Mutations in the gene of the hepatic glucose-6-phosphate transporter cause glycogen storage disease type 1b. In this disease, the altered glucose homeostasis and liver functions are accompanied by an impairment of neutrophils/monocytes. However, neither the existence of a microsomal glucose-6-phosphate transport, nor the connection between its defect and cell dysfunction has been demonstrated in neutrophils/monocytes. In this study we have characterized the microsomal glucose-6-phosphate transport of human neutrophils and differentiated HL-60 cells. The transport of glucose-6-phosphate was sensitive to the chlorogenic acid derivative S3483,N-ethylmaleimide, and 4,4′-diisothiocyanostilbene-2,2′-disulfonic acid, known inhibitors of the hepatic microsomal glucose-6-phosphate transporter. A glucose-6-phosphate uptake was also present in microsomes from undifferentiated HL-60 and Jurkat cells, but it was insensitive to S3483. The treatment with S3484 of intact human neutrophils and differentiated HL-60 cells mimicked some leukocyte defects of glycogen storage disease type 1b patients (ie, the drug inhibited phorbol myristate acetate–induced superoxide anion production and reduced the size of endoplasmic reticulum Ca2+ stores). Importantly, the treatment with S3484 also resulted in apoptosis of human neutrophils and differentiated HL-60 cells, while undifferentiated HL-60 and Jurkat cells were unaffected by the drug. The proapoptotic effect of S3483 was prevented by the inhibition of nicotinamide adenine dinucleotide phosphate oxidase or by antioxidant treatment. These results suggest that microsomal glucose-6-phosphate transport has a role in the antioxidant protection of neutrophils, and that the genetic defect of the transporter leads to the impairment of cellular functions and apoptosis.
Introduction
Glucose-6-phosphatase (G6Pase) catalyses the common terminal reaction of gluconeogenesis and glycogenolysis, hence it plays a major role in the maintenance of blood glucose homeostasis.1,2 G6Pase is expressed mainly in the liver and in the kidney where it is associated with the endoplasmic reticulum (ER) and functions as a multicomponent system.2 The system is composed of the enzyme protein with an intraluminal active site and transporters for the entry of substrate glucose-6-phosphate (G6P) and for the exit of the products, phosphate and glucose.2,3Both the enzyme and glucose-6-phosphate transporter (G6PT) are already known at the molecular level.2,4,5 The deficiency of the G6Pase activity causes type 1 glycogen storage disease (GSD-1).2,6 The genetic deficiency of the enzyme protein is termed GSD type 1a,2,7 and mutations affecting the G6PT gene cause GSD type 1b.2,5,8-13 While the clinical feature in GSD-1a is dominated by metabolic alterations and hepatorenal symptoms, in GSD-1b polymorphonuclear neutrophils (PMNs) and monocytes are also affected.2,7 Both neutropenia and functional defects of PMNs and monocytes have been observed in most GSD-1b patients, who are therefore affected by severe infectious complications in addition to the disturbance of glucose homeostasis.2,7,14 PMN defects in GSD-1b patients include a reduction in respiratory burst, chemotaxis, phagocytosis, and Ca2+ signaling.14-19 Moreover, alterations of several other biochemical parameters—glucose phosphorylation, calcium mobilization, and hexose uptake and transport—have been described as the possible background of the functional defects.20-22The etiology of neutropenia and PMN dysfunction is not known. However, bone marrow aspirates performed on some GSD-1b patients with neutropenia have revealed hypocellularity with a myelocyte-erythrocyte ratio of less than 3:1 and maturation arrest beyond the myelocytic stage.14 15 These findings suggest that, at least in a portion of the patients, impaired granulopoiesis and/or increased apoptosis may underlie the observed neutropenia.
Since PMNs have no detectable G6Pase activity,2,7 in these cells G6PT should have a different role than that in the liver where it is functionally coupled to the G6Pase enzyme. Although the presence of proteins encoded by the G6PT gene has not yet been demonstrated, the mRNA(s) are present in PMNs.10 Moreover, microsomal G6P transport has never been investigated in leukocytes. It has been hypothesized that G6PT might have a sort of function as a G6P sensor11 or it could favor calcium sequestration in the ER lumen.23 Such roles have not been proven and would not explain why other nonhepatic cells expressing G6PT are apparently unaffected in GSD-1b.
In the present work 2 topics have been addressed. Does a functional G6P transporter exist in the ER of PMNs? If so, can a connection be found between the defective G6P transport and the functional abnormalities of the cells? Since PMNs from GSD-1b patients cannot be obtained in the amount necessary for the measurement of their microsomal G6P transport, the transport defect was modeled by the addition of the chlorogenic acid derivative compound S3483, which is a highly specific inhibitor of liver G6PT.24 The results demonstrate that human PMNs and differentiated HL-60 cells possess a microsomal G6P transport similar to that of the liver. The bona fide inhibition of the transporter, as a result of treating intact cells with S3483, led to the inhibition of superoxide anion production, the impairment of ER Ca2+ pool, as well as to an antioxidant-sensitive apoptosis. The results suggest that G6PT is necessary for the antioxidant protection of the ER lumen of PMNs and that, in its absence, pro-oxidant effects can lead to functional defects and apoptosis.
Materials and methods
Cells
Human PMNs were prepared from blood of healthy volunteers by dextran sedimentation followed by Percoll gradient centrifugation according to the procedure described in Hjorth et al.25Contaminating red cells were removed by hypotonic lysis. Cells were finally resuspended in a Hanks balanced salt solution (HBSS) with the following composition: 138 mM NaCl, 5.3 mM KCl, 0.44 mM KH2PO4, 4 mM NaHCO3, 0.3 mM Na2HPO4, 1.26 mM CaCl2, 0.5 mM MgCl2, 0.4 mM MgSO4, and 5.6 mM glucose. Preparations contained more than 95% PMNs and cell viability (evaluated by trypan blue exclusion method) exceeded 97%.
Human myeloid HL-60 cells (supplied by Chiron-Biocine, Siena, Italy) were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum, penicillin (50 units/mL), streptomycin (50 μg/mL), and L-glutamine (2 mM) at 37°C in a humidified atmosphere of 5% CO2, 95% air. Differentiation of HL-60 cells was performed by including 1.25% (vol/vol) dimethyl sulfoxide (DMSO) for 6 days in the culture medium.26 The differentiated state was verified by morphologic inspection (results not shown) and by measuring the superoxide anion production upon phorbol myristate acetate (PMA; 1 μg/mL) stimulation. Cell viability (evaluated by the trypan blue exclusion method) exceeded 95%, both in the presence and absence of DMSO.
Preparation of microsomal fractions
Microsomes were prepared from human PMNs, HL-60 cells, and Jurkat cells as reported in Sumimoto et al.27 Briefly, cells were resuspended (2 × 106 cells/mL) in 0.34 M sucrose, 10 mM HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid; pH 7.2), and 0.5 mM MgCl2, including a cocktail of protease inhibitors (1 mM phenylmethylsulfonyfluoride, 1 μM pepstatin A, 1 μM leupeptin, and 1 μg/mL aprotinin), and sonicated 5 times for 15 seconds at 0°C to 4°C. After the addition of 1 mM EDTA (ethylenediaminetetraacetic acid), cell homogenates were centrifuged for 10 minutes at 1000g. Supernatants were then centrifuged for 30 minutes at 20 000g. Microsomes were then recovered by ultracentrifugation for 60 minutes at 150 000g. Rat liver microsomes (from male Sprague-Dawley rats) were prepared as reported in Bánhegyi et al.28Microsomal fractions were resuspended in 100 mM KCl, 20 mM NaCl, 1 mM MgCl2, and 20 mM Mops (3-[N-morpholino]propanesulphonic acid; pH 7.2), including the cocktail of protease inhibitors. The suspensions were rapidly frozen and maintained under liquid N2 until used.
G6P uptake measurements
Microsomal G6P uptake was measured essentially as previously reported.28,29 Microsomes (0.5 mg protein/mL) were incubated in the KCl/Mops buffer in the presence of 1 mM G6P plus D-[14C(U)]G6P (15 μCi/mL [0.555 MBq]) at 22°C. At the indicated time intervals, samples (0.1 mL) were rapidly filtered through cellulose acetate/nitrate filter membranes (pore size 0.22 μm), and filters were washed with 4 mL of HEPES (20 mM) buffer, pH 7.2, containing 250 mM sucrose and 1 mM DIDS (4,4′-diisothiocyanostilbene-2,2′-disulfonic acid). To distinguish the intravesicular and the bound radioactivity, either the pore-forming antibiotic alamethicin (0.02 mg/mL) or detergent deoxycholate (DOC, 0.1%) was added to the incubation mixture.29 The alamethicin/DOC-releasable portion of radioactivity was regarded as intravesicular. In some experiments of G6P uptake, filters were treated after wash with ZnSO4/Ba(OH)2 to separate intravesicular glucose from G6P according to Bánhegyi et al.28 The radioactivity associated to microsomes retained by filters was measured by liquid scintillation counting.
G6Pase activity was measured in native and alamethicin/DOC-permeabilized microsomes at 5 minutes of incubation in the KCl/Mops buffer at 22°C on the basis of [14C]glucose production from [14C]G6P as detailed in Bánhegyi et al.28
Assay of superoxide anion production
Superoxide anion generation by intact cells was measured by a ferricytochrome c reduction assay30 with minor modification. The cells (1.0 × 106/mL) were preincubated in a substrate solution (50 μM ferricytochromec, 5.6 mM D-glucose, and 0.5 mM CaCl2 in the HBSS medium) for 5 minutes at 37°C, and the assay was initiated by the addition of 1 μg/mL PMA. Parallel samples including 30 μM of superoxide dismutase were also run as blanks. Superoxide anion generation by the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity in a cell-free system was measured essentially as in Bromberg and Pick.31 Cell lysates (2.5 × 106 cells/mL in HBSS medium) were preincubated in the presence of 10 μM flavin adenine dinucleotide, 50 μM ferricytochrome c, and 200 μM NADP+ for 5 minutes at 37°C, and the assay was initiated by the addition of 5 mM G6P. Rate of superoxide anion generation was measured by continuously recording the increase in absorbance at 550 nm using a Varian Cary 50 Bio spectrophotometer (Varian, Torino, Italy).
Measurement of cytosolic-free Ca2+ concentration
Cytosolic-free Ca2+ concentration ([Ca2+]i) was measured in cells loaded with the fluorescent indicator fura-2 as previously described.32
Evaluation of apoptosis
Cells (2 × 106) were suspended in 1 mL of the complete RPMI 1640 culture medium and incubated for 60 minutes at 37°C, under a humidified atmosphere of 5% CO2 and 95% air, in the presence of the different drugs as indicated in the individual experiments. At the end of the incubation, cells were recovered by centrifugation and treated (10 minutes at 22°C) with 0.1 mL of serum-free RPMI 1640 medium containing the 2-integrin reagent VIM12 (1 μg/mL) to reveal phosphatidylserine exposure on cell surface, and propidium iodide (20 μg/mL) to stain nuclei. Cells were then rapidly centrifuged (at 1800g for 2 minutes), resuspended in 0.1 mL of serum-free RPMI, placed on a coverslip, and immediately observed with a real-time confocal microscope. A confocal laser imaging system ViewScan DCV-250 (Bio-Rad, Milano, Italy) mounted on a Nikon (Milano, Italy) Eclipse 300 inverted microscope and equipped with an Argon laser was used. Simultaneous excitation at 488 and 514 nm allowed the visualization of the green fluorescence of the reagent VIM12 and of the red fluorescence of the nucleic acid–propidium iodide complex. A minimum of 200 cells was scored for each sample. Apoptotic cells were identified on the basis of the phosphatidylserine exposure on the plasma membrane surface and on morphologic changes. While the phosphatidylserine reagent VIM12 stained all the cells referred to here as apoptotic, some were also permeable to propidium iodide. However, we also considered these cells as apoptotic, since nuclei were fragmented into condensed round-shape bodies, which were eventually located inside cell evaginations. Cells stained only by propidium iodide were not considered apoptotic. These cells never exceeded 5% independently of the treatment used and had no gross changes in nuclear morphology.
Apoptosis of HL-60 cells undergoing DMSO-induced differentiation was evaluated by measuring cytoplasmic histone-associated DNA fragments with a Cell Death Detection enzyme-linked immunosorbent assay kit (Roche Diagnostics, Milan, Italy).
Measurement of cell permeability to the compound S3483
HL-60 cells (3 × 106/mL) in complete RPMI 1640 culture medium were incubated for 5 minutes at 37°C in the presence of S3483 (50 or 200 μM), recovered by centrifugation (at 1800g for 2 minutes), washed twice (at 1800g for 2 minutes), and resuspended with serum-free RPMI 1640 medium at a concentration of 6 × 106 cells per milliliter. After the addition to cell suspension of an equal volume of KCl 150 mM/HCl 0.1 N (pH 1), S3483 was extracted with 5 volumes of ethyl acetate. The organic layer was dried at 50°C under N2 stream, the residue dissolved in DMSO and analyzed by HPLC. Chromatographic conditions were as follows: column, Waters Symmetrie Shield C18 150 × 3.9 mm; mobile phase, 28% acetonitril in ammonium acetate 0.5%; detection, ultraviolet detector at 314 nm. A Waters Alliance high-performance liquid chromatography apparatus equipped with Millennium 2000 software was used (Waters, Milan, Italy).
Results
Microsomal G6P transport in human neutrophils
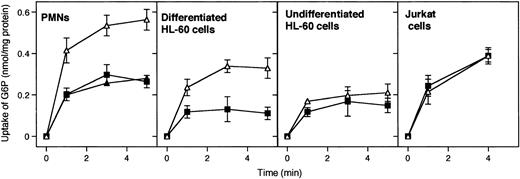
G6P uptake into microsomal vesicles was investigated by using radiolabeled G6P and a rapid filtration method. Figure1 illustrates that G6P uptake into microsomal vesicles was present in human PMNs, undifferentiated and differentiated HL-60 cells, and Jurkat T cells. However, the microsomal G6P uptake was sensitive to the G6PT inhibitor S3483 only in PMNs and differentiated HL-60 cells (Figure 1, upper panels). Little or no inhibition by S3483 was present in undifferentiated HL-60 and Jurkat cells. S3484 concentrations higher than 10 μM did not further inhibit microsomal G6P transport in all cases (see Figure 1 for PMNs; data not shown for differentiated and undifferentiated HL-60 and Jurkat cells).
Effect of the compound S3483 on microsomal G6P uptake.
Microsomes from the indicated cell types were preincubated with solvent alone (1 μL/mL ethanol, ▵), 10 μM S3483 (▪), or 20 μM S3483 (▴) for 5 minutes at 22°C and G6P uptake was then assayed radioisotopically by rapid filtration. Values are means ± SEMs of 3 to 5 independent experiments.
Effect of the compound S3483 on microsomal G6P uptake.
Microsomes from the indicated cell types were preincubated with solvent alone (1 μL/mL ethanol, ▵), 10 μM S3483 (▪), or 20 μM S3483 (▴) for 5 minutes at 22°C and G6P uptake was then assayed radioisotopically by rapid filtration. Values are means ± SEMs of 3 to 5 independent experiments.
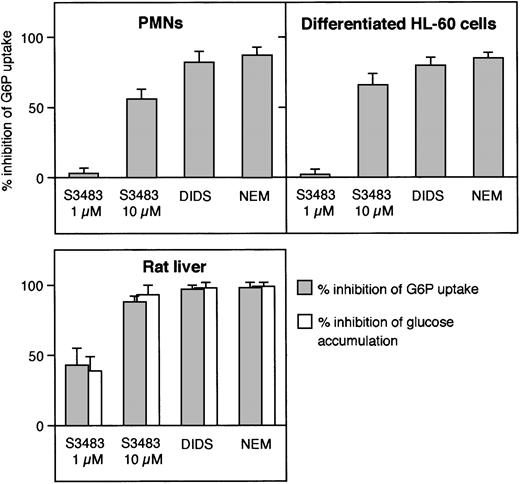
In Figure 2, we have compared the effects of typical inhibitors of G6P transport—S3483,24DIDS,33 and NEM34—in microsomes prepared from PMNs, differentiated HL-60 cells, and rat liver. All 3 inhibitors reduced the level of the steady-state vesicular accumulation of G6P in the 3 microsomal preparations. Higher concentrations of the 3 compounds did not further inhibit G6P accumulation in any case (data not shown; see Figure 1 for PMNs).
Comparison of the effect of G6PT inhibitors in microsomes from human PMNs, differentiated HL-60 cells, and rat liver.
Microsomes from the indicated cell types were preincubated with 10 μM S3483 for 5 minutes at 22°C, 0.5 mM DIDS for 10 minutes at 22°C, or treated with 1 mM N-ethylmaleimide (NEM) as described in Fulceri et al.33 G6P uptake (and the case of liver microsomes, also intravesicular glucose accumulation) was then assayed radioisotopically by rapid filtration. Data are expressed as percent of inhibition. Values are means ± SEMs of 3 to 5 independent experiments.
Comparison of the effect of G6PT inhibitors in microsomes from human PMNs, differentiated HL-60 cells, and rat liver.
Microsomes from the indicated cell types were preincubated with 10 μM S3483 for 5 minutes at 22°C, 0.5 mM DIDS for 10 minutes at 22°C, or treated with 1 mM N-ethylmaleimide (NEM) as described in Fulceri et al.33 G6P uptake (and the case of liver microsomes, also intravesicular glucose accumulation) was then assayed radioisotopically by rapid filtration. Data are expressed as percent of inhibition. Values are means ± SEMs of 3 to 5 independent experiments.
In liver microsomes the G6P taken into the lumen can be converted to glucose (and inorganic phosphate) by the intraluminal G6Pase activity. In transport assays using [14C]G6P, the radioactivity accumulated in the microsomal lumen is therefore a mixture of [14C]glucose and [14C]G6P28,29 because of the high hydrolytic rate of the G6Pase enzyme and the relatively low rate of vesicular efflux of glucose.28,29,35 Consistently, treatment of liver microsomes with G6P transport inhibitors resulted in a decrease in the accumulation of both [14C]G6P and [14C]glucose (Figure 2). In agreement with previous knowledge (Chen and Burchell7), neither [14C]glucose accumulation nor measurable G6Pase activity was present in microsomes from PMNs and HL-60 cells (not shown).
The effect of the inhibition of microsomal G6P transport in PMNs and HL-60 cells
Since PMNs appear to possess a liverlike microsomal G6P transport activity, to model the PMN defects of GSD-1b we treated PMNs and HL-60 cells with S3483. Chlorogenic acid derivatives have been shown to selectively inhibit ER G6P transport, and hence G6Pase activity, not only in isolated microsomes,24 but also in intact hepatocytes and in vivo.36-38 A well-documented defect in PMNs from GSD-1b patients is the reduced ability to produce superoxide anions upon stimulation with PMA.17 18 As shown in Figure3, the pretreatment for 60 minutes of PMNs and differentiated HL-60 cells with 50, 100, or 200 μM S3483 resulted in a dose-dependent inhibition of PMA-stimulated superoxide anion generation in both cell types. The possibility that the drug, or any of the enzymes of the pentose phosphate pathway required for NADPH formation, directly inhibited NADPH oxidase activity, or that the drug reacted with superoxide anions was unlikely. Indeed, no inhibitory effect by S3483 on superoxide anion generation was observed in a cell-free system supplemented with G6P and NADP+(“Materials and methods”). In particular, lysates of differentiated HL-60 cells in the presence of NADP+ and G6P produced 4.1 ± 0.2 and 4.0 ± 0.2 nmol of superoxide anion per minute per milligram protein (means ± SEs, n = 4) with or without 200 μM S3484, respectively.
Effect of the S3483 compound on superoxide anion production in human PMNs and differentiated HL-60 cells.
Cells (1 × 106/mL) were pretreated (1 hour at 37°C) with solvent alone (10 μL/mL ethanol, ○), or with 50 (▿), 100 (▵), or 200 (■) μM S3484. Superoxide anion production was then induced by adding 1 μg/mL PMA. Values shown are means of 4 to 6 independent experiments; SEMs are shown for selected points only.
Effect of the S3483 compound on superoxide anion production in human PMNs and differentiated HL-60 cells.
Cells (1 × 106/mL) were pretreated (1 hour at 37°C) with solvent alone (10 μL/mL ethanol, ○), or with 50 (▿), 100 (▵), or 200 (■) μM S3484. Superoxide anion production was then induced by adding 1 μg/mL PMA. Values shown are means of 4 to 6 independent experiments; SEMs are shown for selected points only.
An impairment of cell Ca2+ signaling has also been previously observed in PMNs from GSD-1b patients.17,21,39Here we observed that treatment with S3483 causes a reduction in the ER Ca2+ pool size in differentiated HL-60 cells, but not in undifferentiated HL-60 or Jurkat cells (not shown). ER Ca2+ pool size was assumed to be reflected by the transient increase in [Ca2+]i induced by adding a supramaximal dose (0.5 μM) of the inhibitor of ER Ca2+ATPases thapsigargin to cells maintained in a Ca2+-free medium.32 In differentiated HL-60 cells treated for one hour with 0, 50, 100, and 200 μM S3483,thapsigargin induced peak [Ca2+]ielevations (nM) of 97 ± 3.9, 75 ± 4.8, 49 ± 5.7, and 25 ± 4.2 (means ± SEs, n = 4), respectively.
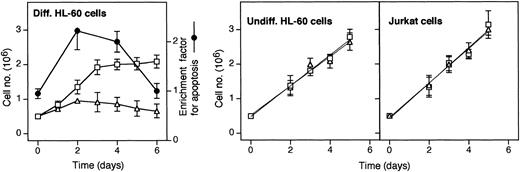
Neutropenia in GSD-1b patients is likely due to a maturation arrest beyond the myelocytic stage.15 Therefore, we investigated the effect of S3483 in a model system of promyelocytic differentiation. To this end, HL-60 promyelocytic cells were differentiated by including DMSO in the culture medium, both in the presence and absence of S3483. As shown in Figure 4, the growth rate of HL-60 undergoing DMSO-induced differentiation was markedly reduced by including 100 μM S3483 in the culture medium. The decrease in growth rate was associated with an increased apoptotic index in S3483-treated cultures. At days 2 and 4, the apoptotic index was at least doubled in the presence of the drug compared with controls (Figure 4, dotted line). On the contrary, the inclusion of S3483 in the culture media did not affect the growth rate of both undifferentiated HL-60 and Jurkat T cells (Figure 4).
S3483 compound inhibits cell growth and induces apoptosis in HL-60 cells undergoing differentiation.
In the left panel, HL-60 cells were cultured in RPMI 1640 complete medium containing 1.25% (vol/vol) DMSO to promote cell differentiation, and including also 100 μM S3483 ▵ or solvent alone (10 μL/mL ethanol, ■); the apoptotic ratio of S3483-treated versus untreated cells, calculated by measuring the levels of cytoplasmic histone-associated DNA fragments with a Cell Death Detection ELISAplus (Roche) kit, is also shown (●). In the middle and right panels, HL-60 and Jurkat cells were cultured in RPMI 1640 complete medium containing 100 μM S3483 ▵ or solvent alone (10 μL/mL ethanol, ■). Values are means ±SEM of 3 to 5 independent experiments.
S3483 compound inhibits cell growth and induces apoptosis in HL-60 cells undergoing differentiation.
In the left panel, HL-60 cells were cultured in RPMI 1640 complete medium containing 1.25% (vol/vol) DMSO to promote cell differentiation, and including also 100 μM S3483 ▵ or solvent alone (10 μL/mL ethanol, ■); the apoptotic ratio of S3483-treated versus untreated cells, calculated by measuring the levels of cytoplasmic histone-associated DNA fragments with a Cell Death Detection ELISAplus (Roche) kit, is also shown (●). In the middle and right panels, HL-60 and Jurkat cells were cultured in RPMI 1640 complete medium containing 100 μM S3483 ▵ or solvent alone (10 μL/mL ethanol, ■). Values are means ±SEM of 3 to 5 independent experiments.
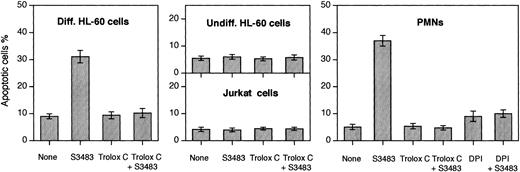
A logical explanation for the data of Figure 4 is that S3483 is selectively proapoptotic in differentiated HL-60 cells. This was indeed the case as demonstrated in Figure 5. Treating differentiated HL-60 cells with 100 μM S3484 did result in a large increase in the number of apoptotic cells, but it had no effect on undifferentiated HL-60 cells and Jurkat cells (Figure 5). The concomitant treatment of differentiated HL-60 cells for 60 minutes with PMA (1 μg/mL) and S3483 resulted in an approximate 80% increase in the number of apoptotic cells (data not shown).
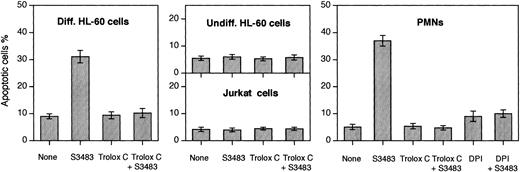
S3483 compound induces apoptosis in differentiated HL-60 cells and PMNs, and DPI and Trolox C prevent the apoptosis induction.
PMNs, differentiated and undifferentiated HL-60 cells, and Jurkat cells (2 × 106) were incubated (at 37°C in 5% CO2/95% air for 60 minutes) in the complete RPMI 1640 culture medium containing the solvent alone (10 μL/mL ethanol) or 100 μM S3483. Where indicated, 10 μM Trolox C or 10 μM DPI was also added to the cell suspension. Cells stained with the 2-integrin reagent VIM12 (to reveal phosphatidylserine exposure on cell surface) and propidium iodide were observed with a real-time confocal microscope. Apoptotic cells were identified on the basis of the phosphatidylserine exposure on the plasma membrane surface and on morphologic changes. A minimum of 200 cells was scored for each sample. Values are means ± SEMs of 3 to 5 independent experiments.
S3483 compound induces apoptosis in differentiated HL-60 cells and PMNs, and DPI and Trolox C prevent the apoptosis induction.
PMNs, differentiated and undifferentiated HL-60 cells, and Jurkat cells (2 × 106) were incubated (at 37°C in 5% CO2/95% air for 60 minutes) in the complete RPMI 1640 culture medium containing the solvent alone (10 μL/mL ethanol) or 100 μM S3483. Where indicated, 10 μM Trolox C or 10 μM DPI was also added to the cell suspension. Cells stained with the 2-integrin reagent VIM12 (to reveal phosphatidylserine exposure on cell surface) and propidium iodide were observed with a real-time confocal microscope. Apoptotic cells were identified on the basis of the phosphatidylserine exposure on the plasma membrane surface and on morphologic changes. A minimum of 200 cells was scored for each sample. Values are means ± SEMs of 3 to 5 independent experiments.
The S3483 compound also promoted apoptosis of human PMNs (Figure 5). To investigate the mechanism of the apoptosis, the level of reactive oxygen species (ROS) was diminished by the block of NADPH oxidase activity or by antioxidant treatment. The NADPH oxidase inhibitor diphenylene iodonium (DPI) rendered PMNs and differentiated HL-60 cells resistant to the proapoptotic effect of S3483. The S3483-induced apoptosis was also prevented by adding the antioxidant compound Trolox C (a water-soluble analog of vitamin E; Sigma, St Louis) to the culture medium.
In intact cells, the active concentrations of the G6PT inhibitor S3483 were at least 10 times higher (Figures 3-5) than those required to inhibit G6P transport in microsomes isolated from PMNs and differentiated HL-60 cells (Figures 1-2). However, this discrepancy could be due to a relatively slow uptake of the drug in these cells. Consistent with this possibility, we observed that HL-60 cells incubated for 5 minutes in the presence of 50 or 200 μM S3483 took up 15.5 ± 1.3 and 23.1 ± 2.2 pmoles/mg cellular protein of the drug (means ± SEs, n = 3), respectively. These values correspond to 3.1 and 4.6 μM intracellular concentrations of the drug assuming a cell water content of 5 μL per milligram protein. After longer incubation times, confident measurements of cell uptake of the drug cannot be obtained, because of the reduced cell integrity.
Discussion
Neutropenia and functional defects in neutrophil and monocyte activities have been repeatedly described in patients with GSD-1b. Although several alterations of cell metabolism and signaling have been reported, the connection between them and the genetic background of the disease has not been clarified. Therefore, in the present work we have addressed 2 topics: the existence of a functional G6PT in the ER of PMNs, and the connection between the defective G6P transport and the functional abnormalities of the cells.
The present study demonstrates the existence of a liverlike G6P transport in microsomes from human PMNs and from differentiated HL-60 cells. The transport of G6P has been reported in ER fractions from various nonhepatic cells,23,29,40 but it has not been extensively characterized. It seems that the characteristics of ER G6P transport in extrahepatic tissues are different from those in liver. In microsomes from fibroblasts and HeLa cells, G6P transport does not show sensitivity to pH 5 pretreatment and chlorogenic acid addition.29 Moreover, these microsomes can transport other phosphoesters in addition to G6P.29 Microsomes from mouse pancreatic islet,41 as well as from undifferentiated HL-60 and Jurkat cells (present study), possess a G6P transport, which is insensitive to the chlorogenic acid derivative S3483. On the contrary, microsomal G6P transport in differentiated HL-60 cells and in human PMNs can be inhibited by S3483, NEM, and DIDS, prototypic inhibitors of liver G6PT (present study). An S3483-inhibitable liverlike microsomal G6P transport has been clearly demonstrated in the kidney,24 which possesses the G6Pase system and is impaired in GSD-1b.2 7 Therefore, it appears that the functional expression of G6PT is indeed restricted to cells/tissues that are primarily impaired in GSD-1b patients (ie, liver, kidney, and PMNs).
The addition of S3483 to intact PMNs and differentiated HL-60 cells resulted in a decrease in PMA-stimulated superoxide anion production and in the ER Ca2+ pool size, as well as in an increased proportion of apoptotic cells. Defects in superoxide anion production and Ca2+ signaling are characteristic of GSD-1b PMNs. On the other hand, the maturation arrest beyond the myelocyte stage of GSD-1b patients could be ascribed to an increase in apoptosis.
Since S3483-dependent apoptosis was prevented by decreasing the level of ROS (either by the inhibition of NADPH oxidase or by antioxidant treatment), one can suppose that a defective ER G6P transport, combined with the endogenous ROS production, is responsible for the increase in apoptosis. It is worth mentioning that granulocyte colony-stimulating factor, used in GSD-1b therapy,14,42 promotes ascorbate uptake and accumulation in PMNs.43 Its beneficial effect might be exerted, at least in part, by an antioxidant-dependent antiapoptotic mechanism. One can also suppose that the proapoptotic effect in GSD-1b PMNs and in PMNs treated with the G6PT inhibitor S3483 is simply due to an overproduction of ROS. However, this is not consistent with the fact that the respiratory burst is defective in GSD-1b PMNs, and that S3483 inhibits superoxide anion production (Figure 3). On the other hand, it is unlikely that the inhibition of superoxide production alone plays a causative role in the S3483-dependent apoptosis; instead, it can be regarded as an early manifestation of the apoptosis.
Alternatively, a self-defense mechanism may be impaired in GSD-1b PMNs. PMNs undergo spontaneous apoptosis due to the production of ROS.44,45 Consequently, redox signaling should be an important determinant of neutrophil apoptosis.46 Although present knowledge of ER redox homeostasis and metabolism is limited, a logical explanation can be that defective G6PT transport alters the luminal redox status in the ER. In liver microsomes, both NADP+-dependent dehydrogenases47-50 and nicotinamide nucleotides51 are present in the lumen; therefore, G6P can be metabolized not only by G6Pase but also by the intraluminal hexose-6-phosphate dehydrogenase.52,53The latter enzyme generates intraluminal NADPH, supporting local reducing reactions. Hexose-6-phosphate dehydrogenase is also present in microsomes from extrahepatic tissues,54 including PMNs (R.F., G.B., and A.B., unpublished observation, March 2002). Therefore, G6PT deficiency may result in a redox shift toward oxidizing conditions in the ER lumen. The S3483-induced impairment of the ER Ca2+ pool (present study) and the impairment of ER-related Ca2+ signaling in GSD-1b PMNs17,21 might also reflect an ER oxidative stress. Various agents disturbing the homeostasis of the ER lumen are known to be proapoptotic, and the ER stress may propagate and lead to cell death. In accordance with this assumption, caspase activation and apoptosis in HL-60 cells caused by oxidative challenges can be prevented by ascorbate and other antioxidants.46,55,56 Very recent observations show that during the granulocytic differentiation, an ER remodeling occurs, affecting the size of the ER and the expression of its chaperones.57 It is tempting to suppose that the defective G6PT hampers the remodeling program by causing maturation arrest and an early apoptosis of PMNs in GSD-1b patients.
The present findings may help to improve the therapy of GSD-1b patients. Antioxidant treatment (eg, tocopherol administration) may moderate neutropenia without adverse effects. It should be noted that the antiapopototic concentration of the water-soluble analog of vitamin E used here (10 μM) is in the physiologic range. For example, a value of 27 μM for tocopherol plasma concentration has been recently reported in humans;58 this concentration can be at least doubled by oral administration of the vitamin.58On the other hand, our observations do not restrict the potential use of chlorogenic acid derivatives as possible candidates for the treatment of type 2 diabetes. Liver G6PT is more sensitive to S3483 than the PMN one. Moreover, human PMNs take up the drug slowly, which is presumably due to the low representation or absence of oatp1, the transporter responsible for its uptake in the liver.59 For therapeutic intervention, chlorogenic acid derivatives can be used in concentrations far lower than those used in the present study.
In conclusion, this report demonstrates the functional presence of G6PT in microsomes from human PMNs and HL-60 cells. The results suggest that microsomal glucose-6-phosphate transport has a role in the antioxidant protection of neutrophils, and that the genetic defect of the transporter leads to impaired cellular functions and apoptosis.
We are grateful to Prof E. Ligeti (Semmelweis University, Budapest) and Prof E. van Schaftingen (Catholic University of Louvain) for their helpful advice. S3483 was kindly provided by Aventis Pharma Deutschland, Metabolic Diseases.
Prepublished online as Blood First Edition Paper, November 7, 2002; DOI 10.1182/blood-2002-08-2576.
Supported by grants of the University of Siena (quota progetti to R.F. and A.B.), the Hungarian Scientific Research Fund (grant nos. T38312 and TS040865), and the Hungarian Ministry of Health (ETT). T.K. and G.B. were recipients of North Atlantic Treaty Organization-Consiglio Nazionale delle Ricerche Research Fellowships to Siena, Italy. T.K. was also supported by an “Eötvös József” State Fellowship.
H.-J. B. has declared a financial interest in a company (Aventis Pharma Deutschland, Metabolic Diseases) whose product (S3483) was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Angelo Benedetti, Dipartimento di Fisiopatologia e Medicina Sperimentale, Università di Siena, Viale Aldo Moro, 53100 Siena, Italy; e-mail:benedetti@unisi.it.