Toxicities of high-dose conditioning regimens have limited the use of conventional unrelated donor hematopoietic cell transplantation (HCT) to younger, medically fit patients. Based on preclinical studies, an HCT approach has been developed for elderly or medically infirm patients with HLA-matched or mismatched unrelated donors. In this study, 52 patients with hematological diseases were included. Most (88%) had preceding unsuccessful conventional HCT or refractory/advanced disease. Patients were treated with fludarabine 30 mg/m2/d from days −4 to −2, 2 Gy total body irradiation on day 0, cyclosporine at 6.25 mg/kg twice daily from day −3, and mycophenolate mofetil at 15 mg/kg twice daily from day 0. Durable donor chimerism was attained in 88% of the patients. By day 28, a median of 100% of CD56+ cells were of donor origin. Granulocyte and T-cell donor chimerism increased to medians of 100% on day 56 and day 180 (range, 55%-100%), respectively. Acute GVHD, grade II, was seen in 42% (CI, 29%-56%); grade III in 8% (CI, 0%-15%); and grade IV in 13% (CI, 4%-23%) of patients; it was fatal in 9%. The 100-day transplantation-related mortality was 11%. Complete remissions, including molecular remissions, were seen in 45% of patients with measurable disease before transplantation. Mortality from disease progression was 27% at one year. With a median follow-up of 19 months, 18 of the 52 patients (35%) were alive and 25% were in remission. HCT from HLA-matched or mismatched unrelated donors can be performed with a reduced intensity conditioning regimen in patients ineligible for conventional HCT.
Introduction
Conventional allogeneic unrelated hematopoietic cell transplantation (HCT) for patients with hematological malignancies involves conditioning with high doses of systemic chemo/radiation therapy.1-3 Regimen-related toxicities have limited the procedure to medically fit patients generally no older than 50 years, with therapy administered on specialized hospital wards. Given that median ages of patients with chronic myelocytic leukemia (CML), acute myelocytic leukemia (AML), chronic lymphocytic leukemia (CLL), multiple myeloma (MM), myelodysplastic syndromes (MDS), and non-Hodgkin lymphoma (NHL) range from 65-70 years, most patients with these diseases are not candidates for conventional unrelated HCT.4 In an attempt to reduce morbidity and mortality associated with allogeneic HCT in elderly patients, reduced-intensity regimens have been developed with the aim to obtain donor engraftment and use graft-versus-tumor effects to eradicate underlying malignancies.5-12 In these protocols, donor engraftment is achieved with regimens conveying different degrees of myelosuppression ranging from minimal to severe. Based on preclinical studies in the canine model, we developed an allogeneic HCT approach with minimal hematopoietic and overall toxicities, in which the burden of tumor eradication has been shifted from cytotoxic therapy to graft-versus-tumor effects.11,13The conditioning regimen consists of 2 Gy total body irradiation (TBI), and both engraftment and graft-versus-host disease (GVHD) are controlled with postgrafting immunosuppression using mycophenolate mofetil (MMF) combined with cyclosporine (CSP). Early results with HLA-identical sibling grafts in elderly or medically infirm patients with hematological malignancies using this regimen have been encouraging, and remissions, including molecular remissions, have been accomplished in more than half of patients receiving transplants.11 Here, we present data extending this approach to patients with HLA-matched and mismatched unrelated donors. In contrast to the transplants with related grafts, patients were given 3 doses of fludarabine in addition to 2 Gy TBI to assure engraftment.
Patients and methods
Patients
Between October 26, 1998, and July 14, 2000, 52 consecutive patients were treated at the University of Leipzig, Fred Hutchinson Cancer Research Center/University of Washington Medical Center and Veteran's Administrations Medical Center in Seattle, WA, Stanford University, CA, and the University of Colorado, CO. Results were analyzed as of September 1, 2001.
Included in this study were patients with hematological diseases treatable by allogeneic HCT who were ineligible for conventional allogeneic HCT because of age (older than 50 years) and/or concomitant diseases (eg, liver cirrhosis, Shwachman-Diamond syndrome) or preceding extensive therapies, such as failed autologous or allogeneic HCT. Exclusion criteria were creatinine clearance ≤ 50 mL/min, cardiac ejection fraction ≤ 30%, bilirubin > 2 × and/or transaminases > 4 × upper limit of normal, diffusing lung carbon monoxide (DLCO) < 35%, and Karnofsky performance score < 50.
Patient characteristics are summarized in Table1. Underlying diseases were MDS (n = 8), including 2 patients evolved to AML, AML (n = 10), acute lymphoblastic leukemia (ALL) (n = 2), CML (n = 12), CLL (n = 3), NHL (n = 6), HD (n = 1), MM (n = 8), Waldenström macroglobulinemia (WM, n = 1), and paroxysmal nocturnal hemoglobinuria (PNH, n = 1). Most patients (n = 46; 88%) were high-risk candidates as defined by relapse after preceding HCT or by refractory/advanced disease. All other patients were considered low risk (n = 6). Overall, 22 patients (42%) had failed at least one previous autologous or allogeneic HCT. Median patient age was 48 years (range, 6-65 years), and median donor age was 35 years (range, 19-58 years). The study protocol was approved by the Institutional Review Boards of the participating institutions (University Hospital, Leipzig, Germany; Fred Hutchinson Cancer Research Center, Seattle, WA; University Hospital Stanford, CA; and University of Colorado, Denver) and written informed consents approved by the Institutional Review Boards were obtained from all patients.
HLA typing
The 52 donor-recipient pairs were selected on the basis of serological matching for HLA-A, B, and C, molecular matching for HLA-DRB1, and in 22 patients for HLA-DQB1. Of donor-recipient pairs, 15 (29%) were HLA mismatched, and 37 (71%) were matched at the antigen level. Retrospective allele level typing was performed on 49 of the 52 donor-recipient pairs for HLA-A, B, C, DQB1, and DPB1 alleles using direct automated fluorescent methods as previously described.14 15 Of the 15 pairs with antigen mismatches, 6 were found to encode additional allele mismatches, and 5 of the 37 HLA antigen-matched pairs were found to be mismatched for one HLA allele. Overall, one or more antigen and/or allele level mismatches were recognized in 20 of the 52 pairs (38%; Table 1). Mismatches by rejection vector (host-versus-graft direction) were present in 19 pairs, including one (n = 12), 2 (n = 6), and 3 (n = 1) mismatches and by GVHD vector (graft-versus-host direction) in 17 pairs including one (n = 9), 2 (n = 6), and 3 (n = 2) mismatches. Results of these studies are shown in Table 1.
Treatment and evaluations
Patients were treated with 3 doses of fludarabine, 30 mg/m2/d, from days −4 to −2 and a single fraction of 2 Gy total body irradiation (TBI) delivered at 0.07 Gy/min from linear accelerators on day 0. In the afternoon of day 0, donor hematopoietic cells were infused. In 39 patients, the source of donor hematopoietic cells were granulocyte colony–stimulating factor (G-CSF)–stimulated peripheral blood cells (PBCs) containing medians of 6.0 × 106 (range, 1.5 × 106-21.6 × 106) CD34+and 3.4 × 108 (range, 0.8 × 108-10.0 × 108) CD3+cells/kg. In 13 patients marrow grafts were infused containing medians of 2.7 × 106 (range, 1.2 × 106-5.2 × 106) CD34+and 0.3 × 108 (range, 0.2 × 108-0.6 × 108) CD3+cells/kg. CSP was administered orally at 6.25 mg/kg twice daily from day −3. CSP levels were targeted to the upper therapeutic range (about 500 ng/mL; Abbott TDX, Abbott Park, IL) until day +35 and then discontinued (one patient). In the subsequent 10 patients, CSP was tapered from day 64 after transplantation until day 180. In the remaining 41 patients, CSP was tapered starting on day 100 through day 180. MMF was given orally at 15 mg/kg beginning in the afternoon of day 0 and then twice daily to day +27 (first 13 patients). In the remaining 39 patients, MMF was given in full doses to day +40 and then tapered through day +96.
Standard prophylaxis against Pneumocystis carinii, fungal infections, toxoplasmosis, and cytomegalovirus (CMV) infections was used.16-18 Patients with chronic GVHD requiring systemic immunosuppressive therapy continued prophylaxis against P carinii and pneumococcal infections. For outpatient transplantations, scheduled follow-up included 2 to 3 clinic visits per week for the first month, and then once or twice weekly or as clinically indicated thereafter.
The degrees of donor chimerism among peripheral blood T cells, granulocytes, and nucleated marrow cells were assessed at days 28, 56, 84, 180, and 360 after HCT using fluorescence in situ hybridization to detect X and Y chromosomes for recipients of grafts from sex-mismatched donors, and polymerase chain reaction (PCR)–based analyses of polymorphic microsatellite regions for recipients of sex-matched transplants.19 In patients who received transplants at the University of Leipzig, donor chimerism was also evaluated on day 14 after HCT.
The primary study end point was mixed chimerism on day 28, defined as between 5% and 95% peripheral blood donor T cells. Disease evaluations were performed monthly. Donor lymphocyte infusions (DLIs) were given to 6 patients for relapse/disease progression between 57 and 382 (median, 135) days after HCT (median, 10 × 106/kg CD3+ cells infused). Of the 6 patients, 4 received a second dose DLI (median, 43 × 106/kg CD3+cells).
Acute and chronic GVHD were graded as described.20,21Disease responses were assessed using standard criteria. PCR-based techniques were used to test for bcr-abl transcripts (CML),22 clonal immunoglobulin rearrangements (lymphoid malignancies), or other chromosomal translocations. Toxicities were determined using the Common Toxicity Criteria, Version 2.0.
Statistical analyses
Survival and progression-free survival were estimated by the Kaplan-Meier method. Cumulative incidence estimates were calculated for acute GVHD, relapse, and mortality from various causes.23Risk factors for acute GVHD, rejection, relapse, and survival were analyzed using proportional hazards regression models, treating death prior to acute GVHD, rejection, or relapse as a competing event. Rejection was treated as a competing event for analysis of acute GVHD and as a time-dependent covariate for analyses of death and relapse. The factors considered for survival, TRM, and relapse were disease risk, diagnosis, Class I and Class II mismatch, and rejection. The multivariate models were constructed in a stepwise fashion, and no more than 2 variables ever entered any of the models. All Pvalues were derived from likelihood ratio statistics and were 2-sided.
Results
Peripheral blood cell changes, allogeneic engraftment, and graft rejection
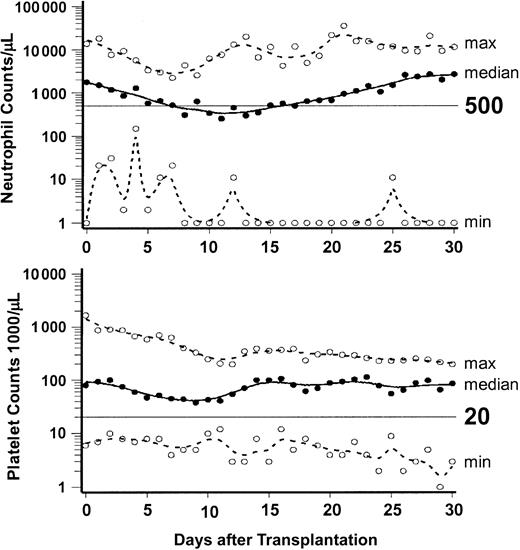
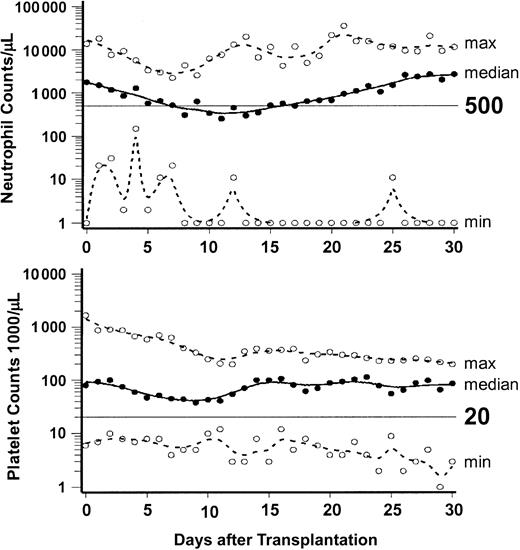
Figure 1 summarizes peripheral blood neutrophil and platelet changes to day +30 after HCT. A median of 4 units (range, 0-166 units) of platelets and a median of 8 units (range, 0-34 units) of red blood cells were transfused. Nine patients (17%) did not require any transfusions, 16 did not require platelet transfusions, and 9 did not require red blood cell transfusions. No adverse hemolytic reactions occurred in 32 blood group (18 minor and 14 major) incompatible transplantations. Median hospital stay for 22 patients treated in the US and eligible for outpatient therapy was 16 days (range, 0 to 56 days), while the 30 patients who received transplants in Germany and were ineligible for outpatient treatment had a median hospital stay of 40 days (range, 13 to 122 days).
Engraftment after unrelated HCT.
Neutrophil and platelet changes after HCT. Graphs show the medians (solid lines) and ranges (broken lines) of neutrophil and platelet counts of the 52 patients from day 0 through day 30. ○ indicates the minimum and maximum values on each day.
Engraftment after unrelated HCT.
Neutrophil and platelet changes after HCT. Graphs show the medians (solid lines) and ranges (broken lines) of neutrophil and platelet counts of the 52 patients from day 0 through day 30. ○ indicates the minimum and maximum values on each day.
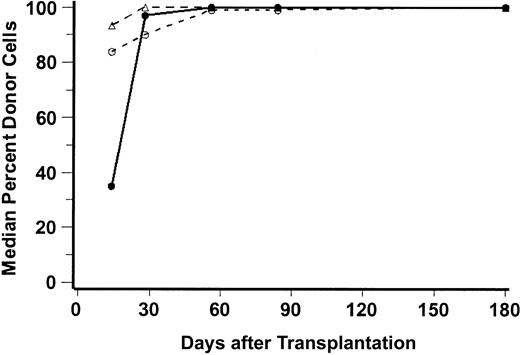
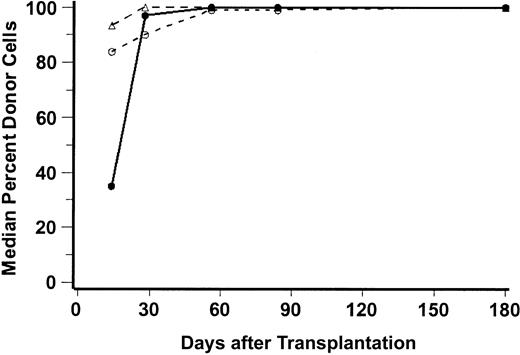
Complete donor chimerism (100% donor cells) was generally attained by day 28 for natural killer (NK) cells, by day 56 for granulocytes, and by day 180 for CD3+ cells. Figure2 illustrates the median percentages of peripheral blood donor CD3+ cells, CD56+ cells, and granulocytes through day 180, and Table2 shows the percentages of donor CD3 cells on days 28 and 56 for each individual patient.
Percent donor chimerism after unrelated HCT.
Median percentages of donor peripheral blood CD56+ cells (NK-cells, ▵), CD3+ cells (T-lymphocytes; ○), and CD15+ cells (granulocytes; ●) are shown for the first 180 days after transplantation. The numbers of patients analyzed at days 28, 56, 84, and 180 were, respectively, 45, 40, 37, and 27 for T cells; 46, 40, 38, and 26 for granulocytes; and 27, 21, 19, and 15 for NK cells.
Percent donor chimerism after unrelated HCT.
Median percentages of donor peripheral blood CD56+ cells (NK-cells, ▵), CD3+ cells (T-lymphocytes; ○), and CD15+ cells (granulocytes; ●) are shown for the first 180 days after transplantation. The numbers of patients analyzed at days 28, 56, 84, and 180 were, respectively, 45, 40, 37, and 27 for T cells; 46, 40, 38, and 26 for granulocytes; and 27, 21, 19, and 15 for NK cells.
Of the 52 patients, 3 died too early to be evaluable for engraftment. Of the remaining 49 patients, 6 (12%; CI, 5%-25%) rejected their grafts between 21 and 56 days after HCT and had autologous marrow recoveries. Factors predictive of graft rejection in univariate analysis were low T-cell contents in the grafts (P = .04) and diagnosis of MDS (P = .03). Trends were observed for low numbers of transplanted CD34+cells (P = .06) and lack of preceding cytotoxic chemotherapy (P = .08). There were no correlations between rejection and HSC source (PBSC vs BM), patient or donor ages, and sex.
Regimen-related toxicities and infections
None of the patients experienced new-onset alopecia. Mild to moderate nausea due to MMF/CSP was common. Reversibly elevated serum creatinine or bilirubin levels ascribed to CSP were the most frequent toxicities (Table 3).
Septicemias were encountered in 8 patients (15%; CI, 7%-28%). These were associated with pneumonia (n = 5), enteritis (n = 5), and/or sinusitis (n = 3). Three patients had toxoplasmosis, and one had pericarditis and pneumonia from Legionella pneumophila. One patient died of septicemia and one of pneumonia; all other infections were successfully treated with antibiotics. One patient died 624 days after transplantation after urosepsis and hydronephrosis caused by prostate hypertrophy.
Nine patients had proven or suspected pneumonias of fungal origin after HCT; 5 of these were successfully treated. Of the 4 patients with persisting pneumonia, one died of relapse, 2 died with GVHD, and one died after a second transplantation following failure of myeloid engraftment of the first.
HSV infections were observed in 6 patients (12%; 4%-23%), CMV reactivations/infections in 15 (29%; CI, 17%-43%), suspected viral pneumonitis in 4, varicella zoster virus (VZV) infection in 1, HHV6 infection in 1, Epstein-Barr virus (EBV) lymphoproliferative disease in 1, and suspected viral encephalitis in 3 patients. Lethal viral infections were not observed.
Acute GVHD
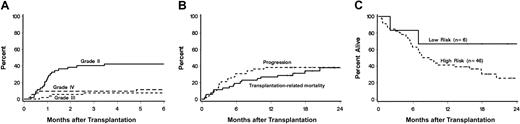
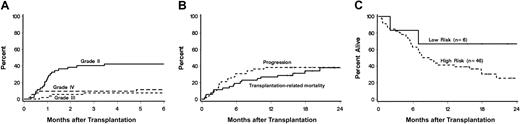
Thirty-three patients (63%; CI, 50%-77%) developed acute GVHD, which was grade II in 22 (42%;CI, 29%-56%), grade III in 4 (8%; CI, 0%-15%), and grade IV in 7 (13%, CI, 4%-23%; Table 2 and Figure3A). Acute GVHD was diagnosed at a median of 30 days (range, 8-284 days) after HCT and affected skin (n = 32), gut (n = 20), and liver (n = 10). Neither recipient and donor ages, recipient and donor sex, HSC source, underlying diseases, nor preceding treatment were predictive for acute GVHD. However, a correlation was suggested between HLA-class I disparity (P = .06) and grades III-IV acute GVHD.
Acute GVHD (A), relapse and transplantation-related mortality (B), and overall survival (C) after unrelated HCT.
Panel A shows the cumulative incidences of acute grades II, III, and IV GVHD among all 52 patients. Panel B shows the probabilities of disease progression and transplantation-related mortality for all 52 patients. Panel C shows survivals among high-risk and low-risk patients.
Acute GVHD (A), relapse and transplantation-related mortality (B), and overall survival (C) after unrelated HCT.
Panel A shows the cumulative incidences of acute grades II, III, and IV GVHD among all 52 patients. Panel B shows the probabilities of disease progression and transplantation-related mortality for all 52 patients. Panel C shows survivals among high-risk and low-risk patients.
In most patients, GVHD responded to CSP and standard courses of methylprednisolone.24 In 5 patients, OKT3 was used because of steroid-resistant GVHD.
Incidence of chronic GVHD
Of 43 evaluable patients, 13 (30%) developed chronic GVHD requiring systemic immunosuppressive therapy.21 Eight patients died from complications associated with either acute or chronic GVHD (15%).
Status of underlying diseases
Table 2 summarizes the results.
Patients with graft rejection.
Of the 6 rejecting patients, 3 died from disease progression, and 3 died from infections after second HCT: one in complete remission (CR), one in partial remission (PR), and one with stable disease.
Patients with sustained engraftment.
Thirty-three patients had measurable disease before HCT, and 15 (45%) of these patients achieved CR at variable times after HCT. Two patients with ALL receiving transplants in second CR died in CR with infections with or without GVHD. Of 6 AML patients receiving transplants in first, third, or fourth CR, 2 were alive in CR, while 4 died in CR from transplantation complications. Of 4 AML patients with persistent disease who received transplants, 2 were alive in CR, one died following urosepsis in CR, and one died of relapse. Of 3 patients with CLL, 2 were alive in CR, while one died in CR from suicide. Of 10 patients with CML who received transplants in first or second chronic phase, accelerated phase, or blast crisis, 2 were alive in CR, 2 died in CR with complications from GVHD, 4 died from disease progression or relapse, and 2 died early (one from thrombotic thrombocytopenic purpura [TTP] and one with acute GVHD) without being evaluable for the underlying disease. Of 7 patients with NHL, 3 were in sustained CR after transplantation, 2 relapsed and are alive, one died of disease progression, and one died in CR of veno-occlusive disease/acute respiratory distress syndrome (VOD/ARDS). One patient with WM had stable disease, developed de novo AML of host cell origin, and died during induction therapy of his AML. Of 5 patients with MDS, 2 were alive, one in CR and one with stable disease, and 3 died: one with sepsis, one with pneumonia, and one with relapse. Of 7 patients with MM, 2 were alive in relapse, and 5 died, 4 either directly or indirectly from relapse and one with acute GVHD. The patient with PNH was alive and free of disease.
DLI
Six patients received DLI. One patient with AML achieved CR, relapsed, and did not respond to a second DLI. One patient with CML progressed to blast crisis after DLI and died. Of 4 patients with MM, 3 responded with transient PR, and one patient had no response.
Survival and causes of death
With a median follow-up of 19.3 months (range, 15.3-28.1 months), 18 of the 52 patients (35%) were alive. Of these, 13 were in CR, 1 had stable disease, and 4 have relapsed or progressed. Twelve patients (23%) died of causes related to transplantation, including GVHD (n = 8), pneumonia (n = 1), TTP (n = 1), VOD/ARDS (n = 1), and infection and multiorgan failure (n = 1). Among the remaining 22 patients who died, fourteen (27%) died from relapse/progression of their underlying diseases; one committed suicide on day 482 after HCT; one died from suspected myocardial infarction on day 156; one died from hemorrhage due to a congenital Dieulafoy arterial malformation25 on day 144; 3 died after rejection, disease recurrence, and second allogeneic HCT from infections; one died after induction therapy of de novo AML in host cells; and one died after urosepsis 624 days after transplantation. The median survival was 271 days.
Figures 3B and 3C show survival, progression, and transplantation-related mortality. Overall, survival was 44% (CI, 31%-58%) at 1 year. For low-risk patients, survival was 67% (CI, 29%-100%), and for high-risk patients, 41 (CI, 27%-56%). There was no statistically significant difference in survival between HLA-matched and mismatched patients (Tables 1 and 2). Overall mortality directly related to the first unrelated donor transplantation was 29 (CI, 17%-41%) at one year. Mortality from disease progression was 27% (CI, 15%-39%) at one year. In a multivariate analysis, rejection (P = .03) and HLA class II mismatch (P = .04) adversely affected survival. Not surprisingly, rejection was a risk factor for relapse (P = .02).
Discussion
This study extends previous observations made in HLA-identical siblings to include grafts from HLA-matched or mismatched unrelated donors. None of the patients were eligible for conventional HCT, either because of age (median age, 48 years) or medical contraindications. Most (88%) were considered high-risk patients, and 42% had failed previous autologous or allogeneic HCT. The study results allowed several conclusions.
First, the regimen was well tolerated and minimally toxic without the typical side effects of conventional HCT. Of 3 patients who died within the first 3 weeks, 2 died from pre-existing infections, which had made them ineligible for conventional HCT, and the third had a long history of chemotherapy, radiation therapy, and 2 unsuccessful high-dose conventional autologous HCTs in addition to developing hyperacute GVHD. The day-100 transplantation-related mortality was 11%. This compared favorably to the 29% day-100 mortality among younger (median age, 20 years) patients with acute leukemias given conventional unrelated HCT26 and to the 41% day-100 mortality among younger patients (median age, 28 years) given conventional allogeneic HCT after preceding failed autologous HCT.27 In addition, even though many patients had abnormally low granulocyte and platelet counts before transplantation, severe protracted pancytopenias were generally not encountered after transplantation. Because of the mild myelosuppression of the current regimen, many patients were managed either as outpatients or in regular hospital rooms and generally did not require hematopoietic growth factor support as has been reported by others.9,12 Transfusion needs were significantly lower than those among patients given conventional HCT.28Considering the patients' ages, the extent of preceding therapies, and their pre-existing organ dysfunction or infections, the regimen had acceptable toxicities. The observed 100-day transplantation-related mortality of 11% compared favorably to those in another recent study, which ranged from 37.4%-87.5%, depending on the reduced-intensity conditioning regimen used and on whether hematopoietic cell donors were related or unrelated.9 In a second study of much younger patients (median age, 17 years [range, 8-48 years] ) undergoing reduced-intensity unrelated HCT, 2 (12.5%) of 16 patients died of transplantation-related complications within 100 days of HCT.10 A third recent study of 47 patients undergoing unrelated HCT showed a day-100 mortality of 14.9%. In this study, patient ages were lower (median, 44 years; range, 18-62 years) than in the current study, and patients with a life expectancy of < 8 weeks were excluded.12
Second, sustained allogeneic engraftment was achieved in 88% of evaluable patients, and graft rejections (n = 6) did not lead to fatal pancytopenias. Rejections occurred predominantly among patients with MDS and those with low T-cell contents in their grafts. They were characterized by either no appearance of donor cells (n = 5) or initial appearance and subsequent gradual disappearance of such cells within 56 days of HCT (n = 1). In all rejecting patients, the underlying diseases persisted and progressed, leading either directly or indirectly (failed second HCT) to death. With the limited data available, there was no discernable correlation between HLA-mismatching and rejection. We previously reported a 5% graft rejection rate among patients (median age, 35 years) with CML in chronic phase (CP) given conventional unrelated HCT,1 a rate that would appear lower than the one reported here (12%), although others have described rejection rates of 15.5% for CML patients3 and of 13% for ALL patients29 after conventional HCT, though the relatively high rate of rejection among CML patients could be due to the use of T-cell–depleted grafts among some of the patients. Others carried out unrelated grafts after reduced intensity conditioning regimens and reported either no graft rejection10 or provided no detailed information.9 Among current patients with engraftment, conversion from host to all-donor hematopoietic cells was generally complete between 3 and 6 months after HCT. Ways to avoid graft rejection in future protocols include the use of grafts with high T-cell contents and modified protocols that convey a higher degree of pretransplantation immunosuppression to overcome transfusion-induced sensitization in patients with MDS who often had no preceding chemotherapies. Optimizing MMF therapy using level-adapted dosing might be another promising approach.
Third, 42% of evaluable patients developed grade II; 8%, grade III; and 13%, grade IV acute GVHD. There was a suggested correlation between GVHD and HLA class I disparity. The current 63% rate of GVHD was comparable to rates previously reported in younger patients with acute leukemias (82%) and CML in CP (77%-89%) given conventional HCT1,26; however, the current 21% rate of grades III-IV GVHD appeared lower than the previously reported rates of 35%-47%. None of the patients with grade II and III acute GVHD died from this complication; 9% of evaluable patients died with grade IV acute GVHD. While this value appeared lower than previously reported after conventional HCT, improved GVHD control remains a critical future research objective. Chronic GVHD requiring therapy developed in 30% of patients with durable engraftment. The relatively benign course of GVHD among these elderly patients with unrelated HLA-matched and -mismatched HCT compared to conventional HCT could be the result of potent immunosuppression by the combination of MMF/CSP, although contributions of the low-intensity pretransplantation conditioning, for example, lack of tissue injury and “cytokine storm”30 and the initial mixed donor-host chimerism, also might be important. A study of unrelated HCT after a conditioning regimen containing a purine analog and melphalan reported 23% grade II and 39% grade III-IV acute GVHD, and deaths related to GVHD were observed in 27.5% of patients.9 Another study using a purine analog and melphalan in combination with CAMPATH-1H monoclonal antibody conditioning reported 14.9% grade II and 6.4% grade III-IV acute GVHD and 8% limited chronic GVHD.12 A comparison of current results with unrelated donors to those previously reported with related donors given the same regimen11 shows higher incidences of acute GVHD (63% vs 47%) and nonrelapse mortality (29% vs 6.7%). Further reduction in the incidence of GVHD might be achieved in the future by optimizing the duration of immunosuppressive therapy after transplantation and by using HLA allele–matched donors.
Fourth, no clear relationship emerged between HLA antigen or allele level mismatching and graft rejection, though a suggested correlation between HLA class I mismatch and GVHD was found. Also, HLA class II mismatch adversely affected survival.
Finally, graft-versus-tumor responses occurred among patients with sustained grafts. Of patients with measurable disease before HCT, 45% attained CR, including 6 of 16 (38%) evaluable patients with preceding failed high-dose autologous or allogeneic HCT. It is unlikely that low-dose TBI and fludarabine were responsible for the CR given that those patients, for the most part, had failed to respond to more specific and intensive therapies, including the high-dose regimens used for HCT. Thus, the current findings are most consistent with graft-versus-tumor effects. These were seen early after transplantation, especially in patients with acute myeloid leukemia, where patients in persistent disease reached remissions within a few weeks after transplantation. NK cells with killer cell inhibitory receptor (KIR) mismatches might have played a role in these rapid-onset graft-versus-tumor reactions, which were usually not associated with GVHD.31 Correlations of KIR mismatches with clinical outcome will be analyzed in current donor-patient combinations. In other diseases such as CML or CLL, graft-versus-tumor effects required months to induce remissions and became evident often only after discontinuation of immunosuppression. Selective tumor cell killing induced by tumor-specific T cells might have played a role in these remissions, and this is currently under study. Relapse mortality was comparable in the present study of unrelated HCT (25%) and in the previously published study of related HCT (27%),11 though there was a greater number of high-risk patients in the current study. The role of DLIs in current transplantations is still unclear. Only a minority of patients received DLIs, and all of them had only transient responses. In the future, more selective tumor cell killing may be accomplished through focusing T-cell immunity against tumor antigens or minor histocompatibility antigens with hematopoietic restriction.32-34
In conclusion, this phase 1 study shows that fludarabine and 2 Gy TBI in combination with cyclosporine and MMF are sufficient to obtain engraftment in most patients with unrelated HLA-matched and partially mismatched donors. Phase 2 studies will evaluate the usefulness of the graft-versus-tumor effect for individual hematological malignancies.
We thank the medical, nursing, laboratory, data processing, and secretarial staff of the participating institutions for their important contributions to this study, including the careful and dedicated care of the patients.
Prepublished online as Blood First Edition Paper,October 3, 2002; DOI 10.1182/blood-2002-05-1340.
Supported in part by grants CA78902, CA49605, HL36444, CA18221, CA18029, CA15704, AI49213, and HL03701 awarded by the National Institutes of Health, Department of Health and Human Services, Bethesda, MD; a grant from the Gabrielle Rich Leukemia Foundation (P.A.M., D.G.M.); and support from the Laura Landro Salomon Endowment Fund (F.R.S.). Chimerism studies in patients treated at University of Leipzig were supported by a grant from Amgen, Lucerne, Switzerland.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Dietger Niederwieser, Division of Hematology and Oncology, University of Leipzig, Philipp Rosenthalstr 23-25, D-4103 Leipzig, Germany; e-mail:dietger@medizin.uni-leipzig.de.