More than 70 years ago, Alexander Fleming discovered lysozyme and proposed that nonpathogenic bacteria fail to cause disease because they are very susceptible to destruction by lysozyme, an enzyme that is one of the principal proteins of phagocytes. Although much has been learned about the effects of lysozyme in vitro, its biological role in vivo has not been determined. We examined transgenic mice deficient in lysozyme M after challenge by the normally nonpathogenic and highly lysozyme-sensitive bacterium Micrococcus luteus. Despite partial compensation by newly expressed lysozyme P in macrophages, lysozyme M–deficient mice developed much more severe lesions than wild-type mice. The tissue injury was due to the failure of lysozyme M–deficient mice to inactivate peptidoglycan, resulting in an intense and prolonged inflammatory response. Our data indicate that tissue injury is normally limited by prompt degradation of bacterial macromolecules that trigger innate immunity and inflammation.
Introduction
In the 1920s, Alexander Fleming described lysozyme as a bactericidal factor of human and other animal tissues and secretions.1 He also discovered and named a yellow bacterium, Micrococcus lysodeikticus (now M luteus), that was highly susceptible to lysozyme-mediated killing. After exploring the susceptibility of other bacteria to lysozyme, Fleming proposed that M luteus and certain other bacteria were nonpathogenic because they were readily destroyed by lysozyme, which was ubiquitous in infected tissues.
More recent investigations showed that lysozyme is one of the principal components of both the primary (azurophil) and secondary (specific) granules of neutrophils,2,3 and the major secretory product of macrophages.4 It is a 14-kDa cationic enzyme whose common natural substrate is peptidoglycan, a copolymer of N-acetyl muramic acid (NAM) and N-acetylglucosamine (NAG) crosslinked with short peptide links. Peptidoglycan is the exoskeletal component of bacterial cell walls that provides bacteria with shape and mechanical rigidity. While lysis of peptidoglycan may not directly kill bacteria, it makes them highly susceptible to subsequent osmotic and other mechanical stress. Like many other highly cationic proteins, lysozyme has been reported to have a bactericidal activity independent of its enzymatic activity.5 In part, lysozyme may damage bacteria by displacing cryptic autolytic enzymes that are stored in bacterial cell walls for remodeling during and after cell division.6 7
Unlike humans, who have a single lysozyme gene, mice have two8: one encoding lysozyme M, found in leukocytes and various epithelial secretions, and another encoding lysozyme P, normally expressed in intestinal Paneth cells. In humans and other mammals, lysozyme is one of the most abundant proteins both in phagocytes and in epithelial secretions. In view of its limited known biologic function, its abundance and ubiquitous distribution in animal tissues has presented an apparent paradox. The role of lysozyme as a bactericidal effector in vivo was suggested by studies of mice transgenic for rat lysozyme under the control of a lung-specific promoter from human surfactant protein C. These mice had about 6.6- and 17-fold as much lysozyme enzymatic activity as control mice in airway fluid, and manifested somewhat increased killing during lung infections with group B Streptococcus and Pseudomonas aeruginosa.9
In the present study, we used lysozyme M–deficient mice (lys M−/−) to explore the role of lysozyme in host defense and the regulation of inflammation.
Materials and methods
Transgenic mice
Lysozyme M–deficient mice were prepared as described previously,10 by homologous recombination of enhanced green fluorescent protein (EGFP) and neo (neomycin resistance gene) into the first exon of the lysozyme M gene with the subsequent removal of lox-flanked neo gene by passage through cre-expressing mice. The resulting transgenic mice contained contributions from both C57Bl6 and 129Sv strains. Accordingly, the control mice included the nontransgenic parental strains C57Bl6 and 129Sv, as well as their F1 and F2 crosses.
Bacteria
M luteus American Type Culture Collection (Manassas, VA) 4698 (Fleming) was obtained from ATCC and grown from single colonies in trypticase soy broth (Difco, Becton Dickinson Company, Sparks, MD) for 48 hours at 37°C with shaking to OD600 = 1.0-1.5. The bacteria were collected by centrifugation at 1800g for 10 minutes and suspended in sterile phosphate-buffered saline (PBS) by vigorous pipetting and vortexing. For injection into mice, the volume of the suspension was adjusted to generate a concentration of bacteria 20 times that which would yield OD600 = 1.
Peptidoglycan
For peptidoglycan preparation, M luteus were grown to confluence on trypticase soy broth (TSB) agar plates inoculated by 200 μL of bacterial suspension. The bacteria were harvested with a glass rod into PBS and peptidoglycan was purified as previously described.11
Subcutaneous infection and inflammation model
Male and female mice (6-8 weeks old) were anesthetized with 2% inhaled isoflurane, the backs were shaved with an electric razor, and the skin cleansed with 70% ethanol. Bacterial suspension (0.2 mL) or 0.1 mL of 2 mg/mL peptidoglycan in PBS was injected subcutaneously into the right flank with a 25-gauge needle. The mice were weighed daily, photographed when lesions developed, killed with inhaled isoflurane at various stages of lesion development, and necropsied. The skin was incised with scissors in the midline, grasped with rat-tooth forceps, and pulled back over the site of injection. The exposed lesion was photographed, the purulent material was harvested with a curette and disrupted by a motor-driven homogenizer, and the homogenate was assayed by quantitative culture on TSB plates and analyzed for lysozyme activity. The digital photographs were analyzed using ImageJ software (National Institutes of Health, Bethesda, MD) to measure the areas of the lesions. Alternatively, the lesions were biopsied, fixed in 10% formalin in PBS, and processed for hematoxylin and eosin (H&E) stain or immunostain with antilysozyme antibodies prepared in our laboratory by immunization of rabbits with lysozyme M or lysozyme P obtained from recombinant baculovirus-infected insect cells.12 Since the antibodies generated against each of the lysozyme forms were strongly cross-reactive against the other form, they could be used interchangeably. To enable observation of the EGFP content of the inflammatory exudates, the lesion was curetted and smears were prepared on glass slides and mounted in PBS for immediate viewing and photography.
Bronchoalveolar lavage (BAL)
The 6- to 8-week-old mice were killed with an overdose of inhaled isoflurane and the lungs and trachea were exposed. Flexible Tygon tubing (0.060-inch outer diameter, 0.020-inch inner diameter; St Gobain Performance Plastics, Akron, OH) was inserted into a midline incision (1.5-2.0 mm) in the trachea and secured with a nylon ligature. Lungs were lavaged with 5 mL normal saline containing 5 mM EDTA (ethylenediaminetetraacetic acid). Cells were promptly sedimented at 300g for 10 minutes, washed with 5 mL PBS, and resuspended in RPMI 1640 + 10% fetal calf serum (FCS). Total leukocytes were counted using a hemacytometer and a fraction of the total cells were centrifuged onto lysine-coated microscope slides, using a CytoSpin3 (Shandon, Cheshire, England) at 800 rpm for 10 minutes, and stained with DiffQuik (Dade Behring, Newark, DE) to determine the proportion of alveolar macrophages. Alveolar macrophages were more than 95% of total leukocytes.
Peritoneal exudate lavage
Mice anesthetized with isoflurane were injected with 2 mL 3% thioglycolate (Sigma, St Louis, MO) intraperitoneally. After 5 hours the mice were killed with an overdose of isoflurane, and the peritoneal cavity was washed with 8 mL of ice-cold 1 × Hanks Balanced Salt Solution (HBSS) + 0.3% EDTA. Cells from 2 mice (4 mL total volume) were overlaid on 5 mL Ficoll, centrifuged at 900gfor 30 minutes, and processed and characterized as described for those recovered from BAL fluid. Neutrophils were more than 85% of total leukocytes.
Fluorescence-activated cell sorting of GFP+ and GFP− mononuclear cell populations
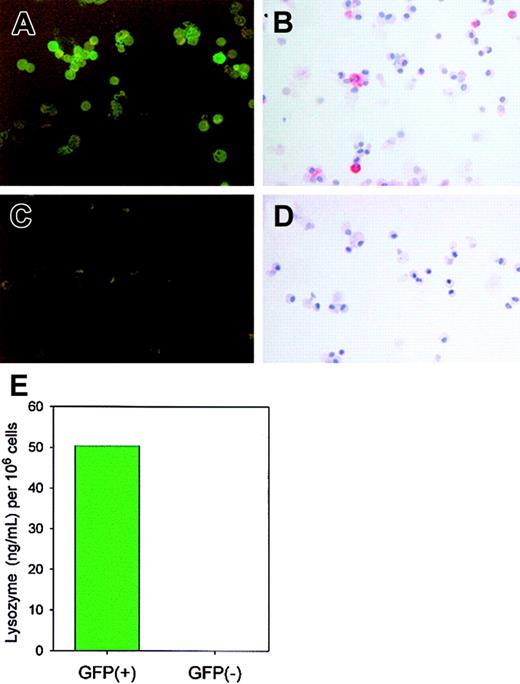
BAL was performed on 15 lys M−/− mice and 5 F2 control mice. For each strain of mouse, cells were pooled, washed twice in RPMI 1640 + 10% FCS, and resuspended to a density of 107 cells/mL in RPMI1640 + 10% FCS. Approximately 106 total cells were sorted on a FACStarPLUSflow cytometer (Janis V. Giorgi Flow Cytometry Laboratory, Los Angeles, CA) on the basis of GFP fluorescence intensity, and GFP+and GFP− cells were collected separately in RPMI 1640 + 20% FCS. A sample of the collected cells was centrifuged onto lysine-coated microscope slides using a CytoSpin3 (Shandon) at 800 rpm for 10 minutes. The expression of GFP was confirmed by epifluorescence on a Labophot microscope (Nikon, Tokyo, Japan) and the expression of lysozyme was confirmed by immunocytochemistry, using 1:1000 rabbit antilysozyme P antisera and Fast Red chromogenic detection (Sigma).
Lysozyme assay
Lysozyme activity was determined by agarose radial diffusion assay with dried M luteus as a substrate and quantified using a standard curve generated with recombinant murine M and P lysozyme standards.13
Electron microscopy
Immediately after infected mice were killed, the lesions were excised and fixed for 2 hours in 2% glutaraldehyde in 0.8M Na-cacodylate with 0.2% calcium chloride (CaCl), pH 7.35, at room temperature. They were then washed in the cacodylate buffer and dehydrated in graded ethanol (50%, 75%, 90%, and 100%). Samples were embedded in Epon (Ted Pella, Redding, CA). Ultrathin sections were cut with Sorvall MT6000 (Boeckeler Instrument, Tucson, AZ), stained with uranyl acetate and lead citrate, and viewed and photographed at 80 keV on a JEOL model 100XC electron microscope (JEOL, Peabody, MA).
Statistics
Comparisons between groups of mice were analyzed by ttest (pairwise) or one-way analysis of variance (ANOVA) (Tukey test). For data that were not normally distributed, logarithmic transformation or Mann-Whitney test were used.
Results and discussion
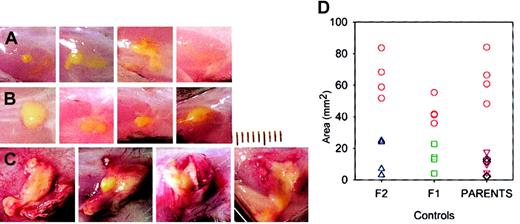
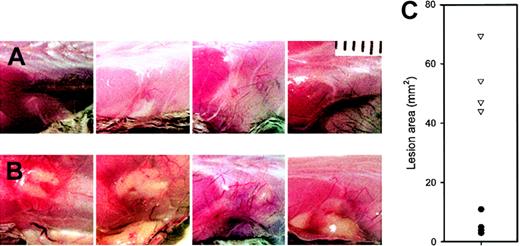
Conventionally housed lysozyme M–deficient mice appeared healthy. Within one day after subcutaneous injection of M luteus, the mice showed localized swelling and redness at the injection site, which resolved by day 2 in control mice (C57Bl6, 129Sv, C57Bl6 × 129Sv F1 and F2) but were much more prominent and persistent in lys M−/− mice. At autopsy 2 to 5 days after injection, the lesions appeared inflamed and purulent in lys M−/− mice but not in control mice (Figure 1), and had a significantly larger area in lys M−/− mice: 58 ± 16 mm2 (mean ± SD, n = 12) than in any of the control groups: C57Bl6 ×129Sv F1, 13.5 ± 7.7 mm2(n = 4); C57Bl6 × F2, 14.8 ± 11.3 mm2(n = 4); C57Bl6, 7.3 ± 5.8 mm2 (n = 4); 129Sv, 11.3 ± 5.5 mm2 (n = 4); P ≤ .002 for all comparisons by t test, regardless of whether the lys M−/− mice were only compared with contemporaneous controls or pooled. Since very similar large, inflamed, and purulent lesions were also produced in lysozyme M–deficient mice by M luteus that were heat-killed by 10 minutes exposure to 100°C (lys M−/−, 45.9 ± 26.8 mm2 [n = 4], compared with C57Bl6, 5.4 ± 2.6 mm2 [n = 4], and 129Sv, 9.3 ± 8.0 mm2 [n = 4]; P = .024 and .04, respectively, by t test), we examined the effect of purified M luteus peptidoglycan, the natural substrate for lysozyme (Figure 2). At 48 hours after injection, lys M−/− mice had much more inflamed and larger lesions than F2 controls (54.2 ± 10.7 mm2 vs 5.8 ± 3.4 mm2 [n = 4 per group];P < .001 by t test).
M luteus produces larger and more inflamed lesions in lys M−/− mice.
Each panel is a photograph of a lesion in an individual mouse on day 5. (A) C57Bl6 mice; (B) 129Sv mice; (C) lys M−/− mice. M luteus bacterial suspension is bright yellow. Lesions in lys M−/− mice show more inflammation, as evidenced by greater volume of white exudate, more redness, and numerous visible blood vessels. Each division on the scale represents 1 mm. (D) Areas of lesions in lys M−/− mice (red circles) compared with controls F2 (blue triangles) and F1 (green squares) and parental strains C57Bl6 (black diamonds) and 129Sv (purple triangles).
M luteus produces larger and more inflamed lesions in lys M−/− mice.
Each panel is a photograph of a lesion in an individual mouse on day 5. (A) C57Bl6 mice; (B) 129Sv mice; (C) lys M−/− mice. M luteus bacterial suspension is bright yellow. Lesions in lys M−/− mice show more inflammation, as evidenced by greater volume of white exudate, more redness, and numerous visible blood vessels. Each division on the scale represents 1 mm. (D) Areas of lesions in lys M−/− mice (red circles) compared with controls F2 (blue triangles) and F1 (green squares) and parental strains C57Bl6 (black diamonds) and 129Sv (purple triangles).
Purified
M luteus peptidoglycan produces larger and more inflamed lesions in lys M−/− mice. Each panel is a photograph of a lesion in an individual mouse on day 2. (A) F2 controls; (B) lys M−/− mice. (C) Areas of lesions in lys M−/− mice (▿) compared with F2 controls (●). Note that lesion areas are significantly larger in lys M−/− mice than in F2 controls (P < .001).
Purified
M luteus peptidoglycan produces larger and more inflamed lesions in lys M−/− mice. Each panel is a photograph of a lesion in an individual mouse on day 2. (A) F2 controls; (B) lys M−/− mice. (C) Areas of lesions in lys M−/− mice (▿) compared with F2 controls (●). Note that lesion areas are significantly larger in lys M−/− mice than in F2 controls (P < .001).
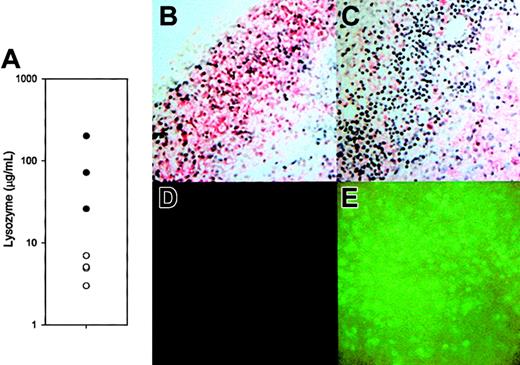
To determine the effect of the disruption of the lysozyme M gene on lysozyme expression, we examined the amount and distribution of lysozyme in lesions induced by live M luteus (Figure 3A-C). At 48 hours after infection, lys M−/− mice had an average of 15-fold lower concentrations of lysozyme in their lesions than did F2 controls (Figure 3A). Although in control sections from wild-type mice, both polymorphonuclear leukocytes (neutrophils) and mononuclear leukocytes stained for lysozyme (Figure 3B), the neutrophils of lys M−/− mice did not express lysozyme (Figure 3C). Surprisingly, cells expressing lysozyme were clearly visible in the immunostained sections from lys M−/− mice, and they were tentatively identified as macrophages because they were larger, mononuclear, and often foamy cells. As expected, the inflammatory cells in the lesions of lys M−/− mice produced EGFP (Figure 3E compared with Figure3D), confirming that the lesions contained abundant cells that expressed the knockout construct.
Lysozyme activity and distribution in lesions.
(A) Lysozyme concentration 48 hours after M luteus injection in tissues of lys M−/− mice (○; n = 4) compared with F2 control mice (●; n = 3). The difference between the geometric means, 4.79 μg/mL vs 72.4 μg/mL, is significant atP = .004 (t test, after logarithmic transformation). (B-C) Sections of lesions from an F2 control and lys M−/− mouse, respectively, immunostained with antibody reactive with lysozymes M and P (red stain, blue hematoxylin nuclear counterstain). Note that most inflammatory cells in the lesions of F2 mice are reactive with antilysozyme antibody, but in lys M−/− lesions inflammatory cells with polymorphonuclear morphology (neutrophils) are not reactive. (D-E) Lesions of F2 mice show only background fluorescence, while those of lys M−/− mice express abundant EGFP from the knockout construct.
Lysozyme activity and distribution in lesions.
(A) Lysozyme concentration 48 hours after M luteus injection in tissues of lys M−/− mice (○; n = 4) compared with F2 control mice (●; n = 3). The difference between the geometric means, 4.79 μg/mL vs 72.4 μg/mL, is significant atP = .004 (t test, after logarithmic transformation). (B-C) Sections of lesions from an F2 control and lys M−/− mouse, respectively, immunostained with antibody reactive with lysozymes M and P (red stain, blue hematoxylin nuclear counterstain). Note that most inflammatory cells in the lesions of F2 mice are reactive with antilysozyme antibody, but in lys M−/− lesions inflammatory cells with polymorphonuclear morphology (neutrophils) are not reactive. (D-E) Lesions of F2 mice show only background fluorescence, while those of lys M−/− mice express abundant EGFP from the knockout construct.
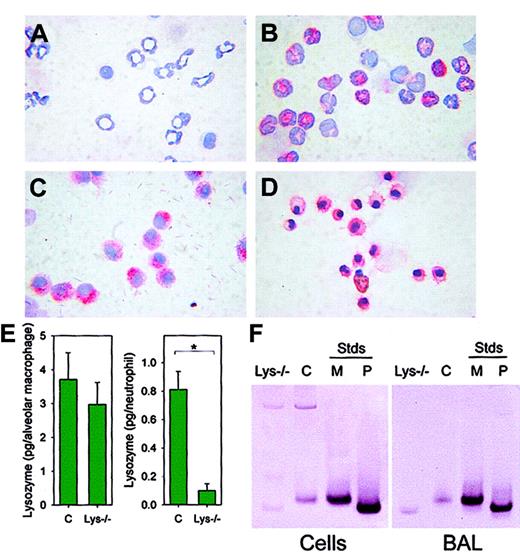
We next assayed the expression of lysozyme in isolated neutrophils and macrophages (Figure 4) of lysozyme M–deficient mice and F2 control mice by immunostaining with an antibody that recognizes both lysozyme M and lysozyme P. As expected, neutrophils from lys M−/− mice did not contain immunostainable lysozyme (Figure 4A). However, alveolar macrophages from lys M−/− mice stained for lysozyme with intensity similar to that of control macrophages (Figure 4C). In F2 control mice, both neutrophils (Figure 4B) and alveolar macrophages (Figure 4D) stained for lysozyme. By lysoplate analysis, the concentrations of lysozyme in BAL (> 95% macrophages) from lysozyme M–deficient mice and F2 control mice were not significantly different (n = 4-6,P = .521; Figure 4E, left panel), while the concentration of lysozyme in peritoneal lavage (> 85% neutrophils) was significantly lower in lysozyme-deficient mice than in F2 controls (n = 3, P = .006; Figure 4E, right panel). Additionally, the concentration of lysozyme contained within each macrophage, and not secreted into the BAL fluid, was determined by lysoplate to be similar for a pool of 8 F2 wild-type mice (2.1 pg per macrophage) and 8 lysozyme M–deficient mice (3.2 pg per macrophage). Western blot analysis of BAL macrophages from lysozyme M–deficient mice, using rabbit antimouse lysozyme antisera, showed that when lysozyme M was absent, lysozyme P was expressed instead (Figure 4F). As determined by immunocytochemistry and lysoplate analysis, the protein expression and enzymatic activity of lysozyme correlated with GFP expression (Figure5), so that cells that did not express the GFP construct under the influence of the lysozyme M promoter did not express lysozyme P either. This suggests that the biological mechanisms that would normally induce the expression of lysozyme M were instead stimulating lysozyme P production in the lys M−/−mice. Our results further indicate that the deficiency of lysozyme M in lys M−/− mice is only partially alleviated by compensatory expression of lysozyme P in macrophages, and possibly in other cell types, but not in neutrophils. This explains the observed 20-fold decrease in lysozyme enzymatic activity in the subcutaneous lesions, in which neutrophils predominated.
Alveolar macrophages of lys M−/− mice express lysozyme P.
Peritoneal exudate neutrophils were from (A) lys M−/−mice and (B) F2 wild-type mice. Alveolar macrophages were from (C) lys M−/− mice and (D) F2 wild-type mice. Cells were stained with antilysozyme antibody and counterstained with hematoxylin. Reactive cells stained red. Note that lysozyme is present in alveolar macrophages but absent in neutrophils of lys M−/−mice. (E) Concentration of lysozyme in lung lavage (> 95% macrophages; pg/cell; left panel) and peritoneal exudate lavage (> 85% neutrophils; pg/cell; right panel). Error bars indicate SEM; asterisk, significant difference (P = .006) in lysozyme concentration. (F) Western blot analysis with antilysozyme antibody indicates that 1.4 × 105 alveolar macrophages (left panel) or 100 μL BAL cell-free supernatant (right panel) from wild-type mice (“C”) contain only lysozyme M, while 1.4 × 105 alveolar macrophages or 100 μL BAL cell-free supernatant from lys M−/− mice contain only lysozyme P. M and P represent recombinant lysozyme M and P standards (Stds, 200 ng).
Alveolar macrophages of lys M−/− mice express lysozyme P.
Peritoneal exudate neutrophils were from (A) lys M−/−mice and (B) F2 wild-type mice. Alveolar macrophages were from (C) lys M−/− mice and (D) F2 wild-type mice. Cells were stained with antilysozyme antibody and counterstained with hematoxylin. Reactive cells stained red. Note that lysozyme is present in alveolar macrophages but absent in neutrophils of lys M−/−mice. (E) Concentration of lysozyme in lung lavage (> 95% macrophages; pg/cell; left panel) and peritoneal exudate lavage (> 85% neutrophils; pg/cell; right panel). Error bars indicate SEM; asterisk, significant difference (P = .006) in lysozyme concentration. (F) Western blot analysis with antilysozyme antibody indicates that 1.4 × 105 alveolar macrophages (left panel) or 100 μL BAL cell-free supernatant (right panel) from wild-type mice (“C”) contain only lysozyme M, while 1.4 × 105 alveolar macrophages or 100 μL BAL cell-free supernatant from lys M−/− mice contain only lysozyme P. M and P represent recombinant lysozyme M and P standards (Stds, 200 ng).
GFP+, but not GFP−, cells express enzymatically active lysozyme P.
Cells (> 95% alveolar macrophages) from BAL of 15 lysozyme M–deficient mice were pooled and sorted by FACS based on the presence or absence of GFP. GFP expression was confirmed by epifluorescence microscopy (panel A, GFP+ cells; panel C, GFP−cells). By immunostaining with antimouse lysozyme antibody, only GFP+ cells (B), not GFP− cells (D), expressed lysozyme, and only the GFP+ fraction contained enzymatically active lysozyme as determined by lysoplate analysis (E).
GFP+, but not GFP−, cells express enzymatically active lysozyme P.
Cells (> 95% alveolar macrophages) from BAL of 15 lysozyme M–deficient mice were pooled and sorted by FACS based on the presence or absence of GFP. GFP expression was confirmed by epifluorescence microscopy (panel A, GFP+ cells; panel C, GFP−cells). By immunostaining with antimouse lysozyme antibody, only GFP+ cells (B), not GFP− cells (D), expressed lysozyme, and only the GFP+ fraction contained enzymatically active lysozyme as determined by lysoplate analysis (E).
The 2 murine lysozyme genes M and P encode proteins that differ by only 6 amino acid substitutions in the mature region. The genes are located in tandem within 5 kb of each other and are thought to have arisen by duplication 30 to 50 million years ago.14 The activation of the normally inactive P lysozyme gene in macrophages is a striking phenomenon, especially considering that only a small part of the M gene is modified, by substitution with enhanced green fluorescent protein (EGFP) at the exon 1–intron 1 junction. This results in the transcription of EGFP instead of the disrupted lysozyme M gene. The insertion may also alter the DNA conformation and transcription of regions downstream from lysozyme M, some of which are regulatory.15 How these or other changes activate lysozyme P gene in macrophages is a fascinating but unanswered question.
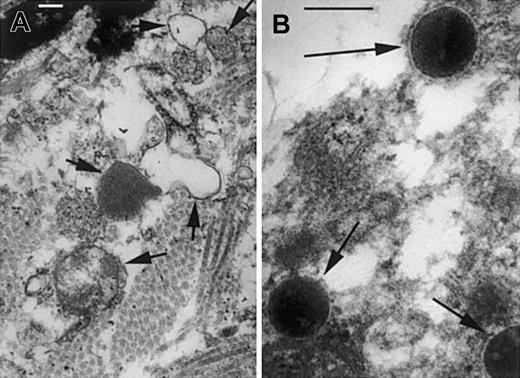
Lysozyme catalyzes the degradation of cell wall peptidoglycan by hydrolysis of the glycosidic bond between its 2 major repeating components, N-acetylmuramic acid (NAM) and N-acetylglucosamine (NAG). As shown by electron microscopy of infected lesions at 6 hours after infection, the normally very rapid digestion of M luteuscell walls is impaired in lys M−/− mice compared with F2 control mice (Figure 6). This impairment may affect not only the survival of bacteria (see next paragraph in this section) but also the evolution of the inflammatory response. Peptidoglycan and some of its fragments induce inflammation in mammals by activating the toll-like receptor TLR-2,16,17 by activating classical and alternative complement pathways,18-20 and probably also by as yet unknown pathways that involve peptidoglycan recognition proteins (PGRPs).21-26 The inflammatory responses are terminated by hydrolysis and inactivation of the peptidoglycan and its active fragments, a process that requires lysozyme and possibly other enzymes as well.27 28 We surmise that even partial deficiency of lysozyme concentration and activity is sufficient to produce a delay in the enzymatic degradation of peptidoglycan that contributes to prolonged and more severe inflammatory response to purified peptidoglycan as well as to live or heat-inactivated M luteus.
Cell wall structure of
M luteus is disrupted in lesions of F2 control mice but preserved in lys M−/− mice. At 7 hours after infection, the infected lesions of F2 controls (A) contain bacterial cell wall fragments and ghosts (short arrows), but lys M−/− mice (B) show bacteria with well-preserved cell walls and normal electron-dense cytoplasm (long arrows). Bars, 0.2 μm.
Cell wall structure of
M luteus is disrupted in lesions of F2 control mice but preserved in lys M−/− mice. At 7 hours after infection, the infected lesions of F2 controls (A) contain bacterial cell wall fragments and ghosts (short arrows), but lys M−/− mice (B) show bacteria with well-preserved cell walls and normal electron-dense cytoplasm (long arrows). Bars, 0.2 μm.
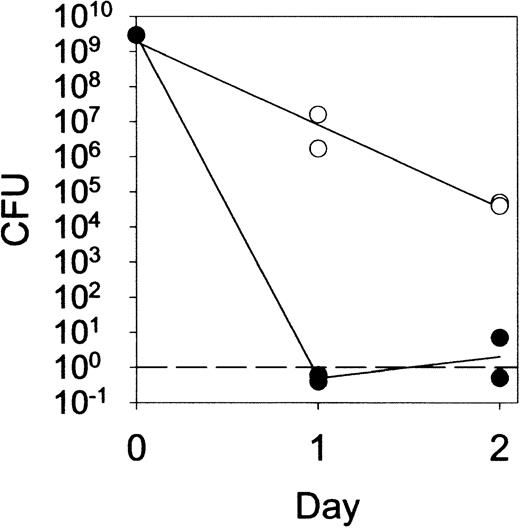
In addition to hydrolysis of peptidoglycan, lysozyme has been shown to kill a variety of bacteria by both enzymatic and nonenzymatic mechanisms.5-7 29 Although routine cultures of 5-day lesions induced by live M luteus were sterile in lys M−/− mice as well as in controls (data not shown), quantitative cultures of fluid from 1-day or 2-day lesions (Figure7) revealed a significant impairment of bacterial killing in lys M−/− mice. Nevertheless, even lys M−/− mice were able to reduce the bacterial CFU counts by about a hundred-fold by 24 hours and 10 000-fold by 48 hours. By day 5, when few if any viable bacteria remained in the lesions of lys M−/− mice or control mice, the lesions of lys M−/− mice were highly inflamed while those of control mice were not. We conclude that impaired killing of bacteria was unlikely to contribute appreciably to the observed tissue pathology. The close similarity of the lesions induced by live and heat-killed M luteus supports this conclusion.
Prolonged survival of
M luteus in lesions of lys M−/− mice compared with F2 control mice. The infected tissue was removed with a curette, weighed, and homogenized, and the M luteuscount determined by colony-forming assay with a threshold of 1 colony-forming unit (CFU) per lesion.
Prolonged survival of
M luteus in lesions of lys M−/− mice compared with F2 control mice. The infected tissue was removed with a curette, weighed, and homogenized, and the M luteuscount determined by colony-forming assay with a threshold of 1 colony-forming unit (CFU) per lesion.
Moreover, in previous unpublished pilot studies (October 2001), we explored the resistance of lys M−/−mice in a model of subacute dermal infection with Staphylococcus aureus.30 In this model, the visible skin lesions and transient weight loss of lysozyme-deficient mice were intermediate to those of the 2 parental strains, indicating that the lysozyme M–deficient mice had no generalized immunodeficiency when challenged by pathogenic bacteria. However, it is possible that studies with additional microbes will reveal selective deficits in host defense against specific pathogens.
Interestingly, severe and prolonged inflammation in lesions that contain few or no live microbes is also a prominent feature of another phagocyte defect, chronic granulomatous disease (CGD), both in the human disease and its transgenic mouse model. Phagocytes of patients with CGD do not produce superoxide and manifest not only delayed killing of phagocytized microbes but also impaired ability to degrade microbial macromolecules.31 Compared with wild-type mice, mice with CGD respond to heat-killed Aspergillus fumigatuswith increased pulmonary injury and inflammation.32 Rapid degradation and inactivation of microbial macromolecules that are recognized by innate host defense mechanisms appears to be an essential function of neutrophils and macrophages. Therapeutic augmentation of lysozyme and other enzymes that neutralize proinflammatory microbial components may be useful in preventing tissue damage caused by bacterial infections.
We acknowledge the expert technical assistance of Iris Williams in performing FACS analyses.
Prepublished online as Blood First Edition Paper, October 31, 2002; DOI 10.1182/blood-2002-07-2319.
Supported by grants from the Cystic Fibrosis Foundation (T. Ganz and A.M.C.) and NIH R01 CA89590-01 (T. Graf).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Tomas Ganz, 37-055 CHS, Department of Medicine, David Geffen School of Medicine, University of California, Los Angeles, CA 90095-1690; e-mail:tganz@mednet.ucla.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal