been demonstrated in cell lines exposed to both direct PI3K inhibitors (eg, wortmannin) and FTIs.95,96 FTI-induced apoptosis may indirectly occur through 2 distinct (but likely interrelated) mechanisms—first, by impeding Ras protein farneyslation and subsequent growth factor-driven PI3K activation; and second, by blocking the effects of AKT-2 on integrin activation, thereby abrogating cell-cell adhesion pathways of cell survival.95
RhoB
The Rho proteins are Ras-related GTP-binding proteins that coordinate growth factor-induced assembly of intracellular focal adhesions and actin stress fiber formation.97 It has been demonstrated that expression of Rho GTPases is increased in human cancer cells as compared with nonmalignant cells.98 The RhoB isoform is an endosomal, short-lived protein that is expressed at highest levels during the S-phase of the cell cycle.99,100 Posttranslational prenylation occurs in these proteins, leading to both farnesylated and geranylgeranylated forms.101,102 As such, Rho may be a relevant target for FTI therapy. Indeed, RhoB appears crucial for oncogenic ras transformation, as dominant inhibitory RhoB mutants are capable of suppressing H-ras-induced transformation of cell lines.103 Increasing evidence now suggests that RhoB mediates the antineoplastic effect of FTIs. To this end, it has been shown that FTI treatment blocks RhoB farnesylation, leading to an increase in the geranylgeranylated (GG) form of RhoB via a preferential increase in geranylgeranyltransferase type I (GGT-I) activity and subsequent cell growth inhibition.104,105 Interestingly, the growth inhibitory and apoptotic effects elicited by FTIs in ras-transformed cells can be abrogated by the introduction of ectopic forms of RhoB, further implicating a role for RhoB as an important target of FTIs.106,107 There is also indirect evidence lending credence to RhoB as a primary oncogenic target of FTIs. For example, it has been observed that normal cells are much less susceptible to FTI-induced apoptosis and inhibition than ras-transformed cells.108 Combined with the fact that elevated levels of GG-RhoB are inconsequential to normal cell growth, while at the same time able to promote phenotypic reversion in FTI-treated malignant cells,104 it is conceivable that RhoB is an important functional target of FTIs, perhaps more so than Ras itself. Somewhat to the contrary, however, one group of investigators found that farnesylated and geranylgeranylated RhoB are both potent tumor growth inhibitors, and that FTI-directed prenylation changes of RhoB may not account for the antitumor effects of these agents.109
Mitotic proteins
The inhibition of protein farnesylation interrupts the functions of diverse proteins that help to move the cell through its division cycle in an orderly and productive fashion. FTIs may also impede the farnesylation and function of certain proteins, such as the kinetochore-binding centromeric proteins (CENPs) E and F, which exert their maximal effects in the G2 and M phases of the cell cycle and which are critical to the orderly completion of mitosis.110,111 The mechanisms by which FTIs impair the full execution of mitosis, however, remain poorly defined. One possibility is that unfarnesylated CENPs are not capable of localizing to and associating with microtubules.111 Alternatively, using human lung cancer cells, Crespo et al112 demonstrated that FTIs can inhibit formation of a bipolar spindle, which, in turn, is crucial to proper chromosome alignment at the metaphase plate. This effect culminates in an inability of the FTI-treated cell to progress through mitosis, with resultant accumulation and arrest in prometaphase.112
Furthermore, FTIs can induce checkpoint arrest at G2/M in both a p53-dependent and -independent fashion.111,113,114 These effects can occur in Ras-activated tumor cells, such as astrocytoma cells, CML cells expressing the p210 BCR-ABL protein, and human p190 BCR-ABL-positive acute lymphocytic leukemia (ALL) cells.113,115,116
Preclinical studies of FTIs in leukemia and myeloma
Although FTIs were introduced into clinical trials for hematologic malignancies prior to extensive preclinical testing in these specific diseases per se, much important in vitro and in vivo work is being done that will ultimately augment knowledge about these agents and how they may be optimally used in the clinic.
Myeloid leukemias
Preclinical data are suggestive that FTIs can inhibit leukemic cell growth in vitro, albeit with limitations. Morgan et al117 evaluated a panel of several FTIs for growth inhibition of myeloid leukemia cells. Although not all agents tested were inhibitory, 2 of these compounds induced both a retardation of colony growth and apoptosis in several cell lines tested, including the NB4 cell line, in which the Ras-MAPK pathway is strongly activated at baseline.117 Postulating that FTI resistance in these leukemic cells may be related to preferential geranylgeranylation of K-Ras and N-Ras, the same group has exposed leukemic cells to a combination of FTIs and geranylgeranyltransferase inhibitors (GGTIs); preliminary results suggest an additive effect on cell growth inhibition and apoptosis when these compounds are combined.118 Further evidence regarding the ability of FTIs to inhibit Ras-driven hematopoietic cells in a limited fashion can be found in a report by Mahgoub et al.59 In that study, the orally administered CAAX peptidomimetic FTI L-744832 failed to inhibit in vivo growth of Ras-hyperfunctional (Nf1-deficient) hematopoietic cells in mice, as determined by peripheral blood leukocyte count and spleen size.59 Although H-Ras prenylation was inhibited in these experiments, N-Ras and K-ras were unaffected, implying that selective farnesylation inhibition of only one Ras isoform may not be sufficient to induce antineoplastic effects in tumors driven more preferentially by the N-Ras or K-ras isoforms. These findings are consistent with the other previously mentioned findings that have suggested a higher rate of FTI resistance in cells mutated by K-ras or N-ras.19,23 It should be noted, however, that these observations do not imply that FTIs are inactive against hematopoietic cells with a malignant phenotype, only that Ras may not be the exclusive target. Clinical data, as will be shown, further substantiate this point.
Preclinical studies are beginning to demonstrate that CML is a very relevant clinical target for FTI-based therapy. Recently reported in vitro data from one group indicate that both peptidomimetic and nonpeptidomimetic FTIs (including R115777) can inhibit proliferation and induce apoptosis in CML cell lines.119 Another group demonstrated that the nonpeptidomimetic FTI SCH66336 strongly inhibited both colony formation and proliferation of a BCR-ABL-transformed murine cell line, in conjunction with decreased levels of GTP-bound Ras. In addition, mice injected with this leukemogenic cell line and subsequently treated with SCH66336 were effectively rescued from developing acute leukemia, whereas untreated mice all developed fatal leukemia shortly thereafter.115 A logical extension to these findings would be to study the effects of combining FTI therapy with the clinically proven BCR-ABL inhibitor STI-571. Indeed, preliminary results of such preclinical work to this end were recently reported by Topaly et al,120,121 who have shown that a variety of FTI agents in combination with STI-571 appear to synergistically inhibit growth of both CML cell lines and primary samples. Other myeloproliferative disorders may also be appropriate disease targets for FTI therapy. One study preliminarily indicated that when R115777 was added to peripheral blood mononuclear cells obtained from patients with myeloid metaplasia, progenitor colony formation was significantly inhibited.122
Multiple myeloma
An emerging role is developing for FTIs in multiple myeloma, and recently reported preclinical data have supported this development. One group has published results on the effect of R115777 on both primary myeloma cells and myeloma cell lines.123 Herein, R115777 induced apoptosis in these cells, albeit at somewhat higher concentrations than those achieved in phase 1 studies. Of interest was also the fact that IL-6 did not rescue myeloma cell lines in the presence of FTI.123 Preliminary results from in vitro studies suggest that the proapoptotic effect induced by R115777 is related to the inhibition of AKT phosphorylation and that the degree of FTI resistance correlated with higher levels of phosphorylated AKT in these cells.124 Hence, although many questions persist regarding the precise mechanisms by which FTIs are active in multiple myeloma, clinical trials in multiple myeloma have commenced, supported in part by these preclinical observations.
Clinical trials in hematologic malignancies
Acknowledgment of FPT as a relevant biologic target along with reproducible antitumor activity in preclinical models has spawned the development of FTIs in the clinic. Specifically, in hematologic malignancies, a plethora of clinical trials are being undertaken. Clinical studies in hematologic malignancies, because of relatively simple acquisition of pathologic tissue from blood or bone marrow, afford a unique opportunity not only to assess antitumor activity but also to further identify the relevant biologic targets that may be inhibited by these compounds. Active clinical trials of FTIs in hematologic disorders are outlined in Table 1.
Active clinical trials of FTIs in hematologic malignancies (as of June 2003)
Sponsor . | Lead institution . | Agent . | Phase . | Disease . | Disease status . | Endpoints . |
|---|---|---|---|---|---|---|
| NCI | University of Maryland | R115777 | 2 | AML, high-risk MDS | Untreated | Response rate, survival, FTase inhibition, angiogenesis, MAPK inhibition, AKT inhibition |
| NCI | University of Maryland | R115777 | 2 | AML, MDS | After remission | Overall survival, disease-free survival |
| NCI | University of Chicago | R115777 | 1 | Advanced hematologic malignancies | Refractory | Toxicity, FTase inhibition, clinical activity |
| NCI | NCI | R115777 | 1 | Pediatric leukemias | Refractory | MTD, pharmacokinetics |
| NCI | Stanford University | R115777 | 1/2 | Myeloproliferative disorders | Progressive | Toxicity, WBC response, erythroid response, cytogenetic response |
| MD Anderson | MD Anderson | R115777 + imatinib mesylate | 1 | CML | Chronic-phase, imatinib failure | MTD, toxicity |
| Johnson & Johnson | International | R115777 | 2 | AML | Post-remission | Overall survival, disease-free survival |
| Johnson & Johnson | National | R115777 | 2 | High-risk MDS | 1 or fewer prior therapy | Response rate, toxicity |
| Schering-Plough | National | SCH66336 | 1/2 | AML, CML, ALL, MDS | 3 or fewer prior therapies | Safety and tolerability, pharmacokinetics, FTase inhibition, clinical activity |
Sponsor . | Lead institution . | Agent . | Phase . | Disease . | Disease status . | Endpoints . |
|---|---|---|---|---|---|---|
| NCI | University of Maryland | R115777 | 2 | AML, high-risk MDS | Untreated | Response rate, survival, FTase inhibition, angiogenesis, MAPK inhibition, AKT inhibition |
| NCI | University of Maryland | R115777 | 2 | AML, MDS | After remission | Overall survival, disease-free survival |
| NCI | University of Chicago | R115777 | 1 | Advanced hematologic malignancies | Refractory | Toxicity, FTase inhibition, clinical activity |
| NCI | NCI | R115777 | 1 | Pediatric leukemias | Refractory | MTD, pharmacokinetics |
| NCI | Stanford University | R115777 | 1/2 | Myeloproliferative disorders | Progressive | Toxicity, WBC response, erythroid response, cytogenetic response |
| MD Anderson | MD Anderson | R115777 + imatinib mesylate | 1 | CML | Chronic-phase, imatinib failure | MTD, toxicity |
| Johnson & Johnson | International | R115777 | 2 | AML | Post-remission | Overall survival, disease-free survival |
| Johnson & Johnson | National | R115777 | 2 | High-risk MDS | 1 or fewer prior therapy | Response rate, toxicity |
| Schering-Plough | National | SCH66336 | 1/2 | AML, CML, ALL, MDS | 3 or fewer prior therapies | Safety and tolerability, pharmacokinetics, FTase inhibition, clinical activity |
NCI indicates National Cancer Institute; WBC, white blood cell.
AML
Clinical testing and application of FTIs in hematologic malignancies are most developed for AML, in which the results of an initial phase 1 trial have provided a foundation for continuing clinical exploration of these agents. In that recently published phase 1 trial, eligible patients included those with relapsed or refractory AML, although a small number of patients with ALL and CML were enrolled as well.125 With the use of the FTI R115777 (ZARNESTRA; Johnson & Johnson PRD, Titusville, NJ), patients were dosed in cohorts from 100 mg twice daily up to 1200 mg twice daily, receiving treatment for a planned 21 consecutive days every 4 weeks. Several important findings emerged from that study. First of all, as expected, overall toxicity (with the exception of hematologic toxicity at the higher dosing levels) was limited, although dose-limiting neurologic toxicity was observed at the 1200-mg, twice-daily dosing level. Another key finding from that study was that drug accumulated in bone marrow cells in a dose-dependent manner and remained present at sustained levels throughout the duration of administration. A somewhat unexpected, although welcome result, was an overall response rate of 30%, including 2 complete responses. Interestingly, responses occurred across all dosing cohorts and did not invariably correlate with the degree of FTase inhibition in sampled leukemic cells, despite the fact that more consistent inhibition of FTase overall was seen at doses of 300 mg twice daily and higher. Certainly, this observation challenges both the validity of the assays and the notion that a specific degree of enzyme inhibition is necessary to achieve clinical response. There was also a trend toward a higher response rate in patients whose leukemia demonstrated active (phosphorylated) MAPK at baseline than in those patients in which this activity did not occur.125 Another somewhat unexpected finding in that trial was the absence of detectable K-ras or N-ras mutations in any of the patient samples, although this observation likely substantiates claims that response to FTI occurs independently of ras mutational status. Although that trial left some questions unanswered, it certainly provided impetus for further development of FTIs in the AML setting. To this end, a large multicenter phase 2 trial using R115777 in relapsed or refractory AML was undertaken; the preliminary results of which have been recently reported. It was observed that 17% of evaluable patients with relapsed disease had a reduction in the bone marrow blasts to less than 5%.126 DNA microarray analysis has also been incorporated into that trial, to delineate gene expression patterns between responders and nonresponders. Further clinical data, including response durations and survival, will be updated in the coming months.
In a somewhat different patient population, we have recently reported preliminary data from a phase 2 trial of oral R115777 at 600 mg twice daily in previously untreated poor-risk AML and MDS.127 In that trial, patients who refused or were poor candidates for standard cytoreductive induction-type chemotherapy were eligible. In addition to assessing clinical response, that trial has undertaken the study of biologic endpoints in leukemic cells, including the expression of HDJ-2 (as a surrogate of farnesylation inhibition) and activated MAPK and AKT in leukemic cells. To date, an overall clinical response rate of 34% has been observed, including 12 complete responses of varying durability. Although hematologic toxicity was frequent and expected, severe nonhematologic toxicities (with the exception of febrile neutropenia) were infrequently encountered, and death during therapy was a rare event. Of note have been the relatively frequent occurrences of reversible skin rash and low-grade neurologic toxicity, observations that are not unique to that trial.
Preliminary results are also being reported from several early-phase clinical trials of FTI therapy in advanced hematologic malignancies inclusive of, but not primarily restricted to, AML.128-131 One such study, piloted by Cortes et al,129 using the oral, nonpeptidomimetic FTI SCH66336, reports that only 1 of 19 patients with AML achieved a pathologic bone marrow response. A phase 1 study of that same agent in advanced myeloid malignancies demonstrated achievement of adequate serum concentrations and reliable inhibition of HDJ-2 farnesylation.130 Another early-phase trial of that agent reported that 1 of 2 patients with secondary AML had a major bone marrow response and that none of the responders carried a ras mutation.131
MDS
Several studies have been undertaken to determine the activity of FTIs in MDS. Kurzrock et al132,133 have reported preliminary results from both phase 1 and phase 2 studies of R115777 in MDS of all subtypes. In the phase 1 study, dose-limiting toxicity (DLT; fatigue) occurred at 900 mg twice daily, whereas objective responses (hematologic improvement or partial responses) were observed in 33% of patients, with varying degrees of treatment-associated myelosuppression. No correlation between ras mutational status and response was detected.132 The phase 2 trial used R115777 at 600 mg twice daily, once again in patients with MDS of all subtypes.133 There was clear activity demonstrated, as documented by 2 patients that had complete responses, neither of which had an activating ras mutation. However, toxicity that necessitated removal from study was observed in 4 of 16 evaluable patients, raising the possibility that drug-induced toxicity may affect the overall response rate and that lower dosing may be appropriate for cohorts of patients with MDS.133 The difference in toxicity rates between the MDS trial and the AML trials may have several explanations, including differences in patients' comorbidities at baseline, previous treatment histories, age, or protocol criteria for removal from study; nonetheless, this discrepancy clearly deserves further attention. Again, data regarding response durability and survival are immature at this time and are expected to be updated in the coming months.
Other FTIs have also been studied in MDS. A preliminary report of a phase 2 study of SCH66336 demonstrated erythroid or platelet responses in 3 of 15 patients with MDS, without severe toxicities.129 Results using the BMS-214662 FTI compound were also recently reported in a subgroup of patients with MDS.128 In that phase 1 trial, 2 of 6 patients with high-grade (refractory anemia with excess blasts [RAEB] or RAEB in transformation [RAEB-T]) MDS experienced more than 50% reduction in bone marrow blasts, whereas a third of the patients achieved hematologic improvement, as demonstrated by platelet count normalization. This agent was also very well tolerated, with no severe toxicities reported.128
Myeloproliferative diseases
There is growing experience with the use of FTIs in myeloproliferative disorders, particularly CML. As discussed previously, CML is a rational disease target in that the BCR-ABL oncoprotein uses signaling cascades, such as Ras, that likely depend on intact FTase activity. The first available clinical data regarding FTIs for CML were recently reported by Cortes et al,134 in which 7 of 22 patients with previously (and often heavily) treated CML achieved complete or partial, albeit transient, hematologic response to R115777. Most responses occurred in patients with chronic-phase disease, and 4 minor cytogenetic responses occurred as well. The toxicity profile was similar to that reported in other trials, with fatigue, skin rash, myelosuppression, and neuropathy among the most common.134 In a similar trial, SCH66336 was used in patients with CML in chronic or accelerated phase, in whom STI-571 had failed. Preliminary results indicated a hematologic response in 2 of 12 patients, with evidence of increased peripheral blood myeloid maturation in several others.135 Gotlib et al136 have also reported the use of FTI therapy in a small number of patients with STI-571 refractory CML. Early results from this trial demonstrate a hematologic response in 3 of 4 patients, although only 1 of these responses was maintained beyond 1 cycle of therapy.136 Included in the preliminary results of some of the above-mentioned trials were patients with other myeloproliferative diseases, including myelofi-brosis and chronic myelomonocytic leukemia. In the series by Cortes et al,134 2 of 6 patients with myelofibrosis experienced a reduction in spleen size by more than 50% following therapy with R115777. Similarly, Gotlib et al136 observed sustained or nonsustained hematologic responses in 5 of 7 patients with undifferentiated myeloproliferative disorders. No activating ras mutations were reported in any of the patients enrolled on that trial to date.
Multiple myeloma
As in the case of myeloproliferative disorders, limited clinical data have been reported regarding FTIs in multiple myeloma. The recent report from Cortes et al134 included 10 patients with refractory multiple myeloma, who received R115777, only one of whom experienced a minor reduction in serum monoclonal paraprotein. A preliminary report from Alsina et al137 demonstrated disease stabilization (as manifested by 0%-25% decrease in paraprotein marker) in 50% of evaluable patients with advanced, heavily pretreated multiple myeloma, several of whom have been maintained on therapy for 4 cycles or longer. Although these and other studies may indicate a lack of an overt response by strictly defined criteria, a stable disease pattern should not be necessarily looked on as undesirable, particularly in patients who have been previously heavily treated and in those with indolently behaving disease.
Future directions
FTIs exhibit encouraging signs of clinical activity in patients with advanced hematologic malignancies. New settings in which single-agent FTI therapy should ideally be investigated include previously untreated disease and minimal residual disease. To this end, as outlined previously, a trial of R115777 in patients with previously untreated poor-risk AML and MDS has been commenced, in which the median age at time of enrollment was 74 years.127 It is now widely accepted that patients with newly diagnosed AML who are older than age 60 experience extremely poor long-term outcomes with standard therapy, largely because of both high relapse rates and significant treatment-related mortality.138-142 One asset of this class of agents and other signal transduction inhibitors is a toxicity profile that is very acceptable, the implications of which may be profound. Currently, the paradigm for successful therapy in aggressive malignancies such as AML is intensive, myelosuppressive chemotherapy. This model is predicated largely on the inability to safely administer multiple, successive courses of chemotherapy. Agents such as FTIs might be administered on a chronic dosing schedule, either alone or in combination with other pharmacologic compounds, with the aim of maintaining the underlying disease in a clinically controlled state.
On another front, it has become evident that postremission chemotherapy for AML in elderly patients or those with other poor-risk features fails to extend either survival or remission duration.143,144 Similarly, in multiple myeloma, maintenance therapy to extend disease remission or survival has been largely unsuccessful. Such a disease setting seems optimal for investigating new agents such as FTIs. Indeed, both phase 2 and phase 3 trials using R115777 are being planned for patients with AML in first or subsequent complete remission.
Another avenue of exploration is the combination of FTIs with chemotherapy. To this end, there are limited data to suggest synergistic or additive effects between the 2. One early study suggested that combining FTI with paclitaxel, a microtubule depolymerization inhibitor, led to synergistic inhibition of cell growth in vitro, implying that inhibition of protein farnesylation may deregulate a mitotic checkpoint, secondarily resulting in enhanced mitotic sensitivity to paclitaxel.145 Further evidence to the potential additive effect of combination therapy comes from Sun et al,57 who reported that combining FTI-2148 with chemotherapy (cisplatin, paclitaxel, gemcitabine) had an additive inhibitory effect on the in vivo growth of human lung adenocarcinoma in nude mice. In AML cell lines, preliminary data indicate growth inhibition synergy between FTI L-744832 and topoisomerase II inhibitors, as well as an increased level of apoptosis with the combination.146 Clearly, a major obstacle to successful combination therapy is the great heterogeneity by which FTIs may inhibit cell cycle progression. For this reason, it is critical to understand which part of the cell cycle machinery is inhibited by a specific FTI in a specific disease setting. It seems likely, for example, that an FTI which blocks entry of the cell into S-phase would not act synergistically or additively with a chemotherapeutic agent that is S-phase specific. However, an FTI that inhibits cell progression through the G2/M phase might be useful in combination with an M-phase-specific agent, such as a taxane or epipodophyllotoxin. Ultimately, the most effective combination regimen will need to take these biologic aspects of antitumor activity into consideration.
In sum, a rapidly evolving understanding of the intracellular signaling apparatus has placed FTIs at the forefront of clinical research trials in hematologic malignancies. Yet, it is clear that a great amount of work remains to be done to elucidate the mechanisms by which these cancers respond to FTIs and the optimal settings in which they do so. A future intriguing strategy for optimizing the use of FTIs in the clinical arena is the potential for combination with other signaling inhibitors. The rationale for such an approach may be based on the knowledge that malignant hematopoietic cells are governed by a vast and often redundant array of signaling networks, creating a situation wherein a single pharmacologic agent is unlikely to greatly interrupt the cellular proliferative and survival processes. Although such an approach offers new opportunities to potentially overcome traditional mechanisms of drug resistance, only limited preclinical data exist at this time to document synergistic or additive effects between FTIs and other agents. The full potential of FTI therapy ultimately will depend on the development of new clinical trials aiming to study unique dosing schedules and various disease stages, as well as the continued study of biologic markers that are affected by FTIs or whose presence may predict for responsiveness to these compounds.
Prepublished online as Blood First Edition Paper, August 14, 2003; DOI 10.1182/blood-2003-02-0633.
Supported in part by a grant from the National Cancer Institute (grant U01-CA69854) (J.E.K.) and research funding from Janssen Research Foundation (J.E.L. and J.E.K.).
We gratefully acknowledge the major contributions from Joseph Rosenblatt, MD; John Wright, MD, PhD; David End, PhD; and Scott Kaufmann, MD, PhD; that have greatly facilitated the development of FTI therapy in hematologic malignancies.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal