The allergic reaction begins with the antigen-induced aggregation of occupied high-affinity IgE receptors expressed on mast cell surface, their activation, and the release of proinflammatory mediators that cause the “early phase” of this process. In addition, mast cell activation induces the onset of a “late phase” reaction characterized by the tissue infiltration of inflammatory cells, mainly eosinophils. We have hypothesized that during the late phase mast cells interact with and are activated by eosinophils. Here we report that highly purified human lung mast cells became responsive to eosinophil major basic protein (MBP) when in coculture with human lung fibroblasts. In addition, cord blood–derived mast cells maintained in coculture with 3T3 fibroblasts released more histamine and prostaglandin D2 (PGD2) compared with cells maintained in suspension. The fibroblast-derived membrane form of stem cell factor (SCF) was found to be involved in the mast cell increased responsiveness to MBP. In fact, cord blood–derived mast cells cocultured with 3T3 in the presence of antisense for SCF or cocultured with fibroblasts that do not express the membrane form of SCF were inhibited in their histamine-releasing activity toward MBP. In addition, this form of SCF induced the expression of a pertussis toxin–sensitive Gi protein, Gi3 that interacts with MBP to trigger mast cell non-IgE–dependent activation in a manner similar to other cationic compounds such as compound 48/80. Mast cell responsiveness to eosinophil mediators is a potentially novel evidence for an alternative pathway of allergen-independent activation able to contribute to the perpetuation of allergy.
Introduction
The allergic process is initiated when antigen causes the aggregation of occupied high-affinity IgE receptors (FcεRI) expressed on the mast cell surface. This event is followed by the immediate release of proinflammatory mediators that contribute to the early phase of the allergic process.1 In addition, mast cell degranulation induces the onset of a second “late phase” reaction, 4 to 8 hours later, characterized by the tissue infiltration of inflammatory cells, mainly eosinophils.2 We have hypothesized that during the late phase of the allergic reaction mast cells interact with and are activated by eosinophils.3,4In fact, we have shown that antigen-challenged rat peritoneal mast cells release histamine following incubation with the eosinophil mediator major basic protein (MBP) by a mechanism similar to that of IgE-independent stimuli induced by cationic compounds such as compound 48/80 and substance P.4
The importance of mast cell activation induced by eosinophil mediators has been underappreciated because isolated human lung–derived and skin-derived mast cells do not release histamine on incubation with MBP.5 According to their protease content, human lung mast cells belong to the tryptase-positive subtype of mast cells (MCT).6 In contrast to the tryptase- and chymase-positive subtype of mast cells (MCTC) found, for example, in the skin and intestinal submucosa,6MCT are well-known for their unresponsiveness to non-IgE–dependent activation.7 However, mast cell populations can express significant variation in numbers, phenotype, and function, in different anatomic locations and even in the same tissue under the microenvironment influence, particularly during the course of inflammatory responses.1 Therefore, we believe that analysis of a particular mast cell population in vitro may not necessarily reflect its behavior in vivo. Indeed, we have demonstrated that the coculture of rat or human lung mast cells with 3T3 fibroblasts, a system that mimics mast cell microenvironment in vivo, prolongs mast cell survival and increases mast cell responsiveness to IgE-dependent activation.8,9 In addition, mouse mast cells derived from bone marrow, the rodent counterpart of human MCT, acquired responsiveness to IgE-independent stimuli when in coculture with 3T3 fibroblasts.10 Moreover, under these conditions, bone marrow–derived mast cells changed their phenotype toward connective tissue mast cells as exemplified by the increase in their histamine content and a marked increase in biosynthesis of proteoglycans that bear heparin.11
In the present study, we investigated whether highly purified human lung mast cells in coculture with human lung fibroblasts become responsive to MBP. Mast cell responsiveness to eosinophil mediators is potentially novel evidence for an alternative pathway of allergen-independent activation able to contribute to disease. We also compared the susceptibility of cord blood–derived mast cells (CBMCs) to IgE-independent activation in suspension and in coculture conditions, and we defined a novel role for the fibroblast-derived membrane stem cell factor (SCF) in this effect.
Materials and methods
Human CBMCs
Mononuclear cells were isolated from umbilical cord blood as previously described.12 Cells were seeded at 106 cells/mL minimum essential medium (MEM-α) containing 10% (vol/vol) heat-inactivated fetal calf serum (FCS), 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mMl-glutamine, 10 μg/mL ribonucleases (Biological Industries, Beith Haemek, Israel), 100 ng/mL SCF (a generous gift from Amgen, Thousand Oaks, CA), 10 ng/mL interleukin 6 (IL-6; Peprotech, Rocky Hill, NJ), and 3 × 10−7 M prostaglandin E2 (PGE2; Sigma Chemicals, St Louis, MO). Half of the culture medium was replaced every week. CBMCs were harvested for the experiments between 6 and 9 weeks of culture when more than 95% of the cells were stained metachromatically with toluidine blue and positively stained for both tryptase and chymase as assessed by flow cytometry.
Human lung mast cell isolation and purification
Human lung mast cells were isolated from normal tissue of patients undergoing surgery for lung emphysema and carcinoma using an enzymatic method.13 Human lung mast cells were purified up to 90% via selection of c-kit+ cells by magnetic cell separation (Dynalbeads, Dynal, Oslo, Norway). Human lung mast cells were cultured at a concentration of 106/mL in Dulbecco modified Eagle medium (DMEM; Biological Industries) containing 10% vol/vol FCS, 100 U/mL penicillin, 100 μg/mL streptomycin, and 2 mM l-glutamine (DMEM+) and supplemented with 100 ng/mL SCF.
Written consent was obtained from all the volunteers according to the guidelines established by the Tel Hashomer (cord blood) and Assaf Harofeh (lung biopsies) Medical Center Human Experimentation Helsinki Committees, Israel.
Rat peritoneal mast cell isolation and purification
Rat peritoneal mast cells were obtained from Sabra rats by peritoneal lavage and purified as previously described (metrizamide gradient, 22.5%, > 96% purity).8 The rats were cared for according to the Guidelines of the Animal Committee of the Hebrew University of Jerusalem, Israel.
Human mast cell–fibroblast cocultures
Human lung mast cells and CBMCs were seeded on confluent monolayers of the human lung fibroblast cell line (MRC 5) and Swiss albino mouse embryonic 3T3 fibroblast cell line (3T3; American Type Culture Collection, Rockville, MD), respectively, in 24 wells at a density of 5 × 104/0.5 mL DMEM+. For some experiments CBMCs were seeded on either Transwell membranes (0.4-μm pore size; Nalge Nunc International, Naperville, IL) to separate them from 3T3 fibroblasts, or embryonic fibroblasts fromSl/Sld mutant mice (kindly provided by Dr T. Jippo, Department of Pathology, Osaka University Medical School, Japan), or 3T3 fibroblasts treated overnight with SCF sense and antisense. Culture media of CBMCs was always supplemented with 100 ng/mL SCF. Human mast cells were maintained in coculture for 4 days prior to activation. This coculture time was determined as optimal in preliminary kinetics experiments.
SCF antisense treatment of 3T3 fibroblasts and human mast cell–fibroblast cocultures
Oligodeoxynucleotides end-capped with 2′-O-methyl RNA substitutions at three 3′-terminal positions were used at 0.1 pM by preincubating 3T3 fibroblast overnight before and during CBMC coculture. This antisense concentration was shown to significantly inhibit the level of membrane SCF protein as assessed by confocal microscopy. Sense oligonucleotide at the same concentration did not affect SCF expression. Sequences for sense and antisense oligodeoxynucleotides have been previously published elsewhere.14 During antisense therapy, 100 ng/mL SCF was added to CBMCs cocultured with 3T3 fibroblasts to support CBMC viability. Therefore, in these cultures only the influence of the membrane form of SCF on CBMCs was selectively suppressed.
Purification of human eosinophil MBP
MBP was purified from eosinophils obtained from patients with marked eosinophilia as described.15 16 The purified protein was stored at −70°C, and samples were thawed immediately before use. MBP was judged pure by its banding pattern on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) after staining with Coomassie brilliant blue R. Protein concentrations were determined using the appropriate E277 values.
Human mast cell activation and mediator assays
Cultures were incubated in Tyrode buffer containing 0.1% gelatin (Tg), 1.8 mM CaCl2, 0.9 mM MgCl2(Tg++) for 30 minutes with one of the following: 2 μg/mL anti-FcεRI-α chain 22E7 (kindly provided by Dr J. P. Kochan, Hoffman La Roche, Nutley, NJ); 2, 5, and 10 μg/mL compound 48/80 (Sigma Chemicals, Rehovot, Israel); MBP at 0.001, 0.01, 0.1, 0.1, and 10 μM for human lung mast cells, and at 0.001, 0.01, 0.1, 1, 2, and 5 μM for CBMCs. To assess the effects of pertussis toxin (Ptx), cultures were incubated for 2 hours in Tg++containing 1 μg/mL Ptx (Sigma, Israel) and then activated as described. Histamine was measured in the culture supernatants and in sonicated cells by a radioenzymatic assay.17 Histamine release was calculated as a percentage as follows: histamine in supernatant/(histamine in supernatant + histamine in cells) × 100. Generation of cysteinyl-leukotrienes (cys-LTs) in supernatants was measured with an enzyme-linked immunosorbent assay (ELISA) for LTC4/D4/E4 (Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom). For this ELISA, the cross-reactivity with LTC4 is 100%, LTD4is 100%, LTE4 is 70%, and LTB4 is 0.3%. Generation of prostaglandin D2 (PGD2) in the supernatants was measured with an ELISA for PGD2 (Cayman Chemical, Ann Arbor, MI). For the PGD2 ELISA, the cross-reactivity with thromboxane B2(TXB2), PGF2a, or PGE2 is less than 0.01%. Cys-LT and PGD2 concentrations were calculated according to manufacturer's instructions and the results expressed in ng/106 cells.
Detection of mast cell viability
Mast cell viability was assessed by trypan blue exclusion test. Mast cells were examined blindly 10 minutes, 60 minutes, and 24 hours after incubation with the different activators immediately following addition of 0.1 mL trypan blue (0.4%; Sigma, Israel). The percentage of viable cells was calculated as follows: percentage of viable mast cells = (no. of trypan blue-negative cells/no. of total cells) × 100. Viability of mast cells was always more than 85% in the experiments.
SDS-PAGE immunoblot analysis
Lysates were prepared from 3 × 106 cells as described.18 After protein concentration was determined by Bradford method,19 samples were resolved in 10% SDS-PAGE under reducing conditions and transferred to polyvinylidene difluoride membranes. Gi3α purified from bacterial lysates was used as positive control (Santa Cruz Biotechnologies, Santa Cruz, CA). The membranes were incubated for 1 hour at room temperature with 1 μg/mL rabbit antihuman Gi3α (Santa Cruz Biotechnologies). After the membranes were washed, the proteins were detected with secondary immunopure goat antirabbit antibodies conjugated with horseradish peroxidase (1:5000; Pierce, Rockford, IL) followed by enhanced chemiluminescence (ECL; Amersham Pharmacia Biotech). Bands were scanned and their density calculated as follows: band area × (band intensity of the sample − background intensity).
Confocal laser microscopy
Fibroblasts were seeded on 12-mm cover glasses until confluency. Human lung mast cells and CBMCs (106 cells/0.5 mL) were seeded on these fibroblasts and maintained in coculture as described. Control cells consisted of rat peritoneal mast cells that constitutively express Gi3α. Cells were fixed and permeabilized as previously described.19 Staining was performed by incubating the cells first with 5 μg/mL rabbit antihuman Gi3α (Santa Cruz Biotechnologies) for 1 hour at room temperature. After washings with phosphate-buffered saline (PBS), slides were incubated with 20 μg/mL fluorescein isothiocyanate (FITC)–conjugated goat antirabbit IgG antibodies (Chemicon, Temecula, CA) for 1 hour at room temperature. Negative controls consisted of slides in which only the second antibody was added. Slides were examined using a × 63 objective under a Zeiss LSM 410 confocal laser scanning system attached to the Zeiss Axiovert 135 M inverted microscope with × 63/1.2 C-Apochromat water immersion lens (Carl Zeiss, Thornwood, NY).
Statistical analysis
Data are expressed as mean ± SEM of at least 3 independent experiments. Parametric analysis (ANOVA, followed by Tukey-Kramer post hoc test) was used to compare the effects. In both cases,P < .05 was considered statistically significant.
Results
Human lung mast cells in coculture with fibroblasts become responsive to eosinophil MBP
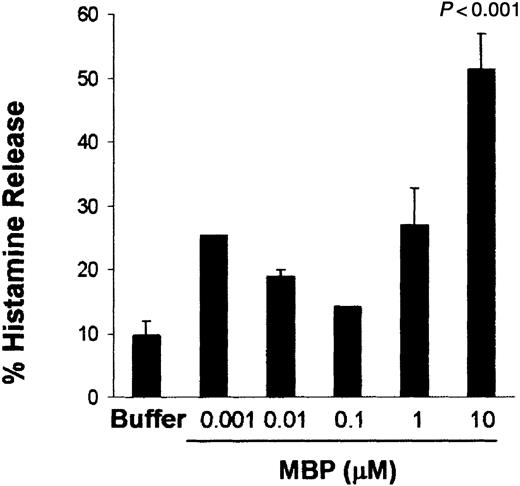
To evaluate whether human lung mast cells can be activated by MBP under fibroblast influence, human lung mast cells were cocultured for 4 days with human lung fibroblasts. Human lung mast cells cocultured with human lung fibroblasts released histamine in a concentration-dependent fashion with a maximum effect at 10 μM MBP (51.4% ± 5.5% histamine release; P < .001; n = 5; Figure1).
Human lung mast cells cocultured with human lung fibroblasts are activated by MBP with a dose-dependent pattern.
Human lung mast cells (90%-99% mast cells) were maintained in coculture with human lung fibroblasts for 4 days before activation. Histamine release was assessed after 30 minutes of incubation with MBP at the concentrations indicated. Data are the means of ± SEM of 5 experiments performed in triplicates. P < .001 compared with cells incubated in buffer alone.
Human lung mast cells cocultured with human lung fibroblasts are activated by MBP with a dose-dependent pattern.
Human lung mast cells (90%-99% mast cells) were maintained in coculture with human lung fibroblasts for 4 days before activation. Histamine release was assessed after 30 minutes of incubation with MBP at the concentrations indicated. Data are the means of ± SEM of 5 experiments performed in triplicates. P < .001 compared with cells incubated in buffer alone.
At the same range of MBP concentrations, human lung mast cells maintained in suspension in the presence of SCF (100 ng/mL) did not release significant levels of histamine compared with buffer-incubated cells (data not shown). The increased reactivity of cocultured human lung mast cells toward MBP cannot be related to a mast cell change in phenotype toward MCTC. In fact, as assessed by confocal microscopy, after coculture the majority of human lung mast cells were still tryptase-containing rather than tryptase/chymase-containing cells (data not shown). This finding is in agreement with a previous study carried out by Dvorak et al.21
Human CBMCs in coculture with 3T3 fibroblasts are more responsive to IgE-independent stimuli than when cultured in suspension
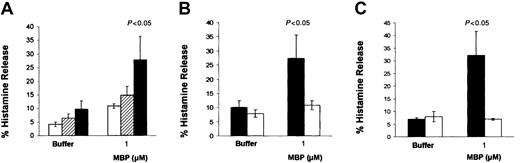
The addition of either MBP (1 μM) or compound 48/80 (5 μg/mL) to CBMC cultures induced the release of relatively low percentages of histamine, that is, 16.1% ± 1.4% and 19.7% ± 1.9%, respectively (P < .05; n = 26; data not shown). Therefore, the modest effect of MBP and 48/80 on mast cell histamine release prompted us to search for culture conditions in which CBMC susceptibility to IgE-independent activation could be increased.10 CBMCs cocultured with 3T3 fibroblasts released 2 times more histamine (60.8% ± 6.7%) than CBMCs maintained in suspension (31.8% ± 3.6%) when activated by MBP (1 μM; P < .05; n = 5). CBMCs cocultured with 3T3 released even higher percentages of histamine when the MBP concentration was increased to 2 μM (28.6% ± 4.5% histamine release for CBMCs maintained in suspension versus 73.7% ± 5.6% for CBMCs cocultured with 3T3; P < .0001; n = 3). Similarly, compound 48/80 (5 μg/mL) induced a 2.9-fold increase in histamine release from CBMCs cocultured with 3T3 (P < .05; n = 5; Figure2A). In contrast to these effects, no differences in histamine release were obtained when CBMCs cocultured with 3T3 were activated immunologically by the anti-FcεRI-α chain antibody, 22E7 (2 μg/mL).
CBMCs cocultured with 3T3 fibroblasts increased their responsiveness to MBP.
CBMCs (> 90% mast cells) were maintained in suspension (■) or in coculture with 3T3 fibroblasts (▪) in the presence of soluble SCF (100 ng/mL) for 4 days before activation. (A) Histamine release was assessed after 30 minutes of incubation with the activators at the concentrations indicated. Data are the means ± SEM of 5 experiments performed in triplicate. (B-C) PGD2 production and release was assessed after 30 minutes of incubation with the activators at the concentrations indicated. Data are the means ± SEM of 2 experiments performed with CBMCs from 2 different donors.P < .05 compared with cells maintained in suspension.
CBMCs cocultured with 3T3 fibroblasts increased their responsiveness to MBP.
CBMCs (> 90% mast cells) were maintained in suspension (■) or in coculture with 3T3 fibroblasts (▪) in the presence of soluble SCF (100 ng/mL) for 4 days before activation. (A) Histamine release was assessed after 30 minutes of incubation with the activators at the concentrations indicated. Data are the means ± SEM of 5 experiments performed in triplicate. (B-C) PGD2 production and release was assessed after 30 minutes of incubation with the activators at the concentrations indicated. Data are the means ± SEM of 2 experiments performed with CBMCs from 2 different donors.P < .05 compared with cells maintained in suspension.
PGD2 release was also enhanced following coculture with 3T3 fibroblasts. In fact, CBMCs from 2 different donors activated with MBP (1 μM) generated 7.7 ± 1.1 and 88.9 ± 39.8 ng PGD2/106 cells (P < .001), whereas the same CBMCs maintained in suspension produced only 1.0 ± 0.6 and 10.5 ± 0.9 ng PGD2/106cells, respectively (Figure 2B-C).
In contrast to PGD2 production, CBMCs activated by MBP did not generate a significant amount of cys-LTs both in suspension and in coculture conditions (data not shown). In control experiments, no cys-LT or PGD2 was released from 3T3 fibroblasts that had been incubated with 22E7 antibodies (2 μg/mL), 48/80 (5 μg/mL), or MBP (1 μM).
The fibroblast-derived membrane form of SCF is responsible for priming human mast cells to IgE-independent activation
To assess whether a soluble fibroblast-derived factor might be responsible for the high mast cell susceptibility to IgE-independent activation, CBMCs were incubated with conditioned media obtained from CBMCs cocultured with 3T3 fibroblasts or were separated from 3T3 fibroblasts using Transwell membranes. Under both culture conditions, mast cell responsiveness to MBP (1 μM) was significantly lower than that of CBMCs cocultured directly on 3T3 (Figure3A). This suggests that a fibroblast membrane-associated factor rather than a soluble one is responsible for the fibroblast influences on CBMCs. The membrane-bound form of SCF has shown to partially mediate the effects of murine lung fibroblasts and human endothelial cells on murine bone marrow–derived mast cell responsiveness to IgE-dependent activation and human intestinal mast cell survival and proliferation, respectively.14,22Therefore, we investigated whether membrane SCF expressed on the fibroblasts during coculture is responsible for the increased susceptibility of human mast cells toward non-IgE–dependent activation. For this purpose, the fibroblast expression of the membrane form of SCF was modulated using antisense oligonucleotides for SCF. CBMCs cocultured with 3T3 and treated with antisense SCF oligonucleotides (0.1 pM) released 83.3% less histamine than sense-treated cells when activated by MBP (1 μM;P < .05; n = 3; Figure 3B). Antisense-treated cells were also completely inhibited in their production of PGD2after stimulation with MBP (1 μM; data not shown). Similar results were obtained when CBMCs were cocultured with embryonic fibroblasts from Sl/Sld mutant mice that produce the soluble but not the membrane form of SCF (Figure 3C).23
Effects of the membrane form of SCF on mast cell histamine release induced by MBP.
CBMCs were maintained in the following conditions in the presence of soluble SCF (100 ng/mL) for 4 days before activation: (A) with conditioned medium of CBMCs and 3T3 fibroblast cocultures (■), in Transwell membranes to separate them from 3T3 fibroblasts (▪), in coculture with 3T3 fibroblasts (▨); (B) in coculture with 3T3 fibroblasts previously treated overnight with 0.1 pM SCF sense (▪) or SCF antisense (■); (C) in coculture with embryonic fibroblasts from Sl/Sld mutant mice (■) or with 3T3 fibroblasts (▪). Histamine release was assessed after 30 minutes of incubation with the activators at the concentrations indicated. Data are the means ± SEM of 3 experiments performed in duplicate. P < .05 compared with CBMCs cocultured with 3T3 fibroblasts (A,C) or with sense-treated cells (B).
Effects of the membrane form of SCF on mast cell histamine release induced by MBP.
CBMCs were maintained in the following conditions in the presence of soluble SCF (100 ng/mL) for 4 days before activation: (A) with conditioned medium of CBMCs and 3T3 fibroblast cocultures (■), in Transwell membranes to separate them from 3T3 fibroblasts (▪), in coculture with 3T3 fibroblasts (▨); (B) in coculture with 3T3 fibroblasts previously treated overnight with 0.1 pM SCF sense (▪) or SCF antisense (■); (C) in coculture with embryonic fibroblasts from Sl/Sld mutant mice (■) or with 3T3 fibroblasts (▪). Histamine release was assessed after 30 minutes of incubation with the activators at the concentrations indicated. Data are the means ± SEM of 3 experiments performed in duplicate. P < .05 compared with CBMCs cocultured with 3T3 fibroblasts (A,C) or with sense-treated cells (B).
The fibroblast-derived membrane form of SCF induces Gi3 expression in human mast cells
We next determined one of the possible mechanisms by which mast cells in coculture with fibroblasts increase their responsiveness to IgE-independent activation. It is known that IgE-independent secretagogues stimulate rat peritoneal mast cells by directly activating Gi3, a Ptx-sensitive Gi protein that leads to mast cell exocytosis and eicosanoid generation.24 25 Therefore, we investigated the expression of Gi3 protein in CBMCs in suspension and after coculture. As shown in Figure 4A, coculture of CBMCs with 3T3 fibroblasts resulted in a marked increase (6.2-fold) in the expression of the α subunit of Gi3 compared with CBMCs maintained in suspension as determined by immunoblotting. Preincubation with Ptx significantly inhibited histamine and PGD2released by MBP-activated CBMCs (75.4% and 94.4% inhibition, respectively).
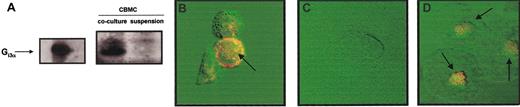
CBMCs cocultured with 3T3 fibroblasts increased their expression of Gi3α protein.
(A) SDS-PAGE immunoblot was performed with lysates of CBMCs maintained in suspension or cocultured with 3T3 fibroblasts for 4 days in the presence of soluble SCF (100 ng/mL). Gi3α purified from bacterial lysates was used as positive control. Confocal microscopy images of Gi3α protein under orange fluorescence (FITC) showing: (B) membrane localization in CBMCs maintained in coculture with 3T3 fibroblasts for 4 days in the presence of SCF (100 ng/mL), (C) no expression in CBMCs maintained in suspension for 4 days in the presence of SCF (100 ng/mL), and (D) perinuclear localization in freshly isolated rat peritoneal mast cells. The displayed figures are from a representative single experiment of 3. Arrows indicate Gi3α expression with the mast cells. Original magnification B-D, × 63.
CBMCs cocultured with 3T3 fibroblasts increased their expression of Gi3α protein.
(A) SDS-PAGE immunoblot was performed with lysates of CBMCs maintained in suspension or cocultured with 3T3 fibroblasts for 4 days in the presence of soluble SCF (100 ng/mL). Gi3α purified from bacterial lysates was used as positive control. Confocal microscopy images of Gi3α protein under orange fluorescence (FITC) showing: (B) membrane localization in CBMCs maintained in coculture with 3T3 fibroblasts for 4 days in the presence of SCF (100 ng/mL), (C) no expression in CBMCs maintained in suspension for 4 days in the presence of SCF (100 ng/mL), and (D) perinuclear localization in freshly isolated rat peritoneal mast cells. The displayed figures are from a representative single experiment of 3. Arrows indicate Gi3α expression with the mast cells. Original magnification B-D, × 63.
In confocal microscopy images, Gi3α expression appeared to be highly localized in the plasma membrane of the CBMCs cocultured with 3T3 fibroblasts (Figure 4B). Similar results of increased presence of Gi3α were obtained in human lung mast cells cocultured with human lung fibroblasts (data not shown). Immunoblotting analysis of CBMCs maintained in suspension did not reveal any expression in Gi3α (Figure 4C). Freshly isolated rat peritoneal mast cells, as expected, constitutively expressed Gi3α24 (Figure 4D).
As assessed by immunoblotting and further densitometric analysis, CBMCs cocultured with 3T3 and treated with antisense for SCF expressed less Gi3α than the sense-treated cocultures (2.4-fold; Figure5A) and less Gi3α than the untreated cocultured mast cells (3.7-fold; Figure 4A). A similar decrease in Gi3α expression was observed in confocal microscopy images of antisense (Figure 5B) compared with sense-treated cocultures (Figure 5C). Furthermore, the same cocultures treated with antisense for SCF were unresponsive to MBP (Figure 3B).
Effects of the membrane form of SCF on the mast cell expression of Gi3α protein.
SDS-PAGE immunoblot (A) and confocal microscopy images of Gi3α protein under orange fluorescence (FITC) were performed with CBMCs maintained in the presence of soluble SCF (100 ng/mL) and cocultured for 4 days with 3T3 fibroblasts previously treated overnight with 0.1 pM SCF sense (B) or SCF antisense (C). The displayed figures are from a representative single experiment of 3. Original magnification B-C, × 63.
Effects of the membrane form of SCF on the mast cell expression of Gi3α protein.
SDS-PAGE immunoblot (A) and confocal microscopy images of Gi3α protein under orange fluorescence (FITC) were performed with CBMCs maintained in the presence of soluble SCF (100 ng/mL) and cocultured for 4 days with 3T3 fibroblasts previously treated overnight with 0.1 pM SCF sense (B) or SCF antisense (C). The displayed figures are from a representative single experiment of 3. Original magnification B-C, × 63.
Discussion
In this work, we have demonstrated for the first time that highly purified human lung mast cells are responsive to the eosinophil mediator MBP when in coculture with fibroblasts. Coculture with fibroblasts is an in vitro culture system that more closely resembles in vivo conditions than the in vitro culture system usually used, that is, keeping the cells in suspension. Furthermore, CBMCs cocultured with fibroblasts were more responsive to MBP and compound 48/80 compared with the same cell population maintained in suspension.
It has been shown that incubation of rat peritoneal mast cells with native MBP and eosinophil cationic protein (ECP) but not eosinophil-derived neurotoxin (EDN) results in a concentration-dependent histamine release that requires both [Ca2+] and metabolic energy.26 In addition, we have recently demonstrated that IgE-desensitized rat peritoneal mast cells release histamine following incubation with MBP. The mechanism causing this release is similar to that of IgE-independent stimuli induced by cationic compounds such as compound 48/80 and substance P.4 However, human mast cell responsiveness toward eosinophil mediators remains less extensively investigated as yet. A previous study has shown that only partially purified human heart mast cells, but not human lung and skin mast cells, released histamine, tryptase, and PGD2 when incubated with ECP and MBP.5 In the present study, we took into account that mast cell functionality/phenotype can be dramatically affected by the microenvironment.1 For example, murine bone marrow–derived mast cells that are mucosal-type mast cells, in coculture with 3T3 fibroblasts, changed their phenotype toward connective tissue mast cells, the rodent counterpart of MCTC, and acquired responsiveness to IgE-independent stimuli in these culture conditions.10,11 Importantly, mast cells lost their responsiveness to IgE-independent activation when the cocultures are dispersed.27 This observation suggests that mast cell functionality or phenotype (or both) may be rapidly altered once the cells are isolated from the tissue influence. This might be in line with the lack of responsiveness of freshly isolated human lung mast cells toward IgE-independent activation. In the present study, we found that human lung mast cells respond to MBP after coculture with human lung fibroblasts, whereas freshly isolated and highly purified human lung mast cells are unresponsive to the same stimulus. Moreover, CBMCs that were only slightly responsive to MBP when challenged in suspension, on coculture with 3T3 fibroblasts became more responsive to this activation and released more histamine and PGD2, but not cys-LTs. Similar results were found with another cationic secretagogue, compound 48/80.
The preferential production of PGD2 over cys-LTs by mast cells when activated by IgE-independent stimuli can be attributed to the larger requirement for Ca2+ by the lipoxygenase than by the cyclooxygenase pathway to exert their enzymatic activities.28 In fact, it is known that the increase in intracellular Ca2+ concentration, after mast cell IgE-independent activation, is derived mainly from intracellular stores and is lower than that induced after IgE-dependent activation, derived from both extracellular and intracellular stores.29 In agreement with this, we observed that CBMCs cocultured with 3T3 fibroblasts produced and released more cys-LTs than PGD2after IgE-dependent activation (130 ± 43.0 pg/mL cys-LTs compared with cells maintained in activation buffer that released only 20.0 ± 5.0 pg/mL cys-LTs). Therefore, the increase in PGD2 generation during mast cell coculture with fibroblasts is not compensating a possible down-regulation of the signaling pathways that lead to cys-LT production.
Besides the low calcium requirements of PGD2production,28 up-regulation in the cyclooxygenase (COX) expression may occur during mast cell coculture with fibroblasts. Indeed, it has been recently shown that murine bone marrow–derived mast cells in coculture with fibroblasts express higher levels of prostaglandin endoperoxide synthase mRNA and protein after stimulation with compound 48/80.10 A similar change in arachidonic acid profile due to an induction of leukotriene C4 synthase expression has been recently reported in CBMCs exposed to IL-4.30 However, in preliminary experiments we could not detect COX-2 up-regulation in CBMCs cocultured with 3T3 fibroblasts (data not shown).
Some of the fibroblast influences on mast cells such as survival, proliferation, maturation, and IgE-dependent activation are known to be mediated by SCF.31-34 Furthermore, SCF induces eosinophil recruitment in the murine model of allergic airway inflammation by priming mast cells to synthesize higher levels of eotaxin, a strong eosinophil chemoattractant factor.35 This together with our observation of SCF produced by eosinophils,20 are strong evidence of the importance of SCF in allergic inflammation.
SCF is normally found in both soluble and membrane forms as a result of differential splicing and proteolytic cleavage.36 The differential effects of the soluble and membrane form of SCF on mast cells have not yet been extensively studied. However, the involvement of a fibroblast membrane-derived factor, rather than a soluble one, prompted us to consider SCF in its membrane form as the possible regulator of mast cell responsiveness to IgE-independent stimulus. Indeed, mast cells cocultured with fibroblasts in the presence of the soluble, but not the membrane, form of SCF released much less histamine and PGD2 when incubated with MBP. In contrast, mast cell survival and adhesion to the fibroblasts in the same culture conditions were not affected by the absence of the membrane form of SCF (data not shown). Similarly, it has been shown that administration of exogenous soluble SCF to Sl/Sld mice increased mast cell numbers indicating differential effects of the 2 forms of SCF on mast cell responses.37
SCF triggers its biologic effects by binding to its receptor, c-kit, a member of the type III receptor tyrosine kinase family.37-39 The existence of different isoforms of c-kit for soluble and membrane forms of SCF has not been reported. However, several works have provided evidence that the membrane forms of some growth factors are involved in signaling events distinct from those mediated by the diffusible forms even when acting on the same receptor. For example, growth and survival regulation have been attributed specifically to the membrane form of the heparin-binding epidermal growth factor (EGF)–like growth factor (proHB-EGF), rather than to the diffusible, processed HB-EGF isoform.40 Moreover, the membrane form of SCF has been shown to induce a prolonged activation of c-kit, inducing more efficient signals for the survival and differentiation of the factor-dependent myeloid cell line MO7e.41 In accordance with this, we observed that the membrane form of SCF up-regulates the expression of the α subunit of Gi3. This Gi3 overexpression may contribute to mast cell responsiveness to the IgE-independent activator, MBP. Indeed, similar correlation between Gi3 expression and responsiveness to compound 48/80 was found in quercetin-treated rat peritoneal mast cells.42 Gi3 is expressed both in the Golgi apparatus and in the plasma membrane. It is the plasma membrane-bound form of Gi3 that appears to facilitate regulated exocytosis because it is more accessible to interact with cationic secretagogues.24 In correlation with this, we found that Gi3α expression appeared to be highly localized in the plasma membrane of CBMCs. Besides this observation, it is possible that other changes in IgE-independent signaling can be induced during mast cell–fibroblast coculture as well, for example, a decrease in the rate of Gα guanosine triphosphatase (GTPase) activity that regulates Gi3-mediated exocytosis.43
In conclusion, we demonstrated that human lung mast cells and human CBMCs can be activated by an allergen-independent eosinophil-derived stimulus. This event can feasibly occur during the late phase of the allergic process when mast cells encounter the infiltrated and activated eosinophils. Mast cell responsiveness to eosinophil mediators is specifically influenced by the membrane form of SCF.
Together these findings are important in the understanding of microenvironment influences on mast cell functionality that dictate either the resolution or the aggravation of a pathologic condition through the interaction with other inflammatory cells such as eosinophils or structural cells such as fibroblasts.
We wish to thank Prof Ronit Sagi-Eisenberg and Prof Hermona Soreq for helpful discussions. We also thank Dr Mark Tarshish for technical assistance with confocal microscopy images and Mrs Madelyn Segev for her excellent secretarial assistance.
Prepublished online as Blood First Edition Paper, October 10, 2002; DOI 10.1182/blood-2002-05-1488.
Supported by a grant from the Aimwell Charitable Trust (United Kingdom). A.M.P. is supported by a grant from the Neville Boland Perpetual Endowment Fund in Allergy Related Research. F.L.-S. is affiliated with the David R. Bloom Center of Pharmacy at The Hebrew University of Jerusalem (Israel). G.J.G. is supported by AI-09728 from the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Francesca Levi-Schaffer, Department of Pharmacology, School of Pharmacy, Faculty of Medicine, The Hebrew University, Jerusalem 91120, Israel; e-mail:fls@cc.huji.ac.il.