Abstract
All-trans retinoic acid (ATRA)—induced growth arrest of myeloid cells is associated with a sequential regulation of cyclins and cyclin-dependent kinase inhibitors (CKIs), which modulates the cell cycle machinery and inhibits the G1-S phase progression. ATRA treatment of myeloid cells induces up-regulation and tyrosine phosphorylation of Stat1, a member of the STAT (signal transducer and activator of transcription) transcription factor family that has been implicated in growth arrest in response to interferons. We have previously shown that ATRA-induced cell cycle arrest is dependent on tyrosinephosphorylated Stat1. In this study, we show that there is a basal level of Stat1 Ser727 phosphorylation in U-937 cells, which is transiently increased in response to ATRA treatment. Using Stat1Ser727Ala-expressing sublines, we provide evidence that Ser727 phosphorylation of Stat1 is required for ATRA-induced growth arrest. To shed further light on the role of Stat1 in ATRA-induced cell cycle arrest, cyclin and CKI expression was analyzed during ATRA treatment in U-937 sublines expressing Stat1Ser727Ala and Stat1Tyr701Phe. Our results show that Ser727/Tyr701-phosphorylated Stat1 plays a key role as a prerequisite for the ATRA-induced down-regulation of c-Myc; cyclins A, B, D2, D3, and E; and the simultaneous up-regulation of p27Kip1, associated with arrest in the G0/G1 phase of the cell cycle. (Blood. 2003;102:254-261)
Introduction
All-trans retinoic acid (ATRA)1 is a powerful inducer of terminal differentiation and cell cycle arrest of several myeloid cell lines in vitro. An in vivo corollary of this biologic principle has been demonstrated by the successful treatment of acute promyelocytic leukemia (APL) by ATRA, resulting in differentiation and apoptosis of the leukemic blasts.1 The process of terminal differentiation is tightly coupled to growth arrest in the G0/G1 phase of the cell cycle. Cell cycle progression depends on the activity of cyclin-dependent kinases (CDKs), which, in turn, can be regulated by cyclins and CDK inhibitors (CKIs).2 ATRA-induced growth arrest of myeloid cells involves the sequential down-regulation of cyclins E, D3, A, and B and the up-regulation of CKIs p21WAF1/CIP1 and p27Kip1.3-6 However, the ATRA-induced signaling pathways involved in modulating the cell cycle machinery are largely unknown.
ATRA acts through a family of nuclear receptors, retinoic acid receptors (RARs) and retinoic X receptors (RXRs), which activate the transcription of retinoic acid response element (RARE)—regulated target genes on ligand binding.7 Subsequent to RAR and RXR activation, downstream signaling events involve up-regulation and/or activation of other signal transducers, including STAT (signal transducer and activator of transcription) proteins, interferon regulatory factor 1 (IRF-1), interferon-α—stimulated gene factor 3 γ (ISGF3γ)/IRF-9, phosphoinositide 3-kinaseγ, extracellular regulated kinase 2 (ERK2), p38 mitogen-activated protein (MAPkinase), and Rac1.8-15 In vitro studies have shown that activation of some of these factors, including Stat1, a member of the STAT family, are required for ATRA-induced differentiation and growth arrest of myeloid cells.14,16 Also, the characterization of an ATRA-resistant APL cell line, which fails to activate Stat1, supports a requirement for this transcription factor in ATRA-induced differentiation.17
STAT proteins have previously been shown to regulate proliferation, both positively and negatively, through the up- or down-regulation of cyclins and CKIs. Stat1 is induced by many cytokines and growth factors and has been implicated in growth arrest in response to interferon γ (IFN-γ), IFN-α, and endothelial growth factor (EGF).18-20 Evidence of a direct link between Stat1 and cell cycle control has also been provided by the reported ability of Stat1 to regulate the expression of the p21 promoter, in response to EGF and IFN-γ.20 The activity of Stat1 is regulated by phosphorylation on Tyr701 by Janus kinases (JAKs), important for its dimerization, translocation to the nucleus, and binding to DNA.21 A second phosphorylation, of Ser727, is required for transcriptional responses to certain inducers, eg, IFN-γ, but not to others, eg, IFN-α.22,23 In accordance with this requirement, both Stat1 Tyr701 and Ser727 phosphorylation are necessary for growth arrest in response to IFN-γ, whereas IFN-α—induced growth arrest requires only Tyr701 phosphorylation.18 More recent data show that Ser727 phosphorylation of Stat1 is important mainly for the regulation of a subset of IFN-γ—induced genes.24 Interestingly, IFN-γ—induced transcriptional suppression of c-Myc, a transcription factor involved in proliferative responses, was suggested to be dependent on both Tyr701 and Ser727 phosphorylation of Stat1.25
ATRA induces terminal differentiation and growth arrest of U-937 cells.26,27 We have previously shown that ectopic expression of dominant-negative Stat1Tyr701Phe inhibits this process, supporting a role for Stat1 in the signaling events leading to cell cycle arrest.16 In this report, using U-937 cells stably expressing Stat1Ser727Ala, we provide evidence that Stat1 Ser727 phosphorylation is required for ATRA-induced growth arrest in the G0/G1 phase of the cell cycle. To determine the mechanism by which ATRA-induced Stat1 activation controls the cell cycle machinery, the regulation of CKIs and cyclins was investigated in U-937 cells expressing phosphorylation-deficient (Tyr701Phe or Ser727Ala) Stat1. Our data suggest that Stat1 is involved in the regulation of ATRA-induced G0/G1 arrest through down-regulation of c-Myc; cyclins A, B, D3, and E; and the concomitant up-regulation of p27 protein levels.
Materials and methods
Cell culture, all-trans retinoic acid induction
U-937-128,29 cells and transfected sublines were maintained in RPMI-1640 medium supplemented with 10% fetal bovine serum (Sigma, St Louis, MO), glutamine, and antibiotics in a humidified 5% CO2 in-air atmosphere at 37°C. All-trans retinoic acid (Sigma) was dissolved in ethanol/dimethyl sulfoxide (DMSO) (1:5) at 50-mM concentration and stored in aliquots at —70°C. Inductions were done with exponentially growing cells using 1 μM ATRA for the indicated time periods.
[3H]-thymidine incorporation assays
For analysis of DNA synthesis, triplicate cultures were induced as indicated. Aliquots of cells were labeled for 6 hours with 0.0074 MBq (0.2 μCi) [3H]-thymidine and harvested using a Titertek cell harvester (Skatron, Norway). Incorporated [3H]-thymidine was measured by liquid scintillation counting using a 1214 Rackbeta Liquid Scintillation Counter (LKB/Wallac, Turku, Finland).
Cell cycle analysis
Preparation of nuclei and analysis of cell cycle phase distribution was performed according to Vindelov et al.30 Briefly, cells were induced with 1 μM ATRA for the indicated time, washed once in phosphate-buffered saline (PBS), treated with 0.03 mg/mL trypsin (Sigma) for 10 minutes at room temperature. Then RNase A (0.08 mg/mL; Sigma) and Trypsin inhibitor (0.5 mg/mL; Sigma) were added, and cells were incubated for an additional 10 minutes at room temperature. Finally, prepared nuclei were stained with propidium iodide (PI; 0.2 mg/mL; Sigma). Stained nuclei were analyzed using a fluorescence-activated cell sorter (FACScan; Becton Dickinson, Mountain View, CA) and MacCycle software (Phoenix Flow Systems, San Diego, CA).
RNA isolation, RNAse protection assay, and Northern blot analysis
Total RNA was isolated using the RNAgents Total RNA Isolation System (Promega, Madison, WI) according to the manufacturer's instructions. RNAse protection assay was done with 7 μg total RNA using the RiboQuant Multi-Probe RNase Protection Assay system (Pharmingen, Becton Dickinson) according to the manual and 32 P-UTP (uridine triphosphate; Sigma) labeled multitemplate probes (Pharmingen). Multitemplate probes hCC-2 (p130, pRb, p107, p53, p57, p27, p21, p19, p18, p16, p14/15, L32, glyceraldehyde phosphate dehydrogenase [GAPDH]) and hCYC-1 (cyclin A, cyclin B, cyclin C, cyclin D1, cyclin D2, cyclin D3, cyclin A1, L32, GAPDH) were used to analyze expression of CKI and cyclin mRNA. RNAse protection assays (RPAs) were repeated at least 3 times, and one representative experiment is shown. For Northern blot analysis, 10 μg/well total RNA was fractionated in an agarose gel containing 1% formaldehyde and was subsequently transferred onto a Duralon-UV membrane (Stratagene, Cedar Creek, TX). [32P]-labeled probes were prepared (Megaprime DNA labeling systems; Amersham, Uppsala, Sweden) using an EcoRI-ClaI fragment spanning the third exon of the c-myc gene cDNA and a HindIII-XbaI fragment of a GAPDH cDNA. Hybridization was performed using the QuikHyb hybridization solution (Stratagene) according to the manufacturer's recommendations.
Cell lysates and Western blot analysis
Cells were collected by centrifugation at 1500 rpm; washed once with PBS; lysed in 1% NP-40, 0.1 M Tris (tris(hydroxymethyl)aminomethane)—HCl, pH 8.0, 0.15 M NaCl, and 5 mM EDTA (ethylenediaminetetraacetic acid) with complete protease inhibitor (Boehringer Mannheim, Mannheim, Germany), 1 mM DTT (dithiothreitol), 1 mM PMSF (phenylmethyl sulfonyl fluoride), 0.1 mM Na3Vo4, 10 mM NaF, 50 μM NaMoO4, and 1 mM ZnCl2; and incubated on ice for 15 minutes. Cell lysates were spun 15 minutes at 14 000 rpm in a chilled microcentrifuge, supernatants were collected, and the protein content was determined using the BioRad protein assay (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer's recommendations. Extracts were fractionated on Novex NuPage (4%-12%, 10%) precast gels using the Novex electrophoresis and blotting system (Novex, San Diego, CA). The membrane (Hybond-C extra; Amersham) was blocked in 5% dry milk in Tris-buffered saline with 0.1% Tween-20 (TTBS), or 1% dry milk and 1% bovine serum albumin (BSA) in TTBS when the pSer727 antibody was used, for 1 hour at room temperature. Incubation with primary antibody was done at 4°C overnight, and then the membranes were washed 5 times in TTBS and incubated with secondary horseradish peroxidase (HRP)—linked antibodies (DAKO, Glostrup, Denmark) for 1 hour at room temperature. After washing the membranes extensively in TTBS, antibody binding was detected using enhanced chemiluminescence (ECL) plus (Amersham Pharmacia Biotech, Uppsala, Sweden). Primary antibodies used were α-Stat1 (C-111), α-cyclin A (C-19), α-cyclin B (H-20), α-p27 (C-19), α-cyclin D2 (C-17), α-cyclin D3 (C-16), α-cyclin E (HE12), α-actin (I-19) (Santa Cruz Biotechnology, Santa Cruz, CA), α-pSer727-Stat1 (a kind gift from Dr Pavel Kovarik, Vienna, Austria), and the Phosphoplus Rb (Ser780, Ser795, Ser807/811) antibody kit (Cell Signaling Technology, Beverly, MA). Western blots were repeated 3 times with separate extract preparations, and one representative experiment is shown.
Immunoprecipitation
Cell lysates were prepared as described in “Cell lysates and Western blot analysis,” and equal amounts of protein were incubated with α-Myc antibodies (N-262) (Santa Cruz Biotechnology) at 4°C overnight and protein G-Sepharose beads at 4°C for 3 hours. The immunocomplexes were washed 3 times in lysis buffer and boiled at 95°C for 5 minutes prior to electrophoresis on Novex NuPage (4%-12%, 10%) precast gels using the Novex electrophoresis and blotting system (Novex). Western blot analysis was done as described in “Cell lysates and Western blot analysis,” using biotin-coupled C-33 monoclonal pan-Myc antibodies (Santa Cruz Biotechnology) followed by streptavidin-HRP conjugates. The C-33 antibodies were conjugated with 10 μg biotin-X-NHS (Calbiochem-Novabiochem, La Jolla, CA) per milligram antibody in 0.1 M NaHCO3, pH 7.4, 0.1 M NaCl for 1 hour at room temperature followed by dialysis in PBS.
Establishment of U-937 sublines
U-937 cells were transfected with a pCIneo vector (Promega) carrying Glu-Glu-tagged Stat1 (Ser727Ala) cDNA (a kind gift from Prof J. K. Chung, Taejon, Korea), through electroporation (220 V, 960 μF). Selection with 1000 μg/mL G418 started 48 hours after transfection, and transfectants were collected 3 to 4 weeks later. Selected sublines were tested for Stat1 expression by Western blot analysis using an α-Stat1 monoclonal antibody (C-111; Santa Cruz Biotechnology). Two separate U-937-pCIneo-Glu-Glu-Stat1 (Ser727Ala) transfectants (designated Ser727Ala.4 and Ser727Ala.5) were chosen and used throughout this study. The Stat1.2 and Tyr701Phe.8 sublines have previously been described.16
Results
Stat1 is phosphorylated on Ser727 in untreated U-937 cells, and the phosphorylation is further increased by ATRA
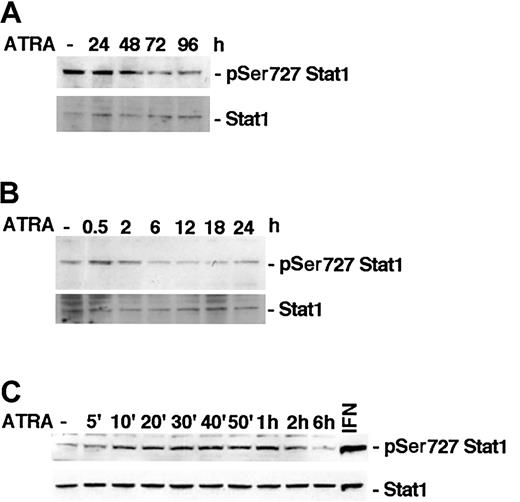
It has previously been reported that ATRA induces phosphorylation of Stat1 Ser727 in NB-4 cells within 48 hours of stimulation.15 To determine if ATRA treatment affects Stat1 serine phosphorylation in U-937 cells, lysates of treated or untreated cells were tested by Western blot using specific antibodies directed toward Ser727-phosphorylated Stat1. We did not detect an increase in Stat1 Ser727 phosphorylation after long-time ATRA stimulation in U-937 cells, but, conversely, an apparent reduction (Figure 1A). However, in untreated U-937 cells a high basal level of Ser727-phosphorylated Stat1 was detected, and it was transiently increased by ATRA, peaking after 30 minutes of treatment (Figure 1B). Increased phosphorylation was detected within 10 minutes of ATRA treatment and was sustained up to 2 hours after stimulation (Figure 1C). Vehicle did not induce serine or tyrosine phosphorylation of Stat1 in repeated experiments (data not shown). The transient increase in phosphorylation was relatively weak compared with the high basal level of Stat1 Ser727 phosphorylation detected in untreated cells and compared with the phosphorylation induced by IFN-γ (Figure 1, 2C).
ATRA treatment induces an early transient phosphorylation of Stat1 Ser727 in U-937 cells. (A-C) U-937 cells were treated with ATRA for 0 to 96 hours (A), 0 to 24 hours (B), or 0 to 6 hours (C). Lysates were prepared and analyzed by Western blot using antibodies directed against Ser727-phosphorylated Stat1. The blots were stripped and reprobed with antibodies targeted against total Stat1. A lysate of U-937 cells induced by 100 U IFN-γ for 30 minutes was included as a positive control for Stat1 Ser727 phosphorylation (C).
ATRA treatment induces an early transient phosphorylation of Stat1 Ser727 in U-937 cells. (A-C) U-937 cells were treated with ATRA for 0 to 96 hours (A), 0 to 24 hours (B), or 0 to 6 hours (C). Lysates were prepared and analyzed by Western blot using antibodies directed against Ser727-phosphorylated Stat1. The blots were stripped and reprobed with antibodies targeted against total Stat1. A lysate of U-937 cells induced by 100 U IFN-γ for 30 minutes was included as a positive control for Stat1 Ser727 phosphorylation (C).
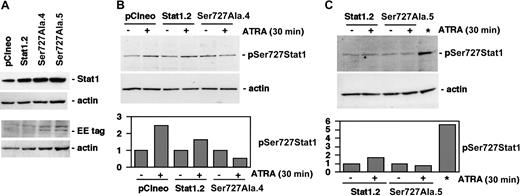
Established U-937 sublines constitutively express exogenous Stat1Ser727Ala. (A) The level of Stat1 protein in pCIneo, Stat1.2, Ser727Ala.4, and Ser727Ala.5 cells was determined by Western blot using antibodies directed against total Stat1. The presence of exogenously expressed Stat1Ser727Ala protein was confirmed by Western blot analysis using antibodies specific for the Glu-Glu tag. (B-C) U-937 sublines expressing wt Stat1 (Stat1.2), empty vector (pCIneo), or Stat1Ser727Ala (Ser727Ala.4, Ser727Ala.5) were incubated with ATRA for 30 minutes; lysates were prepared and analyzed by Western blot using antibodies directed against Ser727-phosphorylated Stat1. A lysate of Stat1.2 cells induced by 100 U IFN-γ for 30 minutes was included as a positive control for Stat1 Ser727 phosphorylation (*). Quantitation of Stat1 Ser727 phosphorylation, normalized to actin expression, is shown in the lower panels (fold induction).
Established U-937 sublines constitutively express exogenous Stat1Ser727Ala. (A) The level of Stat1 protein in pCIneo, Stat1.2, Ser727Ala.4, and Ser727Ala.5 cells was determined by Western blot using antibodies directed against total Stat1. The presence of exogenously expressed Stat1Ser727Ala protein was confirmed by Western blot analysis using antibodies specific for the Glu-Glu tag. (B-C) U-937 sublines expressing wt Stat1 (Stat1.2), empty vector (pCIneo), or Stat1Ser727Ala (Ser727Ala.4, Ser727Ala.5) were incubated with ATRA for 30 minutes; lysates were prepared and analyzed by Western blot using antibodies directed against Ser727-phosphorylated Stat1. A lysate of Stat1.2 cells induced by 100 U IFN-γ for 30 minutes was included as a positive control for Stat1 Ser727 phosphorylation (*). Quantitation of Stat1 Ser727 phosphorylation, normalized to actin expression, is shown in the lower panels (fold induction).
Establishment of U-937 sublines stably expressing Stat1Ser727Ala
We have previously reported that ATRA-induced growth arrest is inhibited by expression of Stat1Tyr701Phe, demonstrating the requirement of Tyr70-phosphorylated Stat1 for this process.16 In view of previous findings, demonstrating the need for Stat1 Ser727 phosphorylation for growth arrest in response to IFN-γ and EGF,18,19 we wanted to investigate if ATRA-induced cell cycle arrest additionally required Ser727-phosphorylated Stat1. To address this question, U-937 sublines expressing Glu-Glu—tagged Stat1Ser727Ala were established. Stat1 levels in Stat1Ser727Ala-expressing sublines (designated Ser727Ala.4 and Ser727Ala.5) were comparable to a previously established subline expressing wild-type (wt) Stat1 (designated Stat1.2) and greatly exceeded levels of endogenous Stat1 in the vector-transfected subline (designated pCIneo) (Figure 2A). The presence of exogenously expressed Stat1 was confirmed by Western blot using antibodies directed toward the Glu-Glu tag (Figure 2A). The ATRA-induced early increase in Stat1 Ser727 phosphorylation was inhibited by exogenous expression of Stat1Ser727Ala and induced normally in previously established sublines expressing wild-type Stat1 (Stat1.2) or empty vector (pCIneo) (Figure 2B-C).16 However, expression of Stat1Ser727Ala did not affect the considerable basal serine phosphorylation of endogenous Stat1 in untreated cells, nor the nuclear accumulation of Tyr701-phosphorylated Stat1 in response to ATRA (data not shown).
Expression of Stat1Ser727Ala inhibits ATRA-induced G0/G1 arrest in U-937 cells
To test whether the expression of a Ser727 phosphorylationdeficient Stat1 affected ATRA-induced growth arrest, U-937 sublines expressing Stat1Ser727Ala, pCIneo, or Stat1 were induced by ATRA or left untreated, and cell numbers were counted daily (Figure 3A). As previously demonstrated,16 U-937 cells expressing either empty vector construct or wild-type Stat1 were growth-arrested by ATRA within 48 hours of treatment. However, Stat1Ser727Ala-expressing cells continued to proliferate after ATRA induction. Cell counting data were confirmed by measurement of thymidine incorporation showing that the ATRA-induced decrease in DNA synthesis was inhibited in Stat1Ser727Ala-expressing sublines (Figure 3B). Finally, analysis of the cell cycle distribution after ATRA treatment was performed using FACS analysis of propidium iodide—labeled nuclei. Although pCIneo and Stat1.2 sublines showed a substantial reduction of the percentage of cycling (S + G2/M) cells after 72 hours of ATRA treatment, consistent with the G1 accumulation previously described for U-937 parental cells,31 ATRA did not induce G0/G1 arrest to the same extent in Stat1Ser727Ala-expressing sublines (Figure 3C). At 96 hours after induction, a reduction in cycling cells was also noted in Ser727Ala.4 and Ser727Ala.5 sublines, but the effect was clearly less pronounced than that observed in pCIneo and Stat1.2 cells.
Expression of Stat1Ser727Ala in U-937 cells inhibits ATRA-induced growth arrest. (A) pCIneo, Stat1.2, Ser727Ala.4, and Ser727Ala.5 cells were seeded at 100 000 cells/mL in the presence of ATRA and counted daily. Results are shown as the percentage of untreated control cells. Mean ± SD (n = 3). (B) Cells were incubated with ATRA for 48 hours, and DNA synthesis was determined by measuring [3H]-thymidine (TdR) incorporation correlated to the total cell number. Data for incorporated [3H]-TdR per cell are shown. Mean ± SD (n = 3). (C) The distribution of cells in different cell cycle phases was determined by flow cytometry of propidium iodide—stained nuclei after 72 and 96 hours of ATRA induction. The graph shows the percentage of cells in the S + G2/M phases. Mean ± SD (n = 3). Representative cell cycle profiles (72 hours after induction) are shown in the right histograms.
Expression of Stat1Ser727Ala in U-937 cells inhibits ATRA-induced growth arrest. (A) pCIneo, Stat1.2, Ser727Ala.4, and Ser727Ala.5 cells were seeded at 100 000 cells/mL in the presence of ATRA and counted daily. Results are shown as the percentage of untreated control cells. Mean ± SD (n = 3). (B) Cells were incubated with ATRA for 48 hours, and DNA synthesis was determined by measuring [3H]-thymidine (TdR) incorporation correlated to the total cell number. Data for incorporated [3H]-TdR per cell are shown. Mean ± SD (n = 3). (C) The distribution of cells in different cell cycle phases was determined by flow cytometry of propidium iodide—stained nuclei after 72 and 96 hours of ATRA induction. The graph shows the percentage of cells in the S + G2/M phases. Mean ± SD (n = 3). Representative cell cycle profiles (72 hours after induction) are shown in the right histograms.
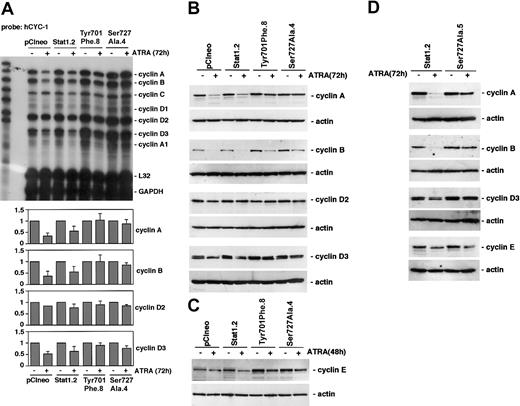
Inhibition of ATRA-induced cell cycle arrest by phosphorylation-deficient Stat1 proteins is associated with an impaired down-regulation of cyclin A, cyclin B, cyclin D3, and cyclin E
To investigate the mechanism by which Stat1 regulates ATRA-induced cell cycle arrest, we analyzed the expression of cyclins in sublines expressing pCIneo, Stat1, and Stat1Ser727Ala, during this process. We also included a previously established U-937 subline expressing Stat1Tyr701Phe (designated Tyr701Phe.8), which also fails to undergo ATRA-induced cell cycle arrest.16 Sublines were grown with or without ATRA for 72 hours, total mRNA was prepared, and RNAse protection assay (RPA) analysis was done using the Pharmingen h-CYC-1 multitemplate probe (Figure 4A). In agreement with previous studies on ATRA-induced differentiation of U-937 cells, pCIneo- and Stat1-expressing U-937 sublines down-regulated mRNA levels of cyclins A, B, D2, and D3 after ATRA treatment.6 Expression of either Stat1Tyr701Phe or Stat1Ser727Ala resulted in maintained cyclin A, B, D2, and D3 mRNA levels during ATRA-induced differentiation. Western blot analysis confirmed that the ATRA-induced down-regulation of cyclins A and B, and the slight reduction in cyclins D2 and D3, evident after 72 hours of treatment, was partially inhibited by expression of either phosphorylation-deficient form of Stat1 (Figure 4B). We have previously identified cyclin E down-regulation as an important early event in ATRA-induced growth arrest of U-937 cells, coinciding with the onset of cell cycle arrest.6 Interestingly, cyclin E down-regulation was impaired in Tyr701Phe.8 and Ser727Ala.4 cells after 48 hours of ATRA induction (Figure 4C). As the basal level of cyclin B appeared to be raised in Ser727Ala.4 and Tyr701Phe.8 cells, the experiment was repeated using another U-937 subline expressing Stat1Ser727Ala, Ser727Ala.5. Similar to Ser727Ala.4 and Tyr701Phe.8 cells, ATRA-induced down-regulation of cyclins was impaired in Ser727Ala.5 cells, but the basal level of cyclin B was found to be comparable to that in Stat1.2 cells (Figure 4D).
ATRA-induced down-regulation of cyclins A, B, D3, and E is inhibited by expression of Stat1Ser727Ala or Stat1Tyr701Phe. (A) U-937 sublines were induced by ATRA for 72 hours, and total mRNA was prepared. The cyclin mRNA levels after ATRA treatment were analyzed by RPA using the hCYC-1 multitemplate probe. The lower panels show quantitation of cyclin mRNA expression that was found to be appreciably changed by ATRA treatment, normalized to GAPDH (fold induction). Mean ± SD (n = 3-4). (B-C) The protein levels of cyclins A, B, D2, and D3 in pCIneo, Stat1.2, Tyr701Phe.8, and Ser727Ala.4 cells were determined after 72 hours of ATRA stimulation by Western blot analysis (B), and the level of cyclin E was analyzed after 48 hours of treatment (C). (D) Expression of cyclins A, B, D3, and E in Ser727Ala.5 cells (C) were determined after 72 hours of ATRA stimulation by Western blot analysis.
ATRA-induced down-regulation of cyclins A, B, D3, and E is inhibited by expression of Stat1Ser727Ala or Stat1Tyr701Phe. (A) U-937 sublines were induced by ATRA for 72 hours, and total mRNA was prepared. The cyclin mRNA levels after ATRA treatment were analyzed by RPA using the hCYC-1 multitemplate probe. The lower panels show quantitation of cyclin mRNA expression that was found to be appreciably changed by ATRA treatment, normalized to GAPDH (fold induction). Mean ± SD (n = 3-4). (B-C) The protein levels of cyclins A, B, D2, and D3 in pCIneo, Stat1.2, Tyr701Phe.8, and Ser727Ala.4 cells were determined after 72 hours of ATRA stimulation by Western blot analysis (B), and the level of cyclin E was analyzed after 48 hours of treatment (C). (D) Expression of cyclins A, B, D3, and E in Ser727Ala.5 cells (C) were determined after 72 hours of ATRA stimulation by Western blot analysis.
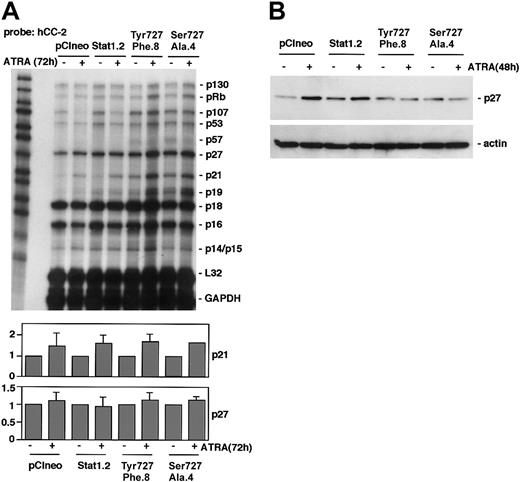
Expression of Stat1Ser727Ala or Stat1Tyr701Phe inhibits ATRA-induced up-regulation of p27Kip1 but not p21WAF1/CIP1
To determine if expression of phosphorylation-deficient Stat1 disturbed ATRA-induced regulation of CKIs, RPA analysis was performed using the Pharmingen h-CC2 multitemplate probe. In accord with previous observations in U-937 cells,5 p21 was up-regulated in response to ATRA treatment in pCIneo and Stat1.2 cells (Figure 5A). This ATRA-induced increase in p21 expression was also noted in U-937 sublines expressing phosphorylationdeficient Stat1Tyr701Phe or Stat1Ser727Ala, along with an increased basal expression of p21 mRNA. This finding points to a Stat1-independent regulation of p21, consistent with the findings of an RARE motif in the p21 promoter.5 The mRNA levels of p27 were not significantly changed by ATRA treatment in repeated experiments. However, ATRA-induced up-regulation of p27 protein in U-937 cells occurs by a posttranscriptional mechanism between 24 and 48 hours of treatment.6 Therefore, p27 expression was analyzed by Western blot. Although p27 was up-regulated in pCIneo and Stat1.2 cells after 48 hours of ATRA treatment, p27 levels remained low in sublines expressing Stat1Tyr701Phe or Stat1Ser727Ala (Figure 5B).
Expression of Stat1Ser727Ala or Stat1Tyr701Phe in U-937 cells inhibits ATRA-induced up-regulation of p27 but not p21. (A) pCIneo, Stat1.2, Tyr701Phe.8, and Ser727Ala.4 cells were incubated with ATRA for 72 hours or left untreated. Total mRNA was prepared and subjected to RPA analysis using the hCC-2 multitemplate probe. The lower panels show quantitation of p21 and p27 mRNA expression, normalized to GAPDH (fold induction). Mean ± SD (n = 3-4). (B) Cells were grown with or without ATRA for 48 hours; lysates were prepared and analyzed by Western blot using antibodies directed against p27.
Expression of Stat1Ser727Ala or Stat1Tyr701Phe in U-937 cells inhibits ATRA-induced up-regulation of p27 but not p21. (A) pCIneo, Stat1.2, Tyr701Phe.8, and Ser727Ala.4 cells were incubated with ATRA for 72 hours or left untreated. Total mRNA was prepared and subjected to RPA analysis using the hCC-2 multitemplate probe. The lower panels show quantitation of p21 and p27 mRNA expression, normalized to GAPDH (fold induction). Mean ± SD (n = 3-4). (B) Cells were grown with or without ATRA for 48 hours; lysates were prepared and analyzed by Western blot using antibodies directed against p27.
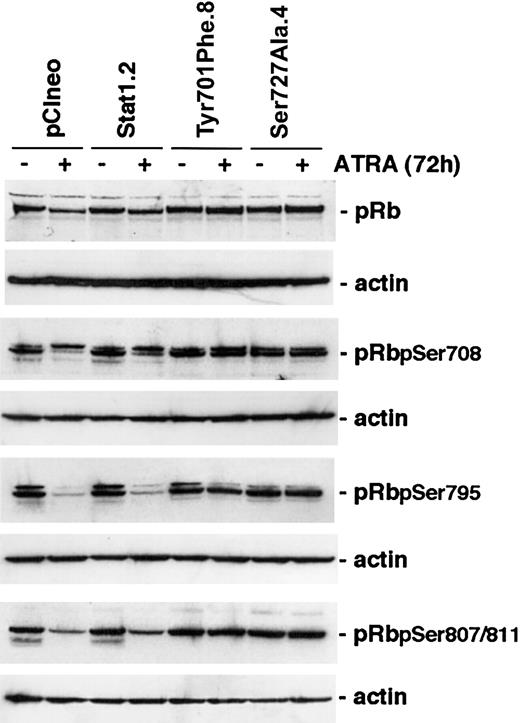
ATRA-induced dephosphorylation of pRb is inhibited in U-937 sublines expressing phosphorylation-deficient Stat1 proteins
Cyclins and CKIs regulate cell cycle progression through the activation or inactivation of CDKs. A key substrate for CDK-mediated serine phosphorylation is pRb that, through the interaction with E2F, regulates target genes important for S-phase entry.2,32 ATRA treatment of myeloid cells has previously been shown to inhibit phosphorylation of pRb.6,33 Because constitutive expression of Stat1Tyr701Phe or Stat1Ser727Ala in U-937 sublines inhibited both ATRA-induced up-regulation of p27 and down-regulation of cyclins A, B, D3, and E, we investigated whether the inhibition of growth arrest was at least partly due to maintained pRb serine phosphorylation during ATRA treatment. Cells were induced by ATRA for 96 hours or left untreated, lysates were prepared, and the levels of total pRb and phosphorylated forms of pRb important for E2F binding (Ser780 and Ser795)34 or binding to c-Abl (Ser807/811)35 were determined by Western blot analysis. Notably, the decline in the levels of Ser780-, Ser795-, and Ser807/811-phosphorylated pRb, in response to ATRA induction, was inhibited in U-937 sublines expressing either phosphorylationdeficient form of Stat1 (Figure 6).
The level of hyperphosporylated pRB is maintained after ATRA treatment in cells expressing Stat1Ser727Ala or Stat1Tyr701Phe. U-937 sublines were induced by ATRA for 72 hours, whole cell lysates were prepared, and the levels of total pRb or Ser780-, Ser795-, or Ser807/811-phosphorylated pRb were determined by Western blot analysis using phosphospecific antibodies.
The level of hyperphosporylated pRB is maintained after ATRA treatment in cells expressing Stat1Ser727Ala or Stat1Tyr701Phe. U-937 sublines were induced by ATRA for 72 hours, whole cell lysates were prepared, and the levels of total pRb or Ser780-, Ser795-, or Ser807/811-phosphorylated pRb were determined by Western blot analysis using phosphospecific antibodies.
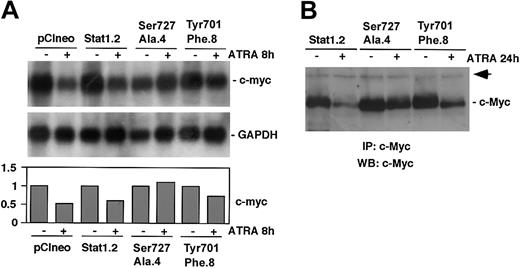
ATRA-induced down-regulation of c-Myc is impaired by expression of Stat1Ser727Ala or Stat1Tyr701Phe
We have previously reported that ATRA-induced differentiation of U-937 cells is associated with an early down-regulation of c-Myc.6,36 In addition, we have shown that exogenous expression of c-Myc blocks ATRA-induced differentiation and growth arrest in U-937 cells37 and impairs regulation of cyclin E and p27.6 Finally, Stat1 has been shown to negatively regulate c-Myc expression in response to interferons, a process that was suggested to be dependent on Stat1 Ser727 phosphorylation.25 In view of these findings we investigated if expression of Stat1Tyr701Phe or Stat1Ser727Ala affected ATRA-induced down-regulation of c-Myc. Although a slight reduction of c-myc mRNA expression was observed after 8 hours of ATRA treatment in the U-937 subline expressing Stat1Tyr701Phe (Tyr701Phe.8), and no decrease was noted in Stat1Ser727Ala-expressing cells (Ser727Ala.4), the mRNA level of c-myc was more sharply reduced in cells expressing empty vector (pCIneo) or a high level of wt Stat1 (Stat1.2) (Figure 7A). The level of c-Myc was determined by immunoprecipitation followed by Western blot analysis. Consistent with the mRNA data, the level of c-Myc was only slightly reduced after 24 hours of ATRA treatment in cells expressing phosphorylation-deficient Stat1, whereas a significant reduction was observed in cells expressing wt Stat1 (Figure 7B).
ATRA-induced down-regulation of c-Myc is inhibited by expression of Stat1Tyr701Phe or Stat1Ser727Ala. (A) pCIneo, Stat1.2, Ser727Ala.4, and Tyr701Phe.8 cells were induced by ATRA for 8 hours or left untreated. Total RNA was prepared, and the levels of c-Myc and GAPDH mRNAs were determined by Northern blot analysis. Quantitation of c-myc expression, normalized to GAPDH, is shown in the lower panel (fold induction). (B) Stat1.2, Ser727Ala.4, and Tyr701Phe.8 cells were induced as described earlier, and whole cell lysates were prepared. Equal amounts of protein were subjected to immunoprecipitation using α-c-Myc antibodies. The immunocomplexes were collected and analyzed by Western blot analysis using biotin-coupled pan-Myc antibodies and streptavidin-HRP. The arrow indicates an unknown protein that is unspecifically coprecipitated by the α-c-Myc antibodies and serves as an internal control.
ATRA-induced down-regulation of c-Myc is inhibited by expression of Stat1Tyr701Phe or Stat1Ser727Ala. (A) pCIneo, Stat1.2, Ser727Ala.4, and Tyr701Phe.8 cells were induced by ATRA for 8 hours or left untreated. Total RNA was prepared, and the levels of c-Myc and GAPDH mRNAs were determined by Northern blot analysis. Quantitation of c-myc expression, normalized to GAPDH, is shown in the lower panel (fold induction). (B) Stat1.2, Ser727Ala.4, and Tyr701Phe.8 cells were induced as described earlier, and whole cell lysates were prepared. Equal amounts of protein were subjected to immunoprecipitation using α-c-Myc antibodies. The immunocomplexes were collected and analyzed by Western blot analysis using biotin-coupled pan-Myc antibodies and streptavidin-HRP. The arrow indicates an unknown protein that is unspecifically coprecipitated by the α-c-Myc antibodies and serves as an internal control.
Discussion
Terminal differentiation of hematopoietic cells requires the sequential activation of multiple signaling pathways, linking morphologic maturation to a permanent arrest in the G0/G1 phase of the cell cycle. We have demonstrated that disruption of the Stat1 signaling pathway, through expression of either Stat1Tyr701Phe or Stat1Ser727Ala, inhibits ATRA-induced down-regulation of cyclins A, B, D3, and E and up-regulation of p27Kip1.We find sustained levels of phosphorylated pRb and continued cell cycle progression associated with this failure to regulate cyclins and p27Kip1 expression. In addition, down-regulation of c-Myc, a key event in the process of terminal differentiation and growth arrest, was impaired in sublines expressing phosphorylation-deficient Stat1. Our results suggest that Stat1 phosphorylation on both Tyr701 and Ser727 are important steps in the ATRA-induced pathways leading to modulation of the cell cycle machinery and G0/G1 arrest.
Previous work has demonstrated an increase in Stat1 Tyr701 phosphorylation in response to ATRA treatment of myeloid cells.12,16 Our data further show a rapid phosphorylation on Stat1 Ser727 within 10 minutes of ATRA treatment. A previous study reported up-regulation of Stat1 Ser727 phosphorylation after 48 hours of ATRA induction in NB-4 cells.15 We did not observe a similar induction in U-937 cells, rather Ser727 phosphorylation appeared to be reduced at later times. Instead, we detected a high basal level of Stat1 Ser727 phosphorylation that is transiently increased, peaking at 30 minutes of ATRA treatment. The requirement for Stat1 Ser727/Tyr701 phosphorylation differs between Stat1-containing complexes. Expression of Stat1Ser727Ala in Stat1-deficient U3A cells can rescue biologic responses to IFN-α (Stat1/Stat2/p48), but not IFN-γ (Stat1/Stat1), whereas Stat1 Tyr701 phosphorylation is required for both inducers.18,38 In contrast, Stat1Tyr701Phe can function in complex with IRF-1 to activate transcription of the LMP2 gene.39 Also, both Stat1Ser727Ala and Stat1Tyr701Phe restored constitutive caspase expression in U3A cells.40 Phosphorylation on Stat1 Ser727 has been demonstrated to mediate differential induction of target genes in response to IFN-γ.24 Serine phosphorylation of Stat1 increases its association to MCM5, a member of the mini chromosome maintenance family, which enhances the transcriptional response to IFN-γ.41 In addition, Ser727-phosphorylated Stat1 interacts with BRCA1 to induce transcription of a subset of IFN-γ target genes.42 The strong phenotype of Stat1Ser727Ala-expressing cells suggests a requirement for Ser727-phosphorylated Stat1 in Stat1-containing complexes induced by ATRA. Because we observe only a slight increase in Ser727 phosphorylation after ATRA induction, and as the basal phosphorylation remains high, we find it unlikely that the reduction of the transient increase by constitutive expression of Stat1Ser727Ala is the underlying mechanism for the failure to arrest in G0/G1. However, as tyrosine phosphorylation of Stat1 occurs independently of serine phosphorylation,43 the high ratio between Stat1Ser727Ala and endogenous Stat1 in these cells may result in nuclear accumulation of tyrosine-phosphorylated Stat1Ser727Ala. The dominant-negative effect of Stat1Ser727Ala in these cells is thus more likely to depend on incorporation of phosphorylation-deficient Stat1 in ATRA-induced signaling complexes, rather than a complete block of Ser727 phosphorylation of endogenous Stat1.
The changes in cyclin expression associated with ATRA-induced differentiation occur in a temporally ordered fashion. A sharp down-regulation of cyclins A and B occurs after 72 hours of ATRA induction in U-937 cells, when a large percentage of the cells is arrested in the G0/G1 phase of the cell cycle, whereas cyclin D3 levels are gradually decreased and cyclin D2 levels remain high during differentiation.6 However, changes in the cyclin E and p27 levels precede the down-regulation of cyclins A, B, and D3, coinciding with, and perhaps triggering, the onset of ATRA-induced cell cycle arrest of U-937 cells. These changes are also associated with growth arrest in the related myeloid cell lines HL-60 and NB-4.6 As cyclin E activates CDK2, and p27 inhibits that same complex, the parallel decrease in cyclin E levels and increase in p27 would be expected to repress CDK2 activity and thus inhibit S-phase entry.2 Interestingly, expression of either Stat1Tyr701Phe or Stat1Ser727Ala repressed ATRA-induced changes in cyclin E and p27 levels, resulting in maintained phosphorylation of Rb and the ultimate failure of the cells to become growth-arrested. However, it should be noted that down-regulation of cyclins was not completely abrogated by expression of phosphorylation-deficient Stat1, suggesting that other signals may participate in ATRA-induced cell cycle control.
The impaired ATRA-induced down-regulation of c-Myc observed in Stat1Tyr701Phe- and Stat1Ser727Ala-expressing sublines is consistent with previous reports, showing that suppression of c-Myc in response to interferons is dependent on both Tyr701- and Ser727-phosphorylated Stat1.25 Interestingly, c-Myc has been demonstrated to regulate cyclin E-CDK2 activity by 2 separate mechanisms: through induction of cyclin E expression and by inhibiting p27 association with cyclin E-CDK2 complexes.44,45 In turn, cyclin E-CDK2 complexes have been demonstrated to regulate p27 protein levels through phosphorylation of Thr187,46 which is necessary for proteosome-dependent degradation of p27.47 Consistent with this, constitutive expression of c-Myc in U-937 cells inhibits both ATRA-induced growth arrest and the associated regulation of cyclin E and p27.6 It is tempting to speculate that Stat1-dependent down-regulation of c-Myc may trigger down-regulation of cyclin E and up-regulation of p27, inhibiting S-phase progression and initiating ATRA-induced cell cycle arrest. Down-regulation of cyclin A and cyclin B may follow as a direct consequence of accumulation of cells in the G0/G1 phase of the cell cycle.
Taken together, our data suggest that Stat1-dependent regulation of c-Myc, cyclin E, and p27 are important early events in ATRA-induced growth arrest of U-937 cells and that Stat1 may direct differentiation-associated growth arrest by acting upstream of multiple cell cycle regulators.
Prepublished online as Blood First Edition Paper, March 13, 2003; DOI 10.1182/blood-2002-10-3149.
Supported by the Swedish Cancer Society; the Swedish Society for Medicine; and the Göran Gustafsson, Magnus Bergvall, HKH Lovisa/Thielman, Hans Von Kantzow, Åke Wiberg, and King Gustaf V's 80-year foundations.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Lina Dimberg for critical reading of the manuscript, and Drs F. Bahram, P. Kovarik, T. Decker, and J. K. Chung for generously providing reagents.

![Figure 3. Expression of Stat1Ser727Ala in U-937 cells inhibits ATRA-induced growth arrest. (A) pCIneo, Stat1.2, Ser727Ala.4, and Ser727Ala.5 cells were seeded at 100 000 cells/mL in the presence of ATRA and counted daily. Results are shown as the percentage of untreated control cells. Mean ± SD (n = 3). (B) Cells were incubated with ATRA for 48 hours, and DNA synthesis was determined by measuring [3H]-thymidine (TdR) incorporation correlated to the total cell number. Data for incorporated [3H]-TdR per cell are shown. Mean ± SD (n = 3). (C) The distribution of cells in different cell cycle phases was determined by flow cytometry of propidium iodide—stained nuclei after 72 and 96 hours of ATRA induction. The graph shows the percentage of cells in the S + G2/M phases. Mean ± SD (n = 3). Representative cell cycle profiles (72 hours after induction) are shown in the right histograms.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/1/10.1182_blood-2002-10-3149/5/m_h81334550003.jpeg?Expires=1769244270&Signature=Wj797i5eZMbNGIvyEYc69~uOq5aeA3JL1imCpRZ7wS7acCnZ98oAK2NhqVgA3A-Io~VNvbYIoanYhHcFXTVWv3w6nEJd7rpYczqBMxzyKt1MqMHtsq4mko3iSYcZ0g9MPR~FhBJOA9I2F1br2sfY7BP6Vtb7PSYW5Osl1urILMNSjI1tk4cp-QuSgg8Dog3wQCfiyLvxNVPFTuEBJUORYCSm-0YRftKrByPXk~gVTDlhIluowUSzuf6~uu7SEQHWt3D70TENVr3BiPgFAPtlm3YrKmxr-fLha~4Y6DQGLtIeLYhiHtu~dJsGW7UnJqGoGpl562EgfL8slaYnKOztaQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)