The pathogenicity of Plasmodium falciparum is due to the unique ability of infected erythrocytes (IRBCs) to adhere to vascular endothelium. We investigated whether adhesion of IRBCs to CD36, the major cytoadherence receptor on human dermal microvascular endothelial cells (HDMECs), induces intracellular signaling and regulates adhesion. A recombinant peptide corresponding to the minimal CD36-binding domain from P falciparum erythrocyte membrane protein 1 (PfEMP1), as well as an anti-CD36 monoclonal antibody (mAb) that inhibits IRBC binding, activated the mitogen-activated protein (MAP) kinase pathway that was dependent on Src-family kinase activity. Treatment of HDMECs with a Src-family kinase–selective inhibitor (PP1) inhibited adhesion of IRBCs in a flow-chamber assay by 72% (P < .001). More importantly, Src-family kinase activity was also required for cytoadherence to intact human microvessels in a human/severe combined immunodeficient (SCID) mouse model in vivo. The effect of PP1 could be mimicked by levamisole, a specific alkaline-phosphatase inhibitor. Firm adhesion to PP1-treated endothelium was restored by exogenous alkaline phosphatase. In contrast, inhibition of the extracellular signal–regulated kinase 1/2 (ERK 1/2) and p38 MAP kinase pathways had no immediate effect on IRBC adhesion. These results suggest a novel mechanism for the modulation of cytoadherence under flow conditions through a signaling pathway involving CD36, Src-family kinases, and an ectoalkaline phosphatase. Targeting endothelial ectoalkaline phosphatases and/or signaling molecules may constitute a novel therapeutic strategy against severe falciparum malaria.
Introduction
The adhesion of infected erythrocytes (IRBCs) containing mature stages of the parasitePlasmodium falciparum to host endothelium is a key pathogenic process of the infection.1 The resulting sequestration of IRBCs in the microvasculature can lead to tissue hypoxia, metabolic disturbances, multiorgan dysfunction, and ultimately the death of 1 to 2 million patients worldwide annually.2Cytoadherence is mediated by the parasite protein P falciparum erythrocyte membrane protein 1 (PfEMP1)3and endothelial receptor molecules, including CD36,4,5intercellular adhesion molecule 1 (ICAM-1),6 E-selectin, and vascular cell adhesion molecule 1 (VCAM-1).7 Under physiologic flow conditions, IRBCs have been shown to interact synergistically with the different adhesion molecules on microvascular endothelium in a shear-dependent manner,8,9 mimicking the adhesive events of the leukocyte recruitment cascade.10IRBCs tether and roll on ICAM-1,8,9 VCAM-1,8and P-selectin.8 These low-affinity interactions by themselves are not sufficient for the arrest of the rolling cells, but they enhance the subsequent adhesion of almost all clinical parasite isolates tested to CD36.8,9 Peptide-mapping studies on CD36 reveal that residues 145 to 171,11 and to a lesser extent 97 to 110,12 are important for IRBC adhesion, while the critical region of PfEMP1 involved in binding to CD36 is localized to a 179–amino acid sequence within the cysteine-rich interdomain region 1 (CIDR1).13
The above investigations focused on the extracellular domains of the adhesion molecules and the intravasacular events that promote cytoadherence. Adhesion molecules may also serve as receptor-signaling molecules capable of transducing an extracellular signal leading to cellular activation.14 Such signaling activities have been reported for the selectins,15-17 the immunoglobulin superfamily of adhesion molecules,18 and integrins,19 which all play critical roles in leukocyte recruitment. Several lines of evidence suggest that IRBC adhesion might also induce intracellular signaling. IRBC adhesion to CD36 on monocytes induces a respiratory burst,20 and cross-linking of CD36 on monocytes by a monoclonal antibody (mAb) that inhibits IRBC adhesion to CD36 activates both the extracellular signal–regulated kinase 1/2 (ERK 1/2) and the p38 mitogen-activated protein (MAP) kinase pathways.21 IRBCs have also been shown to inhibit the maturation of dendritic cells through an interaction with cell surface CD36.22 23 It is currently not known if adhesion of IRBCs to CD36 constitutes a classical receptor-ligand interaction that leads to endothelial intracellular signaling, or if CD36 merely serves as an attachment point for the parasite with no cellular consequences in the endothelium.
CD36 is a multifunctional scavenger receptor for long-chain fatty acids, collagen, thrombospondin-1 (TSP-1), both native and oxidized low- and high-density lipoproteins (LDLs and HDLs), and apoptotic neutrophils.24 These receptor-ligand interactions can lead to diverse cellular and biologic responses, including uptake of apoptotic bodies, transforming growth factor–β (TGF-β) activation, fatty acid transport, and apoptosis of endothelial cells. Src-family kinases are physically associated with CD36,25,26 and the binding of TSP-1 to CD36 on human dermal microvascular endothelial cells (HDMECs) sequentially activates the Src-family kinase Fyn, caspase-3 like proteases, and the p38 MAP kinase (MAPK) pathway.27 As well, it has recently been demonstrated that a CD36-mediated signaling cascade involving the Src-family kinases Lyn and Fyn is responsible for the inflammatory effects of β-amyloid in mouse peritoneal macrophages and microglial cells.28 An additional feature of platelet CD36 is that it is constitutively phosphorylated in the ectodomain on residue Thr92.12 This phosphorylation results in a low-affinity receptor for TSP-1. Initial CD36–TSP-1 interaction induces platelet degranulation with release of acid phosphatases capable of dephosphorylating the ectodomain of CD36, resulting in higher affinity for TSP-1. Although the 2 forms of CD36 have not been identified in endothelium, the amino acid sequences of platelet and endothelial CD36 are almost identical, and the protein kinase C (PKC)–dependent targeting sequence RGPYTYRVRFLA for Thr92 phosphorylation is conserved in the endothelial protein.29 The colocalization of CD36, dually acylated Src-family kinases, and a glycosylphosphatidylinositol (GPI)–anchored alkaline phosphatase in specific membrane microdomains such as lipid rafts and caveolae30-32 suggest that a change in the phosphorylation state of endothelial CD36 might also occur upon ligand binding and intracellular signal activation.
In this study, we tested whether the adhesion of IRBCs to CD36 on HDMECs activates intracellular signaling pathways that ultimately play a role in modulating subsequent parasite-host cell interactions. We showed that the binding of a recombinant peptide PpMC-179 corresponding to the minimal CD36-binding domain from PfEMP1 resulted in Src-family kinase activation and downstream activation of the MAPK pathway in endothelial cells. Inhibition of Src-family kinases by the selective inhibitor PP1, but not the inhibition of ERK 1/2 or p38 pathways, resulted in a significant reduction of IRBC adhesion in a flow-chamber assay in vitro and in intact human microvessels in a human/severe combined immunodeficient (SCID) mouse model in vivo. The effect of PP1 could be mimicked by levamisole, a specific alkaline-phosphatase inhibitor. Firm adhesion to PP1-treated endothelium was restored by exogenous alkaline phosphatase. These results suggest a more dynamic role for the endothelium in mediating cytoadherence than previously recognized, and a novel intracellular signaling mechanism involving CD36, Src-family kinases, and an ectoalkaline phosphatase that could be exploited therapeutically.
Materials and methods
Tissue-culture and other reagents
Unless otherwise stated, all tissue-culture reagents were obtained from Invitrogen Canada (Burlington, ON). The Src-family kinase–selective inhibitor PP1 and the inactive analog PP3 were purchased from Biomol Research Laboratories (Plymouth Meeting, PA); the p38 inhibitor SKF86002 was purchased from Calbiochem and Novabiochem (San Diego, CA); p38 inhibitor SB203580 from AG Scientific (San Diego, CA); and the ERK 1/2 inhibitors PD098059 and U0126 were from Upstate (Lake Placid, NY). The phosphatase inhibitors sodium orthovanadate (SOV) and levamisole were purchased from Sigma-Aldrich (St Louis, MO). Calf-intestine alkaline phosphatase was from Calbiochem. Enhanced chemiluminescence substrate (ECL) was purchased from Amersham Pharmacia Biotech (Piscataway, NJ).
Parasites
Cryopreserved parasite isolates from adult Thai patients with well-documented P falciparum malaria were thawed and studied during their first cycle in culture as described.8 The collection of specimens was approved by the Ethics Committee of the Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand. Informed consent was obtained according to the Declaration of Helsinki.
Recombinant PpMC-179
The recombinant protein PpMC-179 was expressed in Pichia pastoris and purified as described.33 The purified protein, but not chondroitin sulfate A (CSA-163), a homologous yeast recombinant protein from a non–CD36-binding parasite strain FCR3-CSA,34 bound to CD36 by affinity purification and inhibited the adhesion of IRBCs from a CD-36 binding strain (FMG) to immobilized CD36 in a static binding assay.
Antibodies
The anti-CD36 mAb OKM5, known to inhibit IRBC interactions with the protein,35 was a kind gift from Ortho Diagnostics (Raritan, NJ). Goat antimouse F(ab′)2 was purchased from Pierce Biotechnology (Rockford, IL), the phospho-specific mAb 4G10 from Upstate, and a rabbit polyclonal phospho-specific anti-ERK 1/2 Ab from Promega (Madison, WI). A mAb for total ERK 1/2 from BD BioScience, Transduction Laboratories (Mississauga, ON, Canada) was used as a loading control according to the manufacturer's specifications. Horseradish peroxidase (HRP)–conjugated secondary antibodies were purchased from Amersham Pharmacia Biotech.
Endothelial cell culture
HDMECs were harvested from discarded neonatal human foreskins with the use of 0.5 mg/mL Type IA collagenase (Boerhringer Mannheim Biochemicals, Indianapolis, IN) as described previously.8 The protocol was approved by the Conjoint Health Ethics Board of the University of Calgary. The cells were maintained in endothelial basal medium (EBM) (BioWhittaker, Walkersville, MD), with supplements provided by the manufacturer. Experiments were performed with cells from passages 1 to 5, on which adhesion molecule expression was shown to be stable.6
Parallel plate flow-chamber assay
IRBC–endothelial cell interactions at fluid shear stresses approximating those in the microvasculature were studied by means of a parallel plate flow chamber as described.8 In previous studies, we have established that infusion of a 1% IRBC suspension over endothelial cell monolayers at 1 dyne/cm2 allowed us to optimally visualize the adhesive interactions in real time. A rolling IRBC was defined as one that displayed a typical end-on-end rolling motion at a velocity lower than 150 μm/s, as compared with a centerline red blood cell flow rate exceeding 1000 μm/s, and a velocity exceeding 150 μm/s for noninteracting cells in close proximity to the endothelial monolayer. The flux of rolling IRBCs was determined as the number that rolled past a fixed line on the monitor screen for the duration of the experiment, and was expressed as the number of rolling IRBCs per minute per square millimeter. An IRBC was considered adherent if it remained stationary for longer than 10 seconds. The results were expressed as the number of adherent IRBCs per square millimiter of surface area.
Intravital microscopy
CB-17 SCID/beige mice (Jackson Laboratory, Charles River, NJ) were grafted with split-thickness human skin as described previously under a protocol approved by the Conjoint Ethics Board of the University of Calgary.9 The grafts were allowed to heal for 3 to 4 weeks before being used for intravital microscopy experiments. At the time of experimentation, animals were anesthetized with ketamine and xylazine, and body temperature was maintained at physiologic levels by means of a heating pad and rectal thermometer as previously described. The procedure was approved by the Animal Care Committee, University of Calgary. Briefly, the jugular vein was cannulated for administration of additional anesthetic and boluses of IRBCs. A midline dorsal incision was made from the neck to the lower back, without disrupting the lateral dermal blood supply. The skin was examined by means of an upright microscope (Nikon Optiphot) with a 20 × water immersion objective (Nikon, Tokyo, Japan). To identify human vessels, 100 μg fluorescein isothiocyanate (FITC)-Ulex europaeus (Sigma-Aldrich) was injected intravenously immediately before microscopic examination. IRBCs, but not noninfected red cells, were labeled with the nuclear dye rhodamine 6G (25 μg/mL, 5 minutes). Each 200 μL bolus of cells contained IRBCs at approximately 50% hematocrit and 5% to 7% parasitemia. Images of the labeled IRBCs and human microvessels were visualized by means of a silicon-intensified CCD camera (C-2400-08; Hamamatsu Photonics, Hamamatsu City, Japan) and recorded with a videocassette recorder (VCR) for playback analysis. The numbers of rolling and adherent IRBCs were determined off-line. IRBC rolling was expressed as the rolling flux fraction, determined by counting the IRBCs interacting in an individual vessel during the period of observation and expressing this number relative to the total number of IRBCs passing through the vessel during the same period (determined by frame by frame analysis). IRBCs that remained stationary on the vascular wall for at least 30 seconds were defined as adherent. Adhesive interactions in postcapillary venules in skin grafts were counted.
Western blot analysis
HDMECs were grown in 60-mm tissue-culture dishes (Corning, Corning, NY) and used for Western blot analysis at 1 day after confluence. Confluent HDMEC monolayers were starved in M199 medium with 0.5% bovine serum albumin (BSA) for 4 hours prior to cross-linking. The mAb OKM5 was used as the primary antibody at 5 μg/mL for 30 minutes at 4°C. A goat antimouse F(ab′)2 fragment (5 μg/mL) was used as the secondary Ab to hyperlink the receptor for 15 minutes at 37°C. After 5 minutes, 100 μM SOV was added to the dishes to protect against dephosphorylation. A similar procedure was used to determine if PfEMP1 could directly activate cell-signaling pathways. The recombinant 179-peptide PpMC-179 (2 μM) was incubated with HDMEC monolayers at 37°C for 5, 15, 30, and 60 minutes, and SOV was added as in the antibody cross-linking experiments. In experiments using cell signal inhibitors, HDMECs were pretreated with the inhibitors during the last 30 minutes of cell starvation.
Treated or untreated cells were washed twice with phosphate-buffered saline (PBS), lysed with 200 μL 2 × sodium dodecyl sulfate (SDS) Laemmli sample buffer at 80°C, and scraped off the plate. The samples (100 μL) were loaded into the wells of a 10% SDS–polyacrylamide gel, and electrophoresis was performed at 1100V/h overnight. Proteins were transferred onto nitrocellulose membranes (Schleicher and Shuell, Keene, NH). Membranes were blocked with either 5% nonfat dry milk in Tris (tris(hydroxymethyl)aminomethane)–buffered saline (TBS) (5 mM Tris, 135 mM NaCl, 5 mM KCl) with 0.5% NP-40 and 0.1% Tween-20 for non–phospho-specific Ab, or 5% BSA in TBS with only 0.1% Tween-20 for the phospho-specific Ab. Membranes were blocked for 1 hour at room temperature. Primary Abs were incubated for 1 hour at room temperature in their respective blocking buffers. Membranes were washed extensively with TBS–Tween-20 with or without NP-40 depending on the primary Ab. The appropriate HRP-conjugated secondary Ab was used at 1:20 000 dilution in blocking solution for 30 minutes at room temperature. Washes were carried out as described. The membranes were developed by means of ECL.
Flow cytometry
CD36 expression on untreated HDMECs and on HDMECs treated with various inhibitors was determined by flow cytometry as described.8
Statistical analysis
All data are presented as the mean ± standard error of the mean (SEM). The numbers given for flow-chamber experiments represent the number of independent experiments. The numbers given for in vivo experiments were based on the total number of vessels observed in 1 or 2 grafted mice for each condition.36 Two groups of data from studying the same clinical parasite isolates under different conditions were compared by Student t test for paired samples.P < .05 was considered statistically significant.
Results
Recombinant PfEMP1 peptide activates intracellular signaling pathways via CD36 on HDMECs
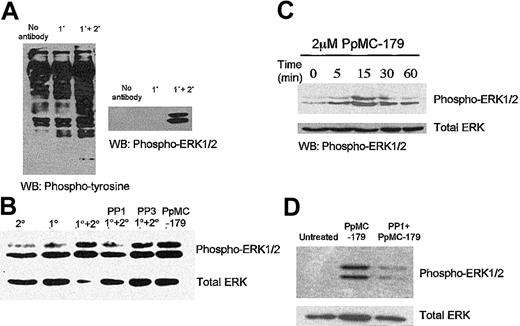
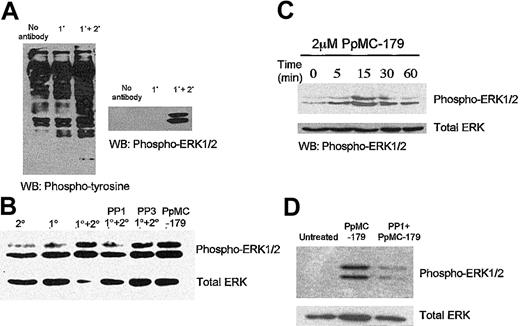
We first used OKM5, an anti-CD36 mAb that inhibits IRBC adhesion, to determine the signaling pathways that might be elicited by IRBCs in human microvascular endothelial cells. Western blot analysis showed that cross-linking CD36 with OKM5 followed by a goat antimouse F(ab′)2 resulted in significant MAPK and ERK 1/2 phosphorylation (Figure 1A). As CD36 in microvascular endothelium associates with a number of Src-family kinases,26 we determined if the ERK 1/2 activation might be a downstream event of Src-family kinase activity. Confluent endothelial monolayers were pretreated for 30 minutes with 10 μM PP1, a selective Src-family kinase inhibitor.37 ERK 1/2 activation by Ab cross-linking was significantly reduced in Src-family kinase–inactivated cells (Figure 1B). PP3, the inactive analog of PP1, had no effect on ERK 1/2 activation.
Activation of intracellular signaling pathways in HDMECs via CD36.
The intracellular signaling pathways in HDMECs were activated via CD36 by the mAb OKM5 and a goat antimouse F(ab′)2 (A) and P falciparum peptide PpMC-179 (C). The activation of CD36 both by cross-linking with antibodies (B) or the parasite peptide (D) was inhibited by the selective Src-family kinase inhibitor PP1 but not the inactive analog PP3. WB indicates Western blot. 1° and 2° refer to primary and secondary antibody, respectively.
Activation of intracellular signaling pathways in HDMECs via CD36.
The intracellular signaling pathways in HDMECs were activated via CD36 by the mAb OKM5 and a goat antimouse F(ab′)2 (A) and P falciparum peptide PpMC-179 (C). The activation of CD36 both by cross-linking with antibodies (B) or the parasite peptide (D) was inhibited by the selective Src-family kinase inhibitor PP1 but not the inactive analog PP3. WB indicates Western blot. 1° and 2° refer to primary and secondary antibody, respectively.
To determine if the PfEMP1-CD36 interaction results in an analogous cell signal in endothelium, we treated endothelial monolayers with PpMC-179, a yeast recombinant protein representing the minimal CD36-binding region of PfEMP1. We found that the PpMC-179–CD36 interaction resulted in phosphorylation of ERK 1/2, similar to the effect of the antibodies. Kinetics studies of the PpMC-179–induced signal further revealed that ERK 1/2 phosphorylation reached a maximum within 15 minutes of interaction (Figure 1C), and could be detected as early as 5 minutes. ERK 1/2 phosphorylation induced by PpMC-179 was inhibited by PP1, consistent with a requirement for Src-family kinase activation (Figure 1D).
Firm adhesion of IRBCs to microvascular endothelium is Src-family kinase dependent
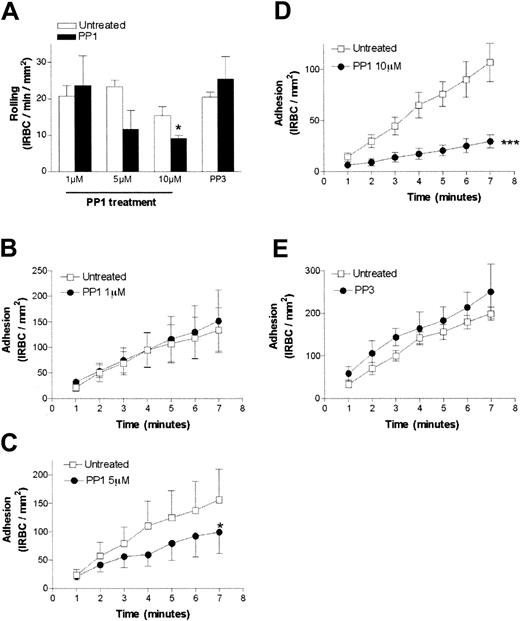
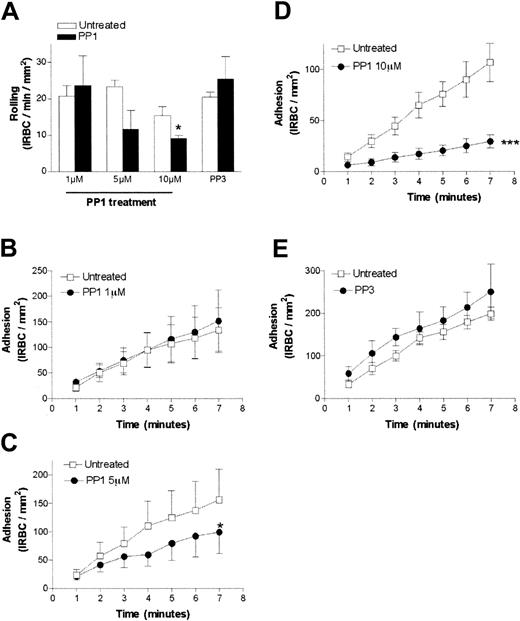
To determine the functional consequence of the Src-family kinase activation, we pretreated HDMEC monolayers with various concentrations of PP1 before the flow-chamber assay. PP1 at 1 μM and 5 μM had no effect on the rolling flux (Figure2A). At 10 μM PP1, rolling flux was reduced from 15.4 ± 2.5 to 9.0 ± 0.8 IRBCs per minute per square millimeter, representing a drop of 41.6% (P < .05). Firm adhesion of IRBCs was also inhibited in a dose-dependent manner, with 1 μM PP1 having no effect, 5 μM PP1 inhibiting adhesion by 37% (from 155.9 ± 54 on untreated cells to 98.7 ± 37.7 IRBCs per square millimeter on treated cells, P < .05), and 10 μM PP1 inhibiting adhesion by 72.4% (from 107.0 ± 18.7 to 29.5 ± 6.3 IRBCs per square millimeter; P < .001) (Figure 2B-D). The inactive analog PP3 at 10 μM had no effect on rolling or adhesion (Figure 2E). These results were obtained with 6 clinical parasite isolates and 3 HDMEC preparations. PP1 did not affect the percentage of endothelial cells expressing CD36 or the total surface expression of CD36 (mean fluorescence intensity) by flow cytometry (data not shown).
The effect of the Src-family kinase inhibitor PP1 on IRBC cytoadherence under flow conditions.
The effects are shown for rolling IRBCs (A) and adherent IRBCs (B-E). Confluent HDMEC monolayers were pretreated with 1, 5, or 10 μM PP1 or 10 μM PP3 (the inactive analog) for 30 minutes at 37°C. IRBCs were infused at 1 dyne/cm2 (n = 3 for 1 and 5 μM PP1 and 10 μM PP3; n = 6 for 10 μM PP1). Values are presented as means ± SEM. *P < .05, ***P < .001.
The effect of the Src-family kinase inhibitor PP1 on IRBC cytoadherence under flow conditions.
The effects are shown for rolling IRBCs (A) and adherent IRBCs (B-E). Confluent HDMEC monolayers were pretreated with 1, 5, or 10 μM PP1 or 10 μM PP3 (the inactive analog) for 30 minutes at 37°C. IRBCs were infused at 1 dyne/cm2 (n = 3 for 1 and 5 μM PP1 and 10 μM PP3; n = 6 for 10 μM PP1). Values are presented as means ± SEM. *P < .05, ***P < .001.
The role of Src-family kinase activity on adhesion in vivo was examined next with the use of the human/SCID mouse model of intact human microvasculature. Skin-grafted animals were untreated or pretreated with either PP1 or PP3 (1.5 mg/kg for 1 hour). This dose of inhibitor had been safely used in a study to examine the protective effect of PP1 against cerebral infarct following an experimentally induced stroke,38 and did not induce changes in shear rate, red blood cell velocity, vessel diameter, leukocyte rolling flux, leukocyte adhesion, or leukocyte emigration in C57BL/6 mice studied in preliminary experiments (data not shown).
We found that PP1 had no effect on the percentage of rolling cells in human microvessels in the skin grafts of 2 treated (21 vessels) compared with 2 untreated animals (6 vessel) (Figure3A). However, Src-family kinase inactivation did decrease IRBC adhesion in vivo from 1.9 ± 0.1 to 0.6 ± 0.1 IRBCs per 100 μm, a change of 68.4% (P < .001) (Figure 3B). The inactive analog PP3 had no effect on rolling or adhesion in one mouse (6 vessels).
The effect of the Src-family kinase inhibitor PP1 on IRBC cytoadherence in a human microvasculature in vivo.
The effects are shown on rolling IRBCs (A) and adhesion IRBCs (B) in a human microvasculature in vivo. Skin-grafted SCID mice were treated with PP1 or PP3 (1.5 mg/kg, 1 hour, ip [intraperitoneall]), and the skin graft was exposed for direct visualization of the adhesive interactions in human microvessels by means of intravital microscopy. Baseline data were obtained from untreated animals. The data are from 6 vessels in 2 untreated mice, 21 vessels in 2 mice treated with PP1, and 6 vessels in 1 mouse treated with PP3. Values are presented as means ± SEM. ***P < .001.
The effect of the Src-family kinase inhibitor PP1 on IRBC cytoadherence in a human microvasculature in vivo.
The effects are shown on rolling IRBCs (A) and adhesion IRBCs (B) in a human microvasculature in vivo. Skin-grafted SCID mice were treated with PP1 or PP3 (1.5 mg/kg, 1 hour, ip [intraperitoneall]), and the skin graft was exposed for direct visualization of the adhesive interactions in human microvessels by means of intravital microscopy. Baseline data were obtained from untreated animals. The data are from 6 vessels in 2 untreated mice, 21 vessels in 2 mice treated with PP1, and 6 vessels in 1 mouse treated with PP3. Values are presented as means ± SEM. ***P < .001.
MAPK inhibitors do not affect IRBC cytoadherence on HDMECs
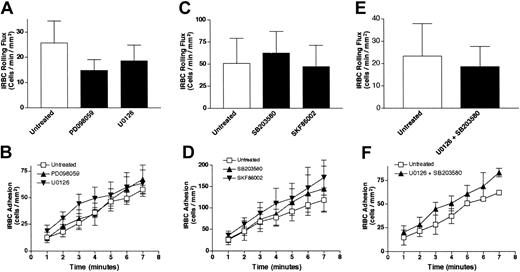
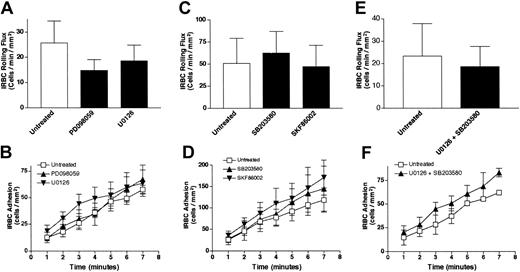
To determine whether MAPK activation has an immediate effect on IRBC adhesion, confluent HDMEC monolayers were pretreated with either PD098059 (25 μM, 30 minutes) or U0126 (10 μM, 30 minutes), both specific inhibitors of the ERK 1/2–MAPK pathway, before being used in flow-chamber assays. These inhibitors did not affect the rolling (Figure 4A) or adhesion (Figure 4B) of 4 different parasite isolates. As the p38 MAPK is activated through CD36 in monocytes by anti-CD36 antibody cross-linking,19 and by TSP-1 binding to CD36 on endothelium,25 the functional effects of 2 p38-MAPK pathway inhibitors, SB203580 and SKF86002, were also studied. Inhibition of the p38 pathway had no effect on IRBC rolling (Figure 4C) and adhesion (Figure 4D). Moreover, a combination of ERK 1/2 (U0126) and p38 (SB203580) inhibitors also had no effect on IRBC rolling (Figure 4E) or adhesion (Figure 4F) on treated HDMECs. The effect of each of the p38 inhibitors and the experiments with a combination of inhibitors were studied with 3 parasite isolates. These results indicate that the activation of the ERK 1/2 and p38 pathways is not directly responsible for the effect of Src-family kinase activation on IRBC adhesion.
The effect of signal transduction inhibitors on IRBC cytoadherence.
Confluent HDMEC monolayers were pretreated with 25 μM PD098059 or 10 μM U0126 (n = 4) (A-B); 10 μM SB203580 or 10 μM SKF86002 (n = 3) (C-D); or a combination of U0126 and SB203580 (E-F) (n = 3). Monolayers were treated with all inhibitors for 30 minutes at 37°C. IRBCs were infused at 1 dyne/cm2. Values are presented as means ± SEM.
The effect of signal transduction inhibitors on IRBC cytoadherence.
Confluent HDMEC monolayers were pretreated with 25 μM PD098059 or 10 μM U0126 (n = 4) (A-B); 10 μM SB203580 or 10 μM SKF86002 (n = 3) (C-D); or a combination of U0126 and SB203580 (E-F) (n = 3). Monolayers were treated with all inhibitors for 30 minutes at 37°C. IRBCs were infused at 1 dyne/cm2. Values are presented as means ± SEM.
Src-family kinases are involved in “inside-out” signaling to regulate an ectodephosphorylation event
Our data suggest that the inhibition of Src-family kinases could inhibit the firm adhesion of IRBCs without changing the level of CD36 expression. Because the phosphorylation state of CD36 appears to be important for ligand binding, and because CD36, Src-family kinases, and a GPI-linked alkaline phosphatase can be present together in caveolae, we investigated whether Src-family kinase activation could be associated with the activity of an ectoalkaline phosphatase (ecto-ALP). Initially, we used a broad-spectrum inhibitor of protein phosphatases, SOV, to pretreat endothelial monolayers for 30 minutes at 5 and 25 μM in 3 experiments. IRBC rolling was significantly decreased on treated monolayers, from 52.5 ± 16.2 to 12.4 ± 6.1 IRBCs per minute per square millimiter at 25 μM (P < .05), but was not significantly affected by 5 μM SOV. Adhesion, however, was significantly inhibited at both concentrations, but was more profound at 25 μM (from 74.3 ± 9.0 to 41.6 ± 12.4 IRBCs per square millimiter for 5 μM SOV,P < .05; and 12.4 ± 2.7 IRBCs per square millimiter with 25 μM SOV, P < .01), representing an overall decrease of 83.3%. SOV did not affect the level of CD36 expression on the endothelial cell surface when assayed by flow cytometry (data not shown). These results suggest that a phosphatase was probably involved in the modulation of IRBC adhesion. However, SOV is a small, soluble molecule that can cross the outer cell membrane, thereby inhibiting both extracellular and intracellular protein phosphatases. In fact, SOV might be acting to block the CD36-signaling pathway by inhibiting the Src-family kinases, in a way similar to the effects of PP1. Src-family kinases require dephosphorylation to become activated,39 40 so it is possible that the effect of SOV was at the level of Src-family kinase activation.
Levamisole, an alkaline phosphatase–specific inhibitor, decreases firm adhesion of IRBCs to HDMECs
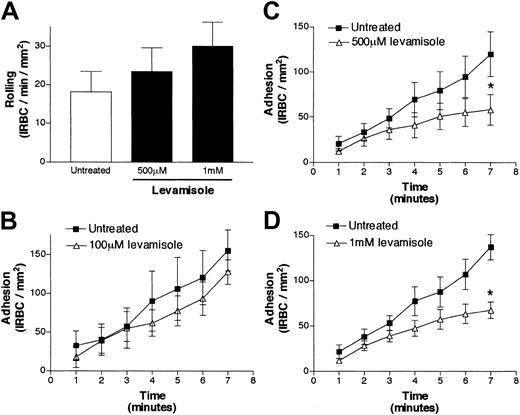
To provide further evidence for an ecto-ALP role in cytoadherence, we used levamisole. Levamisole specifically inhibits purified and cell-bound ALP at physiologic pH without inhibiting the phosphatase activity of any other known membrane enzymes.41 Confluent HDMEC monolayers were pretreated with levamisole (100 μM to 1 mM) for 30 minutes at 37°C. Rolling flux was not significantly altered in the presence of increasing concentrations of levamisole (Figure 5A). On the other hand, adhesion was significantly decreased from 119.9 ± 24.8 to 58.1 ± 17.2 IRBCs per square millimeter at 500 μM levamisole (P < .05) (Figure 5C), and from 137.3 ± 13.9 to 67.7 ± 9.0 IRBCs per square millimeter at 1 mM levamisole (P < .05) (Figure 5D), representing an inhibition of 51% for both drug concentrations. The drug was ineffective at 100 μM (Figure 5B). The results are the mean of 5 experiments with 5 different parasite isolates.
The effect of the specific alkaline-phosphatase inhibitor levamisole on IRBC cytoadherence.
The effects are shown for rolling IRBCs (A) and adherent IRBCs (B-D). Confluent HDMEC monolayers were pretreated with 100 μM to 1 mM levamisole for 30 minutes at 37°C. IRBCs were infused at 1 dyne/cm2 (n = 5). Values are presented as means ± SEM. *P < .05.
The effect of the specific alkaline-phosphatase inhibitor levamisole on IRBC cytoadherence.
The effects are shown for rolling IRBCs (A) and adherent IRBCs (B-D). Confluent HDMEC monolayers were pretreated with 100 μM to 1 mM levamisole for 30 minutes at 37°C. IRBCs were infused at 1 dyne/cm2 (n = 5). Values are presented as means ± SEM. *P < .05.
Exogenous alkaline phosphatase completely restores adhesion in Src-family kinase–inactivated HDMECs
As a second approach to test for the requirement for an ecto-ALP in IRBC adhesion, we treated HDMECs with exogenous ALP. Calf ALP is a large enzyme that would not readily cross the endothelial plasma membrane and should therefore only affect the phosphorylation state of cell surface proteins. We observed that ALP treatment alone (200 U/mL, 30 minutes at 37°C) had no effect on IRBC rolling or adhesion on HDMECs (Figure 6A-B). As shown earlier, treatment of HDMECs with PP1 resulted in a reduction of IRBC adhesion by 58.7%, from 128.3 ± 20.6 to 53.0 ± 20.2 IRBCs per square millimeter (P < .01) (Figure 6C). When ALP was added to PP1-treated HDMECs, the rolling flux was unchanged compared with untreated cells (Figure 6A), but adhesion increased to 180.4 ± 56.5 IRBCs per square millimeter, completely restoring adhesion to normal levels (P < .001 compared with PP1 alone) (Figure 6D). The experiments were performed 5 times with the use of 3 different parasite isolates. These results demonstrate a link between the intracellular signal transduced from CD36 after PfEMP1 engagement and an ectodomain ALP in modulating IRBC adhesion to endothelium.
The effect of exogenous alkaline phosphatase on IRBC cytoadherence.
The effects are shown for rolling IRBCs (A) and adherent IRBCs (B-D). HDMEC monolayers were untreated (B) or pretreated with PP1 (C) or PP1 and calf alkaline phosphatase (200 U/mL Hanks balanced salt solution [HBSS], pH 7.3, for 30 minutes at 37°C) (D). IRBCs were infused at 1 dyne/cm2 (n = 5). Values are presented as means ± SEM. *P < .01, ***P < .001.
The effect of exogenous alkaline phosphatase on IRBC cytoadherence.
The effects are shown for rolling IRBCs (A) and adherent IRBCs (B-D). HDMEC monolayers were untreated (B) or pretreated with PP1 (C) or PP1 and calf alkaline phosphatase (200 U/mL Hanks balanced salt solution [HBSS], pH 7.3, for 30 minutes at 37°C) (D). IRBCs were infused at 1 dyne/cm2 (n = 5). Values are presented as means ± SEM. *P < .01, ***P < .001.
Discussion
In current models of cytoadherence, endothelial adhesion molecules are viewed as mere points of attachment for IRBCs. Regulation of adhesion is believed to be at the level of adhesion molecule expression as modulated by proinflammatory cytokines. The possibility that endothelial cells may play a more dynamic role in the cytoadherence cascade has not been explored. In this study, we demonstrated that IRBC adhesion to the endothelial molecule CD36 represents a ligand-receptor interaction, resulting in cellular responses capable of modulating the adhesion process without affecting receptor expression on HDMECs. Cross-linking CD36 with OKM5, and more specifically with the recombinant peptide PpMC-179, led to the activation of the ERK 1/2–MAP kinase pathway that was dependent on Src-family kinase activity. The PpMC-179 peptide represents the functional binding domain of the parasite cytoadherent ligand PfEMP1, and our finding is therefore the first direct demonstration that PfEMP1 can generate a signal in the host cell.
Our data with selective signaling inhibitors suggest that the outside-in signaling elicited by the initial interaction between IRBCs and CD36 leading to firm adhesion of IRBC is mediated by a PP1-sensitive tyrosine kinase. On the basis of the published observations that Src-family kinases bind to the CD36 cytoplasmic tails in HDMECs,26 and that CD36-mediated signaling involves activation of Src-family kinases in diverse cell types,27,28 we postulate that Src-family kinases are also involved in the IRBC-induced signaling in HDMECs. This family of nonreceptor tyrosine kinases plays critical roles in receptor signaling and cellular communication. The exact mechanism whereby Src-family kinases are activated by CD36 ligation on HDMECs is not known. Dually acylated Src-family kinases are targeted for localization to the plasma membrane owing to palmitoylation and/or myristoylation in their extreme N-terminus.42-44 Much of the Src-family kinase activity therefore lies at the inner leaflet of the plasma membrane in close proximity to the intracellular tails of transmembrane protein receptors such as CD36. It is possible that CD36 ligation by PfEMP1 allows for recruitment and activation of members of the Src-family kinases through phosphorylation at Tyr530 in the carboxyl terminus.43,45 The subsequent ERK 1/2 activation could result from a previously defined pathway in which Src-family kinases activate focal adhesion kinases (FAKs) that subsequently activate Ras, MEK, and ERK.46
Our findings further suggest that activation of Src-family kinases leads to the activation of an ALP, consistent with the fact that compounds known to initiate signal transduction cascades, such as phorbol myristate acetae (PMA) or PMA combined with dibutryl cyclic adenosine 5′-monophosphate (cAMP), increase cell surface phosphatase activity on endothelial cells.47 Although GPI-anchored ALP normally resides in lipid rafts outside of caveolae, it can be induced to move into the caveolae when cross-linked.28 Within the caveolae, the 22-kDa major structural protein caveolin-1 interacts with and regulates the activity of a number of signaling proteins.48 An emerging concept is that caveolin-1 and Src-family kinases might be involved in transmembrane crosstalk with GPI-anchored outer membrane proteins.49 Caveolin-1 could serve as an information conduit interacting with different molecules or different combinations of molecules as they migrate into or out of the membrane microdomain.
In the initial report on CD36 ectodomain dephosphorylation, it was stated that IRBCs bound equally well to phosphorylated and dephosphorylated CD36 protein in a static binding assay.10Preliminary results from our laboratory using purified CD36 in a static binding assay support these findings. However, the results in the present report would suggest that the ectodephosphorylation of CD36 may be critical for firm adhesion of IRBCs to HDMECs under flow conditions in vitro and in vivo. The finding is consistent with the fact that affinity is likely to play a much greater role under physiologic shear stress, particularly on a substratum with much lower site density of CD36 such as HDMECs.8 Differences in the requirement for adhesion to endothelium under static and flow conditions have been elegantly demonstrated with the use of parasites lacking the gene for knob-associated histidine-rich protein 1 (KAHRP1), which forms the core of the electron-dense protrusions (knobs) that anchor PfEMP1 to the erythrocyte membrane. These parasites do not express knobs, and fail to adhere to endothelial cells under flow, but adhere readily under static conditions.50
The functional consequence of an activated ERK 1/2–MAP kinase pathway by PpMC-179 in the endothelium remains to be determined. Inhibition of this pathway had no detectable effect on IRBC adhesion to HDMECs for the duration of the experiment. Inhibition of the p38 MAP kinase pathway also had no effect on IRBC adhesion. However, this lack of effect on adhesion of MAPKs does not exclude an important downstream role for MAPKs in regulating gene expression, including cytokines and chemokines, in response to PfEMP1 ligation of CD36. The occurrence of downstream effects of MAPKs have been reported in monocytes in that the ERK 1/2 and the p38 MAPK pathways are activated in monocytes following CD36 cross-linking with OKM5, and blockade of these pathways resulted in inhibition of phagocytosis of nonopsonic IRBCs.19 In dendritic cells, the binding of IRBCs to surface-expressed CD36 leads to a switch in production of interleukin 12 (IL-12) to IL-10, and an associated inability to activate T cells.21 These findings indicate that the interaction of IRBCs with host cells can result in profound modulation of their normal function. There are currently no data on changes in endothelial cell function induced by IRBC cytoadhesion.
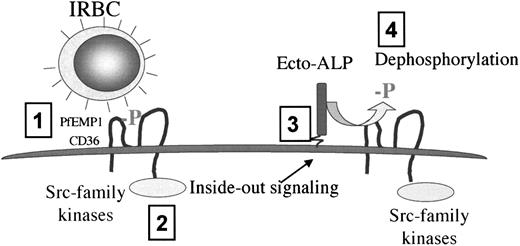
In summary, our data support a novel model of cytoadherence that involves the endothelium in a much more dynamic role than was previously appreciated (Figure 7). This model proposes that the initial attachment of IRBCs to CD36 under flow conditions (step 1) triggers an Src-family kinase-dependent intracellular signal (step 2) that is responsible for increasing subsequent adhesion of IRBCs to CD36 by means of an ecto-ALP (step 3) that dephosphorylates and hence increases the binding affinity of CD36 (step 4). These findings shed new light on the sequestration process and suggest that a small number of strongly adherent IRBCs can activate the endothelium and promote the sequestration of the majority of the parasites. The results further suggest that an ecto-ALP and/or signaling molecules might be an important therapeutic target for antiadhesive therapy.
Proposed model of the regulation of IRBC adhesion to CD36 on HDMECs under flow conditions.
The initial attachment of IRBCs to CD36 (step 1) leads to an Src-family kinase–dependent intracellular signal (step 2) that is responsible for increasing subsequent IRBC adhesion to CD36 by means of an ecto-ALP (step 3) that dephosphorylates and hence increases the binding affinity of CD36 for IRBCs (step 4).
Proposed model of the regulation of IRBC adhesion to CD36 on HDMECs under flow conditions.
The initial attachment of IRBCs to CD36 (step 1) leads to an Src-family kinase–dependent intracellular signal (step 2) that is responsible for increasing subsequent IRBC adhesion to CD36 by means of an ecto-ALP (step 3) that dephosphorylates and hence increases the binding affinity of CD36 for IRBCs (step 4).
The authors are grateful to Dr Ciaran Brady (Malaria Vaccine Development Unit, National Institutes of Allergy and Infectious Diseases, National Institutes of Health, Rockville, MD) for providing the PpMC-179 peptide, and to Dr Robert Lindsay (Department of Surgery, Foothills Hospital, Calgary, AB, Canada) and Dr Caroline Lane (Valley View Family Practice Clinic, Calgary, AB, Canada) for providing skin specimens.
Prepublished online as Blood First Edition Paper, November 27, 2002; DOI 10.1182/blood-2002-09-2841.
Supported by a group grant from the Canadian Institutes of Health Research, and the Alberta Heritage Foundation for Medical Research, Canada. S.M.R. and M.H. are Senior Scholars of the Alberta Heritage Foundation for Medical Research (AHFMR), and S.M.R. holds a Canada Research Chair in Cancer Biology. B.G.Y. is funded by a studentship of the AHFMR.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
May Ho, Department of Microbiology and Infectious Diseases, Health Sciences Centre, Calgary, AB, Canada T2N 4N1; e-mail: mho@ucalgary.ca.

![Fig. 3. The effect of the Src-family kinase inhibitor PP1 on IRBC cytoadherence in a human microvasculature in vivo. / The effects are shown on rolling IRBCs (A) and adhesion IRBCs (B) in a human microvasculature in vivo. Skin-grafted SCID mice were treated with PP1 or PP3 (1.5 mg/kg, 1 hour, ip [intraperitoneall]), and the skin graft was exposed for direct visualization of the adhesive interactions in human microvessels by means of intravital microscopy. Baseline data were obtained from untreated animals. The data are from 6 vessels in 2 untreated mice, 21 vessels in 2 mice treated with PP1, and 6 vessels in 1 mouse treated with PP3. Values are presented as means ± SEM. ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/7/10.1182_blood-2002-09-2841/3/m_h80734098003.jpeg?Expires=1765958218&Signature=CuoDESsckOk6QBJ-~mpBokyAlWJHNoTkbiDvM3j9ajqt4zkPacZ2ZubqYlnSHzkOsuzYVVNOQHBKB1hdBgdI0toQ-VEFl7F6aDFeZ8FH79o6YjlVoMcMT87DRLVva9w5uAZexAWZSMNnUWwWvVLGNZi0Mgo6sKVsqoaLUmAzv5iOrWmaB8-o25pmrZnDfiF7xuiI6k7O4CW5N4DbwgBk-xN9-ZydxGZyB-9FRMflj70OSaAJ-9O9aIAGLzeawfwhnAT7C1aGvA9c8PgqwOmWsEGFXU3sfy22-j7o4OuC0mmIG6vb2ZNj5dP295yRGM80yTHMfLKj2e8SLF5nVpqJYw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 6. The effect of exogenous alkaline phosphatase on IRBC cytoadherence. / The effects are shown for rolling IRBCs (A) and adherent IRBCs (B-D). HDMEC monolayers were untreated (B) or pretreated with PP1 (C) or PP1 and calf alkaline phosphatase (200 U/mL Hanks balanced salt solution [HBSS], pH 7.3, for 30 minutes at 37°C) (D). IRBCs were infused at 1 dyne/cm2 (n = 5). Values are presented as means ± SEM. *P < .01, ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/7/10.1182_blood-2002-09-2841/3/m_h80734098006.jpeg?Expires=1765958218&Signature=YQDC2V51InYvVJF1XFgSw553-tMIHdCHv4tAp96QANTwV0Q~9-SeA~w-3Ob48GS9YR4MyMwgEHS3SlKsya~7bNdqUt7usEpLRdL8qRyyWGO21h8MJQmAHfB2NSKH8MF5JGUADcdZcK~5qQQDeg65-ZqkTFJFbbEzUJOxpfPwO9hDs0PDda9Meu8r4UUmVdUdTeKlyBGawfcqtVGWeqjhu6ubhyzZhB4B77060WS1genyKyi5eMSkuiksD1ZN3P7F9cH96luuZzKK6K3pA71JaFGvLI2YdO5mRWNPRpX8jeGV5a6XUc~xRIcZqpoCmO~ZDZ9XXQ6WVVKtUawy0Hvgtw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Fig. 3. The effect of the Src-family kinase inhibitor PP1 on IRBC cytoadherence in a human microvasculature in vivo. / The effects are shown on rolling IRBCs (A) and adhesion IRBCs (B) in a human microvasculature in vivo. Skin-grafted SCID mice were treated with PP1 or PP3 (1.5 mg/kg, 1 hour, ip [intraperitoneall]), and the skin graft was exposed for direct visualization of the adhesive interactions in human microvessels by means of intravital microscopy. Baseline data were obtained from untreated animals. The data are from 6 vessels in 2 untreated mice, 21 vessels in 2 mice treated with PP1, and 6 vessels in 1 mouse treated with PP3. Values are presented as means ± SEM. ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/7/10.1182_blood-2002-09-2841/3/m_h80734098003.jpeg?Expires=1766370233&Signature=r6wzBqxUZPrkt4uIZNW5LSCulMCSgBShXI-Cb3j4EqWLaRJDl~R1Ws2E3boC3t~wwk3eEuHXS7YqcAq3qxC-e6LuA4ImeqZp~Fj3dmCBkFu0ZcFCMOnuDMgXt9sv78HOQwHLX9TfaVC57CiE7mCbJyABaZ4RpPFyFeXvgvDh4zVZnYd98YV4MbqXAeCxpJz8sA1N7WONlfqmcm7~w-2Id141LOBpHcax7VyYdnFuCoxVybiQh9DoEoYsuHNwPA3RA8~5J~BCRnjhdSEIw-7jc7i~h80Lm~6ZOFpfRK2kHiGgMrLNXlE9uw4DLGEmeHfEszRm54BQAkaniXFJI33ANA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. The effect of exogenous alkaline phosphatase on IRBC cytoadherence. / The effects are shown for rolling IRBCs (A) and adherent IRBCs (B-D). HDMEC monolayers were untreated (B) or pretreated with PP1 (C) or PP1 and calf alkaline phosphatase (200 U/mL Hanks balanced salt solution [HBSS], pH 7.3, for 30 minutes at 37°C) (D). IRBCs were infused at 1 dyne/cm2 (n = 5). Values are presented as means ± SEM. *P < .01, ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/7/10.1182_blood-2002-09-2841/3/m_h80734098006.jpeg?Expires=1766370233&Signature=szyVKIqGJEwKBODBvDG2O0jf4VqltSrRA1yZN7OVI1koyCf8s~kcM8QCHrjHnok7qk1lc~8otY9lcGGTm25WlrkHWOA5tI7ficMyoippM5vpYKaIj0OkFWgqc8dr8JoQ1qQcVU4jypLJKhm6NEFsGwHRwDOjUbBEn2SXuDwnu0PJtxU3X3BD-894P9oH-9gDEB-DtIW~4p2uQlZXj8Ap5fZru8OkvIcny5TV~Oxt6bL0nw1r~d7T85c6G1nL-ho4dzNAtn9MXj5jGYPYe1rwETSWp5xxdst~ECNy3CS1p2cOg~PSpeNSO0Yph~MvLJJxrJzRFS7JJDdcM1Qh-ZZ4ow__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)