Abstract
Platelet-type von Willebrand disease (PTVWD) is a bleeding disorder in which an increase of function mutation in glycoprotein Ibα (GPIbα), with respect to binding of von Willebrand factor (VWF), results in a loss of circulating high molecular weight VWF multimers together with a mild-moderate thrombocytopenia. To better ascertain the specific perturbations in adhesion associated with this disease state, we performed a detailed analysis of the kinetic and mechanical properties of tether bonds formed between PT-VWD platelets and the A1-domain of VWF. Results indicate that the GPIbα mutation, Gly233Val, promotes and stabilizes platelet adhesion to VWF at shear rates that do not support binding between the native receptor-ligand pair due to enhanced formation and increased longevity of the mutant tether bond (k0off values for mutant versus native complex of 0.67 ± 0.11 s-1 and 3.45 ± 0.37 s-1, respectively). By contrast, the sensitivity of this interaction to an applied force, a measure of bond strength, was similar to the wild-type (WT) receptor. Although the observed alterations in the intrinsic properties of the GPIbα–VWF tether bond are comparable to those reported for the type 2B VWD, distinct molecular mechanisms may be responsible for these function-enhancing bleeding disorders, as interactions between the mutant receptor and mutant ligand resulted in a greater stability in platelet adhesion. We speculate that the enhanced cellular on-rate together with the prolongation in the lifetime of the mutant receptor-ligand bond contributes to platelet aggregation in circulating blood by permitting the formation of multiple GPIbα–VWF-A1 interactions. (Blood. 2003;102:152-160)
Introduction
Effective hemostasis relies on the rapid deposition of circulating platelets at sites of vascular injury. Critical to this process is the ability of von Willebrand factor (VWF), a multimeric plasma protein, to form a bridge between components of the subendothelium and the platelet glycoprotein receptor GPIbα.1-3 Surface immobilization of VWF appears to be a prerequisite for subsequent interactions with platelets, as this multimeric protein does not bind appreciably to these cells in the circulation. Once attached, platelets begin to translocate on VWF through interactions with its A1 domain (VWF-A1) until they either encounter reactive substrates such as collagen or are exposed to thrombin, events required for integrin-mediated firm adhesion.4-8
Previously, it has been demonstrated that the ability of VWF to promote both rapid and reversible attachment of platelets in flow, and thus translocation of these cells, is a consequence of the relatively high rate of bond formation and dissociation between the A1 domain and GPIbα.9 Interestingly, kinetic analysis of gain-of-function mutations localized to the VWF-A1 region, collectively known as type 2B von Willebrand disease (VWD), revealed an alteration in the intrinsic properties of these mutant bonds that may account for the observed clinical phenotype. Function-enhancing mutations, however, are not limited to VWF but have also been associated with GPIbα and are termed platelet-type or “pseudo” VWD (PT-VWD).10,11 They occupy a region in the carboxy-terminal flank of this molecule defined as the β-switch that encompasses amino acids 227 to 241.12,13 Incorporation of these mutations is believed to stabilize the interaction with the A1 domain by a mechanism(s) distinct from those associated with type 2B VWD, but functional evidence is lacking to date. Despite these differences, the clinical phenotype and laboratory abnormalities associated with this disorder mimic those seen in type 2B VWD, which include a prolonged bleeding time, borderline thrombocytopenia, and a decrease in the high molecular weight multimers of VWF.14 Patients with either disorder have increased bleeding tendencies that are believed to result from the spontaneous binding of plasma VWF to circulating platelets and the subsequent clearance of these hemostatic elements from blood.
Recently, a limited kinetic analysis of the interaction between a recombinant N-terminal GPIbα fragment (residues 1 to 290) and VWF-A1 domain (residues 498 to 705) was performed.13 Results from this study revealed a dissociation constant (Kd) of about 30 nM for the native receptor-ligand complex, a value 100-fold lower than previous reports.15,16 Interestingly, the alterations in affinity associated with PT-VWD mutations were believed to be due exclusively to changes in on-rate. Yet, functional studies evaluating the interactions of GPIbα either expressed on Chinese hamster ovary (CHO) cells or bound to latex beads with surface-immobilized VWF suggest that the off-rate may be altered as well.17-19 This manifested as a reduction in rolling velocities of cells or particles containing the PT-VWD mutation, Gly233Val, as compared with the native complex. However, disparities in rolling velocities of CHO cells expressing the identical PT-VWD mutation have also been reported.17,18 Moreover, all experiments performed to date have utilized densities of GPIbα and VWF capable of supporting multiple bond formation, which can mimic the effects of increased strength and/or lifetime of the bond between this receptor-ligand pair. Thus, it remains to be determined whether the enhanced phenotype associated with PT-VWD, as compared with the native receptor, is mediated by (1) the ability of mutant form of GPIbα to promote the formation of a greater number of bonds, (2) a prolongation in the lifetime of these bonds, or (3) a direct alteration in the sensitivity of the bond to mechanical loading. Moreover, it has not been demonstrated whether a distinct set of molecular mechanisms can account for the enhanced function associated with PT-VWD versus type 2B VWD.
To investigate the impact of the Gly233Val mutation on GPIbα-VWF interactions, we evaluated the kinetic and mechanical properties of the interactions between PT-VWD platelets and recombinant VWF-A1 domain protein under conditions that limit multiple bond formation. Our results indicate that the mutant form of the receptor contributes to the stability of platelet-VWF interactions by enhancing the rate of bond formation as well as prolonging the duration of its lifetime. Importantly, we demonstrate for the first time that distinct molecular mechanisms govern the increased adhesive properties associated with PT versus type 2B VWD, as interactions between the mutant receptor and mutant ligand resulted in greater stability in platelet adhesion.
Materials and methods
Reagents
Monoclonal antibodies (mAbs) used in experiments included GPIbα–blocking mAb 6D1 and anti-GPIIb/IIIa mAb 7E3 (B. Coller, Rockefeller University, New York, NY), mouse anti–6-HIS mAb (Research Diagnostics, Flanders, NJ), and anti–VWF-A1 mAb AMD-1.9 The generation of Fab fragments and recombinant VWF-A1 histag proteins, as well as coating of latex microspheres (Bangs Laboratories, Fishers, IN) were performed as previously described.9
Laminar flow assays
Platelet-rich plasma was prepared by centrifugation of whole blood obtained from either 5 healthy donors or 2 individuals (patients A and B) with PT-VWD that express the GPIbα mutation Gly233Val.10,11 Approval was obtained from the Washington University institutional review board for these studies. Informed consent was provided according to the Declaration of Helsinki. Platelets were washed twice in Tyrode buffer containing 0.25% human serum albumin (HSA), pH 7.4, resuspended at a concentration of 5 × 107/mL and subsequently perfused over high concentrations of surface-immobilized VWF-A1 proteins (100 μg/mL) or plasma VWF (25 μg/mL) in a parallel plate flow chamber at various wall shear stresses (0.2 to 16 dyne cm-2).9 Platelet attachment and their subsequent forward motion, termed translocation, were recorded on Hi-8 videotape using an inverted Nikon microscope with a plan 10× or 20× objective, respectively.
In flow experiments involving VWF-A1–coated microspheres, purified platelets treated with 10 mM sodium azide, 50 ng/mL prostaglandin E1, and 10 μM indomethacin (Sigma Immunochemicals, St Louis, MO) were immobilized on glass plates coated with Fab 7E3 fragment.9 Platelet coverage of more than 90% of the glass surface area was used in determining the frequency of attachment of VWF-A1–coated beads in flow while a total platelet coverage of less than 10% was used for kinetic assays to ensure bead interactions with only individual platelets. Estimation of the amount of VWF-A1 coupled to beads was determined using mAb AMD-1 and a calibrated microbead system (Flow Cytometry Standards, San Juan, PR) following the manufacturer's instructions. The site density of VWF-A1 on beads coated with 5 μg/mL protein was about 30 sites per square micrometer.
Dissociation rate constants and mechanical strength of transient tether bonds
The duration of tether bonds, the smallest unit of adhesion observable in flow, was estimated for VWF-A1–coated microspheres interacting with surface-immobilized platelets at wall shear stresses ranging from 0.5 to 2.0 dyne cm-2. The lowest coating concentrations of VWF-A1 capable of supporting these brief adhesive events were determined by measuring the percentage of beads (1 × 106/mL) that paused, but did not roll, on antibody-immobilized platelet substrates in flow. Only one tethering event per bead was counted during the observation period.
The dissociation rate constants of transient tethers were calculated for coating concentrations of beads that only supported these transient interactions. The duration of these interactions was measured by recording images from a Nikon X60 DIC objective (oil immersion) viewed at a frame rate of 235 frames per second (fps) (Speed Vision Technologies, San Diego, CA). Dissociation rate constants were determined by plotting the natural log of the number of VWF-A1–coated microspheres that interacted as a function of time after the initiation of tethering.9 The slope of the line = -koff. The force acting on the tether bond was calculated from force balance equations (bead radius of 3.5 μm) satisfied with a tether angle θ of 57.2 degrees.9
Models of pause time distribution
Because platelets were obtained from patients heterozygous for the mutation, we considered the possibility that adhesion could result from either receptor. To this end, we evaluated the 3 most relevant and likely models amenable to experimental and/or theoretical testing to ascertain the relative roles of the wild-type and mutant GPIbα in our experiments. The first of these presumed that all tether bonds formed were mediated by a single mutant GPIb receptor on the surface-immobilized platelet interacting with VWF-A1–coated beads (model 1). The second hypothesis also presumed that all events were initiated by the formation of a single-tether bond but also considered that a fraction of these were the result of interactions between the native complex (model 2). The third and last hypothesis (model 3) presumed that several tether bonds of either the wild-type or mutant variety can mediate platelet adhesion. Inasmuch as these 3 hypotheses are mutually exclusive, statistical rejection of any 2 based upon experimental observations implicitly proves the third.
In this case of the first model, the pause time is the length of time preceding the dissociation of the single mutant tether bond. Because noncovalent interactions between a receptor-ligand pair can behave as a one-step chemical reaction that occurs randomly in time, pause times will also be distributed in a similar manner. In the case where there is only one bond to break, the lifetime of the interaction is exponentially distributed with an average duration of 1/koff,mut, where koff,mut is the dissociation rate constant for the bond.9,20 That is,
Because this equation (equation 1) exactly describes the hypothesis underlying this model (model 1), it can be tested using standard statistical techniques. First, koff,mut must be estimated from a set of experimental pause times at a given wall shear stress. To this end, we employ the statistically robust method of maximum likelihood.9,21 This results in the following formula (equation 2) for the statistical point estimate (SPE) of koff,mut from N experimental pause times9 :
where
A similar procedure was employed for the second alternative hypothesis (model 2) that the single-tether bond connecting the platelet to the surface can be either a wild-type tether bond or a mutant. In this case, equation 2 is the probability that the pause time will be of duration t given that the tether bond is between a mutant GPIbα molecule and VWF. If a fraction q of the transient pauses are mediated by wild-type GPIbα, the probability that a pause is mediated by a single mutant receptor is (1 - q)koff,mut exp(-koff,mutt)dt. Therefore, if this alternative hypothesis is true, the pause time distribution is exactly
If equation 3 (model 2) is the actual pause time distribution for the experimental results, the probability q that the pause is mediated by a wild-type GPIb receptor must be addressed. Although both the mutant and wild-type receptors are assumed to be equally expressed, their association rate constants with VWF-A1 (kon) may differ. Consequently, q would not necessarily be 0.5.
As we have discussed for model 1, a maximum likelihood statistical point estimate for koff,mutt can be computed from experimental data. In this case, its computation is iterative and must be performed using a computer, according to equation 4:
In this process, the dissociation constant for the wild-type receptor (koff,wt) was set to 3.5exp(σFb/kT) as previously obtained for normal platelets at each wall shear stress.9 Because the actual value of q is unknown, a locus of statistical point estimates was obtained for 20 values of q spanning the possible range (0-1). The best value was selected during the KS hypothesis testing of equation 3 as the model that best fit the experimental results.
A third and final hypothesis can be considered (model 3), wherein multiple tether bonds of either the mutant or wild-type (WT) GPIbα can form after the initiation of platelet adhesion. Unlike the previous hypotheses, model 3 does not afford a simple expression for the pause time distribution. However, the pause time distribution can be generated for this model by Monte Carlo (MC) simulation.9 We have included 2 bonding and 2 dissociation steps: 2 for the on and off reaction of WT GPIbα + VWF-A1 ↔ GPIbα–VWF-A1 and 2 for the on and off reaction of mutant GPIbα′ + VWF-A1 ↔ GPIbα′–VWF-A1. In this analysis, the initial bond was chosen randomly in accordance with the preset probability q, which was varied from simulation to simulation. Using a set of association rate constants (kon,mut, kon,wt) and dissociation rate constants (koff,mut, koff,wt), adhesion can be simulated from formation of the first bond to detachment of the platelet. The time between these 2 events is the pause time. By successive simulations, a distribution of pause times is generated at each shear stress, which can be compared with experimental data via the KS test. Therefore, by systematic variation of parameters entered into the MC simulation and subsequent testing of the resulting distributions against experimental data, the best set of rate constants can be obtained. Moreover, this KS testing can reject the hypothesis of multiple bonding.
Statistical analysis
A Student t test was used for multiple comparisons. Statistical significance was set at a P value less than .05.
Results
The impact of the GPIbα mutation Gly233Val on VWF-dependent platelet attachment and translocation
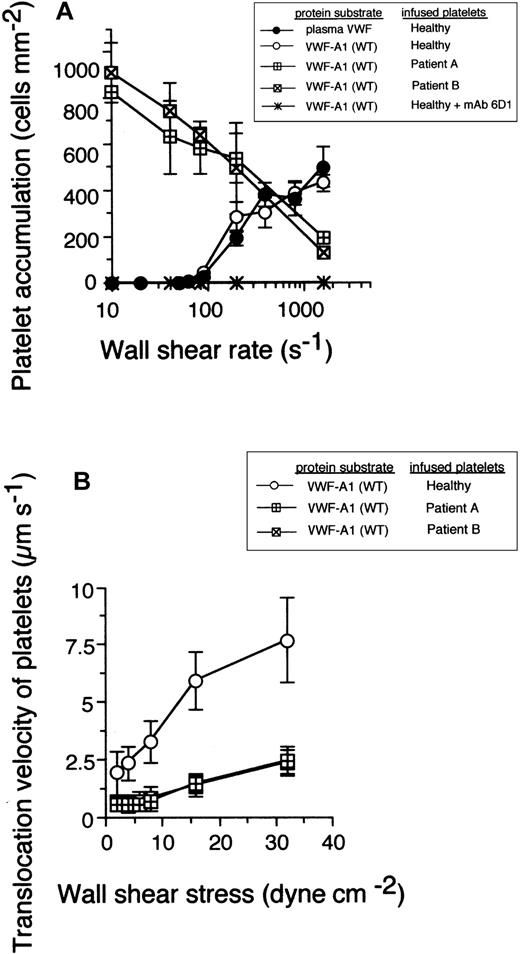
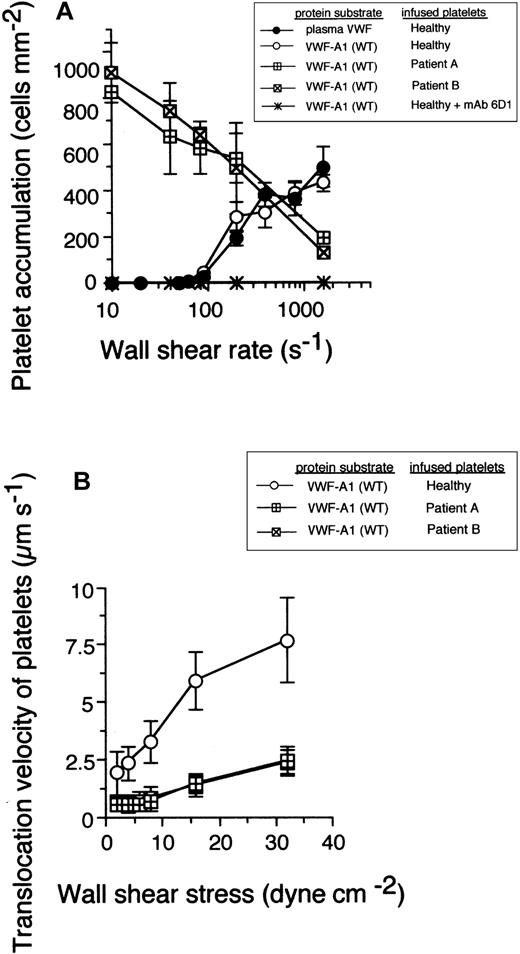
It is been established that successful bond formation between a receptor-ligand pair in flow relies on a balance between the number of encounters that can occur over a defined time and the rate at which a bond can form, parameters affected by shear rate, association rate constant, and receptor-ligand concentrations.22,23 The Gly233Val mutation increased the rate of association of GPIbα: platelets from 2 patients with this bleeding disorder readily attached to and translocated (moved continuously in the direction of flow at least 1 cell diameter) on surface-immobilized VWF-A1 substrate at wall shear rates (less than 100 s-1) that did not promote any interactions with healthy platelets (Figure 1A). At arterial wall shear rates of at least 1000 s-1, platelets expressing the mutant receptor displayed a deficiency in adhesion to high surface density of VWF-A1 as compared with platelets from healthy individuals. This suggests that the Gly233Val mutation may increase the sensitivity of GPIbα to mechanical loading at higher levels of force. The specificity of this adhesive event was demonstrated by the ability of the GPIbα function-blocking mAb 6D1 to inhibit PT-VWD platelets from binding to the WT recombinant protein under all flow conditions tested. The effect of this inherited mutation, however, was not restricted to an increase in the apparent on-rate, because platelet translocation velocities were also reduced by about 3- to 4-fold, suggesting a perturbation in the kinetics that govern bond dissociation as well (Figure 1B).
The impact of the Gly233Val mutation on platelet attachment and translocation velocities. (A) Platelets (5 × 107/mL) purified from 2 patients with PT-VWD or 5 healthy individuals were infused at the indicated wall shear rates through a parallel plate flow chamber apparatus for 5 minutes. The total number of healthy platelets attached to either surface-immobilized plasma VWF (25 μg/mL) or recombinant VWF-A1 protein (100 μg/mL) was determined and compared with PT-VWD platelet interactions with VWF-A1 (mean ± SD; n = 5). The specificity of the interaction was determined by the ability of the GPIbα-blocking antibody, mAb 6D1, to inhibit healthy platelet or PT-VWD platelet (not shown) to A1 substrate. (B) Mean translocation velocities for healthy or PT-VWD platelets (n = 30 to 40 cells) interacting with the immobilized VWF-A1 substrate were determined at the indicated wall shear stresses. Data represent the mean ± SD for 3 to 4 independent experiments performed in duplicate.
The impact of the Gly233Val mutation on platelet attachment and translocation velocities. (A) Platelets (5 × 107/mL) purified from 2 patients with PT-VWD or 5 healthy individuals were infused at the indicated wall shear rates through a parallel plate flow chamber apparatus for 5 minutes. The total number of healthy platelets attached to either surface-immobilized plasma VWF (25 μg/mL) or recombinant VWF-A1 protein (100 μg/mL) was determined and compared with PT-VWD platelet interactions with VWF-A1 (mean ± SD; n = 5). The specificity of the interaction was determined by the ability of the GPIbα-blocking antibody, mAb 6D1, to inhibit healthy platelet or PT-VWD platelet (not shown) to A1 substrate. (B) Mean translocation velocities for healthy or PT-VWD platelets (n = 30 to 40 cells) interacting with the immobilized VWF-A1 substrate were determined at the indicated wall shear stresses. Data represent the mean ± SD for 3 to 4 independent experiments performed in duplicate.
The effect of the Gly233Val mutation on the rate of formation and dissociation of the GPIbα–VWF-A1 tether bond
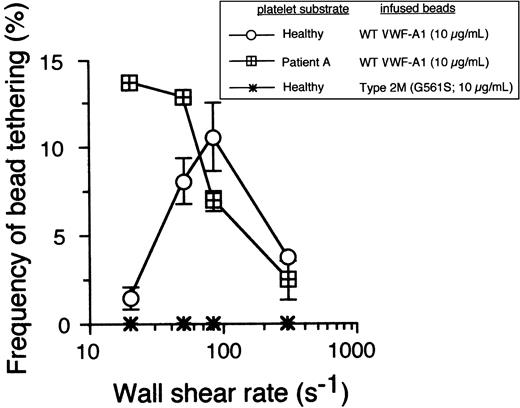
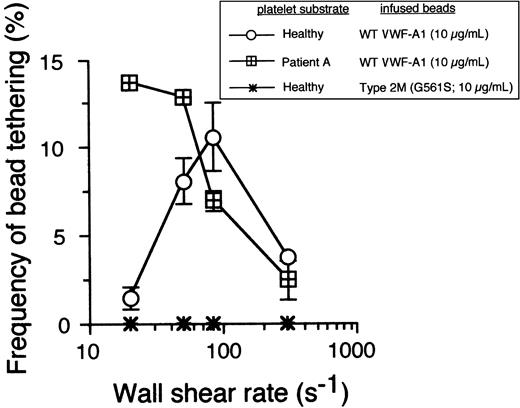
The ability of PT-VWD platelets to attach and translocate at shear rates unable to support healthy platelet interactions is suggestive of enhanced formation and/or increased stability of the receptor-ligand bond. However, stabilization of adhesion may result from either the formation of a greater number of bonds than for the native complex or from a prolongation in the lifetime of the interaction between GPIbα–VWF-A1.24 To more directly measure these kinetic parameters, we next analyzed the formation and dissociation of transient adhesive events, known as tether bonds, formed between microspheres coated with low site densities of VWF-A1 and surface-immobilized platelets. Recently, we have demonstrated the utility of using beads with a uniform size and shape to permit determination of the relationship between wall shear stress and the force directly acting on the GPIbα–VWF-A1 tether bond (Fb),9 a parameter difficult to estimate for discoid-shaped objects such as platelets. This also permits greater hydrodynamic loading rates of bonds due to the projection of the larger bead into a flow stream of higher velocity. Importantly, the use of platelets as the immobilized substrate enables evaluation of GPIbα in its native form (ie, correct orientation and proper posttranslational modification). In this system, the formation of a tether bond is detected as an abrupt halt in the forward motion of a bead in flow, and the lifetime of this quantal unit of adhesion can be determined by measuring the duration that the VWF-A1–coated microsphere remains stationary before resuming a velocity equivalent to a noninteracting particle. These transient adhesive events can be observed over a wide range of wall shear stresses only if the concentration of ligand or receptor is reduced so as to limit multiple bond formation. Importantly, tether bonds also appear to follow first-order kinetics and, as such, permit estimates of dissociation rate constants in zero stress (k0off) as well as the sensitivity of this transient unit of adhesion to the destabilizing effects of hydrodynamic force. At bead coating concentrations of VWF-A1 of 10 μg/mL or less, transient tether bond formation was the only type of interaction observed on either healthy or PT-VWD platelet substrates at wall shear rate tested (Figure 2). Consistent with the dynamic behavior of the PT-VWD platelets perfused over a high density of VWF-A1, the optimal frequency of transient tether bond formation mediated by VWF-A1–coated beads interacting with mutant GPIbα occurred at a shear rate corresponding to the lowest tethering frequency for the WT complex. The specificity of this adhesive event was demonstrated by the inability of microspheres coated with VWF-A1 containing the type 2M mutation Gly561Ser to interact with surface-immobilized platelets under all flow conditions tested.
The cellular on-rate for PT-VWD platelets is higher than that of healthy platelets. Microspheres coated with recombinant VWF-A1 protein at a concentration that did not support translocation were perfused over a confluent monolayer of surface-immobilized healthy or PT-VWD platelets at wall shear rates ranging from 20 to 300 s-1. The frequency of tether formation was determined by quantifying the number of beads that transiently attached to the substrate over 1 minute and subsequently dividing by the measured flux of beads near the wall over the same time period.9 Data represent the mean ± SD of 4 independent experiments.
The cellular on-rate for PT-VWD platelets is higher than that of healthy platelets. Microspheres coated with recombinant VWF-A1 protein at a concentration that did not support translocation were perfused over a confluent monolayer of surface-immobilized healthy or PT-VWD platelets at wall shear rates ranging from 20 to 300 s-1. The frequency of tether formation was determined by quantifying the number of beads that transiently attached to the substrate over 1 minute and subsequently dividing by the measured flux of beads near the wall over the same time period.9 Data represent the mean ± SD of 4 independent experiments.
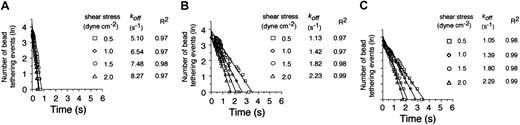
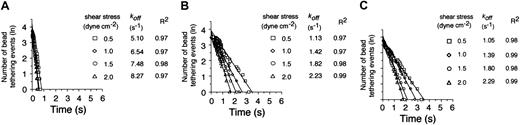
Given this difference in apparent cellular on-rates, we next compared the dissociation kinetics of these transient adhesive events. A coating concentration of VWF-A1 was chosen (5 μg/mL corresponding to 30 molecules/μm2) that supported tether bond formation at wall shear stresses ranging from 0.5 to 2 dyne cm-2. Analysis of the distribution of interactions times between VWF-A1–coated beads and the platelet substrates indicates that more than 95% of all transient tether bonds events fit a straight line, the regressed slope of which corresponded to a single koff (Figure 3). Notably, the cellular off-rates of these quantal units of adhesion were quite similar for the 2 patients but were 3- to 4-fold lower as compared with the native receptor-ligand pair at all wall shear stress tested (Figure 3B-C). Based on these results, it appears that the higher kinetic stability of PT-VWD platelets with the VWF-A1 substrate is also mediated by an increase in the individual tether bond lifetimes or, conversely, a decrease in the cellular dissociation rate. In all cases, increases in wall shear stress resulted in a progressive reduction in the duration of the tether bond lifetime as indicated by the increases in koff.
Kinetics of dissociation of transient tethers. Representative graphs depicting the distribution of interaction times for more than 35 individual transient attachment events (known as tether bonds) that occurred between microspheres coated with VWF-A1 (about 30 molecules per square micrometer) and surface-immobilized platelets purified from healthy individuals (A) or 2 patients with PT-VWD (B-C). Dissociation rate constants were estimated at wall shear stresses of 0.5 to 2.0 dyne cm-2; koff is the negative slope of the linear regression through the experimental data.
Kinetics of dissociation of transient tethers. Representative graphs depicting the distribution of interaction times for more than 35 individual transient attachment events (known as tether bonds) that occurred between microspheres coated with VWF-A1 (about 30 molecules per square micrometer) and surface-immobilized platelets purified from healthy individuals (A) or 2 patients with PT-VWD (B-C). Dissociation rate constants were estimated at wall shear stresses of 0.5 to 2.0 dyne cm-2; koff is the negative slope of the linear regression through the experimental data.
Estimation of the intrinsic kinetics of dissociation and mechanical strength of tether bonds
Previous studies have demonstrated the utility of the Bell model in describing the dissociation kinetics and mechanical properties of adhesive bonds involved in selectin-dependent or GPIbα–mediated attachment of leukocytes and platelets in flow, respectively.9,25-28 Bell proposed that the dissociation rate constant, koff, of a bond under an applied force, Fb, could be given by the equation koff = k0offexp(σFb/kT), where k0off is the dissociation rate constant in the absence of force, kT is the thermal energy, and σ (reactive compliance) is a biomechanical parameter that relates the sensitivity of bond off-rate to an applied force.29 The larger the values for k0off and σ, the shorter the bond lifetime and the more prone the receptor-ligand interaction is to force-driven dissociation (ie, reduced mechanical stability), respectively. Thus, the Bell model permits determination of bond properties that directly govern receptor-ligand interactions in flow, parameters not obtainable by affinity measurements (Kd) alone.30
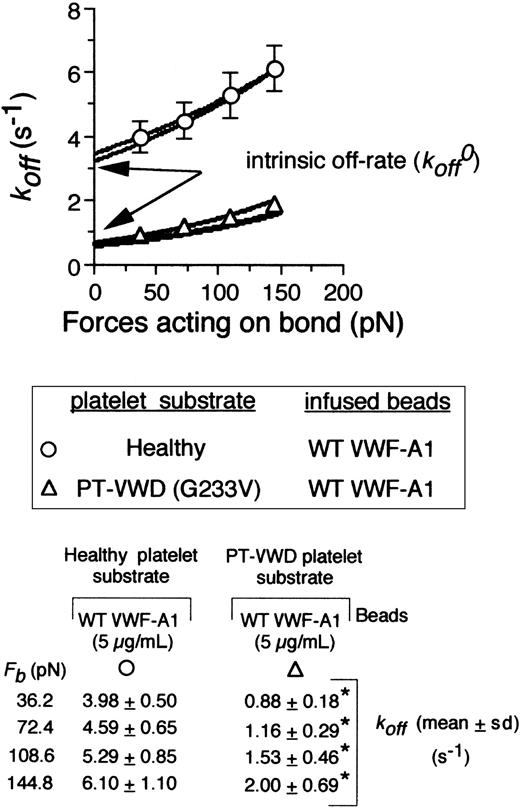
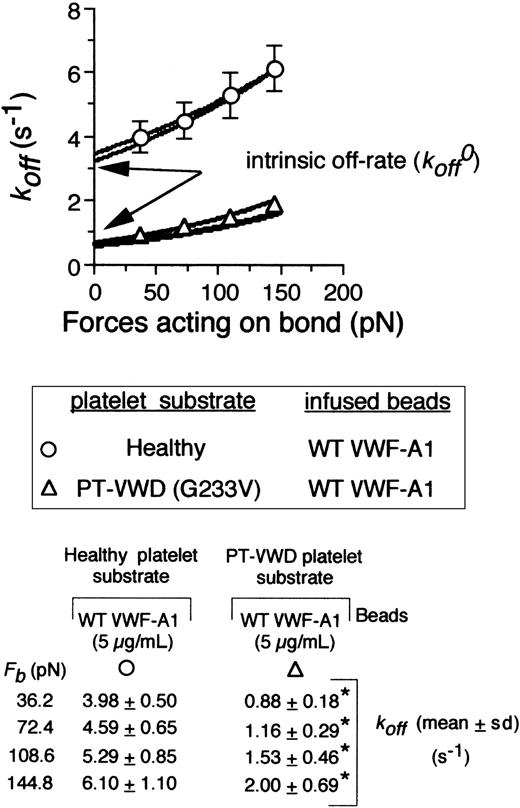
To obtain values for the intrinsic off-rate (k0off) and σ,it was first necessary to demonstrate that our values obtained for the dissociation rate constants at various hydrodynamic forces fit the Bell model. This was confirmed by Monte Carlo (MC) simulation and statistical point estimate (SPE) analysis of the experimental data. Importantly, regression of these analyses fit our data for the dissociation rate constants determined at bond forces ranging from 36 pN to 145 pN (Figure 4, bottom panel). Strong agreement was also observed between the 2 methods. In accordance with the Bell model, dissociation rate constants varied exponentially as a function of force on the tether bond for both the native and mutant complex but were 3- to 4-fold lower for the latter (Figure 4B). Extrapolation of our data to zero force yielded k0off values for the WT and mutant receptors of 3.45 ± 0.37 s-1 and 0.67 ± 0.11 s-1, respectively. By contrast, there was an about 2-fold increase in the reactive compliance for the latter (0.016 ± 0.002 nm [WT] versus 0.031 ± 0.005 nm [mutant]), suggesting that mechanical stability of the tether bond is not enhanced by the inclusion of the Gly233Val mutation.
The effect of shear force on the kinetics and mechanical strength of mutant tether bonds. Cellular dissociation rate constants were obtained for VWF-A1–coated beads interacting with a healthy or PT-VWD platelet substrates. The koff results are plotted as a function of the calculated force on the tether bond, Fb (72.41 × wall shear stress).9 The experimental data were fit to the Bell equation based on Monte Carlo (MC) and statistical point estimate (SPE) analyses. Table shows values for koff as a function of applied force. Data represent the mean ± SD for 5 independent experiments at each wall shear stress tested. *P < .05.
The effect of shear force on the kinetics and mechanical strength of mutant tether bonds. Cellular dissociation rate constants were obtained for VWF-A1–coated beads interacting with a healthy or PT-VWD platelet substrates. The koff results are plotted as a function of the calculated force on the tether bond, Fb (72.41 × wall shear stress).9 The experimental data were fit to the Bell equation based on Monte Carlo (MC) and statistical point estimate (SPE) analyses. Table shows values for koff as a function of applied force. Data represent the mean ± SD for 5 independent experiments at each wall shear stress tested. *P < .05.
Determination of the theoretical model that best represents the mutant GPIbα–VWF-A1 tether bond
Values for koff as a function of wall shear stress, obtained from statistical point estimates (SPEs) of the experimental data and their subsequent fit to the Bell equation, are given in Table 1. As discussed, all 3 models and their corresponding point estimates of koff,mut were assessed for quantitative adequacy using the Kolmogorov-Smirnov (KS) test.31 In 17 of the 20 experimental data sets (5 repeat experiments at each of 4 wall shear stresses), the null hypothesis for model 1 could not be rejected (P > .01), suggesting a good fit with the experimental data. Although 2 theoretical results obtained at 0.5 dyne/cm-2 were rejected by KS testing (P < .01), the model underlying equation 1 could not be rejected for 3 additional sets of data at that shear stress. Model 1 was supported by KS testing of all other data sets at the remaining wall shear stresses tested, as P values for the remaining 17 tests exceeded .05. Thus, the hypothesis that all platelet adhesion observed was mediated by single GPIbα tether bond could not be rejected. In contrast, hypothesis testing of model 2 demonstrated that it was not statistically supported by the experimental observations. In fact, KS testing rejected this model for most of the 20 data sets (P < .01) and for all q except when q = 0, a condition that corresponds to model 1.
The hypothesis that multiple wild-type or mutant GPIbα tether bonds are formed in our experiments (model 3) was tested via Monte Carlo (MC) simulations as discussed. As observed for model 2, the hypothesis underlying model 3 was rejected by KS testing (P < .01 for all experimental data sets; data not shown).
The pause time distributions generated by MC using the best set of parameter estimates (k0off,mut = 0.674 ± 0.108 s-1, σ = 0.031 ± 0.005 nm) agreed with the experimental pause time distributions at all experimental conditions tested for model 1 but not for models 2 and 3. Based upon these results, we can reject the hypothesis that the experimental pause time distribution is affected by the formation of multiple bonds between the platelet GPIbα– and the A1-coated beads as well as the hypothesis that both wild-type and mutant receptors play a role in the adhesion of platelets from our heterozygous patients.
Discussion
By studying the interactions between platelets and VWF under conditions of controlled flow and concentrations of protein that limit multiple bond formation, we have better defined the specific alterations in the kinetics of the GPIbα–VWF-A1 tether bond that may contribute to the clinical phenotype associated with PT-VWD. Specifically, we observed an enhancement in the apparent cellular on-rate as well as a prolongation in the lifetime of the interaction, features known to be critical for stabilizing binding between adhesion receptors and their respective ligands. The higher cellular on-rate manifested as a greater efficiency in attachment of PTVWD platelets to surface-immobilized A1 protein at flow rates that did not support interactions with normal cells (ie, WT GPIbα). This observation was further confirmed by analysis of the frequency of tether bond formation, a measure of association rate, between microspheres coated with low site densities of A1 protein (about 30 molecules per square micrometer) and the platelet substrates. By contrast, a 5-fold decrease in the intrinsic dissociation rate constant was noted for platelets expressing the Gly233Val mutation as compared with the native receptor-ligand pair (k0off values of 0.67 ± 0.11 s-1 versus 3.5 ± 0.37 s-1). This prolongation in tether bond lifetime is consistent with the 3- to 4-fold reduction in platelet and microsphere translocation velocities observed for the mutant receptor. Thus, enhanced bond formation alone does not fully account for the profoundly stronger rolling interactions.24 Interestingly, the reactive compliance (σ) of the mutant tether bond was about 2-fold higher than for native complex (0.031 ± 0.005 nm versus 0.016 ± 0.002 nm, respectively), suggesting that the interaction between GPIbα and VWF-A1 in PT-VWD may be slightly more prone to dissociate upon application of hydrodynamic force and may be expected to be distinguishable only at higher arterial shear stresses. Thus, altered intrinsic kinetic properties of this adhesive interaction and clearly not greater mechanical stability of the tether bond is the defining feature of this bleeding disorder.
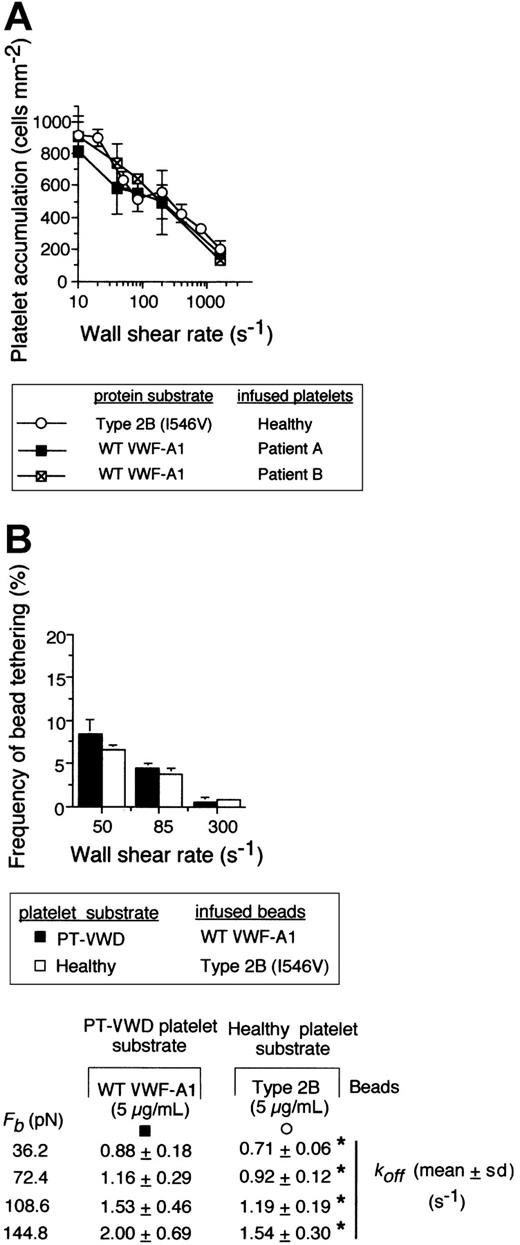
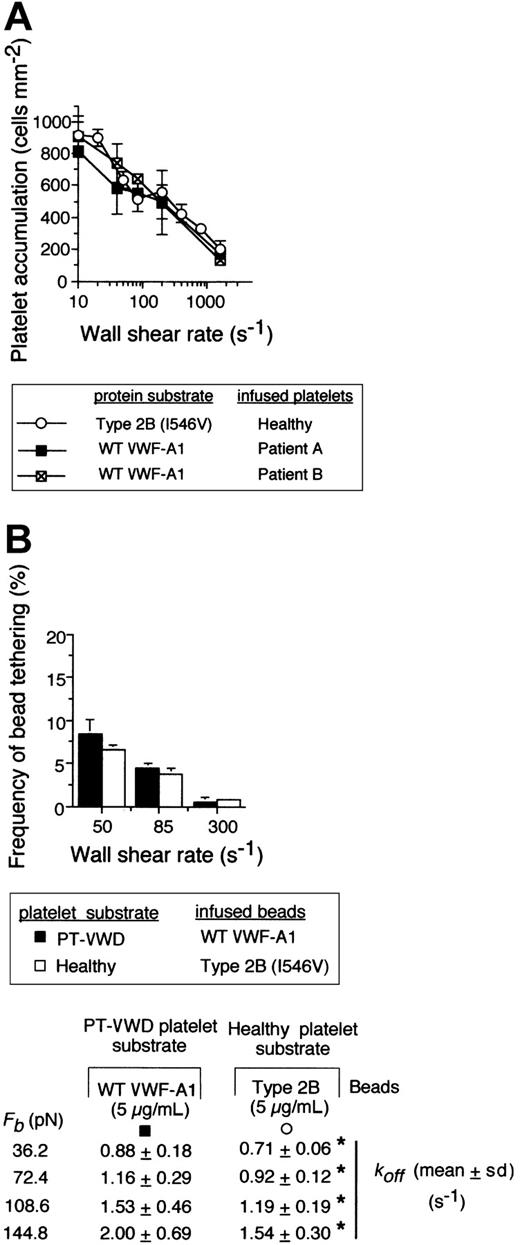
Recently, we have evaluated the kinetics of normal platelet interactions with the type 2B mutation Ile546Val using identical methodologies.9 Remarkably, the number of healthy platelets that accumulated on the mutant substrate and the frequency of type 2B VWF-A1–coated bead tethering to a healthy platelet substrate were comparable to those observed for PT-VWD platelet interactions with WT VWF-A1 (Figure 5A-B). Importantly, the dissociation rate constants were statistically similar at forces ranging from 36.2 to 144.8 pN (Figure 5, bottom panel; P > .1). Comparisons of values for koff at zero force and the reactive compliance as determined by the Bell model are shown in Table 2. Our results suggest that these 2 genetically distinct disease states may resemble one another from a clinical perspective due to similar alterations in rate of tether bond formation and dissociation.
Comparison of the kinetics of the GPIbα–VWF-A1 tether bond for PT-VWD versus type 2B VWD. (A) Platelet accumulation on WT versus type 2B mutant VWF-A1 substrate at the indicated wall shear rates. (B) The frequency of tether bond formation and cellular off-rates (Table 2) for beads coated with either WT or type 2B VWF-A1 protein (5 μg/mL) interacting with the indicated platelet substrate was performed as described in panel A. Data represent the mean ± SD for 5 independent experiments at each wall shear stress tested. *P > .1.
Comparison of the kinetics of the GPIbα–VWF-A1 tether bond for PT-VWD versus type 2B VWD. (A) Platelet accumulation on WT versus type 2B mutant VWF-A1 substrate at the indicated wall shear rates. (B) The frequency of tether bond formation and cellular off-rates (Table 2) for beads coated with either WT or type 2B VWF-A1 protein (5 μg/mL) interacting with the indicated platelet substrate was performed as described in panel A. Data represent the mean ± SD for 5 independent experiments at each wall shear stress tested. *P > .1.
How would an enhanced rate of association and prolongation in the lifetime of these mutant tether bonds explain the clinical phenotype associated with PT-VWD and type 2B VWD? We hypothesize that in the disease states, random collisions between platelets and globular VWF in the circulation could result in a longer-lived GPIbα–VWF-A1 bond than for the WT receptor-ligand pair. The rapid dissociation kinetics of the native complex, however, may preclude additional bonds from forming, thus preventing resting platelets from spontaneously aggregating with plasma VWF in flowing blood. This is supported by previous studies demonstrating that the fast dissociation kinetics of the adhesion molecule L-selectin, a constitutively functional adhesion receptor expressed on leukocytes, are important in preventing the formation of white blood cell aggregates in the circulation through interactions with its ligand, PSGL-1 that is also expressed on these cells.32,33 In the case of PT-VWD and type 2B VWD, the enhanced cellular on-rate and prolongation of receptor-ligand interactions would favor multiple bond formation, thus stabilizing platelet-VWF interactions. This would permit not only multiple platelet interactions with a single high molecular weight VWF molecule, which contains numerous A1 domains, but also the coalescence of the newly formed platelet-VWF aggregates. Depletion of this high molecular species of VWF would effectively reduce the number of A1 molecules available for multiple bonding, thus limiting the disease process. This, in part, may explain the selective loss of the highest molecular weight multimers of VWF and modest, but self-limiting, thrombocytopenia. Activation of other adhesion receptors such as GPIIb/IIIa on platelets as a consequence of multiple GPIbα–VWF interactions may also play a role in aggregate formation if bonds are sufficiently stressed by hydrodynamic forces.34
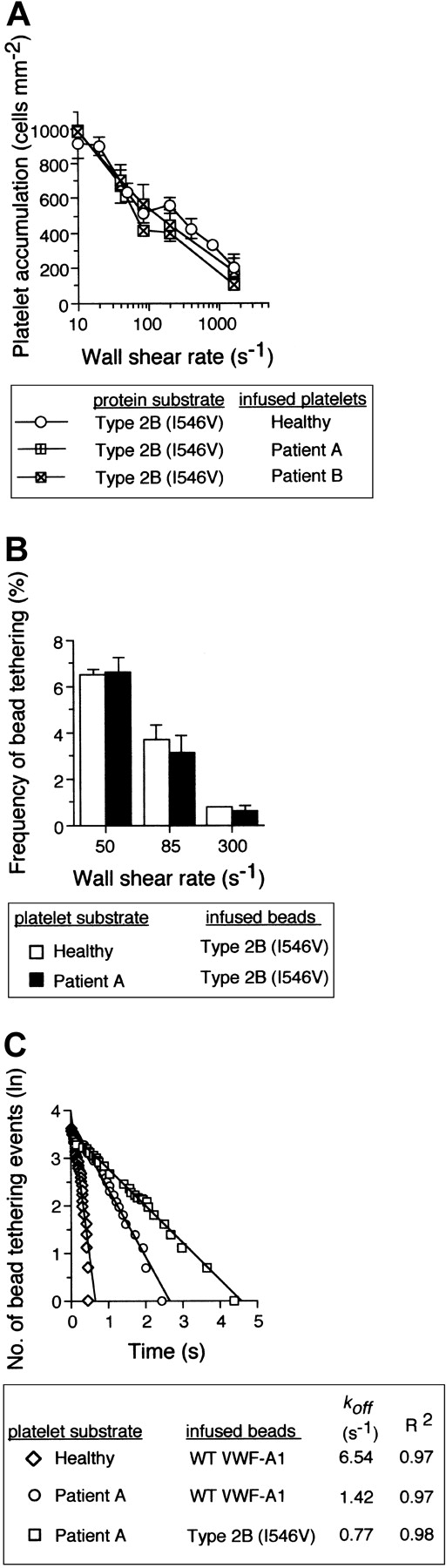
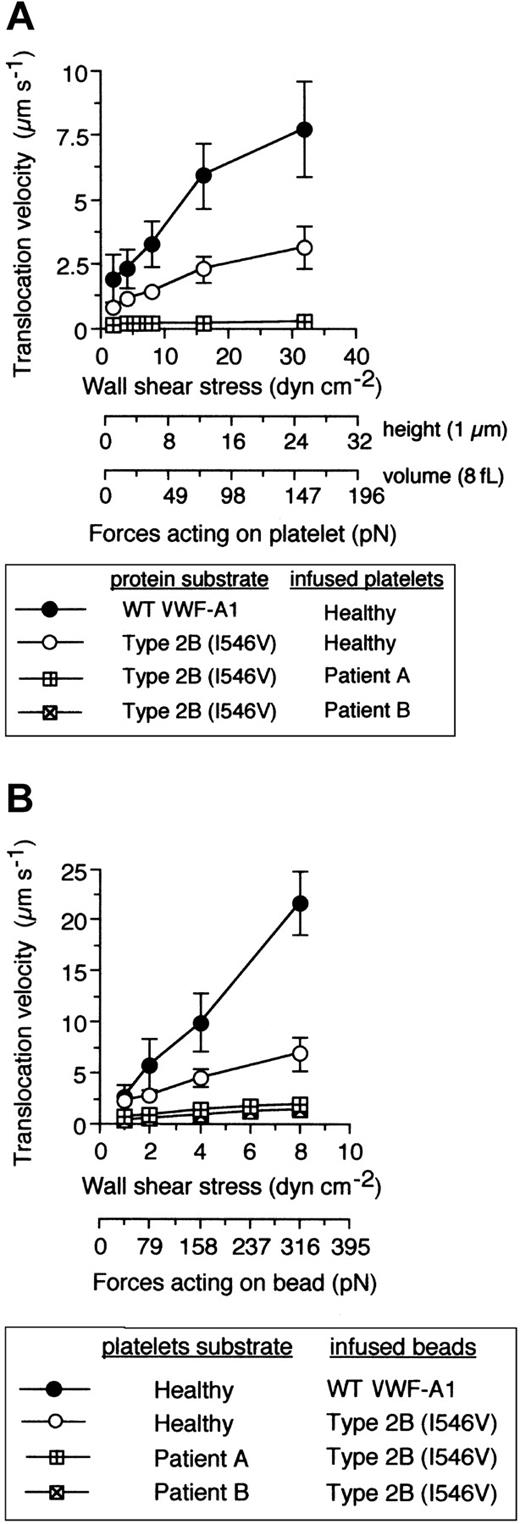
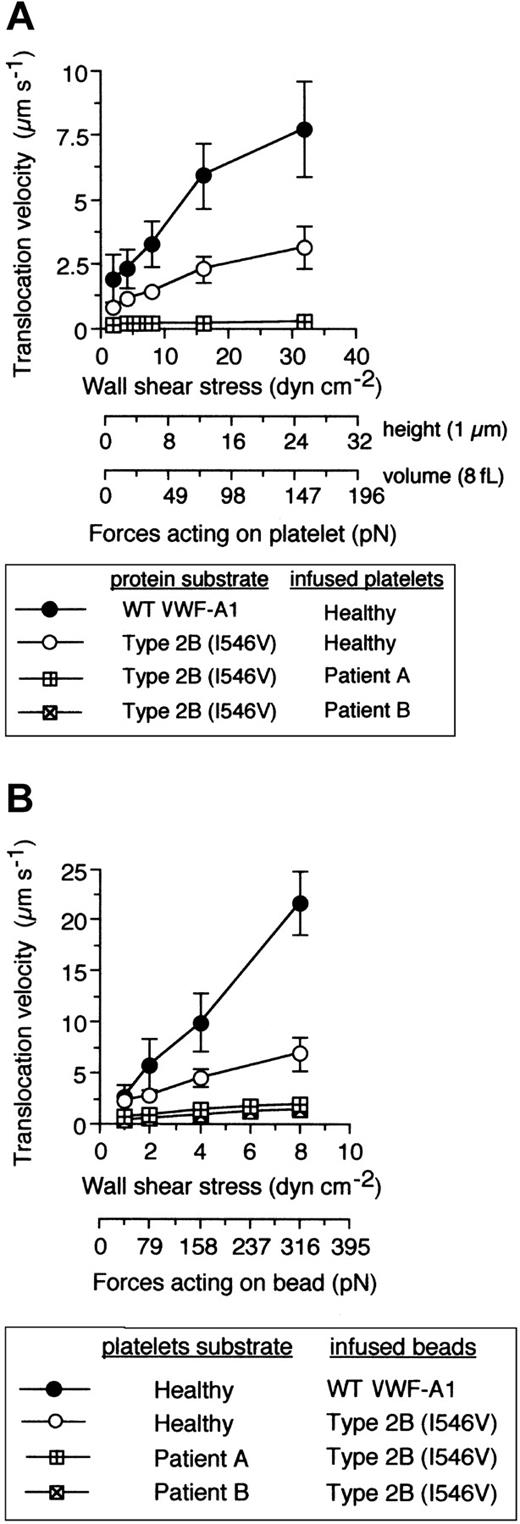
Are the kinetic and biomechanical effects of these genetically distinct mutations on platelet-VWF interactions the result of 2 independent molecular processes? Recently, it has been hypothesized that 2 separate conformation-dependent mechanisms may be responsible for the function-enhancing abilities of mutations associated with either GPIbα or the VWF-A1.13 This was based on analysis of the crystal structure of this receptor-ligand pair that contained the clinically relevant residues, but the significance of the small structural changes associated with the mutant A1 domain remains to be determined.35,36 To evaluate the possibility that these genetically distinct mutations can function independently from one another, platelet attachment, microsphere tethering frequency, and translocation velocities were evaluated as described above using the combination of mutations. Interestingly, PT-VWD platelet attachment to VWF-A1 containing the Ile546Val mutation did not have a profound impact on the quantity of cells bound as compared with either mutation alone (Figure 6A). This lack of further enhancement in attachment was also reflected in the similar level of tether bond formation of microspheres coated with quantities of mutant VWF-A1 insufficient to promote translocation (Figure 6B). Thus, within the limits of our system, this combination of mutations does not result in a greater ability of platelets to initiate interactions with VWF-A1 in flow, a process most influenced by the kon of the interaction. By contrast, we provide the first evidence that that the GPIbα mutation Gly233Val in combination the type 2B mutation Ile546Val does augment the phenotype associated with either PT-VWD or type 2B VWD, as analysis of pause times for the combination of mutations revealed an about 2-fold increase in bond lifetime as compared with the single mutation alone (Figure 6C). This was further confirmed by the reduction in translocation velocities of both PT-VWD platelets on immobilized type 2B substrate and mutant VWF-A1–coated microspheres on surface-bound mutant GPIbα platelets (Figure 7). For instance, an about 15-fold difference in mean translocation velocities of healthy versus PT-VWD platelets (3.12 ± 0.85 μm s-1 versus 0.21 ± 0.06 μm s-1, respectively) on type 2B VWF-A1 was observed at a wall shear stress of 32 dyne cm-2 corresponding to an estimated upper limit of force of about 150 pN (Figure 8). In comparison, an about 4-fold reduction in translocation velocities was observed for type 2B VWF-A1–coated microspheres interacting with PT-VWD platelets at the maximal wall shear stress capable of supporting rolling adhesions (8 dyne cm-2 corresponding to a force of 316 pN). The differences in velocities between platelets and microspheres can be attributed to the reduced forces experienced by the former. Current studies are focused on estimating the degree of alteration in the lifetime of the double mutant tether bond as a function of an applied hydrodynamic force.
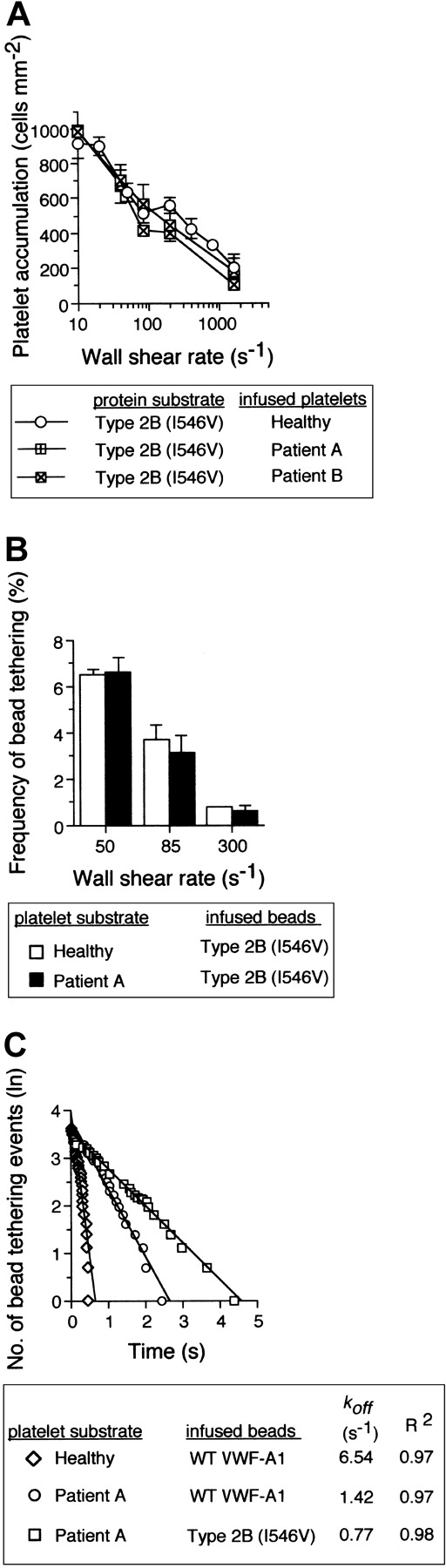
The impact of the type 2B mutant A1, Ile546Val, on PT-VWD platelet attachment and tether bond formation in flow. (A) Platelets (5 × 107/mL) purified from 2 patients with PT-VWD or 2 healthy individuals on 3 consecutive days were infused at the indicated wall shear rates through a parallel platelet flow for 5 minutes. The total number of platelets that attached to surface-immobilized recombinant type 2B mutant protein (100 μg/mL) was determined and compared (mean ± SD; n = 6). (B) Frequency of tether bonds formed between beads coated with a low density of type 2B mutant VWF-A1 and healthy or PT-VWD platelet substrates as a function of wall shear rate (s-1). (C) Representative graph depicting the distribution of interaction times for more than 35 individual transient attachment events that occurred between microspheres coated with WT or type 2B VWF-A1 and surface-immobilized platelets purified from healthy individuals or patient A with PT-VWD. Dissociation rate constants were estimated at wall shear stress of 1.0 dyne cm-2. koff is the negative slope of the linear regression through the experimental data.
The impact of the type 2B mutant A1, Ile546Val, on PT-VWD platelet attachment and tether bond formation in flow. (A) Platelets (5 × 107/mL) purified from 2 patients with PT-VWD or 2 healthy individuals on 3 consecutive days were infused at the indicated wall shear rates through a parallel platelet flow for 5 minutes. The total number of platelets that attached to surface-immobilized recombinant type 2B mutant protein (100 μg/mL) was determined and compared (mean ± SD; n = 6). (B) Frequency of tether bonds formed between beads coated with a low density of type 2B mutant VWF-A1 and healthy or PT-VWD platelet substrates as a function of wall shear rate (s-1). (C) Representative graph depicting the distribution of interaction times for more than 35 individual transient attachment events that occurred between microspheres coated with WT or type 2B VWF-A1 and surface-immobilized platelets purified from healthy individuals or patient A with PT-VWD. Dissociation rate constants were estimated at wall shear stress of 1.0 dyne cm-2. koff is the negative slope of the linear regression through the experimental data.
The combination of PT and type 2B mutations significantly reduces translocation velocities. (A) Mean translocation velocities for interacting healthy or PT-VWD platelets (n = 30 to 40 cells) on the indicated VWF-A1 substrates (100 μg/mL). Data represent the mean ± SD for 3 to 4 independent experiments performed in duplicate. (B) Translocation velocities of WT or type 2B–coated beads (100 μg/mL) on the indicated platelet substrates. Beads that had accumulated on surface-immobilized platelets were subjected to incremental increases in wall shear stress and rolling velocities calculated for a minimum of 50 beads over 10-second intervals. Data represent the mean ± SD for 3 to 4 independent experiments performed in duplicate.
The combination of PT and type 2B mutations significantly reduces translocation velocities. (A) Mean translocation velocities for interacting healthy or PT-VWD platelets (n = 30 to 40 cells) on the indicated VWF-A1 substrates (100 μg/mL). Data represent the mean ± SD for 3 to 4 independent experiments performed in duplicate. (B) Translocation velocities of WT or type 2B–coated beads (100 μg/mL) on the indicated platelet substrates. Beads that had accumulated on surface-immobilized platelets were subjected to incremental increases in wall shear stress and rolling velocities calculated for a minimum of 50 beads over 10-second intervals. Data represent the mean ± SD for 3 to 4 independent experiments performed in duplicate.
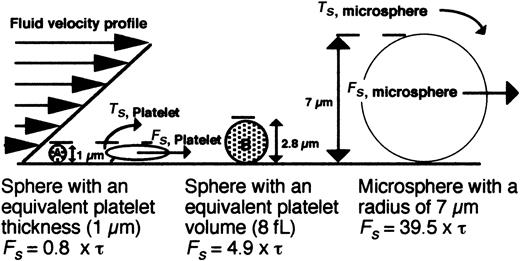
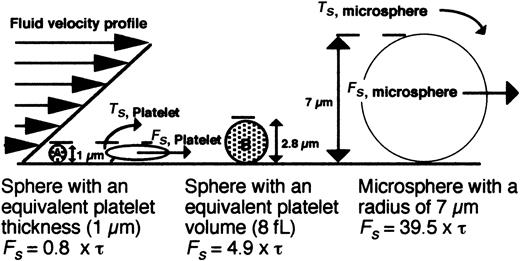
Schematic model depicting the hydrodynamic forces encountered by surface-interacting platelets versus microspheres. The force (Fs) acting on a platelet and a 7-μm bead in shear flow is depicted. Representative hard spheres with diameters proportional to the thickness (A) or volume of a discoid platelet (B) are also shown. The equations of Goldman (Fs = 6πRhCF τ; Ts = 4πR3CF τ) were used to estimate the forces (Fs) acting on a platelet or bead based on a motionless hard sphere in shear flow near a wall, where τ is the shear stress, R is the sphere radius, h is the distance from the center of the sphere to the wall, and CF and CT represent numerical factors that depend on h/R.37 For the case where a cell is tethered to the wall, h = R, CF = 1.7005, and CT = 0.943 99. Because the geometry of a resting platelet is discoid and not spherical, a low and high estimate of the shear force was determined for spheres with either a diameter (1 μm) or a volume (8 fL) equal to that of a platelet.
Schematic model depicting the hydrodynamic forces encountered by surface-interacting platelets versus microspheres. The force (Fs) acting on a platelet and a 7-μm bead in shear flow is depicted. Representative hard spheres with diameters proportional to the thickness (A) or volume of a discoid platelet (B) are also shown. The equations of Goldman (Fs = 6πRhCF τ; Ts = 4πR3CF τ) were used to estimate the forces (Fs) acting on a platelet or bead based on a motionless hard sphere in shear flow near a wall, where τ is the shear stress, R is the sphere radius, h is the distance from the center of the sphere to the wall, and CF and CT represent numerical factors that depend on h/R.37 For the case where a cell is tethered to the wall, h = R, CF = 1.7005, and CT = 0.943 99. Because the geometry of a resting platelet is discoid and not spherical, a low and high estimate of the shear force was determined for spheres with either a diameter (1 μm) or a volume (8 fL) equal to that of a platelet.
Overall, we have identified specific alterations in the property of the GPIbα–VWF-A1 tether bond that may account for clinical phenotype observed for PT-VWD. The higher rate of tether bond formation and prolonged duration of the interaction between GPIbα and the A1 domain of VWF work in concert to promote stability between this receptor-ligand pair and subsequent clearance of the largest multimers of VWF. Moreover, the phenotype associated with the disease state in the heterozygous patient appears to be exclusively mediated by the mutant GPIbα as supported by rigorous statistical analysis of 3 possible tether bond models. The present study extends our previous findings that link an alteration in the intrinsic properties of tether bonds with a genetically distinct bleeding disorder, namely type 2B VWD. Our results also suggest that the mechanical properties of the GPIbα–VWF-A1 tether bond are not enhanced as reflected in the moderately increased values for reactive compliance, a measure of the susceptibility of a receptor-ligand pair to breakage in the presence of an applied force. Lastly, we demonstrate that these clinically relevant mutants alter the kinetics of this interaction through distinct molecular mechanisms that are partially “additive” as indicated by both a further reduction in koff and a near-zero translocation velocity of the Gly233Val mutant platelets on the type 2B mutant VWF-A1 substrate.
Prepublished online as Blood First Edition Paper, March 13, 2003; DOI 10.1182/blood-2003-01-0072.
Supported by National of Institutes of Health grants HL63244-01A1 (T.G.D.) and HL56621 (S.L.D.). T.G.D. and S.L.D. are Established Investigators for the National American Heart Association (grants 02-40009N and 99-40027).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.