Abstract
Platelet factor 4 (PF4), an abundant platelet α-granule protein, accelerates in vitro generation of activated protein C (APC) by soluble thrombin/thrombomodulin (TM) complexes up to 25-fold. To test the hypothesis that PF4 similarly stimulates endothelium-associated TM, we assessed the influence of human PF4 on thrombin-dependent APC generation by cultured endothelial monolayers. APC generated in the presence of 1 to 100 μg PF4 was up to 5-fold higher than baseline for human umbilical vein endothelial cells, 10-fold higher for microvascular endothelial cells, and unaltered for blood outgrowth endothelial cells. In an in vivo model, cynomolgus monkeys (n = 6, each serving as its own control) were infused with either PF4 (7.5 mg/kg) or vehicle buffer, then with human thrombin (1.0 μg/kg/min) for 10 minutes. Circulating APC levels (baseline 3 ng/mL) peaked at 10 minutes, when PF4-treated and vehicle-treated animals had APC levels of 67 ± 5 ng/mL and 39 ± 2 ng/mL, respectively (P < .001). The activated partial thromboplastin time (APTT; baseline, 28 seconds) increased maximally by 27 ± 6 seconds in PF4-treated animals and by 9 ± 1 seconds in control animals at 30 minutes (P < .001). PF4-dependent increases in circulating APC and APTT persisted more than 2-fold greater than that of control's from 10 through 120 minutes (P ≤ .04). All APTT prolongations were essentially reversed by monoclonal antibody C3, which blocks APC activity. Thus, physiologically relevant concentrations of PF4 stimulate thrombin-dependent APC generation both in vitro by cultured endothelial cells and in vivo in a primate thrombin infusion model. These findings suggest that PF4 may play a previously unsuspected physiologic role in enhancing APC generation. (Blood. 2003;102:146-151)
Introduction
The protein C/thrombomodulin (TM) natural anticoagulant system plays an indispensable role in preventing microvascular thrombosis in humans. Newborns homozygously deficient in protein C quickly succumb to widespread microvascular thrombosis, or “purpura fulminans,” at or shortly after birth,1,2 and this pathologic thrombosis can be treated successfully by infusions of protein C (PC).1-3 Proteolytic generation of activated protein C (APC) from its inactive zymogen precursor, protein C, is mediated in vivo by thrombin bound to the endothelial transmembrane protein TM.4 The essential role of endothelial cell TM in generation of APC is shown by the embryonic or immediately postpartum lethal phenotype of massive thrombosis and lethal consumptive coagulopathy that manifests in mice genetically manipulated to have endothelial cell–specific ablation of TM expression.5
Protein C and TM each contain anionic domains potentially capable of electrostatic interactions with cationic proteins. The first is the densely anionic N-terminal γ–carboxyglutamic acid (Gla) domain of protein C. The second results from variable posttranslational modification of amino acid residues 492 and 493 of endothelial cell TM with potentially large amounts of O-linked chondroitin sulfate glycosaminoglycan (GAG), the extent of which varies in an apparently vascular bed–dependent manner.6-8 The existence of these anionic domains has prompted interest in potential interactions of cationic compounds with the TM/protein C system. Polybrene6 and the cationic polypeptides polylysine,9 eosinophil major basic protein,10 and eosinophil peroxidase10 all strongly inhibit both the proteolytic conversion of protein C to APC by the thrombin/TM complex (ie, the protein C–activating cofactor activity of TM) and the ability of TM to prolong the thrombin clotting time (ie, the “direct anticoagulant” action of TM). Thus, these cationic proteins impair the anticoagulant functions of TM and may therefore produce a thrombotic phenotype in vivo. These findings may be relevant to pathologic conditions such as the hypereosinophilic syndrome, which is characterized by both a marked thrombotic diathesis11 and high plasma concentrations of major basic protein derived from activated eosinophils.12
In considering more physiologically relevant cationic proteins that might interact with and modulate the function of the TM/protein C system, we have focused on platelet factor 4 (PF4), an abundant platelet α-granule protein released on platelet activation.13 PF4 tetramerizes at high (> 1 μM) concentrations, and the resulting complex has a strong affinity for heparin and other anionic GAGs.13 Based on the results with other cationic proteins, we hypothesized that PF4 might impair anticoagulant actions of TM and therefore promote a procoagulant phenotype in vessels at sites of platelet activation. We found, unexpectedly, that PF4 instead stimulates the protein C–activating cofactor activity of purified soluble forms of the thrombin/TM complex 4- to 25-fold14 and does not impair the direct anticoagulant effect of TM on thrombin clotting times.15 These studies, performed in vitro with purified soluble TM, raise the possibility that PF4 might also stimulate the function of vascular endothelium–associated forms of TM and so play a role in the physiologic regulation of coagulation.
To test this hypothesis, we assayed the influence of PF4 on APC generation by TM expressed on cultured endothelial cell monolayers derived from 3 different sources. We also performed studies in cynomolgus monkeys, using as our experimental model low-dose infusion of thrombin, a potent stimulus to generation of APC that produces large increases in circulating APC levels accompanied by a pronounced systemic anticoagulant effect.16-18 Our results show that PF4 enhances the protein C–activating cofactor activity of TM both in cultured endothelial cells in vitro and in intact vasculature in vivo.
Materials and methods
Materials
Recombinant human platelet factor 4 was expressed in Escherichia coli and purified as previously described19,20 and was kindly supplied by Ted Maione (Repligen, Cambridge, MA). PF4 was solvated in a vehicle of 150 mM NaCl, 10 mM sodium acetate, pH 5.0. Human protein C and APC were from Enzyme Research Laboratories (South Bend, IN). Human thrombin (specific activity 3000 NIH units/mg protein) and hirudin were from Sigma (St Louis, MO). APC chromogenic substrate S-2366 was from Kabi Vitrum (Franklin, OH). Murine C3 monoclonal anti-APC antibody has previously been characterized.21
Assay of thrombomodulin-dependent APC generation by cultured endothelial cell monolayers
Primary-culture human umbilical vein endothelial cells,10 human dermal microvascular endothelial cells,22 and blood outgrowth endothelial cells23 were established and maintained as previously described. Monolayers grown to confluence in 1-cm2 circular wells were overlaid with 500 μL Hanks buffered salt solution (HBSS) supplemented with 1 mM magnesium and calcium in 20 mM HEPES (N-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid) buffer (pH 7.4;H&Hbuffer) with 0.1% bovine serum albumin, then supplemented with 10 μg/mL human protein C followed by 1 nM human thrombin. Plates were incubated under CO2 at 37°C for 120 minutes, after which APC generation was terminated by the addition of 100 nM hirudin. Plates were then centrifuged at 3000g for 5 minutes and supernatant fluid was aspirated. APC generation was then quantitated by the addition of 500 μM S-2366 chromogenic substrate and initial rate of chromogenic substrate cleavage was assayed at 405 nm on a Thermomax microtiter Vmax plate reader (Molecular Devices, Menlo Park, CA). To control for the TM-independent activation of protein C by thrombin, tissue culture wells without endothelial monolayers were incubated in a similar fashion and these low APC activities were subtracted from those seen in the presence of endothelial cells. For each of the 3 endothelial cell types, experiments were performed on 2 or 3 different primary culture preparations.
Monkey low-dose thrombin infusion protocol
In a randomized, crossover protocol, 6 adult cynomolgus monkeys (Macaca fascicularis) received a 2.5-minute infusion of either recombinant human PF4 or vehicle, followed by a 10-minute infusion of human thrombin. Each monkey was studied twice, once with PF4 and once with vehicle, separated by a 2-week recovery period. On each of the 2 study days, the animals were sedated with ketamine hydrochloride (20 mg/kg, administered intramuscularly) and anesthetized with sodium pentobarbital (20 mg/kg, administered intravenously) as described previously.18 A nonobstructive catheter was inserted into an axillary artery for blood sampling, and the axillary vein was cannulated for administration of PF4, thrombin, and supplemental anesthesia (sodium pentobarbital, 5 mg/kg/h). Arterial pressure was monitored continuously. Either PF4 (7.5 mg/kg) or vehicle (150 mM NaCl,10 mM sodium acetate, pH 5.0) was infused over 2.5 minutes through the axillary vein catheter. Immediately following the infusion of PF4 or vehicle, human α-thrombin (1.0 μg/kg; Enzyme Research Laboratories) was infused over 10 minutes as described previously.18 Blood was collected from the axillary artery catheter before and after infusion of PF4 or vehicle, and at intervals (0-120 minutes) after infusion of thrombin, directly into a 1/10 volume of 3.8% sodium citrate with 30 mM benzamidine (for determination of APC) or 3.8% sodium citrate without benzamidine (for other hemostasis assays). Blood samples were immediately placed on ice and plasma was isolated by centrifugation at 2500g for 30 minutes at 4°C. Additional blood samples were collected for measurement of complete blood count. One additional control monkey received an infusion of PF4 without infusion of thrombin. The protocol was approved by the University of Iowa and Veterans Affairs Animal Care and Use Committees.
Determination of APC by immune capture amidolytic assay
Activated partial thromboplastin time clotting studies
Activated partial thromboplastin time (APTT) was measured in a Diagnostica Stago ST4 kinetic coagulometer (Diagnostica Stago, Parsippany, NJ) with the Platelin L reagent (Organon Teknika, Durham, NC). In studies to determine the extent to which APTT prolongations above baseline depended upon the anticoagulant activity of APC, plasma specimens were supplemented with either 40 μg/mL C3 monoclonal antibody or 40 μg/mL irrelevant immunoglobulin G-1κ (IgG1κ) monoclonal antibody control and clotting times were repeated. Preliminary experiments titrating C3 indicated that this amount of antibody produced nearly complete reversal of APTT prolongation in human plasma spiked with 500 ng/mL human APC (not shown).
Determination of monkey plasma PF4 levels
Monkey plasma PF4 levels were determined using a PF4 enzyme-linked immunosorbent assay (ELISA) kit from Diagnostica Stago according to the manufacturer's instructions.
Statistical methods
APTT and APC responses to thrombin were analyzed by Student 2-way paired t test as well as 2-way repeated-measures analysis of variance with Bonferroni multiple comparison analysis. A value of P < .05 was used to define statistical significance. Values are reported as mean ± SEM.
Results
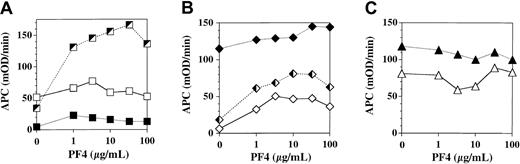
We assessed the influence of PF4 on APC generation by TM expressed on cultured human endothelial monolayers derived from 3 sources: umbilical vein (human umbilical vein endothelial cells, HUVEC), foreskin dermal microvasculature (microvascular endothelial cells, MVEC), and peripheral blood progenitors (blood outgrowth endothelial cells, BOEC; Figure 1). Each curve depicts a separate experiment done on a different primary cell culture preparation under identical conditions. PF4 stimulation of TM-dependent APC generation by monolayers was strikingly variable, even among cell preparations of endothelium derived from the same tissue source. The stimulatory effect of PF4 ranged from no effect to a maximum of 5-fold over baseline for HUVEC and from 1.3-fold to 10-fold over baseline for MVEC; the effect was negligible for BOEC. Thus, PF4 variably stimulates TM-dependent APC generation by some types of cultured endothelium at concentrations less than that found in serum (5-10 μg/mL13 ).
Influence of platelet factor 4 on thrombomodulin-dependent APC generation by cultured endothelial monolayers. Confluent endothelial monolayers derived from human umbilical vein (A), human dermal microvasculature (B), and human peripheral blood progenitors (C) were incubated for 2 hours in the presence of 10 μg/mL protein C, 1 nM thrombin, and the indicated concentration of PF4. APC generation was quenched by addition of hirudin and aliquots of supernatant buffer assayed for APC using the chromogenic substrate S-2366. Each curve within the designated cell type represents a separate experiment done on a separate primary culture preparation using the designated cell type.
Influence of platelet factor 4 on thrombomodulin-dependent APC generation by cultured endothelial monolayers. Confluent endothelial monolayers derived from human umbilical vein (A), human dermal microvasculature (B), and human peripheral blood progenitors (C) were incubated for 2 hours in the presence of 10 μg/mL protein C, 1 nM thrombin, and the indicated concentration of PF4. APC generation was quenched by addition of hirudin and aliquots of supernatant buffer assayed for APC using the chromogenic substrate S-2366. Each curve within the designated cell type represents a separate experiment done on a separate primary culture preparation using the designated cell type.
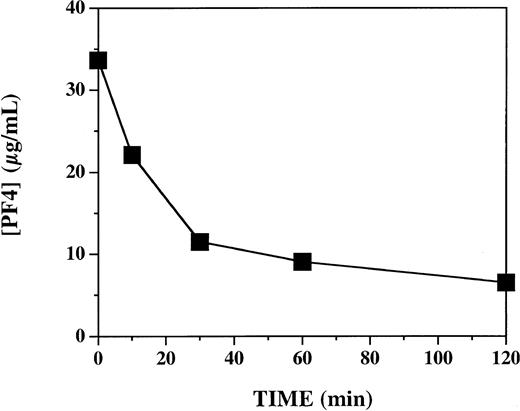
In view of the highly variable effects of PF4 on APC generation in vitro, we directly tested the hypothesis that PF4 accelerates APC generation by intact vascular endothelium in vivo in a primate system. We used an established experimental model in which activation of endogenous protein C is triggered by infusion of human thrombin in cynomolgus monkeys.18 To assess the influence of PF4 in this system, monkeys received either PF4 (7.5 mg/kg) or vehicle buffer intravenously over 2 minutes, immediately prior to a 10-minute infusion of thrombin (1.0 μg/kg). Each of 6 monkeys was studied twice: once after infusion of vehicle and once after infusion of PF4, in random order, 1 to 2 weeks apart. To ascertain whether the plasma levels of PF4 achieved with this protocol were comparable to those previously shown to have activity in vitro, we measured plasma PF4 levels at selected time points after PF4 infusion (Figure 2). Similar to what has previously been reported after infusion of recombinant PF4 into humans,25 there was a decay curve, with levels decreasing from a peak of 33 μg/mL just after the infusion and declining to 7 μg/mL at 120 minutes. These levels are similar to those at which PF4 stimulates APC generation in vitro (Figure 1; also Slungaard and Key14 ).
Plasma platelet factor 4 levels after 2-minute infusion of 7.5 mg/kg PF4 in cynomolgus monkeys. Plasma specimens were assayed by PF4 enzyme-linked ELISA immediately after a 2-minute infusion of 7.5 mg/kg PF4 (T = 0), and 10, 30, 60, and 120 minutes thereafter. Data represent 4 animals (mean ± SEM).
Plasma platelet factor 4 levels after 2-minute infusion of 7.5 mg/kg PF4 in cynomolgus monkeys. Plasma specimens were assayed by PF4 enzyme-linked ELISA immediately after a 2-minute infusion of 7.5 mg/kg PF4 (T = 0), and 10, 30, 60, and 120 minutes thereafter. Data represent 4 animals (mean ± SEM).
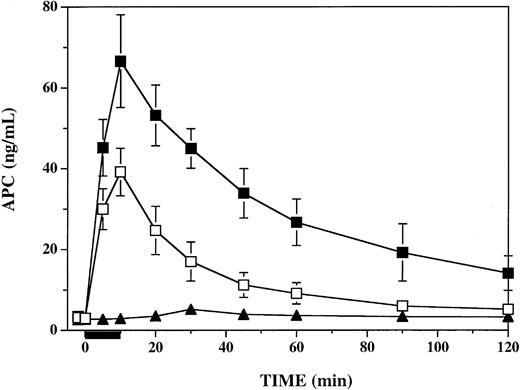
To determine the influence of PF4 on circulating APC activity levels in response to thrombin infusion in this primate model, we assayed plasma specimens from all time points, using a sensitive and specific immune capture amidolytic assay.24 Following infusion of PF4, circulating plasma APC levels at the end of the 10-minute infusion of thrombin were significantly elevated over those seen in vehicle-infused animals (Figure 3). In both groups APC levels peaked at the end of the thrombin infusion at 10 minutes, at which time PF4-treated and vehicle-treated animals had APC levels of 67 ± 5 ng/mL and 39 ± 2 ng/mL, respectively (P < .006). APC levels remained significantly elevated (P ≤ .002) in PF4-treated animals compared with vehicle-treated animals at 30 minutes (40 vs 17 ng/mL), 60 minutes (27 vs 9 ng/mL), 90 minutes (19 vs 6 ng/mL), and 120 minutes (14 vs 5 ng/mL). In a single monkey infused with PF4 alone, APC increased from a baseline of 2.7 ng/mL to a peak of 5.2 ng/mL at 30 minutes, perhaps reflecting PF4 stimulation of the small amounts of endogenous TM-bound thrombin responsible for maintaining baseline circulating APC levels.
Influence of platelet factor 4 on plasma APC levels in thrombin-infused cynomolgus monkeys. Plasma APC was measured by enzyme capture amidolytic assay before, during, and after a 10-minute infusion of 1 μg/kg/min human thrombin, represented by the black bar under the x-axis. For 2 minutes prior to initiation of the thrombin infusion, groups of 6 monkeys were infused with either 7.5 mg/kg PF4 (▪) or a vehicle buffer control (□). A single monkey was infused with PF4 without subsequent thrombin infusion (▴). Data shown are means ± SEM.
Influence of platelet factor 4 on plasma APC levels in thrombin-infused cynomolgus monkeys. Plasma APC was measured by enzyme capture amidolytic assay before, during, and after a 10-minute infusion of 1 μg/kg/min human thrombin, represented by the black bar under the x-axis. For 2 minutes prior to initiation of the thrombin infusion, groups of 6 monkeys were infused with either 7.5 mg/kg PF4 (▪) or a vehicle buffer control (□). A single monkey was infused with PF4 without subsequent thrombin infusion (▴). Data shown are means ± SEM.
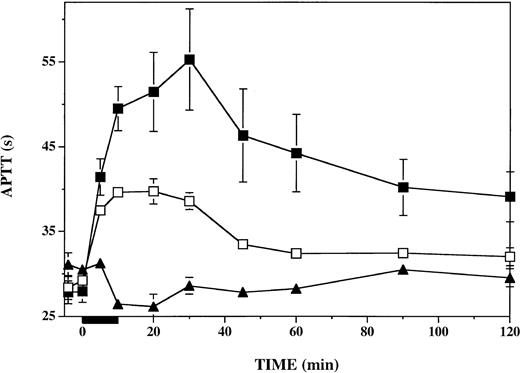
To assess the impact of PF4 on plasmatic coagulation during and after thrombin infusion, we measured the APTT in plasma specimens drawn throughout the protocol. As shown in Figure 4, infusion of PF4 had a negligible effect on the APTT prior to the initiation of the thrombin infusion. In contrast, after thrombin infusion, from 10 through 120 minutes, the APTT was consistently prolonged 2- to 3-fold more after infusion of PF4 than after infusion of vehicle. The APTT (baseline, 28 seconds) was increased by 27 ± 6 seconds in PF4-treated animals and by 9 ± 1 seconds in vehicle-treated animals at 30 minutes (P < .001). Prolongation of the APTT was more than 2-fold greater in PF4-treated than in vehicle-treated animals at all time points from 10 to 120 minutes (P ≤ .04). PF4 infusion alone, in the absence of subsequent thrombin infusion, had a minimal effect on the APTT. Therefore, the longer APTT seen in the presence of PF4 after thrombin infusion was not attributable to any direct effect of PF4 on the APTT.
Anticoagulant response to thrombin infusion in presence or absence of infused platelet factor 4. APTT was measured in plasma specimens at the indicated time points before, during, and after a 10-minute thrombin infusion (black bar under x-axis). Vehicle-infused monkeys (n = 6), □; PF4-infused monkeys (n = 6), ▪. The effect of PF4 infusion in the absence of thrombin infusion was assessed in a single monkey (▴). Data shown are means ± SEM.
Anticoagulant response to thrombin infusion in presence or absence of infused platelet factor 4. APTT was measured in plasma specimens at the indicated time points before, during, and after a 10-minute thrombin infusion (black bar under x-axis). Vehicle-infused monkeys (n = 6), □; PF4-infused monkeys (n = 6), ▪. The effect of PF4 infusion in the absence of thrombin infusion was assessed in a single monkey (▴). Data shown are means ± SEM.
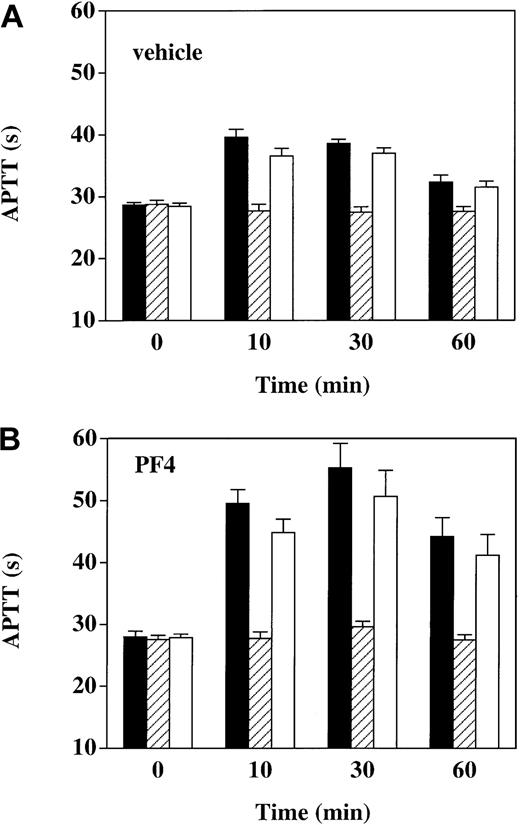
To determine whether prolongations of the APTT seen after thrombin infusion were dependent on the anticoagulant activity of APC, we determined the effect on the APTT of adding C3, a murine monoclonal antibody that blocks the anticoagulant activity of APC23 (Figure 5). In vehicle-infused animals, prior to the start of thrombin infusion, at time 0, the APTT was unaffected by the addition of 40 μg/mL C3 or 40 μg/mL IgG1 isotype control antibody. However, after thrombin infusion (at 10, 30, and 60 minutes), C3 nearly completely reversed the APTT prolongations above baseline, whereas the IgG1 control had a minimal effect. Similarly, APTT prolongations after thrombin infusion in the presence of PF4—though more pronounced than in the presence of vehicle—were also totally reversed by C3. These results show that the APTT prolongation after thrombin infusion, whether in the presence or absence of PF4, was essentially entirely attributable to APC anticoagulant activity.
Reversal of APTT prolongation in thrombin-infused monkeys with anti-APC monoclonal antibody C3. APTT was measured in plasma specimens drawn at the indicated time points under 3 conditions: (1) no addition (black bars); (2) in the presence of 40 μg/mL monoclonal antibody C3, a murine anti-APC IgG1 that reverses the anticoagulant activity of APC (hatched bars); and (3) in the presence of 40 μg/mL irrelevant murine IgG1 monoclonal antibody (open bars). Panel A shows vehicle-infused monkeys (n = 6); panel B, PF4-infused monkeys (n = 6). Data shown are means ± SEM.
Reversal of APTT prolongation in thrombin-infused monkeys with anti-APC monoclonal antibody C3. APTT was measured in plasma specimens drawn at the indicated time points under 3 conditions: (1) no addition (black bars); (2) in the presence of 40 μg/mL monoclonal antibody C3, a murine anti-APC IgG1 that reverses the anticoagulant activity of APC (hatched bars); and (3) in the presence of 40 μg/mL irrelevant murine IgG1 monoclonal antibody (open bars). Panel A shows vehicle-infused monkeys (n = 6); panel B, PF4-infused monkeys (n = 6). Data shown are means ± SEM.
Discussion
PF4, thrombospondin, and β-thromboglobulin are the predominant platelet α-granule proteins. All 3 are cationic and bind heparin with high affinity.26 Although structurally categorized as a C-X-C chemokine in the same family as interleukin 8,27 PF4 is notable for both its striking impotence as a chemotaxin28 and its lack of an identifiable high-affinity chemokine receptor. A variety of biologic activities, including inhibition of hematopoiesis,29-31 inhibition of angiogenesis,20 priming of neutrophil function,32,33 and modulation of immune responses,34 have been attributed to PF4 in vitro. However, its physiologic function in vivo remains uncertain.
Platelets activated by contact with damaged endothelium degranulate and deposit large amounts of PF4 (and therefore presumably also thrombospondin and β-thromboglobulin) on lumenal endothelial cell surfaces,35 the site of TM expression. We hypothesized that these cationic proteins might influence the anticoagulant function of the TM/protein C system. We found that PF4, but not thrombospondin or β-thromboglobulin, could accelerate as much as 25-fold the TM-dependent generation of APC from protein C in the presence of thrombin and purified soluble forms of thrombomodulin.14 The kinetic basis of this phenomenon was a 34-fold decrease in the Michaelis constant (Km) of protein C for the thrombin/TM complex without a significant change in the equilibrium dissociation constant (Kd) of thrombin. PF4 stimulation of TM protein C–activating cofactor activity required the presence of the anionic γ-carboxyglutamic acid (Gla) domain of protein C,14 raising the possibility of an electrostatic interaction between these 2 moieties. Autofluorescence14 and surface plasmon resonance analysis36 confirmed that PF4 binds to (KD 0.37 μM), and induces conformational changes in, protein C in a strictly Gla domain–dependent manner. Western blot analysis of human plasma extracted with avidin agarose in the presence biotinylated PF4 showed that PF4 binds to protein C in a physiologic milieu.36 These studies suggest that PF4 may bind to the Gla domain of protein C and induce a conformational change that enhances protein C affinity for the thrombin/TM complex. Our current findings extend these previous results by demonstrating that PF4 enhances the generation of APC by cell-associated forms of thrombomodulin in vitro and in a thrombin infusion in vivo model, and thus raise the possibility that this phenomenon may constitute a physiologic element of the protein C pathway.
Using cultured monolayers of endothelial cells derived from human umbilical vein endothelium, dermal vascular endothelium, and expanded blood outgrowth endothelial cells, we observed a highly variable but often robust stimulatory effect of PF4 on APC generation by cell-associated TM (Figure 1). The maximal stimulation ranged from none to 10-fold above baseline. Variability was noted even within endothelial cells derived from the same cell source in different primary cell preparations. The dose-response curves in experiments in which PF4 had a stimulatory effect on APC generation by endothelial cells showed that PF4 had a 50% effective concentration (EC50) that was one third to one tenth lower (ie, 1-3 μg/mL) than that of PF4 on purified forms of thrombomodulin.14 Since serum contains 5 to 10 μg/mL PF4,13 this finding supports the possibility that PF4 may play a physiologically relevant role in influencing APC generation in vivo.
The basis for this highly variable effect of PF4 on APC generation by cultured endothelium is unclear. Cell preparation–dependent differences in expression of any of the several components of the TM/protein C system could conceivably be implicated. For example, pronounced vascular bed–specific variability in posttranslational glycanation of TM has previously been demonstrated.7 Either impairment of glycosaminoglycan synthesis in cultured endothelium37 or enzymatic removal of chondroitin sulfate from cell surfaces38 decreases TM affinity for thrombin as much as 10-fold, and PF4 more strongly stimulates APC generation by GAG-positive than GAG-negative forms of purified TM.14 Therefore, endothelial cell preparation–dependent variations in TM glycanation could conceivably modulate the stimulatory effect of PF4 in this system. However, we have found that MVEC are GAG-negative and HUVEC are GAG-positive (not shown), so there is no simple correlation between GAG status of TM and PF4 stimulation of APC generation. The endothelial protein C receptor (EPCR)39 is also variably expressed on endothelial cell vascular beds, being much more heavily expressed in large-vessel endothelium than in microvascular endothelium.40 EPCR strongly influences APC generation in vivo, because blockade of EPCR with an inhibitory monoclonal antibody decreases APC levels by more than 80% in a baboon thrombin infusion model.41 As expression of EPCR, like that of TM and TM glycanation, is vascular bed–dependent, and it functions kinetically in the same manner as does PF4, it is conceivable that these 2 proteins may functionally antagonize or synergize each other. However, MVEC, HUVEC, and BOEC all express EPCR (not shown), and in preliminary experiments there appears to be no obvious relationship between EPCR expression and presence or absence of PF4 stimulation of APC generation. Another possible basis for variability of the PF4 effect on APC generation is variation in non-TM cell surface–associated GAGs, such as heparan sulfate. These possibilities are all the subjects of ongoing investigation.
Our demonstration of a 2- to 3-fold increase in circulating APC levels after thrombin infusion in the presence of PF4 is compatible with, but does not unambiguously establish, the idea that PF4 increases the catalytic conversion of protein C to APC by TM in vivo. In this complex biologic system, other possibilities exist. For example, PF4 may antagonize the inhibitory activity of various plasmatic proteins (eg, the protein C inhibitor) on APC. Indeed, a semilog plot of the decay in APC levels from the termination of the thrombin infusion at 10 minutes through 45 minutes shows an increase in the apparent half-life of APC from 28 to 52 minutes in the presence of PF4 (not shown). However, this change in the rate of decline of APC levels in the presence of PF4 could be at least partly due to increased ongoing APC generation by thrombin-TM. Moreover, when we added APC to monkey plasma and followed its decay over time using the enzyme capture amidolytic assay, we found no evidence for decreased inhibition by plasmatic factors in the presence of PF4 (not shown). Another possibility is that PF4 alters the direct activation of protein C by thrombin in a TM-independent manner, but preliminary experiments in plasma in vitro provide no support for this notion. PF4 could also conceivably alter thrombin clearance kinetics because PF4 has been reported to impair endothelial cell heparin-dependent acceleration of thrombin inactivation by antithrombin III.42 However, this seems unlikely, because we found no significant differences in thrombin-antithrombin complex levels after thrombin infusion with or without prior PF4 infusion (not shown). Given the support of our in vitro data, with both purified and endothelial cell–associated TM, we propose that accelerated catalytic generation of APC by thrombomodulin is at least partly responsible for the increased APC levels we found in vivo in the monkey experiments, but we acknowledge that other mechanisms may apply.
Accompanying the increased APC activity levels after thrombin infusion in the presence of PF4 is an exaggerated anticoagulant response, as assessed by the APTT. Although our experiments with the APC-inhibitory monoclonal antibody C3 show that essentially all of the APTT prolongation is dependent on APC activity (Figure 5), the correlation between APC levels determined by the enzyme capture assay and APTT prolongation becomes skewed at later time points after termination of the thrombin infusion, when APC seems to exert greater anticoagulant activity (compare Figures 3 and 4). This shift in the APC-APTT concentration-response curve is not caused by the presence of PF4, because it also occurs with thrombin infusion alone. The lack of a direct correlation between APTT and APC activity levels may be attributable to other, secondary factors. For example, a specific decrease in factor V levels has been shown to occur after thrombin infusion.18 The interaction of factor V and its various proteolytic products in the regulation of APC activity is complex and includes both pro- and anticoagulant influences.43 It is therefore possible that alterations in levels of factor V and/or its proteolytic products lead to a greater anticoagulant response to APC and this forms the basis for the divergence of APC levels and the APTT prolongations.
In the case of baboons, it has been shown that the anticoagulant laboratory phenotype induced by thrombin infusion is accompanied by a decreased accumulation of platelets and fibrin on a thrombogenic device, thus confirming the functional anticoagulant significance of increased circulating APC.16 Therefore, the results of the present study are compatible with the hypothesis that PF4 serves a natural anticoagulant role that may be relevant in both physiologic and pathologic circumstances. In the immediate vicinity of activated and degranulating platelets, high local concentrations of PF4 could interact with endothelial TM to accelerate local generation of APC. This would both heighten the threshold for generating a stable thrombus and engender a downstream systemic anticoagulant environment that could limit propagation of the thrombus. This mechanism may be endothelial vascular bed–dependent, reflecting variation in posttranslational thrombomodulin glycanation, cell surface glycanation, EPCR status, and other—as yet unenvisioned—modulating influences.
In addition to serving an anticoagulant function by proteolytically inactivating factor Va and factor VIIIa, APC has also been shown to promote fibrinolysis,44-49 exert an anti-inflammatory influence,50 and inhibit apoptosis,51 all of which may contribute to the unique efficacy of APC in reducing mortality in sepsis patients.52 Consequently, if PF4 plays a role in the physiologic stimulation of thrombin-dependent APC generation, the biologic effects of the resulting increases in APC levels would extend beyond prolongation of the APTT to include potential profibrinolytic, anti-inflammatory, and antiapoptotic effects as well. It is conceivable that these effects of PF4 could be exploited pharmacologically for therapeutic effect.
Prepublished online as Blood First Edition Paper, February 27, 2003; DOI 10.1182/blood-2002-11-3529.
Supported by American Heart Association National Grant-in-Aid 94012990 (A.S.) and by Office of Research and Development, Department of Veterans Affairs, grants NIH NS024621 and NIH HL063943 (S.R.L.) and NIH R37 HL52246 (J.H.G).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.