Von Willebrand factor (VWF) is synthesized in endothelial cells, where it is stored in Weibel-Palade bodies. Administration of 1-desamino-8-D-arginine-vasopressin (DDAVP) to patients with type 1 von Willebrand disease and to healthy individuals causes a rapid increase in plasma VWF levels. This increase is the result of stimulated release of VWF from Weibel-Palade bodies in certain beds of endothelial cells. The VWF propeptide (VWFpp) targets VWF to storage granules through a noncovalent association. The nature of the VWFpp/VWF interaction was investigated by using cross-species differences in VWF storage. While canine VWFpp traffics to storage granules and facilitates the multimerization of human VWF, it does not direct human VWF to storage granules. Since storage takes place after furin cleavage, this defect appears to be due to the defective interaction of canine VWFpp and human VWF. To determine the regions within VWFpp and VWF important for this VWFpp/VWF association and costorage, a series of human-canine chimeric VWFpp and propeptide-deleted VWF (Δpro) constructs were produced and expressed in AtT-20 cells. The intracellular localization of coexpressed proteins was examined by confocal microscopy. Two amino acids, 416 in VWFpp and 869 in the mature VWF molecule, were identified as being critical for the association and granular storage of VWF.
Introduction
Von Willebrand factor (VWF) is a multimeric adhesive glycoprotein that mediates platelet adhesion at the site of vascular injury and also serves as the carrier protein of factor VIII (FVIII).1 Decreased synthesis or defects in VWF function cause von Willebrand disease (VWD), a common inherited bleeding disorder.2 VWF is synthesized as a pre-pro-VWF that contains a 22–amino acid (aa) signal peptide, a 741-aa propeptide, and a 2050-aa mature VWF molecule.3 The pro-VWF molecule undergoes extensive posttranslational modifications including dimerization, glycosylation, sulfation, amino-terminal multimerization, and propeptide cleavage.4-6 VWF is synthesized exclusively in endothelial cells and megakaryocytes,7,8 where it is stored together with its propeptide (VWFpp) in regulated storage granules including Weibel-Palade bodies and in platelet α-granules, respectively.9-11 Weibel-Palade bodies contain primarily very high molecular-weight VWF multimers that are the most active for binding to subendothelial tissue and in platelet-platelet interactions.12
The large VWF propeptide, VWFpp, is required for the multimerization and regulated storage of VWF.13,14 The propeptide contains vicinal cysteines in each D domain that may have intrinsic disulfide isomerase activity and catalyze VWF multimer formation.15Deletion of either or both D domains of VWFpp eliminates multimerization and storage of VWF.16 Additionally, VWFpp can independently mediate the assembly of VWF multimers when VWFpp and propeptide-deleted VWF are coexpressed intrans.17 The VWFpp also functions as an intracellular chaperone, trafficking mature VWF multimers to storage through maintained noncovalent association following furin cleavage.18 Previously, we reported differences in cross-species interactions between canine and human VWF. While human and canine propeptide-deleted VWF (Δpro) sort to storage granules when coexpressed in cis or in trans with their respective propeptides, canine VWFpp does not sort human VWF to granules. However, the VWF is normally multimerized and canine VWFpp is stored in granules.
In this study, we have exploited this cross-species storage difference to further define the VWFpp/VWF interaction that is critical for storage of VWF. To identify regions within VWFpp important for association with mature VWF, we investigated whether the replacement of canine VWFpp amino acids with human VWFpp segments would restore granular sorting of human mature VWF. Conversely, we also asked what region within human VWF (Δpro) must contain canine sequence to sort to storage when coexpressed with canine VWFpp. Canine/human chimeric VWFpp or Δpro proteins containing variable portions of canine and human sequence were coexpressed with human Δpro or canine VWFpp, respectively. Intracellular localization of coexpressed proteins was assessed by confocal microscopy. Two amino acids have been identified that are critical for VWFpp/VWF association and granular storage.
Materials and methods
Construction of expression plasmids
The human and canine VWF propeptide (VWFpp) and propeptide-deleted VWF (Δpro) expression vectors were constructed as previously described.18 Chimeric VWFpp expression vectors (Figure 2) were constructed using either unique restriction sites (XbaI, ApaI, BstEII, orBglII) conserved between human (H) and canine (C) VWFpp or engineered BsmBI restriction sites, as previously described.18-20 Base 1 refers to the adenine of the initiator methionine that is amino acid 1 and sequence numbering is continuous through amino acid 2813 (base pair 8439). For example, the chimeric VWFpp H/C-119-VWFpp contains human signal peptide and sequence to Tyr119 followed by canine sequence through the remainder of VWFpp. The C/H-119-VWFpp is the converse and contains canine signal peptide and sequence to Tyr119 followed by human sequence. Other expression vectors were labeled in a similar manner. Three constructs (H/C/H-119/714-VWFpp, C/H/C-119/714-VWFpp, and C/H/C-119/477-VWFpp) containing multiple exchanges of sequence were developed, again based upon conserved restriction sites.
To generate point mutations in the canine VWFpp (C-VWFpp), a first-round polymerase chain reaction (PCR) was performed, using C-VWFpp as a template with mutagenic antisense VWFpp primer in combination with sense primer c-s-74-96. In a separate reaction, a mutagenic sense primer was used with the antisense primer c-a-1956-1937. Two first-round products were generated. A second-round PCR was performed using 1 μL of each first-round product as template with the nested primers c-s-421-442 and c-a-1779-1758. This second-round PCR product was cloned into vector pCR2.1 using the TA Cloning Kit (Invitrogen, Carlsbad, CA) and sequenced to verify introduction of the desired mutation. TheApaI/BssHII cassette (bases 576 to 1751) was substituted for the corresponding ApaI/BssHII fragment of C-VWFpp to create C-VWFpp expression plasmids containing the desired point mutation. A similar strategy was used to introduce a point mutation into human VWFpp (H-VWFpp) to produce H-VWFpp-Arg416Gln.
Plasmids expressing chimeric propeptide-deleted VWF (Δpro) were constructed in a similar manner, using human and canine Δpro plasmids. The H/C-907-Δpro construct expresses a protein containing human signal peptide and mature VWF sequence starting at Ser764 and continuing through Arg907, followed by canine sequence. The H/C-866-Δpro and C/H-866-Δpro were constructed using a strategy based on the enzyme BsaI, similar to the strategy used previously with BsmBI. Point mutations were introduced into human and canine Δpro using 2 rounds of PCR in a strategy similar to that used for VWFpp point mutations.
Mammalian cell culture and transfection
Two cell lines were used in this study: human embryonic kidney cells (HEK293T), which were kindly provided by D. Ginsburg (University of Michigan, Ann Arbor, MI) and mouse pituitary tumor cells (AtT-20/D16v-F2, CRL 1795; American Type Culture Collection, Manassas, VA). Both cell lines were cultured at 37°C in an atmosphere of 5% CO2. AtT-20 cells were grown in Dulbecco modified Eagle medium (DMEM) with high glucose (Life Technologies, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS) and 2 mMl-glutamine. HEK293T cells were grown in Minimal Essential Medium (MEM; Life Technologies) with Earle salts and L-glutamine supplemented with 10% FBS. AtT-20 and HEK293T cells were transiently transfected as previously described.18
Antibodies
Monoclonal antibodies AVW-5, AVW-17, and 105.4 and the polyclonal anti-VWF antibodies were produced by our laboratory. The monoclonals AVW-5 and 105.4 and polyclonal anti-VWF antibodies Edwina and Blynken recognize both human and canine VWF. Anti-VWFpp monoclonal antibodies 239.1 through 239.11 were also produced by our laboratory; 239.1, 239.7, 239.8, and 239.11 recognize canine VWFpp in addition to human, whereas the others recognize only human propeptide.
Immunofluorescent staining
Transfected AtT-20 cells were analyzed for the intracellular location of VWF and VWFpp with immunofluorescence antibody staining and confocal laser scanning microscopy in the Imaging Core of the Medical College of Wisconsin, using a Leica TCS SP2 confocal laser imaging system (Mannheim, Germany). Cells were grown on 25-mm glass cover slips, fixed with 3.7% (vol/vol) buffered formalin, permeabilized in 1% Triton X-100 (in 20 mM HEPES [HCO(3-)-free N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 300 mM sucrose, 50 mM NaCl, 3 mM MgCl2 · 6H2O, pH 7.0), and blocked in 2% normal goat serum in Hanks balanced salt solution (HBSS). Cells were incubated at 4°C overnight in primary antibodies, followed by intensive washes with HBSS. Cells were incubated in secondary antibody for 30 minutes, followed by stringent washes with HBSS. Purified polyclonal anti-VWF antibody, diluted to 5 μg/mL, and a mix of between 3 and 7 anti-VWFpp monoclonal antibodies, diluted to 2 μg/mL each in HBSS/1% bovine serum albumen (BSA), were used as primary antibodies. Secondary antibodies used were goat antirabbit and antimouse IgG (H+L) [F(Ab′)2] fragments conjugated with either AlexaFluor-488 or AlexaFluor-594 (Molecular Probes, Eugene, OR) and diluted to 1:1000 and 1:2000, respectively, in HBSS/1% BSA. Cells were mounted on glass slides with Vectashield (Vector Labs, Burlingame, CA).
Multimer analysis
VWF in the conditioned medium of transfected HEK293T or AtT-20 cells was analyzed by electrophoresis through a 0.8% (wt/vol) HGT(P) agarose (DMC Bioproducts, Rockland, ME) stacking gel and 2% (wt/vol) high gelling temperature (HGT)(P) agarose running gel containing 1% sodium dodecyl sulfate (SDS) for 16 hours at 40 V, using the Laemmli buffer system and Western blotting as previously described.18,21 22
Results
We have exploited the cross-species storage difference to better define the noncovalent association between VWFpp and VWF, using human/canine chimeric VWFpp and Δpro proteins. Human VWFpp sorts both canine and human Δpro to storage granules, whereas canine VWFpp does not sort human Δpro to granules, yet the normal storage of canine VWFpp is maintained and the human VWF is normally multimerized.18 We first focused on identifying regions within the VWFpp important for association with VWF and sought to determine what portion of canine VWFpp must contain human sequence to regain association and storage of human Δpro. Several chimeric VWFpp constructs containing variable portions of human and canine VWFpp sequence were constructed. The amino acid sequences of human and canine VWFpp are 83% identical with an additional 5% conservative substitutions.18 The 64 cysteine residues in VWFpp and the position of these cysteines is absolutely conserved between human and canine, which implies that the folding and overall secondary structures of the human and canine propeptides are probably quite similar. Thus, we expected that the secondary structure of the chimeric VWFpp proteins would be maintained.
To verify that chimeric VWFpp's each contained the necessary structural components for trafficking to storage granules, each construct was first expressed independently in AtT-20 cells that contained an intact storage pathway and correctly synthesized and stored VWF.14,23 24 If a chimeric VWFpp would not sort to granules when expressed independently, we would not expect to observe granular storage of coexpressed Δpro. AtT-20 cells expressing chimeric VWFpp were fixed, immunostained, and examined by confocal microscopy as described in “Methods.” All independently expressed VWFpp's displayed a punctate granular staining pattern similar to wild-type human or canine VWFpp (data not shown), indicating that canine/human chimeric VWFpp's are capable of navigating the storage pathway.
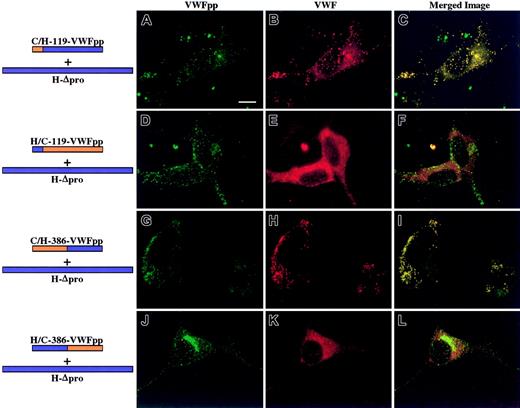
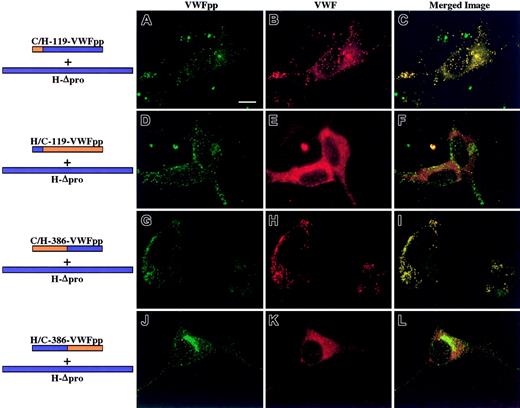
Each chimeric VWFpp was next coexpressed in AtT-20 cells with human Δpro to examine what region within VWFpp must contain human sequence for association with human VWF. To ensure that cotrafficking was due to a noncovalent association rather than a covalent linkage, VWFpp and human Δpro were coexpressed in trans. No evidence of either intracellular or extracellular VWFpp/VWF disulfide bonding could be detected (data not shown). Cotransfected cells were immunostained for VWFpp and VWF and examined by confocal microscopy. AtT-20 cells expressing human Δpro and C/H-119-VWFpp that contains the canine VWF signal peptide and sequence through Tyr119 are shown in Figure 1A-C. We observed a granular staining pattern for both VWFpp (Figure 1A, green) and VWF (Figure 1B, red). Merging the separate images revealed that the 2 proteins were colocalized in granules as shown in yellow (Figure 1C). Previous studies have shown VWF to be excluded from endogenous adrenocorticotropic hormone (ACTH)–containing granules in AtT-20 cells.14 24 We did not observe any colocalization of VWF with endogenous ACTH (data not shown). For every chimeric VWFpp that sorts human Δpro to storage granules, we predicted that the converse VWFpp would not cotraffic human Δpro. The converse construct should not contain the necessary portion of human sequence to associate with human Δpro. The converse of C/H-119-VWFpp is H/C-119-VWFpp. We observed normal granular staining of the chimeric VWFpp, but only a diffuse staining pattern was observed for coexpressed VWF. Merging the images showed no colocalization of the 2 proteins. While C/H-119-VWFpp cotraffics human VWF, the converse construct, H/C-119-VWFpp, does not sort VWF to storage granules, presumably because it does not contain the portion of human sequence necessary for association. The bottom 2 rows of Figure 1 show confocal images obtained for chimeras with sequence exchanges at the beginning of the D2 domain (amino acid 386) coexpressed with human Δpro. When the D2 domain contained human sequence, C/H-386-VWFpp, both the chimeric VWFpp and human VWF were stored colocalized in granules (Figure 1G-I). The converse chimeric VWFpp, H/C-386-VWFpp, did not cotraffic human Δpro to storage, although normal storage of the chimeric VWFpp was maintained (Figure 1J-L). A summary of results of chimeric VWFpp and VWF storage is shown in Figure 2.
Intracellular localization of chimeric VWFpp coexpressed in trans with human propeptide-deleted VWF (Δpro).
AtT-20 cells were transiently transfected with human/canine chimeric VWFpp and human propeptide-deleted VWF (Δpro) to explore what region of VWFpp must contain human sequence to regain association and storage of human Δpro. The expressed chimeric VWFpp is depicted at left, with the portion containing canine sequence in orange and the portion containing human sequence in blue. Transfected cells were fixed, permeabilized, dual-stained as described in “Methods,” and examined by confocal microscopy. Panels A, D, G, and J show cells stained for VWFpp (green). Panels B, E, H, and K show cells stained for VWF (red). The merges of VWFpp and VWF staining are shown in panels C, F, I, and L. Colocalization of VWFpp and VWF is shown in yellow. Expression of C/H-119-VWFpp with human Δpro (A-C) resulted in granular storage of both the chimeric VWFpp (A) and human VWF (B), and the 2 proteins were colocalized (C). The converse chimeric VWFpp, H/C-119-VWFpp, did not sort human VWF to storage (D-F). The chimera C/H-386-VWFpp sorted human VWF to storage granules where they colocalized (G-I). The converse construct, H/C-386-VWFpp, did not traffic human VWF to granules (K-L), although the VWFpp was stored (J). These results demonstrate that the chimeric VWFpp proteins retain the necessary signal(s) for sorting to storage granules and can direct human VWF to storage. Bar, 10 μm.
Intracellular localization of chimeric VWFpp coexpressed in trans with human propeptide-deleted VWF (Δpro).
AtT-20 cells were transiently transfected with human/canine chimeric VWFpp and human propeptide-deleted VWF (Δpro) to explore what region of VWFpp must contain human sequence to regain association and storage of human Δpro. The expressed chimeric VWFpp is depicted at left, with the portion containing canine sequence in orange and the portion containing human sequence in blue. Transfected cells were fixed, permeabilized, dual-stained as described in “Methods,” and examined by confocal microscopy. Panels A, D, G, and J show cells stained for VWFpp (green). Panels B, E, H, and K show cells stained for VWF (red). The merges of VWFpp and VWF staining are shown in panels C, F, I, and L. Colocalization of VWFpp and VWF is shown in yellow. Expression of C/H-119-VWFpp with human Δpro (A-C) resulted in granular storage of both the chimeric VWFpp (A) and human VWF (B), and the 2 proteins were colocalized (C). The converse chimeric VWFpp, H/C-119-VWFpp, did not sort human VWF to storage (D-F). The chimera C/H-386-VWFpp sorted human VWF to storage granules where they colocalized (G-I). The converse construct, H/C-386-VWFpp, did not traffic human VWF to granules (K-L), although the VWFpp was stored (J). These results demonstrate that the chimeric VWFpp proteins retain the necessary signal(s) for sorting to storage granules and can direct human VWF to storage. Bar, 10 μm.
A narrow region within the D2 domain of VWFpp is important for VWFpp/VWF association and storage.
(A) Schematic representation of chimeric human/canine VWFpp and summary of storage and multimerization data. The domain structure of VWFpp is depicted at the top and chimeric VWFpp's are depicted below. Portions of VWFpp containing human sequence are depicted in black and those containing canine sequence in white. The summarized data are for coexpression of the chimeric VWFpp in trans with human Δpro. The area of overlapping human sequence for those chimeric VWFpp's that sorted human Δpro to storage is shown between the 2 vertical lines; it lies between Glu386 and Lys477 in the D2 domain. (B) Sequence comparison of human and canine VWFpp beginning at the D2 domain. The human sequence is listed on the top line, with differences in canine sequence listed below; conserved amino acids are depicted with periods.
A narrow region within the D2 domain of VWFpp is important for VWFpp/VWF association and storage.
(A) Schematic representation of chimeric human/canine VWFpp and summary of storage and multimerization data. The domain structure of VWFpp is depicted at the top and chimeric VWFpp's are depicted below. Portions of VWFpp containing human sequence are depicted in black and those containing canine sequence in white. The summarized data are for coexpression of the chimeric VWFpp in trans with human Δpro. The area of overlapping human sequence for those chimeric VWFpp's that sorted human Δpro to storage is shown between the 2 vertical lines; it lies between Glu386 and Lys477 in the D2 domain. (B) Sequence comparison of human and canine VWFpp beginning at the D2 domain. The human sequence is listed on the top line, with differences in canine sequence listed below; conserved amino acids are depicted with periods.
Since our experiment was designed to identify a gain of function (granular sorting of human Δpro), absence of VWF sorting when coexpressed with a chimeric VWFpp may be indicative of a misfolded VWFpp. While independent trafficking of VWFpp is our first assessment of a functional VWFpp, an additional function of VWFpp is to facilitate multimerization of VWF. Although we have shown that multimerization of VWF is not a prerequisite for granular storage,25 a chimeric VWFpp that neither sorts human VWF to storage nor facilitates VWF multimerization may indicate a structural defect in the VWFpp. Multimerization of VWF was assessed by SDS-agarose electrophoresis (data not shown), and results are summarized in Figure 2. All chimeric VWFpp's sorted to storage granules when expressed individually or coexpressed with human Δpro. For every chimeric VWFpp that sorted VWF to storage, when the converse VWFpp was available, it did not sort VWF to granules. No correlation between VWF multimerization and granular storage was observed.
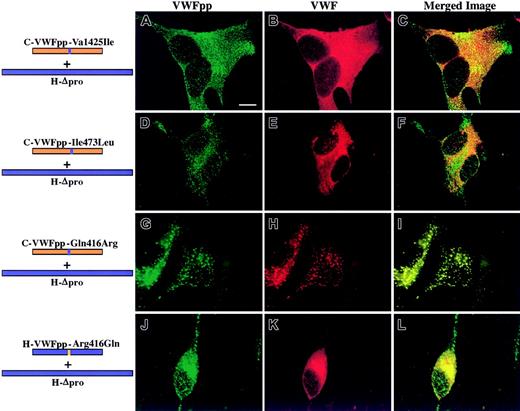
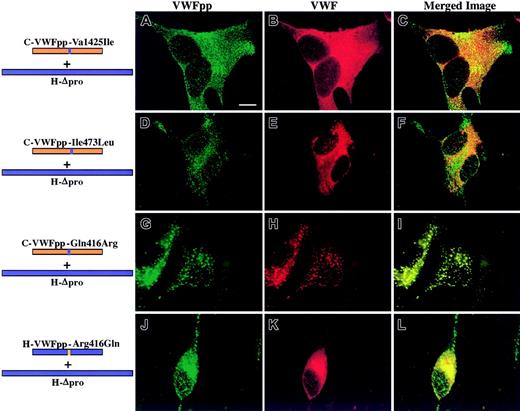
The question that we addressed was what region within VWFpp must contain human sequence to traffic human Δpro to storage. The area of overlapping human sequence of those chimeric VWFpp's that showed a gain of function (VWF storage) is shown in Figure 2. It lies between Glu386 (beginning of the D2 domain) and Lys477. We compared canine sequence with human sequence in this region (Figure 2B). Within this region there are only 10 differences in sequence between the 2 proteins. To investigate the contribution of individual amino acids to VWFpp/VWF association and storage, we mutated a single amino acid in canine VWFpp to the corresponding amino acid found in human VWFpp. The mutated canine VWFpp's were coexpressed in trans with human Δpro as described above. Shown in Figure 3A-F are the confocal images obtained when 2 canine VWFpp's containing conservative substitutions were coexpressed with human Δpro. C-VWFpp-Val425Ile was sorted to granules, whereas the coexpressed human Δpro was not stored but instead showed a diffuse staining pattern (Figure 3A-C). Similarly, C-VWFpp-Ile473Leu did not cotraffic human Δpro to storage, although normal VWFpp storage was observed (Figure 3D-F). In both cases, normal multimerization of VWF was maintained (data not shown). While other substitutions in canine VWFpp's were made that did not confer VWF association and storage, one nonconservative substitution had a profound effect on VWF storage: a glutamine-to-arginine substitution at amino acid 416. Coexpression of C-VWFpp-Gln416Arg with human Δpro resulted in colocalized granular storage of both the mutated VWFpp and VWF (Figure 3G-I). In contrast, the converse, H-VWFpp-Arg416Gln, did not sort human Δpro to storage (Figure 3J-L). The VWF was normally multimerized (data not shown). These results indicate that a single amino acid in VWFpp, amino acid 416, is critical for VWFpp/VWF association and storage.
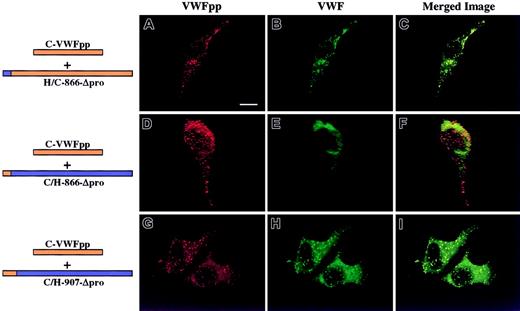
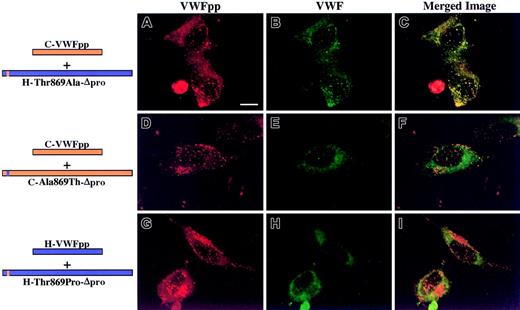
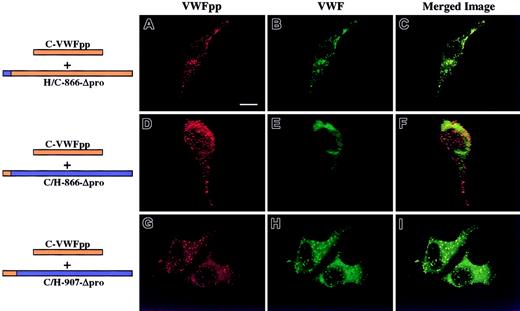
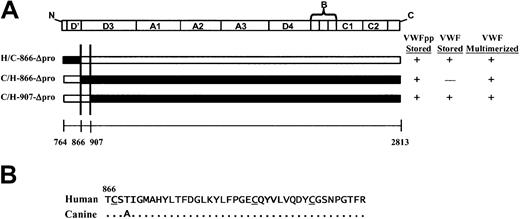
We next addressed the other molecule involved in the noncovalent association, the mature portion of VWF, now examining what region of human Δpro must contain canine sequence to restore association with canine VWFpp, resulting in VWF storage. We focused our attention on the D′ and D3 domains. Previous experiments in our laboratory using VWF truncation mutants demonstrated that a VWF molecule containing intact propeptide, furin cleavage site, and mature VWF sequence through His1221 (near the end of the D3 domain) was stored in granules (data not shown). Since this portion of VWF alone was sufficient for storage, we concentrated our study of full-length chimeras to this region of VWF. Three chimeric Δpro expression vectors were constructed, coexpressed with canine VWFpp and analyzed as described above. The mature VWF sequences of canine and human are 87% identical with an additional 4% conservative substitutions, with the position of 170 cysteine residues absolutely conserved, implying structural similarity.18 The criterion of structural integrity of chimeric Δpro is multimerization of VWF. A chimeric Δpro, H/C-866-Δpro, that contains human signal peptide and mature VWF sequence through the end of the D′ domain (Thr866) was coexpressed with canine VWFpp. Both the canine VWFpp (Figure4A, red) and chimeric VWF (Figure 4B, green) demonstrated a granular staining pattern and were colocalized as depicted in Figure 4C (yellow). In contrast, the converse, C/H-866-Δpro, was not stored in granules, although normal canine VWFpp storage was maintained (Figure 4D-F). A third chimera, C/H-907-Δpro, was also coexpressed with canine VWFpp, as shown in Figure 4G-I. Both the canine VWFpp and the chimeric Δpro were colocalized in granules. In all cases the chimeric Δpro was normally multimerized (data not shown). The area of overlapping canine sequence is shown in Figure 5A. It begins at Thr866 and extends to Ile907. Within this region there is only one amino acid difference in the protein sequence (Figure 5B), a threonine at 869 in human VWF that is alanine in canine. We explored the contribution of the single amino acid by mutating the threonine in human Δpro to the alanine in canine, H-Thr869Ala-Δpro, and coexpressing with canine VWFpp. This single amino acid substitution resulted in a gain of storage. Both the canine VWFpp and the chimeric Δpro were stored in granules, where they colocalized (Figure 6A-C). The converse, C-Ala869Thr-Δpro, was not stored in granules, although normal granular storage of the coexpressed canine VWFpp was observed (Figure 6D-F). We also wanted to confirm the importance of this amino acid in storage of human VWF. A nonconservative mutation, threonine 869 to proline, was made in human Δpro. Coexpression of H-Thr869Pro-Δpro with human VWFpp resulted in loss of granular storage of VWF, with normal granular storage of the human VWFpp (Figure 6G-I). However, normal multimerization of the mutated Δpro was not maintained. Together, these results demonstrate that amino acid 869 is critical for maintaining VWFpp/VWF association and storage.
A single amino acid substitution enables canine VWFpp to store human Δpro VWF.
Canine VWFpp's containing single human amino acid substitutions were coexpressed in trans with human Δpro in AtT-20 cells. Panels A, D, G, and J show cells stained for VWFpp (green). Panels B, E, H, and K show cells stained for VWF (red). The merges of VWFpp and VWF staining are shown in panels C, F, I, and L. Colocalization of VWFpp and VWF produces granules (yellow). In panels A-C, a canine VWFpp with a valine-to-isoleucine substitution at amino acid 425 (C-VWFpp-Val425Ile) coexpressed with human Δpro showed a granular staining pattern for the mutated canine VWFpp (A), but the human VWF was not cotrafficked to storage (B-C). Similarly, a canine VWFpp with an isoleucine-to-leucine substitution at amino acid 473 (C-VWFpp-Ile473Leu) also did not cotraffic human Δpro (E-F), although normal storage of the mutated VWFpp was maintained (D). A single, nonconservative substitution in canine VWFpp at amino acid 416, glutamine to arginine (C-VWFpp-Gln416Arg), resulted in a granular staining pattern for both the mutated VWFpp (G) and human VWF (H). The 2 proteins were colocalized in granules (I). The converse, an arginine-to-glutamine substitution at amino acid 416 in human VWFpp, resulted in a loss of human VWF storage (K-L), with a normal granular staining pattern for the mutated human VWFpp (J). A single amino acid, 416, is important for VWFpp/VWF association and costorage. Bar, 10 μm.
A single amino acid substitution enables canine VWFpp to store human Δpro VWF.
Canine VWFpp's containing single human amino acid substitutions were coexpressed in trans with human Δpro in AtT-20 cells. Panels A, D, G, and J show cells stained for VWFpp (green). Panels B, E, H, and K show cells stained for VWF (red). The merges of VWFpp and VWF staining are shown in panels C, F, I, and L. Colocalization of VWFpp and VWF produces granules (yellow). In panels A-C, a canine VWFpp with a valine-to-isoleucine substitution at amino acid 425 (C-VWFpp-Val425Ile) coexpressed with human Δpro showed a granular staining pattern for the mutated canine VWFpp (A), but the human VWF was not cotrafficked to storage (B-C). Similarly, a canine VWFpp with an isoleucine-to-leucine substitution at amino acid 473 (C-VWFpp-Ile473Leu) also did not cotraffic human Δpro (E-F), although normal storage of the mutated VWFpp was maintained (D). A single, nonconservative substitution in canine VWFpp at amino acid 416, glutamine to arginine (C-VWFpp-Gln416Arg), resulted in a granular staining pattern for both the mutated VWFpp (G) and human VWF (H). The 2 proteins were colocalized in granules (I). The converse, an arginine-to-glutamine substitution at amino acid 416 in human VWFpp, resulted in a loss of human VWF storage (K-L), with a normal granular staining pattern for the mutated human VWFpp (J). A single amino acid, 416, is important for VWFpp/VWF association and costorage. Bar, 10 μm.
Intracellular localization of chimeric Δpro VWF coexpressed in trans with canine VWFpp.
AtT-20 cells were transiently cotransfected with canine VWFpp and human/canine chimeric Δpro to determine what portion of VWF must contain canine sequence to restore association and granular sorting of VWF. Panels A, D, and G show cells stained for VWFpp (red). Panels B, E, and H show cells stained for VWF (green). The merges of VWFpp and VWF staining are shown in panels C, F, and I. The chimeric Δpro, H/C-866-Δpro, was coexpressed with canine VWFpp and demonstrated a granular staining pattern for both VWFpp (A) and the chimeric Δpro (B). The 2 proteins were colocalized in granules (C). The converse chimeric Δpro, C/H-866-Δpro, was also coexpressed with canine VWFpp; it demonstrated a granular staining pattern for VWFpp (D) but only a diffuse staining pattern for VWF (E). Canine VWFpp was coexpressed with a third chimeric Δpro, C/H-907-Δpro. Both the canine VWFpp (G) and chimeric Δpro (H) were stored in granules, where they colocalized (I). When the first 143 amino acids of mature VWF contain canine sequence, association with canine VWFpp is restored and results in granular sorting of VWF. Bar, 10 μm.
Intracellular localization of chimeric Δpro VWF coexpressed in trans with canine VWFpp.
AtT-20 cells were transiently cotransfected with canine VWFpp and human/canine chimeric Δpro to determine what portion of VWF must contain canine sequence to restore association and granular sorting of VWF. Panels A, D, and G show cells stained for VWFpp (red). Panels B, E, and H show cells stained for VWF (green). The merges of VWFpp and VWF staining are shown in panels C, F, and I. The chimeric Δpro, H/C-866-Δpro, was coexpressed with canine VWFpp and demonstrated a granular staining pattern for both VWFpp (A) and the chimeric Δpro (B). The 2 proteins were colocalized in granules (C). The converse chimeric Δpro, C/H-866-Δpro, was also coexpressed with canine VWFpp; it demonstrated a granular staining pattern for VWFpp (D) but only a diffuse staining pattern for VWF (E). Canine VWFpp was coexpressed with a third chimeric Δpro, C/H-907-Δpro. Both the canine VWFpp (G) and chimeric Δpro (H) were stored in granules, where they colocalized (I). When the first 143 amino acids of mature VWF contain canine sequence, association with canine VWFpp is restored and results in granular sorting of VWF. Bar, 10 μm.
A small portion of the D3 domain of VWF is crucial for association with VWFpp.
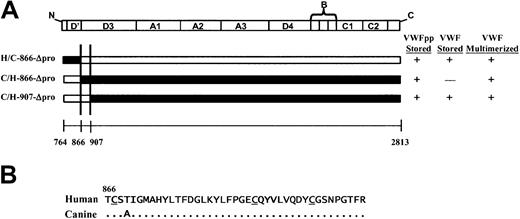
(A) Schematic representation of chimeric human/canine Δpro and summary of storage and multimerization data. The domain structure of Δpro is depicted at the top and chimeric Δpro's are depicted below. Portions of Δpro containing human sequence are depicted in black and those containing canine sequence in white. The summarized data are for coexpression of the chimeric Δpro intrans with canine VWFpp. The area of overlapping canine sequence for those chimeric VWFpp's that sorted to storage with canine VWFpp is shown between the 2 vertical lines; it lies between amino acids 866 and 907 in the D3 domain. (B) Sequence comparison of human and canine Δpro beginning at the D3 domain, amino acid 866. There is only 1 amino acid difference between the human and canine VWFpp sequence in this region.
A small portion of the D3 domain of VWF is crucial for association with VWFpp.
(A) Schematic representation of chimeric human/canine Δpro and summary of storage and multimerization data. The domain structure of Δpro is depicted at the top and chimeric Δpro's are depicted below. Portions of Δpro containing human sequence are depicted in black and those containing canine sequence in white. The summarized data are for coexpression of the chimeric Δpro intrans with canine VWFpp. The area of overlapping canine sequence for those chimeric VWFpp's that sorted to storage with canine VWFpp is shown between the 2 vertical lines; it lies between amino acids 866 and 907 in the D3 domain. (B) Sequence comparison of human and canine Δpro beginning at the D3 domain, amino acid 866. There is only 1 amino acid difference between the human and canine VWFpp sequence in this region.
A single amino acid substitution in human Δpro restores association with canine VWFpp, enabling VWF storage.
In AtT-20 cells, Δpro's containing single amino acid substitutions were coexpressed in trans with canine VWFpp. Transfected cells were examined by confocal microscopy. Panels A, D, and G show cells stained for VWFpp (red). Panels B, E, and H show cells stained for VWF (green). The merges of VWFpp and VWF staining are shown in panels C, F, and I. The substitution of threonine at amino acid 869 to alanine in human Δpro, H-Thr869Ala-Δpro, resulted in a granular staining pattern for both canine VWFpp (A) and the mutated Δpro (B). The 2 proteins were colocalized in granules (C). The converse, an alanine-to-threonine substitution at amino acid 869 in canine Δpro (C-Ala869Thr-Δpro), resulted in a loss of storage of mutated VWF (D-F). Substitution of threonine 869 to proline in human VWF (H-Thr869Pro-Δpro) resulted in a loss of VWF storage (H), with normal granular storage of human VWFpp (G). These results indicate that amino acid 869 in VWF is important for the association with the VWFpp that results in VWF granular storage. Bar, 10 μm.
A single amino acid substitution in human Δpro restores association with canine VWFpp, enabling VWF storage.
In AtT-20 cells, Δpro's containing single amino acid substitutions were coexpressed in trans with canine VWFpp. Transfected cells were examined by confocal microscopy. Panels A, D, and G show cells stained for VWFpp (red). Panels B, E, and H show cells stained for VWF (green). The merges of VWFpp and VWF staining are shown in panels C, F, and I. The substitution of threonine at amino acid 869 to alanine in human Δpro, H-Thr869Ala-Δpro, resulted in a granular staining pattern for both canine VWFpp (A) and the mutated Δpro (B). The 2 proteins were colocalized in granules (C). The converse, an alanine-to-threonine substitution at amino acid 869 in canine Δpro (C-Ala869Thr-Δpro), resulted in a loss of storage of mutated VWF (D-F). Substitution of threonine 869 to proline in human VWF (H-Thr869Pro-Δpro) resulted in a loss of VWF storage (H), with normal granular storage of human VWFpp (G). These results indicate that amino acid 869 in VWF is important for the association with the VWFpp that results in VWF granular storage. Bar, 10 μm.
Discussion
Our laboratory has been investigating the mechanisms involved in trafficking VWF to storage granules. Specifically, we have examined the role of the VWF propeptide, VWFpp, in the processing of VWF. The VWFpp independently traffics to storage granules in AtT-20 cells and endothelial cells.26 VWF that lacks its propeptide is not stored in granules.14,16,27 Furthermore, VWFpp is capable of rerouting a model, unrelated, constitutively secreted protein, C3α, to Weibel-Palade bodies in endothelial cells, where it could be released in response to agonist stimulation.26 In our model of VWF granular sorting, VWFpp contains the necessary signal (linear sequence or conformation) for sorting to storage granules, and mature VWF is cotrafficked to storage through association with VWFpp.
This model of VWFpp/VWF association requires a site or sites within VWFpp for interaction with a separate site or sites within VWF. The 2 proteins maintain a post–furin cleavage association in thetrans-Golgi network, and both proteins are subsequently sorted to storage granules. Mature VWF and VWFpp are found in an equimolar ratio in Weibel-Palade bodies.28,29 The association between these 2 proteins is also pH dependent. At low pH in the presence of calcium, conditions similar to those found in thetrans-Golgi network,30,31 mature VWF and VWFpp are noncovalently associated, while at pH 7.4 this association is not maintained.6 Secretory granules have been shown to have a pH more acidic than the trans-Golgi network that would promote the continued association of VWF and VWFpp within the granule.32 Building on our previous studies that demonstrated species differences in VWF storage provided us with a unique opportunity to identify the sequences necessary for VWFpp/VWF association and storage.
The primary sequences of human and canine pro-VWF are 86.2% identical with an additional 4.5% conservative substitutions and conservation of the position of 234 cysteine residues.18 The structural similarity of human and canine VWFpp is reflected in the independent trafficking of each propeptide to storage granules. The domains or conformations involved in multimerization must also be well conserved, since each VWFpp can facilitate folding and multimerization of the opposite species. Subtle differences in secondary structure must exist that render the canine VWFpp incapable of maintaining an association with human VWF to traffic it to storage. It is this cross-species association difference that we have exploited to examine VWFpp interaction with VWF by using chimeras of human and canine VWFpp or Δpro. This approach has allowed a detailed examination of VWFpp/VWF noncovalent association.
Our data indicate that the expressed chimeric VWFpp and Δpro produced functional proteins. All chimeric proteins were efficiently expressed and secreted. In addition, it would be expected that if a chimeric protein were significantly altered structurally, antibody recognition would be lost. We did not observe any loss of antibody recognition of expressed chimeric VWFpp or Δpro. All chimeric VWFpp's that were expressed independently in AtT-20 cells were sorted to storage granules, indicating that chimeric VWFpp's retain the structural features necessary for navigating the regulated storage pathway (Figure2). An additional criterion was that for each chimera, either VWFpp or Δpro, that showed a gain of function (gain of VWF storage), the converse chimera must show a loss of function (loss of VWF storage). Although it was not possible in every case to construct a converse expression vector, in all cases where the converse was available, this criterion was met (Figures 2 and 5).
As discussed above, both canine and human VWFpp facilitate cross-species multimerization of VWF. While loss of multimerization may indicate a structural disruption, it does not directly affect the granular storage of VWF. Other laboratories have suggested multimerization as the driving force for VWF granular storage.27,33 While we do not exclude multimerization as a component of VWF aggregation and storage, our data have demonstrated that multimerization is not a prerequisite for VWF storage. We recently reported a mutation in VWFpp (Y87S) identified in a patient that resulted in loss of multimerization.25 However, the expressed dimeric VWF was stored in granules together with the mutant VWFpp. Other laboratories have also demonstrated granular storage of dimeric VWF species. Previous studies by Wagner et al14demonstrated that C-terminal VWF deletion mutants formed only dimers that were stored in granules. Disruption of the vicinal cysteines in VWFpp also resulted in loss of multimerization of VWF with maintenance of granular sorting.15 In the present study, we did not observe a correlation between VWF multimerization and granular trafficking (Figures 2 and 5). A few chimeric VWFpp's failed to multimerize human VWF but did maintain association and cotrafficked the VWF to storage. Conversely, in many cases normally multimerized VWF was not stored in granules although VWFpp storage was maintained. Only one chimeric VWFpp neither multimerized nor sorted VWF to storage granules.
We identified a single amino acid within VWFpp that conferred gain of function. Mutation of glutamine 416 in canine VWFpp to the arginine found in human VWFpp restored association with human VWF, resulting in VWF granular storage. The importance of this amino acid was confirmed by mutation of the human arginine 416 to glutamine, which resulted in a loss of storage. In a similar manner we identified a single critical amino acid in the mature VWF protein, the threonine at 869 in canine Δpro that is alanine in human Δpro. A gain of function was observed when H-Thr869Ala-Δpro was coexpressed with canine VWFpp. The converse, C-Ala869Thr-Δpro, showed a loss of function. These data indicate that amino acids 416 in VWFpp and 869 in the mature VWF molecule are important in the noncovalent VWFpp/VWF association that is required for the granular storage of VWF. The threonine 869 found in human VWF is an alanine in canine, bovine, porcine, and murine VWF. While the arginine 416–to–glutamine difference between human and canine is a nonconservative substitution, these 2 amino acids are conserved over 5 species. The arginine 416 found in human VWF is conserved in porcine and murine VWF, while the glutamine found in canine VWF is also found in bovine VWF. It appears that VWF residues 416 and 869 are relatively well conserved over several species. We have not examined other interspecies interactions. The amino acid differences between canine and human alone do not explain the pH-dependent association of the 2 proteins, which suggests that additional residues may be involved. Whether Arg416 and Thr869 constitute the site of contact between VWFpp and VWF or merely enable a conformation promoting association is not clear. It is very likely that other amino acids are involved in the site or sites of contact between VWFpp and VWF.
The regulated storage of VWF in Weibel-Palade bodies is important physiologically. The vasopressin analog 1-desamino-8-D-arginine-vasopressin (DDAVP) is commonly used to treat patients with type 1 von Willebrand disease. Administration of DDAVP results in a rapid increase in plasma VWF levels.11,34DDAVP has been shown to stimulate VWF secretion from endothelial cell Weibel-Palade bodies in specific vascular beds.35 The administration of DDAVP to healthy individuals causes a rapid increase in FVIII plasma levels as well as VWF levels.36,37 Although the source of the FVIII released in response to DDAVP has not been definitively determined, it may be released from Weibel-Palade bodies in endothelial cells.38,39 Rosenberg et al40 have shown that FVIII that is transfected into endothelial cells colocalizes with endogenous VWF in Weibel-Palade bodies and is released together with VWF in response to several agonists. Similar studies using AtT-20 cells demonstrated the VWF-dependent nature of this FVIII storage.41 Taken together, these data suggest that defining the mechanisms involved in the trafficking of VWF may have a significant impact on the biology of FVIII. Additionally, VWF storage or lack of storage may affect other Weibel-Palade body proteins, such as P-selectin and tissue plasminogen activator (t-PA). In VWF-deficient mice, P-selectin was no longer found in Weibel-Palade bodies but instead was routed to lysosomes, resulting in defective regulated secretion and leukocyte recruitment.42 Likewise, loss of VWF storage may have an impact on storage and secretion of t-PA, which is stored in Weibel-Palade bodies with VWF.43
This study has identified 2 amino acids in VWF that are important in the trafficking of VWF to storage granules. Whether amino acid 416 in VWFpp or amino acid 869 in mature VWF constitutes a site of interaction or merely enables a conformation necessary for the association is not known. However, these 2 amino acids are clearly important in the VWFpp/VWF association that ultimately results in the granular sorting of VWF. At this time there are no reported patient mutations that affect VWF storage. One might predict that similar, naturally occurring mutations in either VWFpp or mature VWF would result in constitutively secreted multimerized VWF, but VWF storage would not be facilitated. Individuals expressing this type of mutated VWF would be unresponsive to the administration of DDAVP and their platelets (and endothelial cells) would be devoid of VWF storage pools, yet would maintain storage of the propeptide.
Prepublished online as Blood First Edition Paper, October 10, 2002; DOI 10.1182/blood-2002-07-2281.
Supported by National Institutes of Health training grant HL-07209; American Heart Association Postdoctoral Fellowship 0120594Z (S.L.H.); National Blood Foundation Scientific Research Grant (S.L.H.); National Institutes of Health grants HL-44612, HL-33721, and HL-56027 (R.R.M.); and the Clinical Research Center of the Medical College of Wisconsin (M01 RR00058).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Robert R. Montgomery, Department of Pediatrics, Medical College of Wisconsin, 8701 Watertown Plank Rd, Milwaukee, WI 53226; e-mail: bob@bcsew.edu.