EB1089, a novel vitamin D3 analog, has been shown to have cytotoxic and antiproliferative properties in a variety of malignant cells. However, its potential as a treatment for B-cell chronic lymphocytic leukemia (B-CLL) has not been evaluated. EB1089 induced apoptosis in all of the 102 B-CLL samples tested with a mean LD50 (the concentration of EB1089 required to kill 50% of cells) value (± SD) of 2.1 × 10−8 M (± 1.4 × 10−8 M). Furthermore, no significant difference was found in the cytotoxicity of EB1089 in B-CLL samples from previously treated and untreated patients (P = .1637). Induction of apoptosis was associated with a reduction in Bcl-2 and Mcl-1 protein expression, but this was evident only in the apoptotic cells. In contrast, the expression of Bax, p21, and p53 was not altered in the viable or apoptotic cells from either B- or T-lymphocyte lineages. EB1089-induced apoptosis was preceded by activation of p38 mitogen–activated protein (MAP) kinase and suppression of extracellular signal–regulated kinase (ERK) activity, and this was associated with downstream activation of caspase-3. The pancaspase inhibitor (Z-VAD-FMK) and the caspase-9 inhibitor (Z-LEHD-FMK) were able to partially abrogate the apoptotic effects of EB1089 but did not affect the phosphorylation of p38 MAP kinase or the suppression of ERK. The B-CLL cells in the study were shown to highly express vitamin D receptor, but an additional receptor-independent mechanism of cell killing cannot be ruled out at this stage. These findings show that EB1089 is a potent apoptosis-inducing agent in B-CLL cells and may be useful in the treatment of B-CLL patients, particularly those with p53 mutations or drug-resistant disease.
Introduction
B-cell chronic lymphocytic leukemia (B-CLL) is an incurable disease characterized by the clonal expansion of CD5+/CD19+ B cells. Most of the leukemic cells are in the G0/G1 phase of the cell cycle and have a long life span. Therefore, B-CLL is an archetypal example of a malignant condition caused by a failure in cell death mechanisms rather than escape from proliferative control.1 This may be explained, at least in part, by the high levels of the antiapoptotic protein Bcl-2 found in most B-CLL cells and an associated low expression of the proapoptotic protein Bax compared with that of normal peripheral blood B lymphocytes.2 The consequent high Bcl-2/Bax ratios have been associated with in vitro and in vivo resistance to many cytotoxic agents.3-5
The clinical course of B-CLL is variable, but when treatment has been initiated the development of drug resistance often ensues. Therefore, delineation of the biologic mechanisms of drug resistance and the development of novel therapies designed to overcome this clinical problem has become pivotal.
The secosteroid hormone 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) plays an important role in the maintenance of calcium homeostasis mediated through binding to specific intracellular receptors.6,7 The classic target organs of this hormone are the intestines, kidney, and bone, but a number of other tissues not involved in mineral and bone metabolism also possess specific vitamin D3 receptors (VDRs), including normal and neoplastic hematopoietic cells.8,9 The interest in vitamin D analogs in relation to the treatment of human malignancy emerged from the finding that many types of cancers express VDRs, including those of the breast, prostate, pancreas, colon, and bladder, and also leukemias and lymphomas.10-13 It has been demonstrated that 1,25(OH)2D3 is capable of controlling cellular differentiation and proliferation in many cell types and can promote differentiation and inhibit proliferation of cancer cells in vitro.14-16 Furthermore, exposure to 1,25(OH)2D3 will induce apoptosis in human breast cancer and leukemia cell lines.17,18 However, the hypercalcemic effects of 1,25(OH)2D3 limit its potential as a therapeutic agent, but analogs with lower calcemic potential have subsequently been developed. One such analog, EB1089, is 50 to 200 times more potent than vitamin D in inhibiting growth and inducing differentiation of some cancer cell lines.19,20 Recent gene expression profiling experiments of B-CLL cells have identified that the VDR is highly expressed in B-CLL compared with normal B and T lymphocytes.21 This current study was designed to investigate the effects of EB1089 on B-CLL cells in terms of apoptosis induction, Bcl-2 family expression, and cell cycle regulation.
Patients, materials, and methods
Isolation of leukemic and normal lymphocytes
Peripheral blood samples from 102 patients with B-CLL (37 treated and 65 untreated) and 12 age-matched healthy controls were obtained with the patients' informed consent. The treated group of patients had previously received a number of alternative therapeutic regimens, but none had been treated for at least 3 months. Clinical staging was based on the Binet system.22 Peripheral blood lymphocytes were isolated by density centrifugation on Ficoll-Hypaque (Sigma, Poole, United Kingdom) prior to dilution in culture medium.
Cell culture conditions
Freshly isolated peripheral blood lymphocytes (1 × 106/mL) were cultured in Eagle medium (Invitrogen, Paisley, United Kingdom) supplemented with penicillin, streptomycin, and 10% fetal calf serum (FCS). All cultures were incubated at 37°C in a humidified 5% carbon dioxide atmosphere in the presence of EB1089 (10−12 to 10−7 M) with or without the addition of 75 μM final concentration of the cell-permeable caspase inhibitors Z-VAD-FMK, Z-LEHD-FMK, or Z-IETD-FMK (Cambridge Bioscience, Cambridge, United Kingdom). Parallel experiments using chlorambucil (3 × 10−6 M) were also performed in some of the assays. In addition, control cultures were carried out to which no drug was added to cultured B-CLL cells. Cells were subsequently harvested by centrifugation and were analyzed by flow cytometry using the methods outlined below. Experiments were performed either in duplicate or triplicate.
Measurement of in vitro apoptosis
In this study changes in forward light scatter (FSC) and side light scatter (SSC) characteristics were used to quantify apoptotic and viable cell populations as described previously.23,24Typically lymphocytes show a reduction in FSC (a function of cytoplasmic shrinkage) and an increase in SSC (due to increased granularity) when they undergo apoptosis.25 The quantitation of apoptosis using an FSC/SSC gating strategy in conjunction with back gating of CD19+ or CD3+lymphocytes allowed simultaneous acquisition of Bcl-2 family protein expression data in viable and apoptotic B-lymphocyte and T-lymphocyte subpopulations, respectively. All LD50 and LD90values (the concentration of EB1089 required to kill 50% and 90% of cells, respectively) were derived from the dose-response curves. Duplicate samples were assessed using fluorescein isothiocyanate (FITC)-labeled annexin V to confirm the presence of apoptotic cells in the cell cultures and to validate the FSC/SSC quantitation method.26
Flow cytometric analysis of Bcl-2, Bax, Mcl-1, p53, and p21 protein expression
Samples from all 102 patients were analyzed by dual- or triple-color immunofluorescent staining using combinations of Bcl-2, Bax, Mcl-1, p53, and p21 antibodies in conjunction with CD19 (pan B-cell marker) or CD3 (pan T-cell marker). Briefly, 1 × 106 cells were incubated with 10 μL anti-CD19 or CD3, cyanine 5 (Cy5) phycoerythrin (PE)-conjugated antibodies, or isotype-negative controls (DAKO, Ely, United Kingdom). The cells were then fixed using a commercially available kit (DAKO) and resuspended in permeabilization solution together with titration-determined volumes of each antibody or isotype-matched negative control—that is, Bcl-2-FITC, p53-FITC (DAKO), Bax, p21 (Santa Cruz Biotechnology, CA), and Mcl-1 (Pharmingen, San Diego, CA). Subsequently, a PE-labeled secondary antibody was added to the Bax, p21, and Mcl-1–labeled cells (Sigma). The cells were then resuspended in 0.5 mL of 1% paraformaldehyde prior to flow cytometric analysis using a FACScan flow cytometer (Becton Dickinson, CA). From each sample at least 10 000 cells were analyzed and nonspecific binding was excluded by gating using the isotype-negative control antibodies. Gating of the CD19+ (B cells) and CD3+ (T cells) was performed in the analyses to discriminate the apoptosis and specific protein expression in the B- and T-lymphocyte subpopulations. The mean fluorescent intensity (MFI) was calculated for each individual protein using WinMDI software (J. Trotter, Scripps Research Institute, La Jolla, CA).
Vitamin D receptor expression in leukemic and nonleukemic lymphocytes
Vitamin D receptor status in normal and leukemic B lymphocytes and T cells from both healthy age-matched controls and B-CLL patients was quantified using a mouse monoclonal antibody (Santa Cruz Biotechnology) in conjunction with an FITC-labeled secondary antibody (DAKO). Receptor expression was quantified using FACScan flow cytometer (Becton Dickinson) under the experimental conditions described above. Competitive inhibition experiments designed to determine whether EB1089-induced apoptosis was mediated through the vitamin D receptor were performed using a recombinant vitamin D receptor (Upstate Biotechnology, New York, NY) at molar ratios of 10:1 and 100:1 (recombinant receptor: EB1089). EB1089 was preincubated for 30 minutes with recombinant receptor prior to addition to B-CLL cells from 12 separate patients.
Flow cytometric analysis of p38 MAP kinase and ERK phosphorylation
B-CLL cells from 32 patients were assessed for the activation of p38 mitogen–activated protein (MAP) kinase and extracellular signal–regulated kinase (ERK) MAP kinase at 2-hour time intervals following exposure to EB1089 or chlorambucil for 24 hours. In addition, control experiments without drug were also performed. Fluorescein-labeled phosphorylation-specific antibodies were used for measuring the expression of both MAP kinases, p38, and ERK (Santa Cruz Biotechnology) after the cells had been fixed and permeabilized using the methods described above. Samples were also labeled with CD19 as described previously in order to employ a gating strategy that enabled analysis of viable (nonapoptotic) B cells. The p38 MAP kinase inhibitor, SB203580 (Calbiochem, Nottingham, United Kingdom), and the ERK inhibitor, PD98059 (Calbiochem), were used at final concentrations of up to 1 μM to establish whether inhibition of these transduction molecules might modulate the apoptotic responses in B-CLL cells. In addition, experiments were also performed in which Z-VAD-FMK (75 μM final concentration) was added to cell cultures to determine whether the phosphorylation of p38 MAP kinase and the dephosphorylation of ERK were proximal or distal to caspase activation and apoptosis induction in B-CLL cells.
Measurement of caspase-3 activation
B-CLL cells were exposed to EB1089 (10−12 to 10−7 M) for 48 hours and were subsequently harvested by centrifugation. The cells were then fixed and permeabilized as described above and were labeled with 10 μL of an R-phycoerythrin (RPE)-labeled caspase-3 antibody (Pharmingen). The antibody binds only activated caspase-3 and does not recognize procaspase-3. Subsequently, the cellular protein was quantitated by flow cytometry.
Cell cycle analysis
The distribution of cells within the cell cycle was determined by propidium iodide incorporation into DNA. Briefly, aliquots of cells (1 × 106) were harvested by centrifugation and resuspended in 200 μL phosphate-buffered saline (PBS). Aliquots (2 mL) of ice-cold 70% ethanol were then added, and the cells vortexed prior to being cooled to −20°C for 30 minutes. Subsequently, 100 μL of an RNase (Sigma) at a concentration of 1 mg/mL and 100 μL propidium iodide (Sigma) at a concentration of 400 μg/mL were added prior to incubation at 37°C for 30 minutes. The cells were analyzed using a FACScan flow cytometer (Becton Dickinson), and the percentages of cells in the different phases of the cell cycle were quantified using Cylchred version 1.0.2 software (Terry Hoy, University Hospital of Wales, Cardiff, United Kingdom).
Statistical analysis
The data obtained in these experiments were evaluated using the equal variance and paired Student t test, and correlation coefficients were calculated from least squares linear regression plots. LD50 and LD90 values were calculated from line of best-fit analysis of the dose-response curves. All statistical analyses were performed using Graphpad Prism 3.0 software (Graphpad Software, CA).
Results
Induction of apoptosis in B-CLL cells
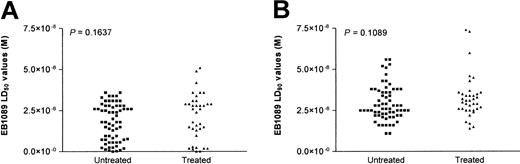
A flow cytometry–based in vitro apoptosis detection assay was used to determine whether EB1089 could induce apoptotic cell death in B-CLL cells. The characteristic changes in the forward and side light scatter resulting from cellular shrinkage described previously were used to define apoptosis.25 In addition, annexin V labeling was also performed to verify the light scatter data. Apoptosis was induced in all of the 102 patient samples following exposure to EB1089 with a mean LD50 value (± SD) of 2.1 × 10−8 M (± 1.4 × 10−8 M) and a mean LD90 value (± SD) of 3.2 × 10−8 M (± 1.2 × 10−8 M). There was no significant difference in the LD50 and LD90 values between the treated and untreated patient groups (Figure 1A and1B, respectively).
Comparison of the cytotoxic effect of EB1089 on treated and untreated B-CLL cells.
The mean LD50 (A) and LD90 values (B) were derived from the flow cytometric apoptosis assay. There was no significant difference in drug sensitivity between the previously treated (n = 37) and untreated (n = 65) patient groups.
Comparison of the cytotoxic effect of EB1089 on treated and untreated B-CLL cells.
The mean LD50 (A) and LD90 values (B) were derived from the flow cytometric apoptosis assay. There was no significant difference in drug sensitivity between the previously treated (n = 37) and untreated (n = 65) patient groups.
Induction of apoptosis in normal age-matched control B and T lymphocytes and T cells from B-CLL patients
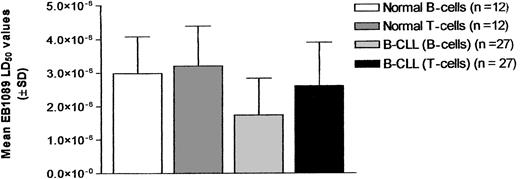
B and T lymphocytes from 12 age-matched healthy controls were assessed for their sensitivity to EB1089-induced apoptosis. In addition, the T lymphocytes from 27 B-CLL patients from the untreated patient group whose T-lymphocyte population was more than 5% of the total lymphocyte population were also analyzed to determine whether EB1089 had differential cytotoxic effects on the various lymphocyte subpopulations. This threshold was chosen to ensure that sufficient T cells could be evaluated; none of the treated patient samples met this criterion and were therefore not analyzed. The T cells from the B-CLL samples showed consistently higher LD50 values than their corresponding malignant B-cell clones (P < .0001, paired t test). In addition, the normal age-matched control B and T lymphocytes demonstrated higher LD50 values than the B-CLL cells (P = .005 and P = .0008, respectively). The relative sensitivities of the various lymphocyte populations to EB1089 are illustrated in Figure2.
Comparison of the cytotoxicity of EB1089 in B-CLL cells and normal lymphocytes.
Cytotoxicity was compared using LD50 values (± SD) derived from in vitro cultures of malignant B cells, T cells derived from B-CLL samples, and the B- and T-lymphocyte subsets from normal age-matched control samples.
Comparison of the cytotoxicity of EB1089 in B-CLL cells and normal lymphocytes.
Cytotoxicity was compared using LD50 values (± SD) derived from in vitro cultures of malignant B cells, T cells derived from B-CLL samples, and the B- and T-lymphocyte subsets from normal age-matched control samples.
Vitamin D receptor status and the role of these receptors in EB1089-induced apoptosis in B-CLL cells
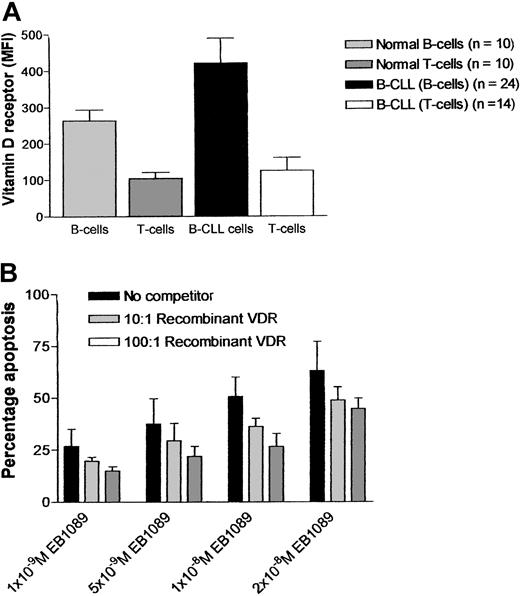
Vitamin D receptor status was assessed in normal and leukemic B lymphocytes and T cells from both healthy age-matched controls (n = 10) and B-CLL patients (n = 24). The expression of vitamin D receptor was markedly higher in B-CLL cells when compared with normal B and T cells as measured by flow cytometry (Figure3A). Competitive inhibition experiments designed to determine whether EB1089-induced apoptosis was mediated through the vitamin D receptor were performed using a recombinant vitamin D receptor at molar ratios of 10:1 and 100:1 (recombinant receptor: EB1089). The experiments showed a consistent reduction in EB1089-mediated apoptosis (range, 19.3%-52.8%; n = 12) but did not conclusively rule out the possibility of an alternative, receptor-independent cell killing mechanism in EB1089-treated B-CLL cells (Figure 3B).
Expression of vitamin D receptor and its involvement in EB1089-mediated apoptosis.
(A) B-CLL cells, T cells from B-CLL samples, and normal B and T lymphocytes were assessed for their expression of vitamin D receptor by immunofluorescent flow cytometric assay. (B) Competitive inhibition studies using recombinant vitamin D receptor EB1089 molar ratios of 10:1 and 100:1 abrogated some of the apoptotic effects of EB1089.
Expression of vitamin D receptor and its involvement in EB1089-mediated apoptosis.
(A) B-CLL cells, T cells from B-CLL samples, and normal B and T lymphocytes were assessed for their expression of vitamin D receptor by immunofluorescent flow cytometric assay. (B) Competitive inhibition studies using recombinant vitamin D receptor EB1089 molar ratios of 10:1 and 100:1 abrogated some of the apoptotic effects of EB1089.
Effect of EB1089 on cell cycle distribution
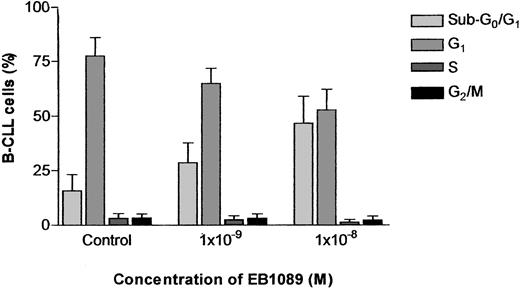
Because the mechanism of EB1089-induced apoptosis in B-CLL cells could be potentially mediated through modulation of the cell cycle, we assessed the effects of EB1089 on the cell cycle distribution of B-CLL cells using flow cytometry. The concentrations of EB1089 used in these experiments caused a concentration-dependent increase in a sub-G0/G1 peak indicative of apoptosis induction. However, when the sub-G0/G1 peak was excluded from the analysis, the percentage of B-CLL cells in G1, S, and G2/M of the cell cycle remained remarkably consistent, indicating that EB1089 did not induce its apoptotic effects through cell cycle perturbations but was capable of inducing apoptosis in B-CLL cells in all phases of the cell cycle (Figure4).
Cell cycle analysis of B-CLL cells cultured with EB1089.
Cells were incubated with the indicated concentrations of EB1089 for 48 hours and then fixed in ice-cold ethanol prior to DNA labeling with propidium iodide. The percentages of cells in the different phases of the cell cycle were measured by flow cytometry. Results represent the means (± SD) of 3 independent experiments.
Cell cycle analysis of B-CLL cells cultured with EB1089.
Cells were incubated with the indicated concentrations of EB1089 for 48 hours and then fixed in ice-cold ethanol prior to DNA labeling with propidium iodide. The percentages of cells in the different phases of the cell cycle were measured by flow cytometry. Results represent the means (± SD) of 3 independent experiments.
EB1089-induced cell death is caspase dependent
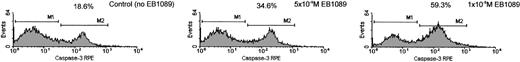
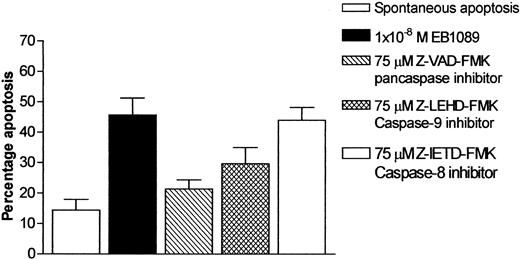
It has recently been reported that caspase-3 is activated during EB1089-induced apoptosis in myeloma cells.27 We therefore set out to investigate whether EB1089 mediated its cell killing effects through the activation of caspase-3 in B-CLL cells. Experiments were conducted in which the active form of caspase-3 was specifically measured using an immunofluorescence-labeled flow cytometric assay. Exposure to EB1089 induced a concentration-dependent activation of caspase-3, as shown in Figure5. Furthermore, only the populations of apoptotic cells showed an increase in activated caspase-3, as confirmed by back gating to forward and side light scatter dotplots and morphologic examination of cytospin preparations. To elucidate further the caspase activation pathway that EB1089 employs in B-CLL cell killing, a series of experiments were performed using caspase inhibitors in order to evaluate their ability to block EB1089-induced apoptosis. The pancaspase inhibitor Z-VAD-FMK at a final concentration of 75 μM significantly inhibited the cytotoxic effects of EB1089 and reduced the numbers of apoptotic cells toward those seen in control cultures incubated without EB1089. The caspase-9 inhibitor (Z-LEHD-FMK) also caused a reduction in EB1089-induced apoptosis at a final concentration of 75 μM, but the caspase-8 inhibitor (Z-IETD-FMK) had little cytoprotective effect, as shown in Figure6.
Caspase-3 activation in B-CLL cells following exposure to EB1089 for 48 hours.
Caspase-3 activity was determined by using a specific active caspase-3-RPE-labeled antibody as described in “Patients, materials, and methods.” The increase in caspase-3 activation (M2) was concentration dependent and was correlated with an increase in apoptotic cell death. Percentages indicate apoptotic cell death at each concentration of EB1089.
Caspase-3 activation in B-CLL cells following exposure to EB1089 for 48 hours.
Caspase-3 activity was determined by using a specific active caspase-3-RPE-labeled antibody as described in “Patients, materials, and methods.” The increase in caspase-3 activation (M2) was concentration dependent and was correlated with an increase in apoptotic cell death. Percentages indicate apoptotic cell death at each concentration of EB1089.
Effect of caspase inhibitors on apoptosis in B-CLL cells following exposure to EB1089 (1 × 10−8 M) for 48 hours.
Apoptosis was quantified using changes in forward and side light scatter. Results are the mean (± SD) of 3 separate experiments. The pancaspase inhibitor, Z-VAD-FMK, and the caspase-9 inhibitor, Z-LEHD-FMK, both demonstrated the ability to abrogate the apoptotic effects of EB1089.
Effect of caspase inhibitors on apoptosis in B-CLL cells following exposure to EB1089 (1 × 10−8 M) for 48 hours.
Apoptosis was quantified using changes in forward and side light scatter. Results are the mean (± SD) of 3 separate experiments. The pancaspase inhibitor, Z-VAD-FMK, and the caspase-9 inhibitor, Z-LEHD-FMK, both demonstrated the ability to abrogate the apoptotic effects of EB1089.
Bcl-2 family expression in B-CLL cells exposed to EB1089
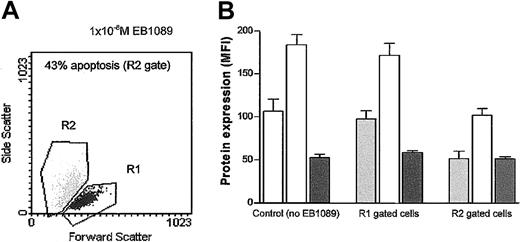
Previous studies have shown that the expression of Bcl-2, Bax, and Mcl-1 significantly influences the ability of B-CLL cells to undergo apoptosis.4,5 28 In this present study their expression was determined in B-CLL cells following 48 hours of incubation in the presence or absence of EB1089. Both Bcl-2 and Mcl-1 were significantly down-regulated in the EB1089-treated cells, whereas Bax expression remained largely unchanged. However, when a gating strategy was employed that enabled discriminatory analysis of viable and apoptotic populations to be performed, only the apoptotic cells showed the down-regulation in Bcl-2 and Mcl-1 expression (Figure7).
Bcl-2 family protein expression in viable and apoptotic subpopulations of B-CLL cells.
Viable and apoptotic cells were identified by using a flow cytometric gating strategy based on changes in forward and side light scatter (A), and a comparison of the Bcl-2 (light gray bars), Mcl-1 (open bars), and Bax protein (dark gray bars) expression in the viable and apoptotic subpopulations of B-CLL cells treated with 1 × 10−8 M EB1089 was made (B). Both Bcl-2 and Mcl-1 were modulated only in apoptotic cells, indicating that changes in their expression is the consequence of apoptosis rather than the cause.
Bcl-2 family protein expression in viable and apoptotic subpopulations of B-CLL cells.
Viable and apoptotic cells were identified by using a flow cytometric gating strategy based on changes in forward and side light scatter (A), and a comparison of the Bcl-2 (light gray bars), Mcl-1 (open bars), and Bax protein (dark gray bars) expression in the viable and apoptotic subpopulations of B-CLL cells treated with 1 × 10−8 M EB1089 was made (B). Both Bcl-2 and Mcl-1 were modulated only in apoptotic cells, indicating that changes in their expression is the consequence of apoptosis rather than the cause.
EB1089 induces p53-independent apoptosis in B-CLL cells
The lack of Bax induction in EB1089-induced apoptotic B-CLL cells was indicative of a p53-independent mechanism of action. Therefore, time course experiments were performed to establish whether p53 protein was induced in EB1089-treated B-CLL cells. In addition, p21 protein expression was also measured, because it is transcriptionally activated in response to p53 up-regulation and may therefore represent an alternative indirect marker of p53 function.29 No evidence of p53 induction was seen in any of the samples tested, and in most cases the expression was not significantly different from the isotype-matched negative control. In addition, p21 expression was not altered following exposure to EB1089 at any of the time points tested. Further evidence for a p53-independent mechanism of action was derived from the fact that one of the patients in this study had previously been reported to have a p53 mutation that was shown to cause defective p53 and p21 induction.30 The sample derived from this patient showed sensitivity to EB1089 indistinguishable from that seen in the other 101 patients in the series. In addition, experiments were also performed in which apoptosis was induced by chlorambucil (3 μM). Under these conditions, most of the B-CLL cells tested showed marked increases in Bax and p21 expression after 8 hours, 12 hours, and 24 hours of exposure to drug. These data demonstrate that most B-CLL cells are capable of mounting a p53 response to an appropriate stimulus and validate the p53-independent nature of EB1089-induced cell killing.
Effect of EB1089 on MAPK activity
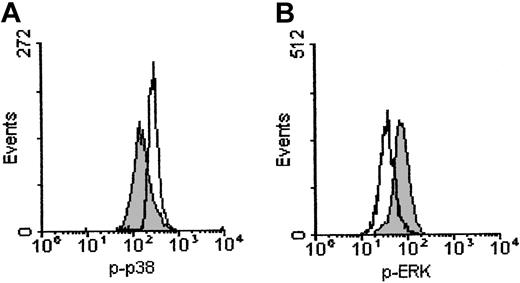
The expression of phosphorylated p38 MAP kinase was markedly increased in B-CLL cells exposed to EB1089 for 12 hours (Figure8A). In addition, the phosphorylated form of ERK was decreased at 12 hours in B-CLL cells treated with EB1089; this occurs before the morphologic changes associated with apoptosis, because it was detected in the gated viable (nonapoptotic) B cells (Figure 8B). Furthermore, these findings were specific for EB1089 because B-CLL cells exposed to chlorambucil showed no increase in phosphorylated p38 MAP kinase or decrease in phosphorylated ERK over the same time period. These findings suggest that EB1089-induced apoptosis may be mediated through the activation of p38 MAP kinase and the suppression of ERK.
Effect of EB1089 on MAP kinase activity in B-CLL cells.
Cells were incubated for 12 hours with 1 × 10−8 M EB1089 and were then stained with FITC-labeled phosphorylation-specific antibodies. (A) The p38 MAP kinase was phosphorylated following exposure to EB1089 (open histogram) when compared with the no-drug controls (shaded histogram). (B) In contrast, phosphorylation of ERK was inhibited by EB1089 (open histogram) when compared with the no-drug controls (shaded histogram).
Effect of EB1089 on MAP kinase activity in B-CLL cells.
Cells were incubated for 12 hours with 1 × 10−8 M EB1089 and were then stained with FITC-labeled phosphorylation-specific antibodies. (A) The p38 MAP kinase was phosphorylated following exposure to EB1089 (open histogram) when compared with the no-drug controls (shaded histogram). (B) In contrast, phosphorylation of ERK was inhibited by EB1089 (open histogram) when compared with the no-drug controls (shaded histogram).
Evaluation of the role of MAP kinases in EB1089-mediated apoptosis
More detailed time course experiments were performed in which B-CLL cells were analyzed for phosphorylation of p38 MAP kinase and dephosphorylation of ERK at 2-hour intervals for 24 hours. Phosphorylation of p38 MAP kinase was observed at all of the early time points (2, 4, 6, 8, and 12 hours) when compared with time 0 controls. Similarly, ERK was shown to be dephosphorylated over the same time period. These events preceded morphologic evidence of apoptosis in these cultures and were not inhibited by the addition of Z-VAD-FMK to the cultures. The p38 MAP kinase inhibitor, SB203580, markedly reduced the phosphorylation of p38 MAP kinase and also prevented the dephosphorylation of ERK at a final concentration of 1 μM. In contrast, the ERK inhibitor, PD98059, caused dephosphorylation of ERK but did not alter the EB1089-induced phosphorylation of p38 MAP kinase (Figure9). Although the addition of Z-VAD-FMK had no effect on the phosphorylation of p38 MAP kinase or the dephosphorylation of ERK, it did cause a marked reduction in apoptosis when these cultures were extended to 48 hours. Similarly, both of the kinase inhibitors caused a reduction in apoptosis in EB1089-treated B-CLL cells over the same time period.
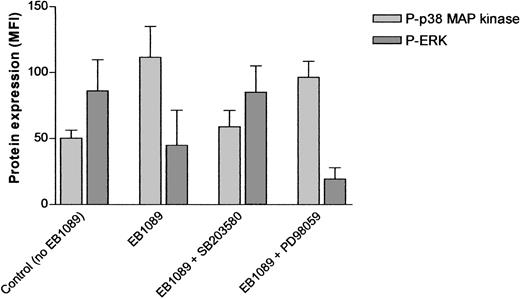
Modulation of MAP kinase activity following exposure to EB1089.
A total of 1 × 10−8 M EB1089 induced marked phosphorylation of p38 MAP kinase and dephosphorylation of ERK after 8 hours in culture. The p38 inhibitor, SB203580, at a final concentration of 1μ M prevented EB1089-induced phosphorylation and downstream dephosphorylation of ERK. In contrast, the ERK inhibitor, PD98059, failed to prevent p38 MAP kinase phosphorylation despite effective inhibition of ERK. Data shown represent the mean (± SD) of duplicate experiments carried out on samples from 12 individual patients.
Modulation of MAP kinase activity following exposure to EB1089.
A total of 1 × 10−8 M EB1089 induced marked phosphorylation of p38 MAP kinase and dephosphorylation of ERK after 8 hours in culture. The p38 inhibitor, SB203580, at a final concentration of 1μ M prevented EB1089-induced phosphorylation and downstream dephosphorylation of ERK. In contrast, the ERK inhibitor, PD98059, failed to prevent p38 MAP kinase phosphorylation despite effective inhibition of ERK. Data shown represent the mean (± SD) of duplicate experiments carried out on samples from 12 individual patients.
Discussion
In this present study we investigated the in vitro effects of the vitamin D3 analog, EB1089, on B-CLL cells taken from 102 patients. EB1089 induced apoptosis in all of the samples tested with a mean LD50 value of 2.1 × 10−8 M (± 1.4 × 10−8 M). Although the cytotoxicity data were impressive, perhaps of more importance was the finding that B-CLL cells appeared to be more sensitive to EB1089 than T cells from the same patient and the B- and T-cell subpopulations derived from age-matched healthy controls. Fludarabine, the most active agent presently available for the treatment of B-CLL, is highly cytotoxic to normal T lymphocytes, resulting in an increased risk of opportunistic infections.31 Differential cytotoxicity is a major goal for the development of novel agents for the treatment of malignancy and, as such, EB1089 shows some promise in this regard. Furthermore, there was no apparent increase in resistance to EB1089 in B-CLL samples derived from previously treated patients when compared with the untreated patient group. This too is a significant finding, because the development of drug resistance in B-CLL is associated with previous treatment and is often pleiotropic in nature.32 The reasons for the differential cytotoxicity are undoubtedly complex, but a recent gene profiling study of B-CLL cells indicated that the vitamin D3 receptor was highly expressed in B-CLL cells when compared with normal B cells, a finding confirmed at the protein level in this study. Therefore, this provides a rationale for the data presented in this paper.21 However, attempts to show that the apoptotic effects of EB1089 were mediated exclusively through the vitamin D receptor were not conclusive and require further investigation. The identification of a suitable blocking antibody or antagonist would certainly precipitate a better understanding of the role of vitamin D receptors in EB1089-induced cell killing.
The fact that p53, p21, and Bax expression was not altered in response to EB1089 indicated that EB1089-induced apoptosis was independent of p53 activation. This was corroborated by the fact that the leukemic cells of one of the patients included in this study had a p53 mutation that resulted in impaired p53 and p21 induction. Samples from this patient showed similar sensitivity to EB1089 when compared with the rest of the patient group, implying that p53 activation was not required for EB1089-induced apoptosis.
Most chemotherapeutic drugs mediate their effects through a p53-dependent apoptotic pathway and, as such, it is common that pleiotropic resistance develops when this pathway becomes impaired.32 33 The mechanisms for this impairment may be multiple, but acquired p53 mutation or dysregulation of the Bcl-2 family has been described. A p53-independent mechanism of apoptosis induction may explain the lack of impact that previous treatment appears to have on B-CLL cell sensitivity to EB1089. In the context of B-CLL, any apoptosis mechanism that circumvents the intracellular overexpression of antiapoptotic members of the Bcl-2 family would be predicted to be useful as part of a treatment strategy. However, EB1089-mediated apoptosis is caspase dependent and appears to work through the mitochondrial pathway of cytochrome c release, caspase-9 activation, and subsequent caspase-3 activation. Bcl-2 family members and possibly other apoptosis-regulating proteins such as the IAP family, at least in principle, could still impact upon this pathway and promote drug resistance.
Previous work has suggested that the response of B-CLL cells to cell death signals is determined, at least in part, by the relative expression of proapoptotic and antiapoptotic Bcl-2 family members and the ability of Bcl-2 to be down-regulated and Bax to be up-regulated.23 It was therefore of considerable interest to investigate whether EB1089 altered the expression of this family of proteins. Bcl-2, Bax, and Mcl-1 expression was measured by flow cytometry both in B-CLL cells cultured with and without EB1089. The initial analysis indicated that both Bcl-2 and Mcl-1 were down-regulated in response to EB1089, whereas Bax expression was not altered. However, when the viable and apoptotic cells were analyzed separately, only the apoptotic cells showed loss of Bcl-2 and Mcl-1 expression. This finding supports the notion that Bcl-2 and Mcl-1 are modulated downward in response to successful apoptotic signaling rather than loss of Bcl-2 and Mcl-1 being a proximal event in apoptosis induction. If the latter were the case, loss of Bcl-2 and Mcl-1 should be evident in a proportion of the viable cells too; that was not evident in this study.
The role of MAP kinase activation in apoptosis has been the subject of many investigations, and it appears that apoptosis induced by a variety of agents may be dependent upon, or regulated by, up-regulation of p38 MAP kinase.34,35 This activation may or may not be associated with activation of Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) depending on the cell type and apoptotic stimuli.35-38 Another MAP kinase, ERK, has been shown to be activated in response to growth factor stimuli and is related to cell survival.35,37 In this context it is of interest that protein kinase C inhibitors inhibited ERK phosphorylation at an early time point during HL-60 cell apoptosis.39Further support for this idea was recently published by Barragan et al, who demonstrated a role for protein kinase C and phosphatidylinositol-3 kinase pathways in the survival of B-CLL cells.40 In this study we found that EB1089 induced a reduction in ERK phosphorylation and a marked increase in p38 MAP kinase phosphorylation in B-CLL cells prior to the morphologic changes associated with apoptosis. These findings are consistent with those previously reported in the myeloma cell line, NCI-H929,27 and indicate that EB1089 induces its effects, at least in part, through the inhibition of ERK-mediated cell survival signaling. Furthermore, the p38 MAP kinase inhibitor, SB203580, prevented phosphorylation of p38 MAP kinase, inhibited the dephosphorylation of ERK, and partially abrogated the apoptotic effects of EB1089. Therefore, it seems likely that EB1089-induced apoptosis is initiated through phosphorylation of p38 MAP kinase, leading to downstream inhibition of ERK. The ERK inhibitor, PD98059, failed to inhibit the phosphorylation of p38 MAP kinase in response to EB1089 despite effective inhibition of ERK, adding further evidence to the upstream position of p38 MAP kinase as a target for EB1089-induced apoptosis.
In conclusion, this study showed that the vitamin D3 analog, EB1089, potently induces apoptosis in B-CLL cells via a novel signal transduction mechanism. This, added to the fact that its mode of action is p53 independent, makes EB1089 a potentially useful agent for the treatment of B-CLL, particularly in those patients who are refractory to conventional chemotherapeutic options. Investigations into the effects of other vitamin D3 analogs are currently being undertaken in our laboratory as well as further elucidation of the role of the vitamin D receptor in potentiating apoptosis in B-CLL cells.
We are grateful to Leo Pharmaceutical Products (Ballerup, Denmark) for the generous gift of the EB1089.
Prepublished online as Blood First Edition Paper, November 21, 2002; DOI 10.1182/blood-2002-07-1984.
Supported in part by a grant from the Welsh Bone Marrow Transplant Research Fund.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Chris Pepper, Department of Haematology, Llandough Hospital, Penlan Road, Penarth, Vale of Glamorgan CF64 2XX, United Kingdom; e-mail:chrisp@llanhaem.demon.co.uk.