Abstract
Gene therapy approaches involving vascular endothelial growth factor (VEGF) to promote therapeutic angiogenesis are under consideration for conditions ranging from ischemic heart disease to nonhealing skin ulcers. Here we make the surprising observation that the transgenic delivery of VEGF to the skin results in a profound inflammatory skin condition with many of the cellular and molecular features of psoriasis, including the characteristic vascular changes, epidermal alterations, and inflammatory infiltrates. Even longstanding psoriatic disease remains dependent on the transgenic VEGF in this model because it can be effectively reversed by the addition of VEGF Trap, a potent VEGF antagonist. Previous attempts to faithfully replicate the psoriatic phenotype through the transgenic delivery of epidermal keratinocyte growth factors or inflammatory mediators generated phenotypes with only partial resemblance to human psoriasis, leaving unanswered questions about the etiology of this disease. The ability of transgenic VEGF to induce a psoriasiform phenotype suggests a new etiology and treatment approach for this disease and further substantiates emerging concerns about possible proinflammatory adverse effects that might be associated with therapeutic attempts to deliver VEGF. (Blood. 2003;102:161-168)
Introduction
Vascular endothelial growth factor (VEGF) is a potent mediator of angiogenesis, prompting recent efforts to therapeutically exploit this factor in conditions involving pathologically decreased blood flow, such as ischemic heart disease and nonhealing skin ulcers. Some recent studies,1-6 however, have raised concerns about whether the delivery of VEGF could also have deleterious consequences. Here we make the surprising observation that chronic transgenic delivery of VEGF to the skin can result in a profound inflammatory condition with many of the cellular and molecular hallmarks of human psoriasis, such as hyperplastic and inflamed dermal blood vessels,7 epidermal thickening (termed acanthosis) with aberrant keratinocyte differentiation,8 and characteristic inflammatory infiltrates.9,10
It has long been known that the reddened appearance of psoriatic skin is caused by hyperplastic dermal blood vessels, that vascular changes occur early in this disease, and that levels of VEGF are elevated in psoriatic skin.7,11-13 In addition, the dermal vessels in psoriasis appear to be highly abnormal in that they are hyperpermeable, contributing to the edema that characterizes psoriatic skin, and in that they express markers of an inflamed vasculature, such as E-selectin, vascular cell adhesion molecule-1 (VCAM-1), and intracellular adhesion molecule-1 (ICAM-1).14-16 In addition, the levels of soluble adhesion molecules in the sera of psoriasis patients, particularly E-selectin, provide a valuable surrogate marker of disease severity and therapeutic efficacy of various treatments.17-20 Despite this, most efforts at understanding the etiology of this disease have focused on the epidermal and inflammatory aberrations. In the abnormally thickened epidermis, the top layer (termed stratum corneum), usually consisting of cornified keratinocytes lacking nuclei, instead contains cells with nuclei (termed parakeratosis). Furthermore, this keratinized upper layer is excessively thickened (termed hyperkeratosis). Most striking, the epidermis produces highly abnormal and characteristic fingerlike projections into the underlying dermis, termed rete ridges. The typical inflammatory cell infiltrate seen in psoriasis is composed of epidermal microabscesses, increased numbers of mast cells, neutrophils and macrophages in the dermis, and activated T cells in the dermis and epidermis.21,22 T-cell subsets achieve a unique distribution as psoriasis evolves, with CD4+ T cells congregating primarily in the dermis while CD8+ T cells migrate from their normal dermal position to the epidermis, which is usually free of leukocytes.23,24
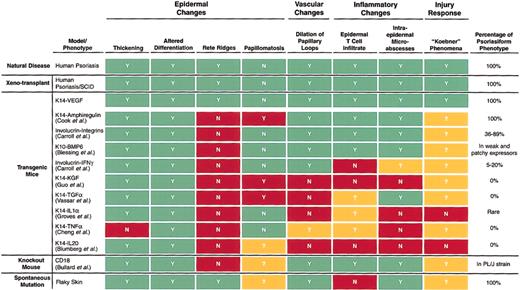
It is clear that psoriatic skin is a hotbed of epidermal growth factors and inflammatory mediators.23,25-30 Supportive evidence of a key role for such mediators comes from patients who respond to immunosuppressive, anti-inflammatory, and antiproliferative therapies such as cyclosporine, methotrexate, tacrolimus, corticosteroids, and ultraviolet-light—activated psoralen. However, extensive efforts aimed at transgenically delivering inflammatory mediators or keratinocyte growth factors to the skin have not completely reproduced the psoriatic phenotype28,31-36 (Figure 1), which has thus far only been faithfully modeled in animals by transplanting human psoriatic skin onto mice with severe combined immunodeficiency disease (SCID)10,23 (Figure 1). Factors such as keratinocyte growth factor, transforming growth factor-α (TGF-α), and interleukin-20 (IL-20) promote some degree of epidermal hyperplasia, in certain cases with associated inflammation, but fail to produce many of the hallmarks of human psoriasis.28,34,37,38 Transgenic delivery of amphiregulin has resulted in the most promising transgenic model, but this model still lacks the characteristic rete ridge projections seen in human psoriasis, and it is also prone to papillomatosis, which is not typical in the human disease29 (Figure 1). In addition, a chronic inflammatory skin condition developed in mice in which CD18 was knocked out, but only when the CD18-deficient 129/Sv mice were backcrossed onto the PL/J strain.39 Again, these mice lacked the rete ridge structures that are highly characteristic of human psoriasis (Figure 1).
Summary of various mouse models and their resemblance to human psoriasis. The top line indicates whether characteristic changes are seen in human psoriasis (Y for yes, N for no). Other models are compared with the human standard and are blocked in green if they match human psoriasis or in red if they do not match. Question marks and the yellow blocks indicate that the feature in question was not examined. Note that only 2 models precisely match human psoriasis in all the features indicated here—a xenotransplantation model in which human psoriatic skin was transplanted onto a SCID mouse (second line) and the K14VEGF transgenic mouse discussed (third line).
Summary of various mouse models and their resemblance to human psoriasis. The top line indicates whether characteristic changes are seen in human psoriasis (Y for yes, N for no). Other models are compared with the human standard and are blocked in green if they match human psoriasis or in red if they do not match. Question marks and the yellow blocks indicate that the feature in question was not examined. Note that only 2 models precisely match human psoriasis in all the features indicated here—a xenotransplantation model in which human psoriatic skin was transplanted onto a SCID mouse (second line) and the K14VEGF transgenic mouse discussed (third line).
The problems with the above transgenic models of psoriasis raise the possibility that there is an upstream predisposition for psoriasis that can somehow be triggered so as to lead to the extremely diverse cytokine and growth factor abnormalities that drive psoriasis and that none of these individual downstream mediators is sufficient to induce the full spectrum of psoriatic disease. Consistent with the notion that psoriasis involves an underlying predisposition, the wounding of asymptomatic skin in psoriatic patients can trigger a complete psoriatic response adjacent to the wound, in a classic reaction termed the Koebner phenomenon.40
Our studies suggest that excess VEGF may provide just such a predisposition by inducing a vascular inflammatory response that then predisposes to more widespread tissue inflammation closely resembling the psoriatic state. We report that young mice transgenically overexpressing VEGF in the skin initially lack overt disease but have a predisposition such that wounding can elicit the psoriatic phenotype, analogous to the Koebner phenomenon in humans. In older transgenic mice, the condition progresses until a profound inflammatory skin condition spontaneously develops with many of the cellular and molecular hallmarks of psoriasis, from characteristic epidermal alterations including dramatic rete ridge formation to the inflammatory infiltrates typical of psoriasis. Even late-stage disease remains dependent on transgenic VEGF because it can be effectively reversed by the addition of a potent VEGF antagonist. The ability of transgenic VEGF to induce a psoriasiform phenotype suggests a new etiology and treatment approach for this disease and further substantiates emerging concerns6 about possible proinflammatory adverse effects that might be associated with therapeutic attempts to deliver VEGF.
Materials and methods
K14-VEGF transgenic mice
A keratin-14 (K14)—based expression vector and a mouse cDNA encoding VEGF164 were used to generate K14-VEGF transgenic mice on the FVB genetic background, as previously described4 ; mice homozygous for this transgene were used throughout the studies described here. All animals in the facility are cared for in accordance with the “Guide for the Care and Use of Laboratory Animals” (National Research Council, 1996).
Tissue processing and immunostaining
Tissue from the K14-VEGF transgenic and wild-type littermate mice used in these studies was matched according to sex, age, and wound site. Fixed sections were immunostained with antimouse platelet-endothelial cell adhesion molecule-1 (PECAM-1) (CD31; BD PharMingen, San Diego, CA), antimouse CD4 (BD PharMingen), antimouse CD8 (BD PharMingen), antimouse F4/80 (Serotec, Oxford, England), or antimouse VEGF (R&D Systems, Minneapolis, MN) following the manufacturer's instructions. Stainings for keratinocyte proliferation and differentiation markers or leukocyte adhesion molecules were performed as previously described41 with rabbit polyclonal antibody against mouse keratin 6 (K6) (Babco, Richmond, CA) and rat monoclonal antibodies against mouse E-selectin (CD62E), ICAM-1 (CD54), and VCAM-1 (CD106; BD PharMingen) using the Vectastain ABC kit (Vector Laboratories, Burlingame, CA).
Histology
Hematoxylin and eosin (H&E) staining and trichrome staining were performed according to protocols previously described.42
Injection of VEGF Trap
VEGF Trap is a fusion of the immunoglobulin 2 domain of human VEGFR1, the immunoglobulin 3 domain of human VEGFR2, and the Fc domain of human immunoglobulin G1 (IgG1), creating a forced homodimer that binds VEGF with high affinity (dissociation equilibrium constant, 1-5 pM) and prolonged in vivo half-life (1-2 days in mice).43 The 6-month-old K14-VEGF homozygous transgenic mice were treated by the systemic administration of VEGF Trap by subcutaneous injection at a site distant from the psoriatic skin. Mice were treated with either 25 mg/kg VEGF Trap or 12.5 mg/kg human Fc, corresponding to an equal molar concentration as a control, using an injection schedule of every 3 days for 12 days that resulted in a total of 4 injections per animal. Mouse ear tissue was harvested on day 14 for subsequent histologic analyses.
Results
Young K14-VEGF transgenic mice display a mild pre-psoriatic phenotype
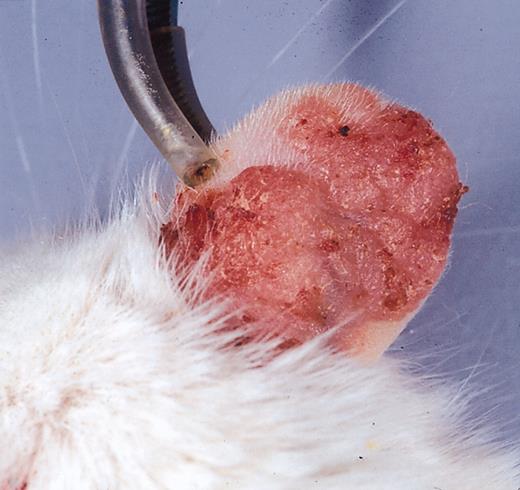
As previously reported, K14-VEGF transgenic mice overexpressing VEGF in the epidermis are fertile and overtly healthy.2,4 However, the ear skin of mice homozygous for this transgene is visibly redder than that of their wild-type FVB littermates. By 3 months, occasional focal skin lesions begin to develop on the ear and, to a lesser extent, on the dorsal and lateral skin. This condition worsens with age such that pronounced skin lesions are observed on the ears, neck, and snout by 5 months of age, with lesions characterized by erythematous and scaly skin (Figure 2). These lesions coincided with sites of highest expression of the VEGF transgene (data not shown).
Psoriasiform phenotype. Erythematous, scaly, and thickened skin lesions with associated edema develop in homozygote K14-VEGF transgenic mice older than 5 months.
Psoriasiform phenotype. Erythematous, scaly, and thickened skin lesions with associated edema develop in homozygote K14-VEGF transgenic mice older than 5 months.
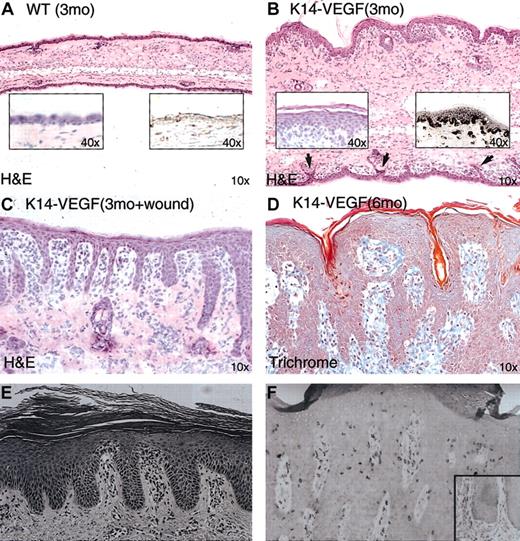
An initial histologic screen of the ear skin from young K14-VEGF transgenic mice, at 3 months of age, revealed a mild and potentially pre-psoriatic phenotype in these young mice using standard H&E staining—that is, the epidermis of these mice exhibited moderate acanthosis (epidermal hyperplasia) (Figure 3B, left inset), focal parakeratosis (keratinocytes in the stratum corneum retain nuclei) (Figure 3B, left inset), and mild rete ridge formation on the ventral ear surface (Figure 3B, arrows), compared with age-matched control littermates (Figure 3A). In the dermal compartment, edema contributing to an approximately 2- to 3-fold increase in tissue thickness was observed in the K14-VEGF mice, as was inflammatory cell infiltration in the subepidermal dermis (Figure 3; compare panels A and B).
Histologic examination of ear skin from K14-VEGF transgenic mice, using H&E-stained tissue sections. (A) Control, wild-type littermate. Left inset shows epidermis at higher magnification; while right inset shows epidermis is negative for VEGF immunostaining. (B)Transgenic mouse (3 months of age) with edema, mild rete ridge formation on the ventral ear surface (arrows), epidermal acanthosis (left inset), and VEGF immunostaining in epidermis and in dermal microvessels (right inset). (C) Wound-induced rete ridge formation in a 3-month-old VEGF transgenic mouse. (D) Six-month-old VEGF transgenic mice showed spontaneous extensive rete ridge formation and anastomosis. (E) Early-stage human psoriasis shown for comparison. (F) Fully developed human psoriasis shown for comparison. Panels E-F reproduced with permission from Elder et al46 and Nickoloff and Wrone-Smith,23 respectively. Original magnifications: × 10 (A-D); and × 40 (insets).
Histologic examination of ear skin from K14-VEGF transgenic mice, using H&E-stained tissue sections. (A) Control, wild-type littermate. Left inset shows epidermis at higher magnification; while right inset shows epidermis is negative for VEGF immunostaining. (B)Transgenic mouse (3 months of age) with edema, mild rete ridge formation on the ventral ear surface (arrows), epidermal acanthosis (left inset), and VEGF immunostaining in epidermis and in dermal microvessels (right inset). (C) Wound-induced rete ridge formation in a 3-month-old VEGF transgenic mouse. (D) Six-month-old VEGF transgenic mice showed spontaneous extensive rete ridge formation and anastomosis. (E) Early-stage human psoriasis shown for comparison. (F) Fully developed human psoriasis shown for comparison. Panels E-F reproduced with permission from Elder et al46 and Nickoloff and Wrone-Smith,23 respectively. Original magnifications: × 10 (A-D); and × 40 (insets).
Consistent with high-level transgenic overexpression of VEGF in the epidermis of these mice, VEGF protein was observed in the epidermis (where it is produced) and on dermal microvessels (where it presumably accumulates after diffusion into the dermis) in patterns (Figure 3A-B; compare right insets), remarkably reminiscent of those seen in human psoriasis.12
Young K14-VEGF transgenic mice exhibit a dramatic Koebner-like psoriatic response to injury
In contrast to the mild changes seen under basal conditions, creation of an excisional wound in the dorsal ear skin of 3-month-old K14-VEGF transgenic mice resulted in dramatic invaginations of the epidermis on the ventral side of the ear apposing the wound. These invaginations resembled the prominent rete ridge structures present in early human psoriasis (Figure 3E) and were observed in all 10 mouse ears used in the wounding study. Five ears were quantified for rete ridge counts (Figure 3C; Table 1). The induction of rete ridge formation in human pre-psoriatic skin has also been documented after skin injury and is termed the Koebner phenomenon.40 Accompanying these rete ridge structures were increased dermal cellularity (inflammatory infiltrate), hyperkeratosis, and focal parakeratosis. These data suggest that to induce a severe psoriatic phenotype in the 3-month-old K14-VEGF transgenic animals, it would be necessary to introduce another stimulus such as wounding. This is consistent with the clinical evolution from pre-psoriatic skin to psoriatic lesion in humans (Koebner phenomenon).
Dramatic psoriasiform epidermal lesions spontaneously develop in older K14-VEGF mice
As the K14-VEGF transgenic mice aged, they spontaneously began to develop dramatic lesions resembling full-fledged psoriasis. K14-VEGF transgenic animals older than 5 months of age developed pronounced epidermal rete ridges and marked cutaneous inflammation (Figure 3D). In these lesions, psoriasiform hyperplasia with elongated rete ridges and anastomosis of neighboring rete ridges was found (Figure 3D), revealing a striking resemblance to fully developed psoriasis in humans (Figure 3F). It is important to note that rete ridge formation is one of the most characteristic and longest-recognized histologic features of human psoriasis and that no other transgenic mouse model results in rete ridge formation (Figure 1).
Hyperplastic and inflamed cutaneous blood vessels in K14-VEGF transgenic mice are similar to those observed in human psoriasis
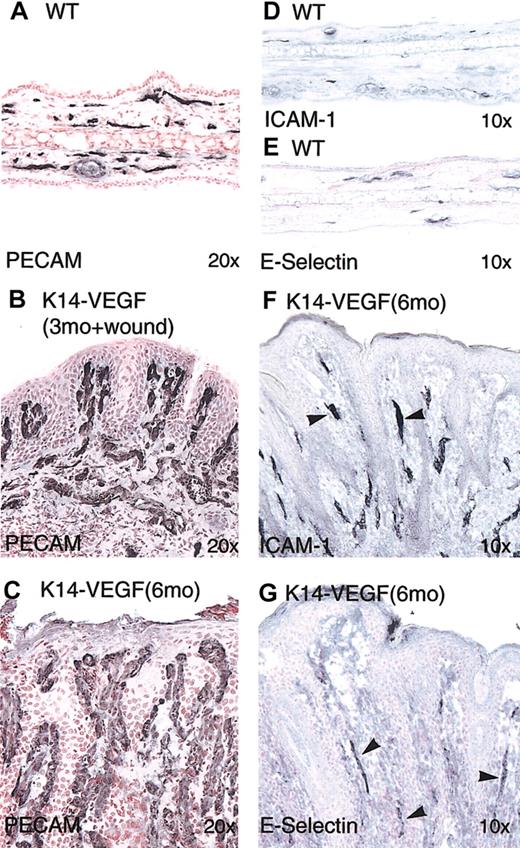
To understand the nature of the visible skin redness in the K14-VEGF transgenic mice, we immunocytochemically stained ear sections with an antibody to an endothelial-cell—specific antigen, PECAM-1. When compared with microvessels in wild-type skin (Figure 4,A) those in 3-month-old K14-VEGF mice with wound-induced psoriasis were obviously dilated and tortuous. The superficial vascular plexus showed that the most prominent angiogenic alterations consisted of vertically oriented vascular tufts in dermal papillae, similar to human psoriasis (Figure 4B). In the unwounded skin of older K14-VEGF mice (6 months of age), these enlarged vessels became more prominent within dermal papillae that were surrounded by a hyperproliferative epidermis undergoing rete ridge anastomosis (Figure 4C). Elongated and enlarged vessels found in the dermal papillae of K14-VEGF mice have a remarkable resemblance to the long, ectatic vessel loops seen in the dermal papillae of human psoriatic skin.
Hyperplastic and inflamed cutaneous blood vessels in K14-VEGF transgenic mice. Immunostaining was performed on cryosections of ear skin from wild-type littermate controls (A, D-E) and transgenic mice (B-C, F-G). PECAM staining showed increased vascular density mostly in the papillary dermis in wound-induced psoriasis in the 3-month-old transgenic mice (B). Enlarged vessels in 6-month-old transgenic mice showed vessels enclosed by anastomosing epidermal rete ridges (C). Immunostaining of E-selectin (G) and ICAM-1 (F) showed positive signals on dermal microvessels in transgenic mice (arrowheads). Original magnifications: × 20 (A-C); and × 10 (D-G).
Hyperplastic and inflamed cutaneous blood vessels in K14-VEGF transgenic mice. Immunostaining was performed on cryosections of ear skin from wild-type littermate controls (A, D-E) and transgenic mice (B-C, F-G). PECAM staining showed increased vascular density mostly in the papillary dermis in wound-induced psoriasis in the 3-month-old transgenic mice (B). Enlarged vessels in 6-month-old transgenic mice showed vessels enclosed by anastomosing epidermal rete ridges (C). Immunostaining of E-selectin (G) and ICAM-1 (F) showed positive signals on dermal microvessels in transgenic mice (arrowheads). Original magnifications: × 20 (A-C); and × 10 (D-G).
Because the K14-VEGF mice exhibited enlarged and tortuous vessels in dermal papillae analogous to those seen in human psoriatic skin, we next explored whether these hyperplastic vessels also exhibited features of vascular inflammation seen in patients with psoriasis. In particular, the induction of specific endothelial cell adhesion molecules is a hallmark of the hyperplastic and inflamed vessels seen in human psoriatic skin lesions, including the induction of E-selectin (CD62E),15 VCAM-1 (CD106),16 and ICAM-1 (CD54).14 Similar to findings in human psoriasis, the expression of these cell adhesion molecules were prominent in hyperplastic vessels in the psoriasiform skin from K14-VEGF mice (Figure 4F-G).
Abnormal epidermal proliferation and differentiation in K14-VEGF mice resembling that seen in human psoriasis
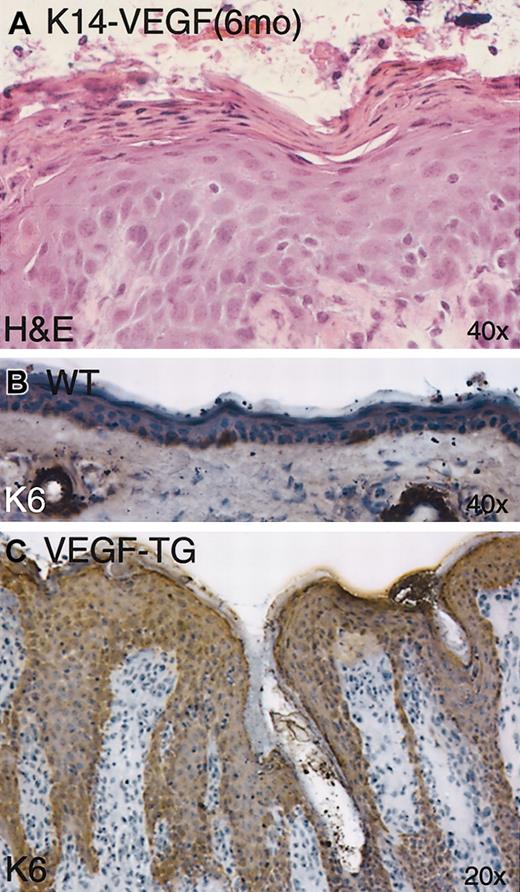
Epidermal analysis of the psoriasiform lesions in K14-VEGF transgenic mice revealed hyperkeratosis (increased thickness of the stratum corneum) and parakeratosis (retention of nuclei in the cornified keratinocytes) (Figure 5A). Human psoriasis is characterized by similar hyperkeratosis and parakeratosis44 and by altered epidermal hyperproliferation, as reflected by thickening of the epidermis and aberrant expression of hyperproliferation-associated keratins K6 and K16 throughout the epidermis, which are normally restricted to sporadic basal keratinocytes and hair follicles.45 Another similarity we found between the psoriasiform lesions of K14-VEGF mice and human psoriasis was the strong expression of K6 throughout the hyperplastic epidermis of K14-VEGF mice (Figure 5C); normal mouse skin, with the exception of hair follicles and occasional basal keratinocytes, did not express K6 (Figure 5B).
Abnormal epidermal proliferation and differentiation in K14-VEGF transgenic mice. Parakeratosis and hyperkeratosis were observed in H&E-stained skin sections from 6-month-old transgenic mice (A). Immunostaining of keratin K6 showed strong up-regulation throughout the epidermis (compare panels B and C) in 6-month-old transgenic mice.
Abnormal epidermal proliferation and differentiation in K14-VEGF transgenic mice. Parakeratosis and hyperkeratosis were observed in H&E-stained skin sections from 6-month-old transgenic mice (A). Immunostaining of keratin K6 showed strong up-regulation throughout the epidermis (compare panels B and C) in 6-month-old transgenic mice.
K14-VEGF mice exhibit epidermal microabscesses and inflammatory infiltrates characteristic of human psoriasis
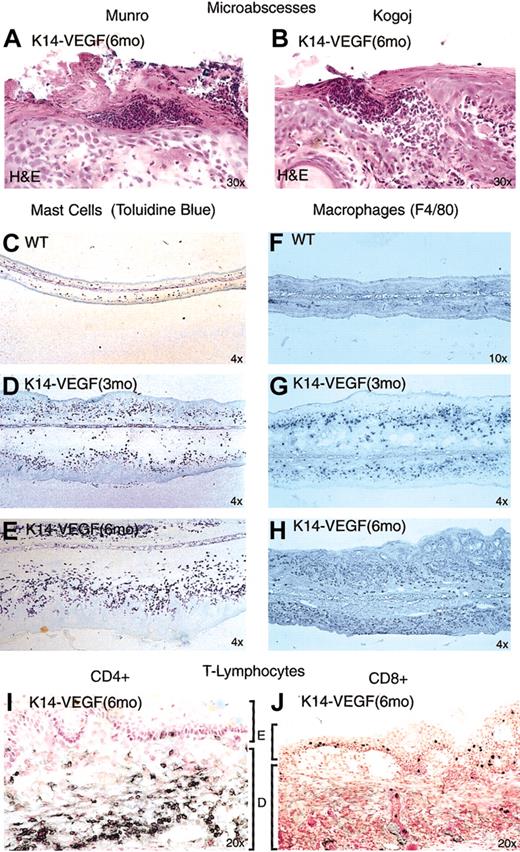
Neutrophil-filled lesions resembling the epidermal microabscesses found in advanced human psoriasis were observed in the epidermis of 6-month-old K14-VEGF transgenic mice (Table 2). One type of microabscess, mimicking the location of Munro microabscesses described in human psoriasis, was localized within the stratum corneum (Figure 6A),46,47 and a second type of microabscess, resembling Kogoj microabscesses seen in human psoriasis, was localized immediately beneath the stratum corneum (Figure 6B).46,47 The presence of microabscesses in human psoriatic skin is a key feature used in the clinical diagnosis of human psoriasis.46,47
K14-VEGF mice exhibit epidermal microabscesses and inflammatory infiltrates characteristic of human psoriasis. (A) Munro-like microabscess. (B) Kogoj-like microabscesses. Progressive increase of mast cell (C-E) and macrophage (F-H) density with 3-month-old (D, G) and 6-month-old (E, H) transgenic mice compared with wild-type littermates (C, F). CD4+ T-lymphocytes were detected primarily in the dermis (I) and CD8+ T-lymphocytes in the epidermis of 6-month-old transgenic mice (J). E indicates epidermis; D, dermis.
K14-VEGF mice exhibit epidermal microabscesses and inflammatory infiltrates characteristic of human psoriasis. (A) Munro-like microabscess. (B) Kogoj-like microabscesses. Progressive increase of mast cell (C-E) and macrophage (F-H) density with 3-month-old (D, G) and 6-month-old (E, H) transgenic mice compared with wild-type littermates (C, F). CD4+ T-lymphocytes were detected primarily in the dermis (I) and CD8+ T-lymphocytes in the epidermis of 6-month-old transgenic mice (J). E indicates epidermis; D, dermis.
Analysis of the inflammatory cell infiltrate in 3-month-old K14-VEGF mice revealed a significant increase in both mast cells (as determined by staining for toluidine blue, which stains mast cell granules; Figure 6D) and macrophages (as determined with an antibody to the murine macrophage marker F4/80 antigen (Figure 6G) when compared with wild-type littermate controls (Figure 6C-F). Further increases in mast cells and macrophages were evident as the animals aged to 6 months (Figure 6E-H).
To assess the number and distribution of CD4+ and CD8+ T-lymphocytes, we immunostained for these cells in the 3- and 6-month-old transgenic animals. Results revealed massive infiltration of CD4+ T-lymphocytes localized primarily to the dermis of 3-month- (data not shown) and 6-month-old transgenic mice (Figure 6I). The overall level of CD8+ T-lymphocytes that infiltrated into the transgenic skin was significantly lower than that of the CD4+ T-lymphocytes. In young K14-VEGF mice, these CD8+ T-lymphocytes were detected in the dermis and the epidermis (data not shown), whereas the CD8+ lymphocytes translocated and became localized to the epidermis in the 6-month-old transgenic mice (Figure 6J; Table 2). This complementary localization of CD4+ versus CD8+ lymphocytes in dermis and epidermis is also characteristic of human psoriatic skin.
Treatment with VEGF Trap normalizes the psoriatic phenotype in K14-VEGF transgenic mice
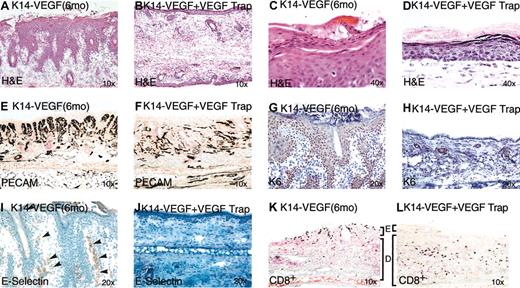
Our data demonstrate that many of the histologic and immunologic hallmarks of human psoriasis appear when VEGF is chronically overexpressed in mouse epidermis. To confirm the role of VEGF in the initiation and maintenance of this psoriatic phenotype and to attempt to ameliorate it, we used a potent VEGF inhibitor, VEGF Trap.43 Six K14-VEGF transgenic mice at 6 months of age with obvious psoriatic lesions were systemically treated with VEGF Trap at a dose of 25 mg/kg every 3 days for 12 days. Although similar lesions in K14-VEGF mice do not regress spontaneously or after control treatments, in 4 of the K14-VEGF mice treated with VEGF Trap, pronounced visual improvement was observed in lesions on gross inspection. The other 2 K14-VEGF mice treated with VEGF Trap displayed moderate improvement. Using enzyme-linked immunosorbent assay (ELISA) to detect antibodies raised against VEGF Trap in these animals, we detected an obvious immune response only in the latter 2 moderate responders, suggesting partial immunoneutralization of VEGF Trap in these mice (data not shown). Histologic evaluation of all 6 K14-VEGF mice treated with VEGF Trap revealed near-complete resolution of the rete ridge elongations (Figure 7A-B), normalization of epidermal architecture and diminution of parakeratosis (Figure 7C-D), and reduction in vascular hyperplasia (Figure 7E-F). In addition, the K6 marker of aberrant epidermal differentiation was normalized by VEGF Trap treatment (Figure 7G-H), as were markers of vascular inflammation E-selectin (Figure 7I-J), ICAM-1 in basal keratinocytes and vasculature (data not shown), and CD8+ T-lymphocyte distribution (Figure 7K-L).
VEGF Trap normalizes the psoriatic phenotype in K14VEGF transgenic mice. Transgenic mice with severe skin lesions were injected with VEGF Trap (25 mg/kg) on days 0, 3, 7, and 12. Tissue was harvested on day 14 for histologic analysis. H&E staining of mouse ear skin treated with VEGF Trap showed clear resolution of rete ridges (compare panels A and B) and decreased parakeratosis/hyperkeratosis (compare panels C and D). Immunostaining with PECAM showed a drop-off of microvessels in the papillary dermis (compare panels E and F). Immunostaining with keratin K6 and E-selectin each showed remarkable down-regulation of signals in the epidermis (compare panels G and H), dermal capillaries (compare panels I and J), respectively. Arrowheads in panel I denote positive staining of blood vessels for E selectin. CD8+ T-lymphocytes shifted localization from the epidermis to the dermis in treated animals (compare panels K and L). E indicates epidermis; D, dermis.
VEGF Trap normalizes the psoriatic phenotype in K14VEGF transgenic mice. Transgenic mice with severe skin lesions were injected with VEGF Trap (25 mg/kg) on days 0, 3, 7, and 12. Tissue was harvested on day 14 for histologic analysis. H&E staining of mouse ear skin treated with VEGF Trap showed clear resolution of rete ridges (compare panels A and B) and decreased parakeratosis/hyperkeratosis (compare panels C and D). Immunostaining with PECAM showed a drop-off of microvessels in the papillary dermis (compare panels E and F). Immunostaining with keratin K6 and E-selectin each showed remarkable down-regulation of signals in the epidermis (compare panels G and H), dermal capillaries (compare panels I and J), respectively. Arrowheads in panel I denote positive staining of blood vessels for E selectin. CD8+ T-lymphocytes shifted localization from the epidermis to the dermis in treated animals (compare panels K and L). E indicates epidermis; D, dermis.
Numerous reports have correlated the level of disease activity in psoriasis with the levels of soluble vascular adhesion molecules in the sera of patients with psoriasis, in particular soluble E-selectin that is presumably cleaved from the surface of inflamed dermal vessels.17-20 Further correlating our animal model with the human condition, we find that serum levels of E-selectin are much higher in K14-VEGF mice (249.42 ± 38.64 ng/mL) than in control mice (37.34 ± 7.06 ng/mL), and, just as important, these increased levels are reduced with VEGF Trap treatment (to 96.74 ± 16.25 ng/mL). Altogether, our data indicate that VEGF is continuously required to maintain the psoriasiform lesions in older K14-VEGF mice and that even longstanding disease can be dramatically reversed with VEGF blockade.
Discussion
The underlying pathogenic mechanism and the key molecule(s) that are causative for psoriasis have not yet been identified. Recent studies for causative agents have focused on molecular mediators of inflammation or keratinocyte growth. However, attempts to mimic human psoriasis by transgenically overexpressing such mediators in mice do not completely recapitulate the human disease in all its pathologic aspects (Figure 1). In addition, the most faithful animal model of psoriasis to date requires the transplantation of human psoriatic skin to SCID mice23 (Figure 1). Our findings lend credence to earlier suggestions that vascular changes might be among the earliest markers of the human psoriatic state.7,11 In particular, our studies suggest that VEGF, which has previously been shown to be dramatically elevated in human psoriatic skin,12 might play a causative role in the vascular changes seen in this disease and also in epidermal and inflammatory alterations. Along these lines, we demonstrate that excess VEGF in the skin is sufficient to create a predisposition to a psoriatic phenotype and that such overexpression eventually leads to the spontaneous development of a psoriasiform condition in mice that recapitulates human psoriasis in many of its features—not only the hyperplastic and inflammatory vascular changes but also the characteristic epidermal alterations and tissue inflammatory cell infiltrates (Figure 1). Additional emerging evidence for a role of VEGF in the etiology of psoriasis comes from recent genetic analyses showing an association between VEGF promoter polymorphisms and the development of psoriatic symptoms (M.D., personal oral communication, November 2002). The induction of psoriasis by VEGF seems to be specific in that skin-specific transgenic delivery of another angiogenic factor, Ang1, does not result in a similar phenotype.1,4
In our model, it is clear that excess VEGF does not immediately cause full-blown disease. It takes up to 5 to 6 months for the development of obvious spontaneous disease. Overexpression of VEGF in the adult animal skin by viral gene transfer does not induce psoriasis because short-term VEGF expression is not sufficient to induce the psoriatic phenotype. In fact, it has been shown that the injection of a nonreplicating adenoviral vector, engineered to express VEGF164, into the ears of athymic mice only temporarily induced the formation of dilated and leaky angiogenic vessels.48 Thus, it seems likely that acute overexpression of VEGF creates a dilated, leaky, and inflamed cutaneous vasculature but that chronic overexpression is necessary to yield a more widespread inflammatory condition in the skin with profound epidermal changes resembling psoriasis. Although it is unclear how VEGF results in such widespread changes, it seems likely that the inflamed vasculature, which exhibits elevations in vascular adhesion molecules such as E-selectin, ICAM-1, and VCAM-1, plays a primary role by promoting the extravasation of inflammatory cells to the skin that then lend their own cytokine and chemokine mediators to the process. This inflammatory infiltrate and the tissue edema promoted by the leaky vessels may well compromise the normal barrier function of the skin, allowing the entry of exogenous antigens and further exacerbating the immune state. The creation of a diverse inflammatory milieu may then secondarily lead to epidermal alterations that seem to occur after the initial vascular and inflammatory changes in our model. Regardless of the mechanism by which chronically elevated VEGF in our model results in a psoriasiform phenotype, the maintenance of this abnormal state remains dependent on VEGF—we show that VEGF blockade late in this process can effectively reverse almost all the observed abnormalities. It should perhaps not be surprising, based on previous studies indicating that VEGF can act as a potent and pleiotropic inflammatory agent, that transgenic delivery of VEGF to the skin can lead to a profound inflammatory skin condition. For example, a recent in vitro study using human umbilical vein endothelial cells (HUVECs) showed that VEGF stimulated the expression of ICAM-1, VCAM-1, and E-selectin through nuclear factor-κB activation.49 VEGF has also been shown to induce monocyte activation and chemotaxis through VEGF receptor-1 (Flt-1), which is expressed on monocytes.50,51 In addition, VEGF has been shown to induce expression of the chemokine IL-8, which is a potent modulator of the transendothelial migration of neutrophils.52
Although we do not yet precisely understand how transgenic overexpression of VEGF eventually leads to a psoriasiform condition in mice, it seems impossible to ignore the possibility that VEGF may play a key causative role in human psoriasis, and it seems important to follow up on this possibility and on the implications for understanding and treating the human disease. Conventional psoriasis treatments that attempt to control the inflammatory response and subsequent epidermal hyperproliferation rely on immunosuppressants and antiproliferative therapy, involving considerable toxicity often without complete resolution. The use of a specific VEGF antagonist, such as VEGF Trap, to eliminate the hyperplastic vascular phenotype, suppress the associated inflammatory state, and reduce the levels of surrogate markers (such as E-selectin) of the disease in human psoriasis may provide a novel therapeutic strategy with minimal adverse side effects. It is also important to note that some existing or emerging therapies for psoriasis may act in part by blocking the VEGF pathway. For example, calcineurin inhibition by cyclosporine or FK-506 may block VEGF production or action,53,54 and tumor necrosis factor-α (TNF-α) and IL-1 seem to be potent inducers of VEGF. This induction may be important for some of their pathologic actions.55,56
Our findings demonstrate that prolonged VEGF overexpression has powerful proinflammatory capabilities in vivo and leads to a skin phenotype resembling human psoriasis. VEGF is likely a key factor in the link between inflammation and angiogenesis. Therefore, its role should be explored in a variety of other inflammatory conditions. Furthermore, our findings substantiate emerging concerns6 about potential adverse effects that might be associated with therapeutic attempts to chronically deliver VEGF for proangiogenic purposes, particularly with regard to its profound proinflammatory capabilities.
Prepublished online as Blood First Edition Paper, March 20, 2003; DOI 10.1182/blood-2002-12-3793.
Supported in part by National Institutes of Health/National Cancer Institute grants CA69184 and CA86410 (M.D.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs Nick Gale and Virginia Hughes for maintaining transgenic mouse lines, Dr Dan Ragland for help with mast cell staining, and Drs Thomas Hawighorst and Jennifer Silva for contributing to the immunostaining. We also thank Scott Staton and Vicki Lan for image processing.