Abstract
Programmed cell death, or apoptosis, is a tightly regulated, naturally occurring process by which damaged or unwanted cells are removed. Dysregulated apoptosis has been implicated in a variety of pathophysiological conditions, including degenerative diseases, tissue remodeling, and tumorogenesis. The decision to live or die results from integration of numerous environmental signals transmitted by specific classes of cell surface receptors that bind hormones, growth factors, or components of the extracellular matrix. Here we show that platelet endothelial cell adhesion molecule-1 (PECAM-1), a homophilic-binding member of the immunoreceptor tyrosine-based inhibitory motif (ITIM) family of inhibitory receptors, functions prominently to inhibit apoptosis in naturally occurring vascular cells subjected to apoptotic stimuli. Murine endothelial cells and human T lymphocytes lacking PECAM-1 were found to be far more sensitive than their PECAM-1—expressing counterparts to multiple death signals that stimulate Bax, a multidomain, proapoptotic member of the Bcl-2 family that plays a central role in mitochondrial dysfunction-dependent apoptosis. In addition, PECAM-1 markedly suppressed Bax overexpression—induced cytochrome c release, caspase activation, and nuclear fragmentation. Amino acid substitutions within PECAM-1's extracellular homophilic binding domain, or within its cytoplasmic ITIM, completely abolished PECAM-1—mediated cytoprotection. Taken together, these data implicate PECAM-1 as a novel and potent suppressor of Bax-mediated apoptosis and suggest that members of the immunoglobulin gene (Ig) superfamily, like cell surface integrins, may also transmit survival signals into blood and vascular cells. (Blood. 2003;102:169-179)
Introduction
Platelet endothelial cell adhesion molecule-1 (PECAM-1; also known as CD31) is a 130-kDa member of the immunoglobulin gene (Ig) superfamily expressed on the surface of circulating platelets and leukocytes and at the intercellular junctions of all continuous endothelium.1-3 Extracellular Ig homology domain 1 possesses homophilic binding properties4,5 and functions to mediate leukocyte transendothelial migration6,7 and angiogenesis,8 while the cytoplasmic domain harbors a functional9-11 immunoreceptor tyrosine-based inhibitory motif (ITIM) that, when tyrosine phosphorylated, has been shown to recruit and activate the protein-tyrosine phosphatase, SHP-2, in a number of cellular systems, including human platelets,12 bovine aortic vascular endothelial cells,13 and rat basophilic leukemia cells.14 Owing, in part, to its cytoplasmic ITIM, PECAM-1 has recently been assigned to the Ig-ITIM family of inhibitory receptors.15
In addition to its role in vascular cell adhesion and signaling, there is growing evidence that PECAM-1 may be able to transduce signals that suppress programmed cell death. The first evidence of a role for PECAM-1 in apoptosis was provided by the studies of Noble et al,16 who found that monocytes promoted the survival of serum-starved endothelial cells. Interestingly, when the anti—PECAM-1 monoclonal antibody (mAb), PECAM-1.3, was included in the monocyte/endothelial cell coculture, the cytoprotective role of added monocytes was lost. Because PECAM-1.3 inhibits PECAM-1 homophilic interactions,4,17 the authors speculated that monocyte PECAM-1—endothelial cell PECAM-1 homophilic interactions might contribute to endothelial cell survival. Further evidence that engagement of PECAM-1 can result in the transduction of a survival signal was provided by studies showing that the rate and extent of serum deprivation—induced apoptosis of endothelial cells is lessened if endothelial cells are first attached to immobilized PECAM-1/IgG 18 or treated with an anti—PECAM-1 monoclonal antibody.19 While the latter investigation, like the study of Noble et al, found a correlation between PECAM-1—induced cell survival and increased transcript levels of the antiapoptotic gene, A1, the molecular mechanisms by which PECAM-1 might exert its cytoprotective effects have not to date been examined.
Two major cell death pathways—termed the extrinsic and intrinsic pathways of apoptosis—exist in mammalian cells (reviewed by Hengartner20 ). The extrinsic pathway is initiated by engagement and aggregation of tumor necrosis factor (TNF) family death receptors (such as CD95/Fas) which, through a series of death domain—containing adaptor molecules, recruit and directly activate cytosolic caspase 8, which in turn converts procaspase 3 to caspase 3—the central executioner of the apoptotic process. This pathway is thought to be largely mitochondrial independent21 —though arguably so.22 In contrast, the intrinsic pathway is intimately tied to the integrity of mitochondria (and other intracellular organelles), the health of which is maintained by a carefully regulated balance of proapoptotic and antiapoptotic members of the B-cell lymphoma (Bcl)—2 family of proteins. In mitochondria of normal, healthy cells, resident antiapoptotic proteins such as Bcl-2 and Bcl-XL effectively bind up and neutralize proapoptotic proteins, including a series of cofactors known as BH3 domain-only molecules,23 and prevent perturbation of the outer mitochondrial membrane. Under conditions of cellular stress (eg, oxidative injury, UV- or x-irradiation, exposure to cytotoxic agents), however, multidomain, proapoptotic proteins residing either in the cytosol (Bax) or within the outer mitochondrial membrane (Bak) become activated and, in the case of Bax, translocate from the cytosol to the membranes of the endoplasmic reticulum and the mitochondria.24,25 A second series of events, likely involving BH3-only domain-containing cofactors, results in allosteric conformational activation of Bak and Bax, including their homo-oligomerization, ultimately resulting in an increase in mitochondrial membrane permeability,23 with subsequent release into the cytosol of mitochondrial intermembrane compartment—resident proapoptotic proteins such as cytochrome c and the second mitochondria-derived activator of caspases/direct IAP-binding protein with low pI (Smac/DIABLO) complex. Liberated cytochrome c assembles with a cytosolic, multidomain protein known as Apaf-1 to form a large, wheellike particle known as an apoptosome, which functions to bind and activate procaspase 926 —a potent activator of effector caspases 3 and 7.27 Released Smac/DIABLO contributes to the apoptotic process by inhibiting a series of endogenous caspase inhibitors known as inhibitors of apoptosis (IAPs), which normally interact with and inhibit the enzymatic activity of the initiator caspase 9 and effector caspases 3 and 7.28 Thus, both the location and the activation state of proapoptotic and antiapoptotic components of the cell death pathway are crucial for controlling cellular decisions about life and death.
Given its purported ability to delay serum starvation—induced endothelial cell death, we sought to examine the mechanism by which PECAM-1 suppresses apoptosis, using a number of well-defined in vivo and in vitro models of programmed cell death. We found that endogenously expressed PECAM-1 confers broad resistance to externally applied, cytotoxic stimuli that activate the mitochondrial-dependent cell death pathway. In addition, enforced expression of wild-type PECAM-1, but not mutant forms of PECAM-1 lacking homophilic binding or signaling capabilities, was sufficient to suppress Bax overexpression—induced cell death. Finally, we found that while PECAM-1 fails to block Bax translocation to mitochondrial membranes, it nonetheless markedly inhibits cytochrome c release from mitochondrial stores as well as subsequent caspase activation and nuclear condensation—all important indices of Bax-mediated apoptosis. Taken together, these data extend the list of cell surface adhesion molecules that are able to send prosurvival signals into cells and support the notion that PECAM-1 may be an important regulator of cell survival in those vascular cells in which it is expressed.
Materials and methods
Antibodies and reagents
Rabbit antihuman Bax (no. 554104) and mouse anticytochrome c (no. 556433) were purchased from Pharmingen/BD Biosciences (San Diego, CA). Mouse antihuman Bcl-2 antibody (Sc 7382) was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-Fas antibody clone CH 11 was obtained from Upstate Biotechnologies (Lake Placid, NY). Rabbit antihuman Bcl-XL antibody (B22630) was from Transduction Laboratories (Lexington, KY). Monoclonal antibodies (mAbs) against Akt (587F11) and serine-phosphorylated Akt (clone no. 4E2) were obtained from Cell Signaling Technology (Beverly, MA). Murine mAb to lactate dehydrogenase (LDH) (no. L7016) was purchased from Sigma Chemical (St Louis, MO), mouse antiproliferating cell nuclear antigen (anti-PCNA) (no. NA103) from Oncogene (Boston, MA), and to F1—adenosine triphosphatase (F1-ATPase) α-subunit (F1α) (no. A-11144) from Molecular Probes (Eugene, OR). PECAM-1—specific mAbs have been extensively characterized and described previously.4
Cell lines
The chicken B-cell line, DT40, and the SHP-2- variant of DT40 were obtained from Riken Cell Bank (Ibaraki, Japan). Wild-type and ITIM-less PECAM-1 transfectants of these cell lines have been developed previously and extensively characterized.9 DT40 cells were maintained at a density of 0.5 × 106 to 1.5 × 106/mL in a humidified, 37°C, 5% CO2/95% air atmosphere.
Jurkat cell lines were cultured in RPMI 1640 medium (BioWhittaker, Walkersville, MD) containing 2 mM l-glutamine (Life Technologies, Gaithersburg, MD), 1 mM sodium pyruvate (Life Technologies), 10 IU/mL heparin (Pharmacia/Upjohn, Kalamazoo, MI), 25 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) (Life Technologies), 50 mg/mL gentamicin (Elkins-Sinn, Cherry Hill, NC), 100 U/mL penicillin (Life Technologies), and 100 mg/mL streptomycin (Life Technologies) containing 10% fetal bovine serum (FBS; Life Technologies). PECAM-1- Jurkat cells were derived by 6 serial fluorescence-activated cell sorts (FACS) of PECAM-1low—expressing cells. For some experiments, Jurkat-negative cells were stably transfected by electroporation with either the mammalian expression vector pcDNA3 or with pcDNA3 containing full-length cDNA encoding wild-type or ITIM-less forms of human PECAM-1. Transfected Jurkat T-cell lines were grown in the presence of 2 mg/mL G418 and maintained in log phase growth at a density of 1 × 105 to 4 × 105/mL. Matrigel-coated 6-well plates and Matrisperse were purchased from Becton Dickinson. Endothelial cell growth supplement was from Sigma.
In vivo thoracic x-irradiation—induced apoptosis
C57BL/6 wild-type and PECAM-1—deficient mice were mildly anesthetized using a combination of ketamine and xylazine (70 and 7 mg/kg, respectively) and then irradiated in custom-made immobilization chambers that allow exposure of the entire lung (bilateral) with shielding of the head, abdomen, and extremities. The radiation source was an orthovoltage machine that uses 225 kilovoltage (peak) (kV(p)) energy, with a 0.2 mm Copper filter in place. All treatments were calibrated by a board-certified radiation oncology physicist to yield a radiation dose rate of approximately 1.69 Gy per minute at a depth of 1 cm below the surface of the mouse (corresponding to the midplane of the mouse thorax). Estimated radiation dose heterogeneity within the mouse lungs is typically 15% or less. The dose of irradiation is a single fraction delivered via single anteroposterior approach. This dose is prescribed at a depth of 2 cm below the anterior surface of the chest (corresponding approximately to midplane), yielding a final dose of 20 Gy. For quality assurance, thermoluminescent dosimeters were placed over the skin of the chest of selected mice to verify correct dose administration. A combined total of 22 wild-type and 26 PECAM-1—deficient mice were irradiated in 2 separate experiments. Following radiation treatment, animals were killed and analyzed at time points ranging from 1 to 48 hours after treatment. The data sets from both experiments were combined to generate the final data set used in Figure 1A-B.
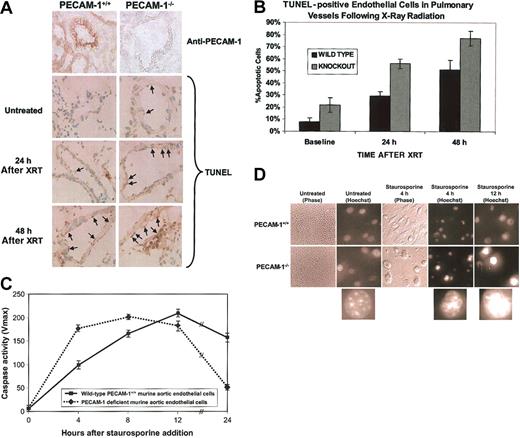
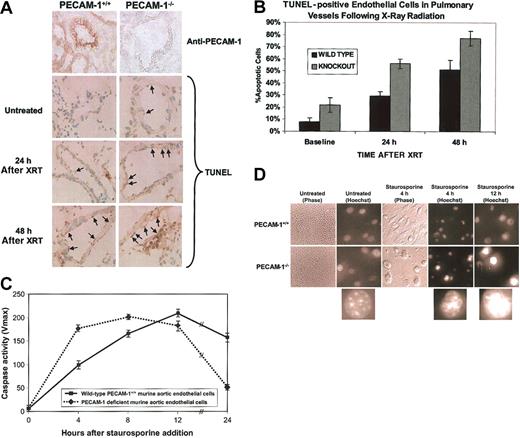
PECAM-1 protects against endothelial cell apoptosis. (A) Endothelial cell apoptosis in PECAM-1+/+ versus PECAM-1-/- following thoracic x-irradiation. The top 2 panels show immunohistochemical evaluation of PECAM-1 expression in pulmonary endothelium. PECAM-1 expression is completely absent from lung tissue in the knock-out animals. The bottom 6 panels show immunohistochemical staining for terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL) to detect fragmented DNA. TUNEL-positive pulmonary vessel endothelial cells have brown staining nuclei. Magnification, × 500. Positive signal is seen as a brown precipitate confined to the nucleus. (B) Quantitative evaluation of TUNEL-positive vessel endothelial cells. Percentage of apoptotic endothelial cells as determined by TUNEL positivity in 15 to 20 randomly selected large pulmonary vessels was evaluated from frozen lung sections at 0, 24, and 48 hours following x-ray irradiation of the thorax. Analysis of variance (ANOVA) yielded a P value of less than .001. (C) Aortic endothelial cells (AECs) derived from wild-type or PECAM-1—deficient C57BL/6 mice were grown in microtiter wells for up to 5 passages. Cell extracts were prepared from one set of wells, normalized for protein content, and their caspase activity determined as described in “Materials and methods.” Following addition of staurosporine, PECAM-1—deficient murine AECs reached near-maximal levels of cytosolic caspase activity nearly 8 hours earlier than did wild-type cells and were nearly all dead within 1 day. Data shown are the mean ± standard deviation of duplicate determination of a single representative experiment of 3 such performed. (D) Phase and fluorescent microscopic analysis of cell death. Nuclear morphology was assessed at the indicated time points using Hoechst dye 33358. A low-power (× 10) view (left panels) of untreated cells shows characteristic endothelial cell cobblestone morphology, while × 40 views of cellular nuclei show initially healthy chromatin, with progressive nuclear condensation with time following addition of staurosporine. This was especially pronounced in PECAM-1-/- AECs. PECAM-1+/+ cells appear much more firmly attached 4 hours after staurosporine treatment than do AECs lacking PECAM-1 (middle panels). Lower insets show magnified (approximately × 100, digitally) representative Hoechst-stained nuclei of AECs at various stages of condensation from PECAM-1—deficient AECs (taken from circled nuclei in the panel directly above).
PECAM-1 protects against endothelial cell apoptosis. (A) Endothelial cell apoptosis in PECAM-1+/+ versus PECAM-1-/- following thoracic x-irradiation. The top 2 panels show immunohistochemical evaluation of PECAM-1 expression in pulmonary endothelium. PECAM-1 expression is completely absent from lung tissue in the knock-out animals. The bottom 6 panels show immunohistochemical staining for terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL) to detect fragmented DNA. TUNEL-positive pulmonary vessel endothelial cells have brown staining nuclei. Magnification, × 500. Positive signal is seen as a brown precipitate confined to the nucleus. (B) Quantitative evaluation of TUNEL-positive vessel endothelial cells. Percentage of apoptotic endothelial cells as determined by TUNEL positivity in 15 to 20 randomly selected large pulmonary vessels was evaluated from frozen lung sections at 0, 24, and 48 hours following x-ray irradiation of the thorax. Analysis of variance (ANOVA) yielded a P value of less than .001. (C) Aortic endothelial cells (AECs) derived from wild-type or PECAM-1—deficient C57BL/6 mice were grown in microtiter wells for up to 5 passages. Cell extracts were prepared from one set of wells, normalized for protein content, and their caspase activity determined as described in “Materials and methods.” Following addition of staurosporine, PECAM-1—deficient murine AECs reached near-maximal levels of cytosolic caspase activity nearly 8 hours earlier than did wild-type cells and were nearly all dead within 1 day. Data shown are the mean ± standard deviation of duplicate determination of a single representative experiment of 3 such performed. (D) Phase and fluorescent microscopic analysis of cell death. Nuclear morphology was assessed at the indicated time points using Hoechst dye 33358. A low-power (× 10) view (left panels) of untreated cells shows characteristic endothelial cell cobblestone morphology, while × 40 views of cellular nuclei show initially healthy chromatin, with progressive nuclear condensation with time following addition of staurosporine. This was especially pronounced in PECAM-1-/- AECs. PECAM-1+/+ cells appear much more firmly attached 4 hours after staurosporine treatment than do AECs lacking PECAM-1 (middle panels). Lower insets show magnified (approximately × 100, digitally) representative Hoechst-stained nuclei of AECs at various stages of condensation from PECAM-1—deficient AECs (taken from circled nuclei in the panel directly above).
In vitro induction and quantitation of apoptosis
Mouse aortic endothelial cells (AECs) from wild-type and PECAM-1—deficient C57BL/6 mice were isolated from mouse aortic explants.29 Fibroblasts were selectively eliminated by growing cultures in d-valine—containing medium for 4 days.30 At approximately 50% confluence, 0.5 μM staurosporine (final concentration) was added to subconfluent passage 3 or 4 AECs to induce apoptosis. Then, 0, 4, 8, 12, and 24 hours after addition of staurosporine, an aliquot of cells was stained with 1% Hoechst dye 33358 and examined microscopically to assess nuclear morphology.31 Preliminary dose-response experiments (not shown) demonstrated that this concentration resulted in 90% cell death within 24 hours. All microscopic evaluations were performed in a double-blinded manner. Another aliquot of the cells was detergent lysed, normalized for protein content, and subjected to caspase activity determination according to a previously established protocol.32 In brief, 20 μg protein was incubated at 37°C with 100 μM benzyloxycarbonyl-Asp-Glu-Val-Asp-AFC (Z-DEVD-AFC) peptide substrate in a total volume of 100 μL caspase buffer (50 mM Tris [tris(hydroxymethyl)aminomethane]—HCl [pH 7.2], 100 mM KCl, 10% sucrose, 1% CHAPS [3-[(3-cholamidopropyl)dimethylammonio]-1-propane-sulfonic acid], 5 mM dithiothreitol [DTT]).33 Liberated AFC was monitored continuously over 5 minutes in a fluorometer (Molecular Devices, Sunnyvale, CA) using an excitation wavelength of 405 nm and an emission wavelength of 510 nm. Emission from each well was plotted against time and linear regression analysis of the initial velocity (slope) for each curve determined to yield total activity.
Jurkat cell apoptosis was induced by placing 5 × 106 cells into 10 mL prewarmed fresh media (RPMI 1640 containing 10% FBS, 25 mM HEPES, 2 mM l-glutamine, 100 U/mL penicillin, 10 μg/mL streptomycin, 1 mM sodium pyruvate, 10 U/mL heparin) and either adding anti-Fas antibody to a final concentration of 10 ng/mL or exposing cells to 100 to 500 J/m2 UV-C radiation. Then, 0, 2, 4, and 6 hours after induction, an aliquot of cells was removed, fixed with 2% formaldehyde, stained with Hoechst dye 33358, and nuclear morphology examined in triplicate, double-blinded samples to determine the percentage of apoptotic cells.
Bax overexpression—induced apoptosis.
Human embryonic kidney (HEK) 293 T cells (1 × 106) were seeded in duplicate 6-well plates and allowed to grow overnight in minimum essential medium (MEM) containing 10% heated-inactivated fetal calf serum, 100 U/mL penicillin, and 100 μg/mL streptomycin. pcDNA3 expression vectors (0.5 to 4 μg per well) encoding Bax, X-linked inhibitor of apoptosis protein (XIAP), wild-type PECAM-1, or selected mutant forms of PECAM-1 have been described previously34,35 and were transiently transfected into the 293 cells using GeneJammer transfection reagent (Stratagene) or, in some cases, FuGENE 6 (Roche Applied Science, Indianapolis, IN). A total of 0.5 μg of a green fluorescent protein (GFP)—encoding expression plasmid was included in all transfections to facilitate evaluation of transfected cells. Twenty hours later, GFP-expressing cells were stained with 1% Hoechst dye to quantify the percentage of apoptotic transfected cells. An aliquot of the cells was washed twice with ice-cold Dulbecco PBS, and intracellular caspase activity was determined as described above. In some experiments, cells were cultured in the absence or in the presence of the phosphatidylinositol-3 kinase (PI-3K) inhibitor wortmannin (100 nM) for another 20 hours. An aliquot of the cells was washed twice with ice-cold phosphate-buffered saline (PBS) and resuspended in lysis buffer (20 mM Tris-HCl, 150 mM NaCl, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride [PMSF], 50 mM sodium fluoride, 0.1% sodium dodecyl sulfate [SDS], 1% Triton X-100). Then, 100 μg protein was subjected to Western blot analysis using antibodies to PECAM-1, Akt, or Ser473-phosphorylated Akt.
Subcellular fractionation and cytochrome c release measurements
Cells were collected by centrifugation at 600g for 10 minutes at 4°C, washed with ice-cold DPBS, and resuspended in 200 μL ice-cold homogenization buffer (250 mM sucrose, 20 mM HEPES [pH 7.5], 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA [ethylenediaminetetraacetic acid], 0.1 mM phenylmethylsulfonyl fluoride, 20 μg leupeptin, and 10 μg aprotinin). Cells were then homogenized by 100 to 200 strokes of a Teflon homogenizer, and the homogenate was centrifuged at 1000g for 10 minutes at 4°C to pellet nuclei. The resulting supernatant was centrifuged at 14 000g for 30 minutes at 4°C to yield a mitochondria-containing heavy membrane pellet and a cytosolic supernatant. Nuclear and heavy membrane pellets were dissolved in 20 μL of 1 × SDS—polyacrylamide gel electrophoresis (SDS-PAGE) sample loading buffer, boiled for 5 minutes, and subjected to immunoblot analysis using the procedure for cytochrome c determination of Yang et al.36 Antibodies to LDH, PCNA, and F1-ATPase α-subunit were used to verify proper enrichment for cytosolic, nuclear, and mitochondria-containing heavy membrane fractions, respectively. Total cell lysates were prepared in ice-cold lysis buffer containing 50 mM NaCl, 25 mM HEPES (pH 7.4), 1 mM EDTA, 1 mM EGTA (ethylene glycol tetraacetic acid), 1 mM PMSF, 10 μg/μL protease inhibitor cocktail (Sigma), and 1% Triton X-100).
Coimmunoprecipitation analysis of PECAM-1—Bax interactions
HEK 293 T cells were transiently transfected with 2 μg Bax-encoding plasmid together with 8 μg wild-type PECAM-1—encoding plasmid. Following transfection, cells were grown for an additional 20 hours in MEM medium containing 50 μM of the caspase inhibitor, zVAD-fmk. Following solubilization in Triton lysis buffer (145 mM NaCl, 0.1 mM MgCl2, 15 mM HEPES, 10 mM EDTA, 2 mM Na3VO4, 0.2 mM leupeptin, 1 mM phenylmethylsulfonyl fluoride, 2% Triton X-100), 100 μg protein was subjected to immunoprecipitation/Western blot analysis using both anti—PECAM-1 and anti-Bax antibodies.
Immunofluorescence
HEK 293 cells grown in 2-well chamber glass slides were transiently transfected with 1 μg GFP—Bax-encoding plasmid together with 4 μg wild-type PECAM-1—encoding plasmid. After transfection, cells were grown in MEM containing 50 μM caspase inhibitor, zVAD-fmk, for 20 hours, washed in DPBS, fixed in acetone-methanol (-20°C, 1:1 solution) for 5 minutes, washed once in 3% bovine serum albumin (BSA)—PBS, blocked for 15 minutes in 3% BSA—PBS, air dried, and incubated with 5 μg/mL of mAb PECAM-1.3 for 1 hour at room temperature. After 3 washes in 2% BSA—0.1% Tween—DPBS, cells were incubated with Texas red—conjugated goat antimouse antibody (1:200) for 1 hour at room temperature. After 3 more washes, the wells were covered with cover slides for fluorescence microscopy analysis.
Results
We used a combined genetic and biochemical approach to investigate the potential for PECAM-1 to regulate apoptosis. In the first model, endothelial cells derived from age- and sex-matched wild-type and PECAM-1—deficient mice were compared for their ability to survive a variety of proapoptotic insults. Anesthetized mice were subjected in vivo to localized x-ray irradiation of their lungs. Lung tissue was harvested 0, 24, and 48 hours after irradiation and endothelial cell apoptosis evaluated by standard terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL) analysis. As shown in Figure 1A-B, the number of apoptotic endothelial cells from PECAM-1—deficient mice was nearly double that observed in wild-type mice (P < .0001) at all time points examined. The cytoprotective effect of PECAM-1 was not specific for lung endothelium or x-ray irradiation—induced insult, because cultured PECAM-1—deficient murine aortic endothelial cells in vitro were also found to be strikingly more susceptible to staurosporine-induced caspase activation than were their wild-type counterparts (Figure 1C). As previously described,37 the onset of apoptosis initiates endothelial cell retraction and eventual detachment due to proteolysis of focal adhesion complexes in endothelial cells. As shown in Figure 1D, PECAM-1-/- cells became detached from their substrate and underwent chromatin condensation—characteristic of cells undergoing apoptosis—much sooner after staurosporine-induced apoptotic stress than did PECAM-1+/+ endothelial cells, reflecting the loss of cytoprotection. Thus, PECAM-1- endothelial cells appear to (1) have lost an important cytoprotective molecule (Figure 1A), (2) activate their caspases faster (Figure 1C) and, as a result, (3) detach more quickly following exposure to apoptotic stimuli (Figure 1D).
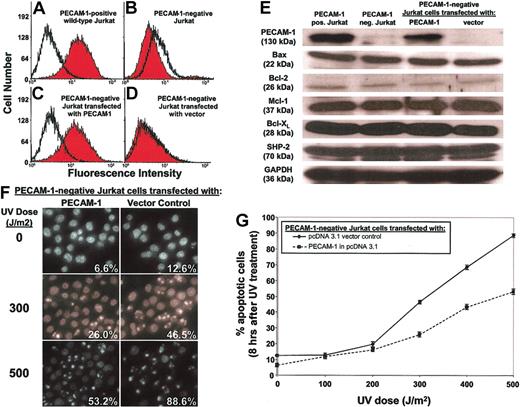
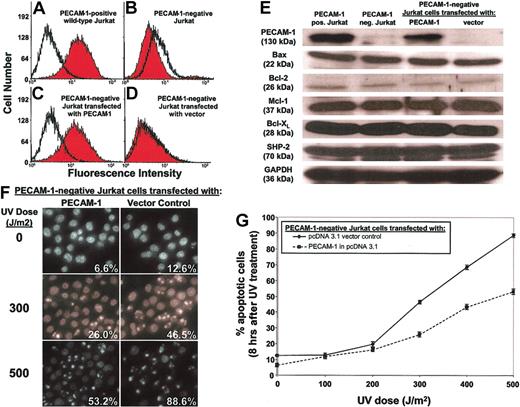
In the second model, we used FACS to prepare from wild-type, PECAM-1+ human Jurkat T lymphocytes (Figure 2A) a PECAM-1- Jurkat T cell line (Figure 2B). The PECAM-1- Jurkat cells were then stably transfected with either the empty mammalian expression vector pcDNA3 or with pcDNA3 containing a full-length cDNA encoding human PECAM-1. The resulting PECAM-1+ (Figure 2C) and PECAM-1- (Figure 2D) rederived Jurkat T cells were then exposed to ultraviolet radiation and apoptotic cell death determined as a function of time. Despite similar levels of Bax, Bcl-2, Mcl-1, Bcl-XL, and SHP-2 (Figure 2E), the presence of PECAM-1 markedly and reproducibly delayed UV-induced cell death (Figure 2F-G). Together with the finding that PECAM-1 also delays endothelial cell death (Figure 1), these data suggest that PECAM-1 confers broad resistance to apoptotic stress in naturally occurring blood and vascular cells that express it.
PECAM-1 affects the rate and extent of apoptosis in human Jurkat T cells. Wild-type, PECAM-1+ Jurkat cells were FACS sorted through 6 rounds of anti—PECAM-1 antibody screening to enrich for cells negative for PECAM-1 expression, finally yielding the PECAM-1+ (A) and PECAM-1- (B) Jurkat cell lines shown. A PECAM-1 cDNA expression vector or a vector control was transfected back into PECAM-1- Jurkat cells to reconstitute genetically identical lines differing only in PECAM-1 expression (C-D) while retaining similar levels of Bax, Bcl-2, Mcl-1, Bcl-XL, and SHP-2 (E). Upon exposure to varying doses of UV irradiation, PECAM-1- Jurkat cells with enforced expression of PECAM-1 were found to be resistant to this proapoptotic stimulus, as judged by nuclear condensation (F-G). Original magnification, panel F: × 40. Data shown in panel G represent the mean ± the standard deviation of triplicate determinations.
PECAM-1 affects the rate and extent of apoptosis in human Jurkat T cells. Wild-type, PECAM-1+ Jurkat cells were FACS sorted through 6 rounds of anti—PECAM-1 antibody screening to enrich for cells negative for PECAM-1 expression, finally yielding the PECAM-1+ (A) and PECAM-1- (B) Jurkat cell lines shown. A PECAM-1 cDNA expression vector or a vector control was transfected back into PECAM-1- Jurkat cells to reconstitute genetically identical lines differing only in PECAM-1 expression (C-D) while retaining similar levels of Bax, Bcl-2, Mcl-1, Bcl-XL, and SHP-2 (E). Upon exposure to varying doses of UV irradiation, PECAM-1- Jurkat cells with enforced expression of PECAM-1 were found to be resistant to this proapoptotic stimulus, as judged by nuclear condensation (F-G). Original magnification, panel F: × 40. Data shown in panel G represent the mean ± the standard deviation of triplicate determinations.
X-ray irradiation, exposure to ultraviolet light, and staurosporine treatment are all thought to induce apoptosis by activating an intrinsic cell death pathway that involves Bax—a proapoptotic, pore-forming member of the Bcl-2 family that plays a central role in mitochondrial-dependent apoptosis.38 When Bax is overexpressed in cells, it translocates from the cytosol to the outer mitochondrial membrane24,39 where, if it reaches high enough concentrations, it overcomes the protective effects of resident antiapoptotic Bcl-2 family members such as Bcl-2 and Bcl-XL.40 The ensuing mitochondrial damage results in the release of, among other mitochondrial constituents, cytochrome c, which catalyzes a downstream caspase activation program that is responsible for the proteolysis of a diverse array of cellular proteins, the destruction of which ultimately leads to the apoptotic phenotype.20
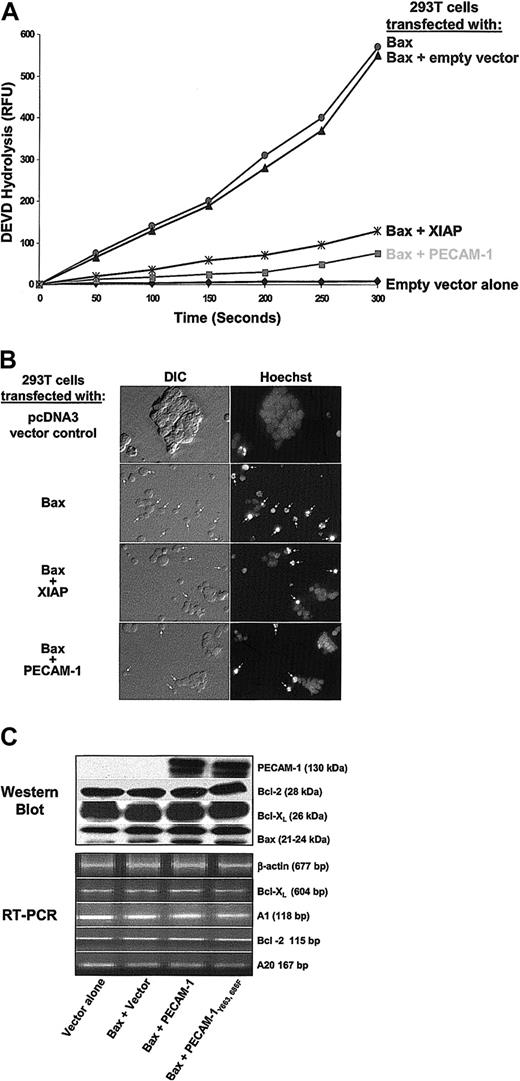
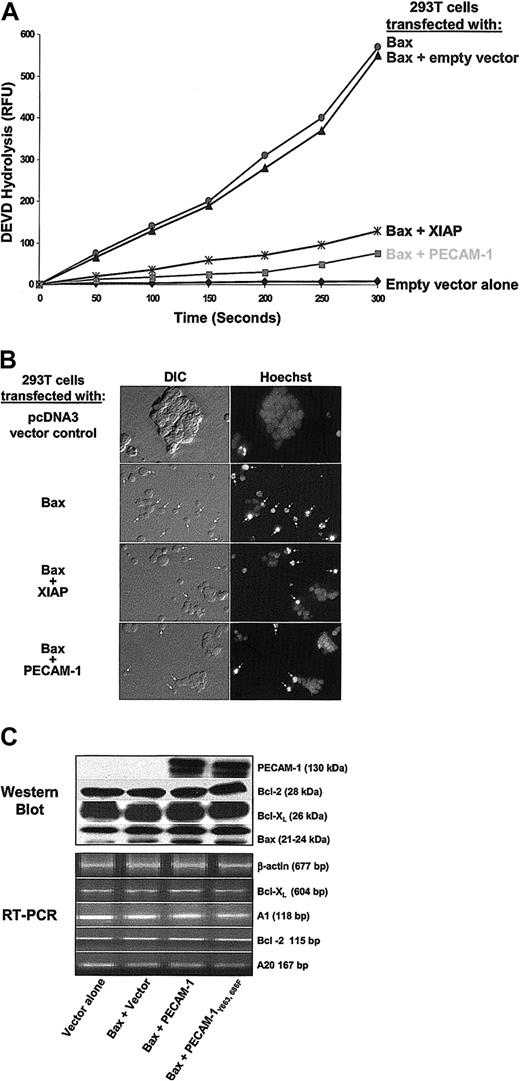
To examine whether PECAM-1 could specifically inhibit Bax overexpression—induced cell death, a plasmid vector encoding full-length, wild-type PECAM-1 cDNA (wtPECAM-1) was cotransfected into HEK 293 T cells together with a cDNA encoding Bax. When 293 T cells are transfected with Bax, they undergo typical apoptosis within 24 hours, as judged by caspase activation and nuclear fragmentation. PECAM-1 markedly suppressed both of these indices of Bax-induced cell death (Figure 3A-B)—an effect that was linearly related to the concentration of PECAM-1 expressed on the cell surface (not shown). Notably, the degree of cytoprotection was at least as good as that conferred by XIAP, a potent cytosolic inhibitor of caspase 3 that is able to prevent programmed cell death in response to a wide variety of proapoptotic stimuli.41 Similar to what we observed in Jurkat T cells (Figure 2), a combination of immunoblot and semiquantitative reverse transcriptase—polymerase chain reaction (RT-PCR) analysis revealed no substantial difference in the expression of Bcl-2, Bcl-XL, Bax, A1, or A20 (Figure 3C), suggesting that the cytoprotective effect of PECAM-1 on Bax-induced 293 cell death is neither due to up-regulation of antiapoptotic, nor to down-regulation of proapoptotic, family members.
PECAM-1 inhibits Bax overexpression—induced apoptosis. HEK 293 cells were transiently transfected with 1 μg of the control vector pcDNA3, with 1 μg of Bax-encoding plasmid alone, or with 1 μg of Bax plasmid together with 4 μg of expression plasmids encoding PECAM-1 or XIAP. (A) PECAM-1 expression suppressed DEVD hydrolysis as effectively as did XIAP, (B) cells coexpressing Bax and PECAM-1 exhibited significantly less nuclear condensation than did cells expressing Bax alone (original magnification, × 40), and (C) there were no major changes in the expression of major proapoptotic or antiapoptotic family members correlating with PECAM-1 expression that could account for PECAM-1—mediated cytoprotection.
PECAM-1 inhibits Bax overexpression—induced apoptosis. HEK 293 cells were transiently transfected with 1 μg of the control vector pcDNA3, with 1 μg of Bax-encoding plasmid alone, or with 1 μg of Bax plasmid together with 4 μg of expression plasmids encoding PECAM-1 or XIAP. (A) PECAM-1 expression suppressed DEVD hydrolysis as effectively as did XIAP, (B) cells coexpressing Bax and PECAM-1 exhibited significantly less nuclear condensation than did cells expressing Bax alone (original magnification, × 40), and (C) there were no major changes in the expression of major proapoptotic or antiapoptotic family members correlating with PECAM-1 expression that could account for PECAM-1—mediated cytoprotection.
Thus, PECAM-1 is a novel and potent inhibitor of Bax-mediated cell death that, unlike other Bax inhibitors, resides neither in the cytoplasm nor the mitochondria but in the plasma membrane of the cell.
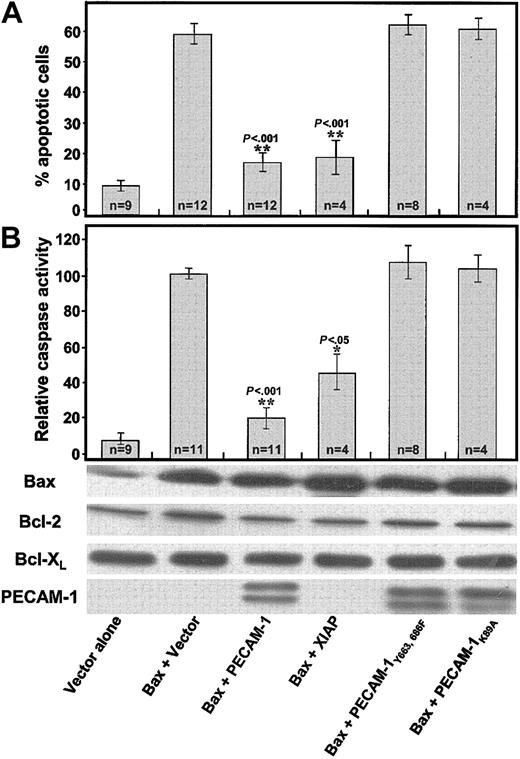
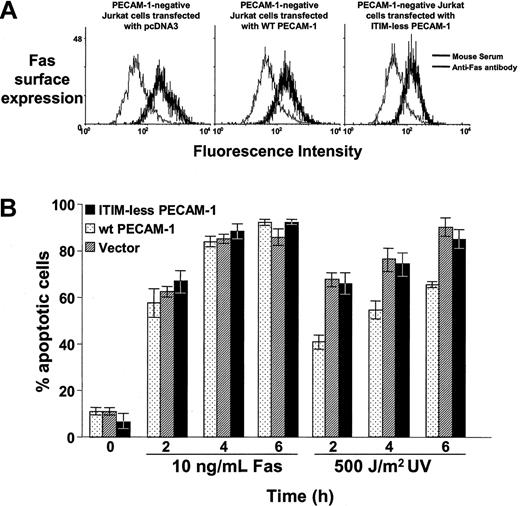
To determine the molecular requirements for PECAM-1's cytoprotective function and to gain further insight into its mechanism of action, we transfected HEK 293 cells with Bax cDNA, together with cDNAs encoding PECAM-1 variants lacking key cytoplasmic or extracellular domain sequences. Unlike wild-type PECAM-1, Lys89Ala PECAM-1, which lacks homophilic binding capacity,5 was unable to suppress Bax overexpression—induced cell death (Figure 4). ITIM-less PECAM-1, which differs from wild-type PECAM-1 in the substitution of phenylalanine for the 2 cytoplasmic tyrosines (Tyr663 and Tyr686) that comprise the PECAM-1 ITIM, also failed to inhibit Bax overexpression—induced apoptosis (Figure 4). Consistent with these findings, wild-type but not ITIM-less PECAM-1 also inhibited UV irradiation—induced apoptosis in Jurkat cells (Figure 5), indicating a similar mode of action for PECAM-1—mediated cytoprotection of endogenous Bax-induced apoptosis. This effect was specific for mitochondrial-dependent, intrinsic apoptosis, because PECAM-1 was found to have no cytoprotective ability against Fas-induced cell death (Figure 5).
Amino acid substitutions within the PECAM-1 extracellular homophilic binding domain, or within its cytoplasmic ITIM, abolish PECAM-1—mediated cytoprotection. The 293 cells were transiently transfected with plasmids encoding the indicated proteins. Twenty-four hours after transfection, cells were either stained for apoptotic nuclei (A) or used to prepare detergent lysates for caspase activity measurements (B), which were carried out essentially as described in the legend to Figure 1. While PECAM-1 effectively suppressed Bax-mediated cell death, expression of an equivalent amount (see the characteristic PECAM-1 doublets in the anti—PECAM-1 immunoblot) of either of 2 mutated forms of PECAM-1, the first differing in only 2 Tyr→Phe amino acid substitutions within PECAM-1's cytoplasmic ITIM and the second containing a single Lys89Ala amino acid substitution within the PECAM-1 extracellular homophilic binding domain, conveyed no cytoprotective effect at all. n represents the number of times each experimental condition was repeated. P values were derived using a paired Student t test. The y-axis (“relative caspase activity”) represents normalized data from up to 11 different experiments, and the data are presented as percentage of caspase activity present in the Bax overexpression—induced control. * and ** indicate P values compared with vector-transfected cells.
Amino acid substitutions within the PECAM-1 extracellular homophilic binding domain, or within its cytoplasmic ITIM, abolish PECAM-1—mediated cytoprotection. The 293 cells were transiently transfected with plasmids encoding the indicated proteins. Twenty-four hours after transfection, cells were either stained for apoptotic nuclei (A) or used to prepare detergent lysates for caspase activity measurements (B), which were carried out essentially as described in the legend to Figure 1. While PECAM-1 effectively suppressed Bax-mediated cell death, expression of an equivalent amount (see the characteristic PECAM-1 doublets in the anti—PECAM-1 immunoblot) of either of 2 mutated forms of PECAM-1, the first differing in only 2 Tyr→Phe amino acid substitutions within PECAM-1's cytoplasmic ITIM and the second containing a single Lys89Ala amino acid substitution within the PECAM-1 extracellular homophilic binding domain, conveyed no cytoprotective effect at all. n represents the number of times each experimental condition was repeated. P values were derived using a paired Student t test. The y-axis (“relative caspase activity”) represents normalized data from up to 11 different experiments, and the data are presented as percentage of caspase activity present in the Bax overexpression—induced control. * and ** indicate P values compared with vector-transfected cells.
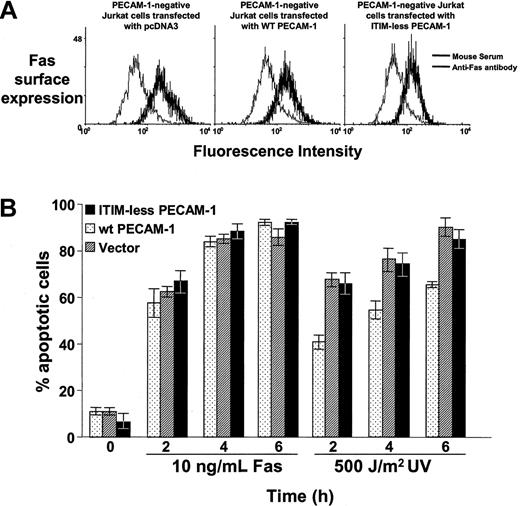
PECAM-1 suppresses mitochondrial, but not Fas-induced, apoptosis. (A) Stable PECAM-1- Jurkat cell lines that had been transfected with empty pcDNA3 vector, wild-type PECAM-1, or ITIM-less PECAM-1 were resuspended in 200 μL DPBS containing 25 μg/mL anti-Fas antibody (mouse IgM, clone CH11, Upstate Biotechnologies) or normal mouse serum. After incubation for 1 hour at 4°C, cells were washed in ice-cold PBS containing sodium azide and incubated with fluorescein isothiocyanate (FITC)—conjugated goat-mouse IgM. After incubation for 30 minutes at 4°C, cells were washed, fixed in 2% paraformaldyhede, and subjected to flow cytometric analysis. All 3 cell lines express similar levels of Fas on their cell surface. (B) Jurkat cells were induced to undergo apoptosis by incubation with 10 ng/mL anti-Fas antibody or by exposure to 500 J/m2 UV-C irradiation, as described in “Materials and methods.” The percent of apoptotic cells was determined by microscopic examination of nuclear morphology of triplicate blinded samples taken at the indicated time points. PECAM-1 suppresses UV-induced, endogenous Bax-mediated apoptosis but has no significant effect on Fas-induced programmed cell death.
PECAM-1 suppresses mitochondrial, but not Fas-induced, apoptosis. (A) Stable PECAM-1- Jurkat cell lines that had been transfected with empty pcDNA3 vector, wild-type PECAM-1, or ITIM-less PECAM-1 were resuspended in 200 μL DPBS containing 25 μg/mL anti-Fas antibody (mouse IgM, clone CH11, Upstate Biotechnologies) or normal mouse serum. After incubation for 1 hour at 4°C, cells were washed in ice-cold PBS containing sodium azide and incubated with fluorescein isothiocyanate (FITC)—conjugated goat-mouse IgM. After incubation for 30 minutes at 4°C, cells were washed, fixed in 2% paraformaldyhede, and subjected to flow cytometric analysis. All 3 cell lines express similar levels of Fas on their cell surface. (B) Jurkat cells were induced to undergo apoptosis by incubation with 10 ng/mL anti-Fas antibody or by exposure to 500 J/m2 UV-C irradiation, as described in “Materials and methods.” The percent of apoptotic cells was determined by microscopic examination of nuclear morphology of triplicate blinded samples taken at the indicated time points. PECAM-1 suppresses UV-induced, endogenous Bax-mediated apoptosis but has no significant effect on Fas-induced programmed cell death.
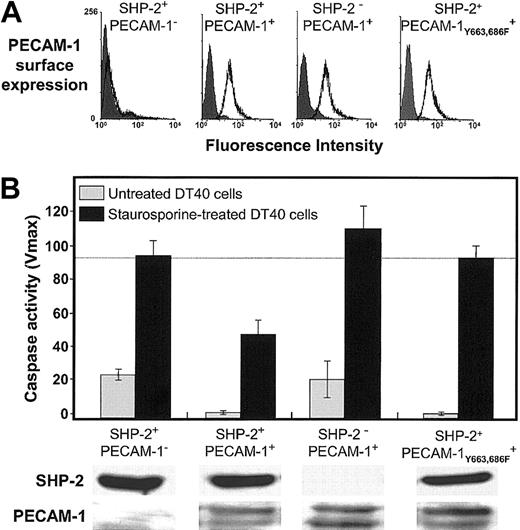
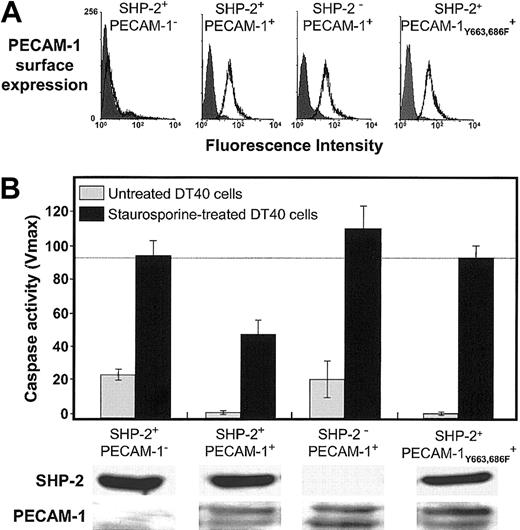
The fact that wild-type PECAM-1 protects against Bax-induced cell death, while ITIM-less PECAM-1 does not (Figures 4 and 5), suggests that an SH2 domain—containing protein might be involved in transmitting PECAM-1—mediated survival signals into the cell. Of the cytosolic signaling molecules that have been reported to bind the cytoplasmic domain of PECAM-1 (“Discussion”), the protein-tyrosine phosphatase, SHP-2, is unique in that it has been previously been shown to interact directly via its 2 SH2 domains with the paired PECAM-1 ITIMs.35 To determine whether a PECAM-1/SHP-2 signaling complex might be required for PECAM-1—mediated cytoprotection, we examined the ability of PECAM-1 to prevent Bax-mediated cell death in DT40 cell lines that have been engineered to differ in their constitutive expression of SHP-2.9 As shown in Figure 6, PECAM-1 suppressed apoptosis only in those cells that also expressed SHP-2. These observations establish the requirement for this protein-tyrosine phosphatase in PECAM-1—mediated cell survival.
Requirement for SHP-2 in PECAM-1—mediated cell survival. DT40 cells and an SHP-2- variant of DT40 expressing the indicated levels of PECAM-1 (A) were induced to undergo programmed cell death by addition of 25 nM staurosporine. Twelve hours later, the cells were lysed and caspase levels determined as in Figures 1 and 4. ITIM-less PECAM-1 (B, far right) fails to prevent Bax-mediated cell death in DT40 cells, similar to the results shown for HEK 293 cells (Figure 4). In the absence of SHP-2 (lower panels), wild-type PECAM-1, even at slightly higher expression levels, fails to protect DT40 cells from apoptosis. Data shown in panel B represent the mean ± the standard deviation of triplicate determinations. All of the bands were derived from a single gel to which equal protein levels were loaded.
Requirement for SHP-2 in PECAM-1—mediated cell survival. DT40 cells and an SHP-2- variant of DT40 expressing the indicated levels of PECAM-1 (A) were induced to undergo programmed cell death by addition of 25 nM staurosporine. Twelve hours later, the cells were lysed and caspase levels determined as in Figures 1 and 4. ITIM-less PECAM-1 (B, far right) fails to prevent Bax-mediated cell death in DT40 cells, similar to the results shown for HEK 293 cells (Figure 4). In the absence of SHP-2 (lower panels), wild-type PECAM-1, even at slightly higher expression levels, fails to protect DT40 cells from apoptosis. Data shown in panel B represent the mean ± the standard deviation of triplicate determinations. All of the bands were derived from a single gel to which equal protein levels were loaded.
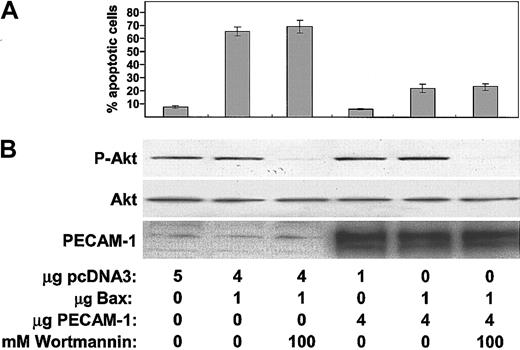
Several years ago, Pellegata et al reported that PECAM-1 could, under certain conditions, be functionally associated with phosphatidylinositol-3 kinase (PI-3K),42 and a recent report by the same laboratory43 found that, in a subset of circulating CD14+, CD34+ hematopoietic precursors, antibody-induced cross-linking of PECAM-1 resulted in PI-3K—dependent activation of the serine/threonine kinase, Akt (also called protein kinase B or PKB). Because Akt is a potent cell survival factor44 and can be associated with and activated by SHP-2,45 we examined whether it might be involved downstream of PECAM-1 in protecting cells from Bax overexpression—induced cell death. As shown in Figure 7, addition of the PI-3K inhibitor, wortmannin, to Bax-transfected 293 cells at a dose that prevented activation of Akt did not at all affect the ability of PECAM-1 to protect cells from Bax overexpression—induced cell death. These data indicate that PI-3K probably is not required for PECAM-1—mediated suppression of mitochondrial-dependent apoptosis.
PECAM-1—mediated protection from BAX-induced apoptosis is independent of the PI-3K/Akt signaling pathway. HEK 293 cells were transfected with control vector pcDNA3, Bax-encoding plasmid, and PECAM-1—encoding plasmid as indicated and then cultured for 20 hours in the presence or absence of 100 nM wortmannin—a PI-3K inhibitor. (A) Quantification of apoptosis. The number of apoptotic nuclei was counted after Hoechst staining and expressed as the percentage of total nuclei of the transfected cells. (B) Aliquots were subjected to Western blot analysis with the indicated antibodies. Note that (1) expression of PECAM-1 did not affect overall Akt antigen levels or its phosphorylation state, (2) wortmannin inhibited Akt phosphorylation as expected, and (3) blocking activation of Akt had no effect on the ability of PECAM-1 to suppress Bax overexpression—induced apoptosis.
PECAM-1—mediated protection from BAX-induced apoptosis is independent of the PI-3K/Akt signaling pathway. HEK 293 cells were transfected with control vector pcDNA3, Bax-encoding plasmid, and PECAM-1—encoding plasmid as indicated and then cultured for 20 hours in the presence or absence of 100 nM wortmannin—a PI-3K inhibitor. (A) Quantification of apoptosis. The number of apoptotic nuclei was counted after Hoechst staining and expressed as the percentage of total nuclei of the transfected cells. (B) Aliquots were subjected to Western blot analysis with the indicated antibodies. Note that (1) expression of PECAM-1 did not affect overall Akt antigen levels or its phosphorylation state, (2) wortmannin inhibited Akt phosphorylation as expected, and (3) blocking activation of Akt had no effect on the ability of PECAM-1 to suppress Bax overexpression—induced apoptosis.
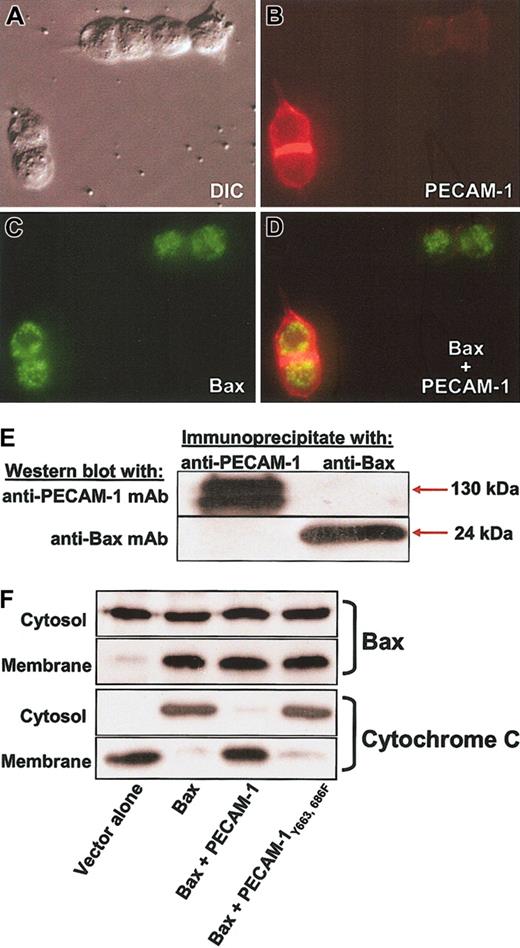
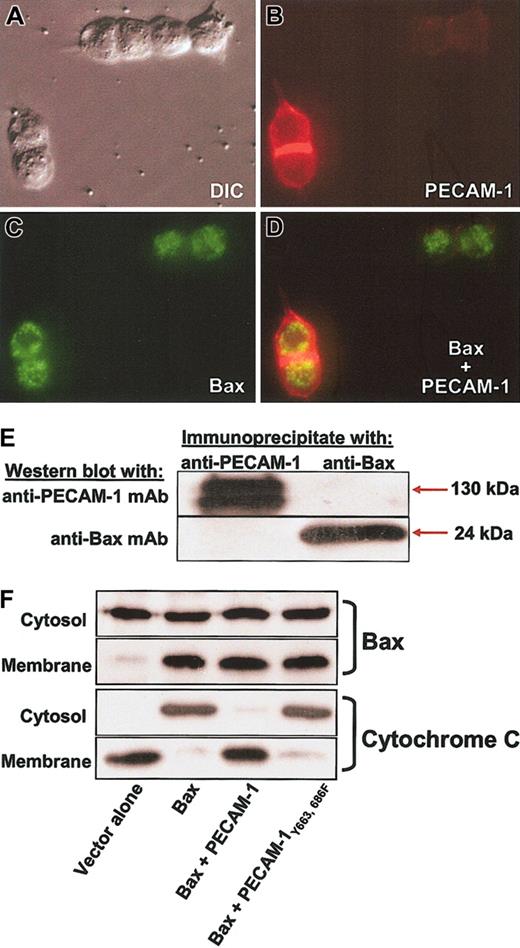
Finally, we examined the ability of PECAM-1 to bind Bax, prevent its translocation to mitochondrial membranes, and inhibit cytochrome c release. Immunofluorescence microscopic analysis of cells cotransfected with PECAM-1 and GFP-Bax failed to show evidence for significant subcellular colocalization (Figure 8A-D), and neither protein was found in immunoprecipitates of the other (Figure 8E). Consistent with these findings, PECAM-1 failed to prevent translocation of Bax from the cytoplasm to the mitochondria (Figure 8F). Despitemitochondrial translocation of Bax, transfected PECAM-1 completely suppressed cytochrome c release—an effect that was dependent on its ITIM tyrosine residues (Figure 8, bottom). Similarly, translocation of endogenous Bax induced by UV irradiation, instead of Bax overexpression, was also not affected by expression of PECAM-1 (Figure 9; Table 1). Despite not affecting Bax translocation in either system, PECAM-1 still significantly suppresses cell death. Taken together, these data suggest that PECAM-1 functions to inhibit Bax-mediated apoptosis by preventing a still-to-be-defined post-Bax translocational event that causes mitochondrial injury, release of cytochrome c, and subsequent cell death.
PECAM-1 is a potent inhibitor of cytochrome c release without blocking translocation of Bax to the mitochondrial membrane. (A) The 293 cells were transfected with PECAM-1, GFP-Bax, or both and analyzed by immunofluorescence microscopy following addition of Texas red—conjugated goat-antimouse IgG to detect cell-surface PECAM-1 (cells were not permeabilized). Expression of PECAM-1 (B) failed to significantly affect the mitochondrial staining pattern of Bax (C), and little or no colocalization was evident in the merged image (D). Original magnification, × 100. (E) Coprecipitation analysis of Bax and PECAM-1. PECAM-1 immunoprecipitates did not contain detectable levels of Bax, and Bax immunoprecipitates were devoid of PECAM-1. (F) Cells transfected as indicated were fractionated into cytosolic and heavy mitochondrial membrane fractions and each fraction analyzed for the presence of Bax and cytochrome c. Overexpression of Bax resulted in significant accumulation in the mitochondria-enriched membrane fraction and concomitant release of cytochrome c into the cytosolic fraction. PECAM-1 suppressed cytochrome c release but failed to prevent translocation of Bax into mitochondria. ITIM-less PECAM-1Tyr663, 686Phe, which is not cytoprotective (Figures 4, 5, 6), failed to block either Bax translocation or cytochrome c release, as expected.
PECAM-1 is a potent inhibitor of cytochrome c release without blocking translocation of Bax to the mitochondrial membrane. (A) The 293 cells were transfected with PECAM-1, GFP-Bax, or both and analyzed by immunofluorescence microscopy following addition of Texas red—conjugated goat-antimouse IgG to detect cell-surface PECAM-1 (cells were not permeabilized). Expression of PECAM-1 (B) failed to significantly affect the mitochondrial staining pattern of Bax (C), and little or no colocalization was evident in the merged image (D). Original magnification, × 100. (E) Coprecipitation analysis of Bax and PECAM-1. PECAM-1 immunoprecipitates did not contain detectable levels of Bax, and Bax immunoprecipitates were devoid of PECAM-1. (F) Cells transfected as indicated were fractionated into cytosolic and heavy mitochondrial membrane fractions and each fraction analyzed for the presence of Bax and cytochrome c. Overexpression of Bax resulted in significant accumulation in the mitochondria-enriched membrane fraction and concomitant release of cytochrome c into the cytosolic fraction. PECAM-1 suppressed cytochrome c release but failed to prevent translocation of Bax into mitochondria. ITIM-less PECAM-1Tyr663, 686Phe, which is not cytoprotective (Figures 4, 5, 6), failed to block either Bax translocation or cytochrome c release, as expected.
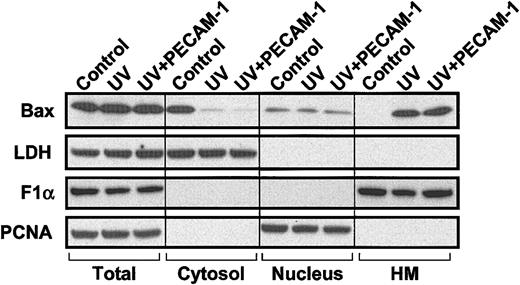
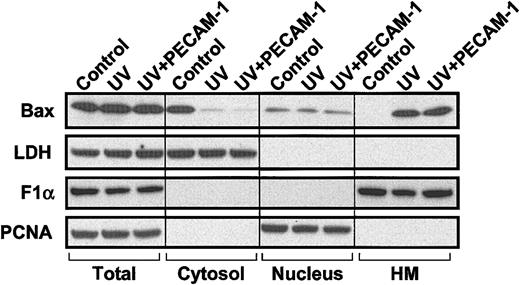
Translocation of endogenous Bax during UV-induced apoptosis is not inhibited by PECAM-1. HEK 293 T cells (107 cells) were transfected with 10 μg pcDNA3-control vector (control and UV) or pcDNA3 encoding wild-type PECAM-1 (UV+PECAM-1). One day following transfection, cells were treated with 200 J/m2 UV-C irradiation. Cells were collected 24 hours later, and subcellular fractionation was performed as described in “Materials and methods” to yield soluble cytosol, nuclei, and heavy membranes (HM) containing mitochondria. Lactate dehydrogenase (LDH), proliferating cell nuclear antigen (PCNA), and FoF1 ATP synthase subunit α (F1α) were used as markers for cytosolic, nuclear, and mitochondrial fractions, respectively. Bax is found predominantly in the cytosol in untreated, vector-transfected control 293 cells but translocates to the heavy membrane fraction following treatment with UV irradiation. PECAM-1 transfection does not inhibit Bax translocation (uppermost right lane) but still suppresses apoptosis by more than 50% (Table 1).
Translocation of endogenous Bax during UV-induced apoptosis is not inhibited by PECAM-1. HEK 293 T cells (107 cells) were transfected with 10 μg pcDNA3-control vector (control and UV) or pcDNA3 encoding wild-type PECAM-1 (UV+PECAM-1). One day following transfection, cells were treated with 200 J/m2 UV-C irradiation. Cells were collected 24 hours later, and subcellular fractionation was performed as described in “Materials and methods” to yield soluble cytosol, nuclei, and heavy membranes (HM) containing mitochondria. Lactate dehydrogenase (LDH), proliferating cell nuclear antigen (PCNA), and FoF1 ATP synthase subunit α (F1α) were used as markers for cytosolic, nuclear, and mitochondrial fractions, respectively. Bax is found predominantly in the cytosol in untreated, vector-transfected control 293 cells but translocates to the heavy membrane fraction following treatment with UV irradiation. PECAM-1 transfection does not inhibit Bax translocation (uppermost right lane) but still suppresses apoptosis by more than 50% (Table 1).
Discussion
The purpose of the present investigation was to determine whether PECAM-1 functions to control survival in the blood and vascular cells in which it is naturally expressed and, if so, to gain insight into the molecular mechanism by which PECAM-1 might be exerting its antiapoptotic effect. Using in vivo and in vitro models of mitochondrial-dependent cell death, we found that normal endothelial cells derived from PECAM-1—deficient mice were more susceptible to x-irradiation— and staurosporine-induced apoptosis than were their wild-type counterparts (Figure 1). Interestingly, in some experiments, PECAM-1+ cells exhibited a lower constitutive level of baseline apoptosis than did PECAM-1- cells (compare Figure 1B), perhaps reflecting increased viability or a lower turnover rate. Consistent with these observations, PECAM-1 was also found to protect against UV irradiation—induced cell death in Jurkat T lymphocytes (Figure 2). Taken together, these data establish PECAM-1 as a cell surface receptor capable of conferring broad resistance to apoptotic stress in naturally occurring vascular cells that normally express it.
PECAM-1 markedly suppressed apoptosis in all models examined in which the intrinsic, mitochondrial-dependent Bax pathway was stimulated. In contrast, expression of PECAM-1 was found to have little or no suppressive effect on the extrinsic pathway of programmed cell death (Figure 5). An indication of the potency of PECAM-1's cytoprotective ability was provided by the finding that it could even block apoptosis induced by Bax overexpression (Figure 3). Interestingly, a Lys89Ala variant of PECAM-1, which lacks homophilic binding capacity, failed to suppress apoptosis (Figure 4). While we are not certain why Lys89Ala PECAM-1 cannot inhibit Bax-induced cell death, it has been shown that functional homophilic binding is required for efficient PECAM-1 border localization46 and also influences the subcellular localization of PECAM-1. Our data are consistent, therefore, with the notion that PECAM-1—PECAM-1 interactions, either in trans between cells, or in cis within the plane of the membrane, may be required to support transmission of a signal to the cytoplasmic domain that initiates one or more antiapoptotic pathways.
The observation that mutations in either the homophilic binding domain or the PECAM-1 cytoplasmic domain are each alone capable of abolishing its antiapoptotic functions (Figure 4) suggests that PECAM-1—mediated homophilic interactions signal to the PECAM-1 cytoplasmic domain to regulate cell survival—a phenomenon that is reminiscent of the ability of cell surface integrins to transduce survival signals into cells.47,48 In that regard, it might be interesting to consider whether the antiapoptotic effect of PECAM-1 may be due to its modulation of integrin-mediated cell-cell or cell-matrix interactions. There is an abundant literature describing the ability of anti—PECAM-1 antibodies49-55 and PECAM-1 oligomerization-inducing reagents56 to modulate integrin function. Integrin engagement, in turn, has the potential to protect cells from a specialized form of apoptosis termed “anoikis” (Greek for “homelessness”). While it is formally possible that PECAM-1—mediated cytoprotection is secondary to its effect on integrin function, several observations argue against this. First, modulation of integrin function by PECAM-1 has been demonstrated largely in artificial systems involving antibody- or small molecule—mediated oligomerization (reviewed by Zhao and Newman56 )—it has been reported in vivo only in relation to regulating the expression of α6β1 during neutrophil transendothelial migration,57 and there are no other obvious integrin activation defects in PECAM-1—deficient mice. Second, following UV irradiation—induced cell injury, both PECAM-1+ and PECAM-1- Jurkat cells adhere somewhat to each other (not shown), but there is no apparent difference in the degree of adhesion that they undergo following UV-induced damage. This would appear to rule out differential gain or loss of cell attachment as the reason for their increased susceptibility to UV irradiation—induced apoptosis. Third, while the signal transduction pathway(s) involved in integrin-mediated cell survival has not yet been fully resolved, Gilmore et al31 found that detachment from extracellular matrix induces rapid translocation of Bax to mitochondria—a pathway that is unaffected by PECAM-1 (Figures 8 and 9; Table 1). Finally, Stupack et al58 recently demonstrated that integrin-mediated adhesion promotes cell survival by disrupting mitochondrial-independent, caspase 8—mediated cell death—a pathway also not affected by PECAM-1. Taken together, it would seem unlikely that PECAM-1 is exerting its antiapoptotic activity via any effects it may be having on integrin activation.
Unlike 2 previous studies in which PECAM-1 engagement was found to be associated with an increase in expression of A1,16,19 we found no evidence in any of the model systems we employed that PECAM-1 up-regulates antiapoptotic, or down-regulates proapoptotic, members of the Bcl-2 family most likely to influence Bax-induced cell death. Based on these findings, we speculate that PECAM-1 affects signal transduction pathways that modulate either the location and/or the activation state of preexisting proapoptotic and antiapoptotic components of the cell death pathway rather than stimulating de novo gene expression of regulatory apoptotic proteins. Gene expression array analysis will be required to determine whether significant PECAM-1—induced transcriptional events contribute to the prosurvival phenotype of PECAM-1—expressing cells.
The observation that the cytoprotective function of PECAM-1 is abrogated by substitution of tyrosine residues 663 and 686 for phenylalanine (Figure 4) is strongly suggestive of the involvement of the PECAM-1 ITIM, or at least this region of the cytoplasmic domain, in PECAM-1—mediated cell survival. ITIM-like motifs have recently been shown to play a role in death receptor signaling, because death receptor cytoplasmic ITIMs have recently been shown to preferentially recruit SHP-1, which, via its ability to negatively regulate Src family kinase-dependent survival signals,59 inhibits prosurvival, tyrosine kinase—dependent signaling pathways. Although the paired ITIM of PECAM-1 is capable of recruiting and activating SHP-1 under certain conditions,60,61 it is much more likely to form a signaling complex with SHP-2, for which its affinity is nearly 5 times greater.61 Our finding that SHP-2 is required for maximal cytoprotection by PECAM-1 (Figure 6) is consistent with growing evidence for at least an indirect role for SHP-2 in cell survival.62,63 Additional studies examining the molecular requirements for the PECAM-1/SHP-2 signaling complex to confer antiapoptotic properties to blood and vascular cells may provide interesting and important insights into the mechanism by which PECAM-1 suppresses mitochondrial-dependent apoptosis.
Perhaps the most intriguing aspect, and possibly that providing the greatest clue to the mechanism by which PECAM-1 exerts its antiapoptotic activity, is the observation that expression of PECAM-1 almost completely abrogates Bax overexpression—induced cytochrome c release from mitochondria—despite having no observable effect on Bax translocation from the cytosol to mitochondria (Figures 8 and 9; Table 1). Although the process by which Bax disrupts mitochondrial membranes is incompletely understood, it appears to be a multistep process involving transformation from an inactive, monomeric Bax molecule located primarily in the cytosol to a conformationally active protein capable of inserting into biologic membranes and dimerizing. The solution structure of Bax64 suggests that membrane insertion and dimerization are both regulated by the most C-terminal of Bax's 9 α helices (helix α 9), which in the inactive conformer lies in close apposition to a hydrophobic pocket that runs diagonally across the back of the molecule. Activation of Bax results in a conformational change that displaces helix α 9 out of the hydrophobic pocket, allowing the C-terminus to enter mitochondrial membranes and simultaneously exposing the hydrophobic pocket such that it can participate in dimer formation. Although still a matter of considerable debate,22 it appears that, following membrane insertion, there is an additional requirement for BH3 domain-only proteins to interact with mitochondrial-localized Bax and Bak23 and serve as terminal effectors of mitochondrial damage and subsequent cytochrome c release. Whether PECAM-1 exerts its antiapoptotic function by tying up one or more BH3-only Bax cofactors that that are required for postinsertion mitochondrial damage23,38 is the subject of current investigation in our laboratory.
One BH3 domain-only protein that plays a prominent role in inducing certain forms of apoptosis is Bid. Might Bid be involved in PECAM-1—mediated cell survival? Early studies by Luo et al65 showed that caspase 8, activated downstream of the Fas death receptor pathway, cleaves the cytosolic precursor form of Bid to yield p15 t(runcated)Bid, which, in model systems involving purified mitochondria, induces oligomerization of multidomain proapoptotic proteins Bak66 and Bax.67 That tBid “activates” Bax and Bak has also been observed in murine embryonic fibroblasts,38 and it is this activity that is thought to account for the sudden increase in mitochondrial permeability that leads to cytochrome c release. Importantly, however, tBid appears to activate Bax and Bak via different mechanisms. In the case of Bak, which resides constitutively in the outer mitochondrial membrane, tBid induces allosteric conformational activation resulting in mitochondrial dysfunction.66 In the case of Bax, Ruffolo et al68 and Wei et al38 both found that the major effect of tBid was to induce cytosolic Bax to translocate and insert as an integral mitochondrial membrane protein (ie, tBid cofactor activity is upstream of Bax insertion).
Several pieces of evidence make it unlikely that PECAM-1 is acting at the level of tBid to exert its antiapoptotic function. First, Bax translocation to mitochondrial membranes—the pathway affected by tBid—is not affected by PECAM-1, even when PECAM-1 is overexpressed (Figures 8 and 9; Table 1). Second, there are a number of instances where Bax does not require tBid as a cofactor to accumulate in mitochondria and induce cytochrome c release,40 including when Bax is overexpressed. As shown in Figures 3 and 4, Bax overexpression—induced cell death, which is tBid independent, is still remarkably inhibitable by PECAM-1. Third, PECAM-1 is not cytoprotective against Fas-induced cell death—the pathway that specifically activates caspase 8 to yield p15 tBid. Finally, if PECAM-1 were to act by suppressing the function of tBid, it would be expected to inhibit both Bak- as well as Bax-mediated apoptosis, and we have found that PECAM-1 has no effect on Bak overexpression—induced cell death (M.S. and S.M., unpublished observations, May 2002). Because Bid-/- cells remain susceptible to staurosporine- or UV-induced cell death,38 it is clear that Bid is not the sole activator of Bax or Bak. Taken together, our data are most consistent with PECAM-1 functioning to enhance cell survival downstream of tBid-enhanced Bax insertion. How and/or if other BH3 domain-only proteins are involved in PECAM-1—mediated cytoprotection is the subject of an ongoing and extensive investigation in our laboratory.
Finally, in addition to protein-tyrosine phosphatases, PECAM-1 has also been reported to be capable of associating with other potentially cytoprotective signaling molecules, including phospholipase Cγ1,69 SHIP,69 Src,70-72 Stats 3 and 5a,73 and β and γ catenins,74,75 raising the possibility that PECAM-1 exerts its antiapoptotic effects by activating one or more prosurvival signaling pathways that intersect with the Bax pathway in ways that are still to be defined. Identification of the downstream effectors that enable PECAM-1 to inhibit Bax-mediated mitochondrial damage will be crucial for understanding how PECAM-1 regulates cell survival in those vascular cells that express it. Independent of its precise molecular mechanism of action, the finding that PECAM-1 is a potent and specific suppressor of Bax-induced apoptosis strongly suggests that this novel member of the Ig-ITIM family may be an important regulator of cell survival in those blood and vascular cells in which it is expressed. Modifying expression levels of PECAM-1 may open new avenues for controlling apoptosis in a host of pathophysiologic conditions.76,77
Prepublished online as Blood First Edition Paper, March 20, 2003; DOI 10.1182/blood-2003-01-0003.
Supported by National Institutes of Health grants HL-44612 (P.J.N.), HL-40926 (P.J.N.), HL-49591 (S.M.A.), and ALA RG-087-N (M.C.-S.).
C.G. and W. S. contributed equally to this work.
Portions of this work were presented in abstract form at the 43rd annual meeting of the American Society of Hematology, Orlando, FL, December 7-11, 2001.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Diane Reis and Sara Hoffman for preparation and characterization of the PECAM-1—deficient Jurkat cell lines and Brian Boylan for preparation of transfected Jurkat cell lines.