HIV infection leads to reduced numbers and increased turnover of CD4+ T cells in blood. However, blood represents only 2% of the total lymphocyte pool, and information about other organs is lacking, leading to controversy about the effects of HIV infection on T-cell homeostasis. Therefore, we have determined phenotype and turnover of lymphocyte subsets in various tissues of macaques. Infection with simian immunodeficiency virus (SIV) resulted in increased proliferation rates of T cells in all organs. Despite reduced CD4 counts in blood, absolute numbers of CD4+ T cells were increased in spleen and lymph nodes and remained stable in nonlymphoid organs such as liver, lung, bone marrow, and brain during the asymptomatic phase, indicative for an altered tissue distribution. In animals killed with first signs of AIDS, total body CD4 counts and proliferation rates had returned to control levels, whereas thymocytes were almost completely absent. Our data show that a drastically increased turnover in the early stages of HIV infection, driven by a generalized immune activation rather than a homeostatic response to CD4+ T-cell destruction, is followed by exhaustion of the regenerative capacity of the immune system.
Introduction
Determining the mechanisms leading to CD4+ T-cell loss in human immunodeficiency virus (HIV)–infected patients is crucial for the understanding of the pathogenesis of AIDS.1 The common picture holds that T-helper cells decline steadily although there may be differences in the rate between various organs.2-7 The observation of a rapid rebound of CD4+ cell numbers in blood after initiation of highly active antiretroviral therapy (HAART)8,9 led to speculation that the infection results in increased peripheral T-cell proliferation. This increased turnover was thought to be a homeostatic response to a massive destruction of T-helper cells, which cannot be compensated for in the long run by the regenerative capacities of the immune system. Subsequently, direct evidence for an increased fractional proliferation rate of blood CD4+ T cells has accumulated,10-18 whereas the effect of HIV infection on total T-cell turnover is still being discussed.19-21 Therefore, other groups postulate decreased production and peripheral turnover as the cause of T-helper cell depletion.13 22 The discrepancies between the various theories are due mainly to a lack of information about the situation in other parts of the body.
So far, the effects of HIV infection on T-cell pool and T-cell turnover have been investigated mostly in blood.10-12,17 However, blood lymphocytes represent only 2% of the total lymphocyte pool, and their composition differs from that within the organs.23,24 From other organs, only information about changes in the proportions of CD4+ T cells and fractional proliferation rates is available.3-5,13,18,25 Absolute figures for the entire body have been extrapolated solely from blood-derived data.8-10,13,15 However, because there is evidence that HIV infection also alters the distribution of lymphocytes between different organs,26 it is necessary to directly determine the absolute numbers of lymphocyte subsets for the various compartments.24 For obvious reasons, this can be achieved only in an animal model. Therefore, we have determined absolute numbers of lymphocyte subsets in 14 different tissues of uninfected and simian immunodeficiency virus (SIV)–infected macaques.
Materials and methods
Animals and viruses
Rhesus monkeys (Macaca mulatta) used for this study were housed at the Deutsches Primatenzentrum, Göttingen, Germany, according to institutional guidelines. They were serologically free of SIV, simian T-lymphotropic virus (STLV), and simian retrovirus (SRV). A total of 32 macaques were infected with either 100 MID50(50% monkey infective dose) of SIVmac251MPBMC (n = 20) or with the viral clone SIVmac239 or closely related pathogenic derivatives of this clone (n = 12). The SIVmac239 derivatives contain point mutations, which do not influence the course of the infection. Eleven uninfected monkeys were used as controls. Monkeys were killed when they became moribund or at predetermined time points (Table1). After exsanguination under anesthesia, the animals were thoroughly perfused with 2 L RPMI via the aorta ascendens. Cell-associated viral loads in blood were determined by limiting dilution coculture of monkey peripheral blood mononuclear cells (PBMCs) and the permanent T cells C81-66 as indicator cells as described.27
Preparation of lymphocytes from different organs
All macroscopically visible inguinal, axillary, submandibular, mediastinal, mesenteric, and mesocolic lymph nodes were prepared from 6 anatomically defined areas as described.27 After carefully removing adhering connective and fat tissue, lymph nodes (LNs) as well as spleen, thymus, liver, lung, bone marrow (BM), brain, intestine, and other organs were weighed. A representative part of these organs was weighed again, and cells were isolated from this piece of tissue as described.3,27 28 Briefly, total numbers of lymphocytes (N) per organ were calculated as follows: n = nW/w, where n is the number of lymphocytes isolated, W the total weight of the organ or LN region, and w the weight of the tissue sample used to isolate the lymphocytes. For the organs, where we did not determine the actual total weight, the following values were estimated: bone marrow, 50 g; gut, 200 g; and blood, 300 mL.
Flow cytometric analysis
Isolated cells from the various organs (about 2 × 105) or citrated blood samples were subjected to 3-color flow cytometry as described recently.29 The following antibodies were used: antimonkey CD3 FN18 (M. Jonker, TNO (Nederlandse Organisatie voor toegepast-natuurwetenschappelijk onderzoek), Rijswijk, The Netherlands) biotinylated using standard techniques, CD20–fluorescein isothiocyanate (FITC) (B1, Coulter, Krefeld, Germany), CD4-phycoerythrin (PE) (OKT4, Ortho, Neckargemünd, Germany; L200, Pharmingen, Heidelberg, Germany), CD8-FITC (RPAT8, Pharmingen), CD29-FITC (4B4, Coulter), and Ki-67-PE (Ki-67, Dako, Hamburg, Germany). After lysing of erythrocytes and fixing the cells with fluorescence-activated cell sorter (FACS) lysing solution (Becton Dickinson, Heidelberg, Germany), bound biotinylated antibodies were detected with streptavidin-coupled CyChrome (Pharmingen, Hamburg, Germany). Cells were analyzed on a FACScan flow cytometer (Becton Dickinson). Quadrants were set according to the staining pattern obtained with isotype-matched control antibodies except for the bimodal distribution of CD29, where markers were set between the 2 CD29low- and CD29high-expressing populations. For determination of absolute numbers of lymphocyte subsets, the proportion of the respective lymphocyte subset within a forward and side light scatter gate including CD3+ T cells, CD20+ B cells, and CD3−CD8+ natural killer (NK) cells was multiplied by the absolute number of lymphocytes obtained with a Coulter counter (blood) or by counting in a Neubauer chamber as described above (organs). Because fewer than 5% of macaque and human T cells in blood and LNs express neither CD4 nor CD8, the respective T-cell subsets negative for 1 of the 2 antigens consist mainly of the reciprocal population. Thus, because the expression of Ki-67 was measured only in combination with CD8, the expression of Ki-67 determined for CD8− T cells will be referred to as expression on CD4+ T cells. Some CD4+ T cells also express the CD8αα homodimer. Because we did not perform double staining for both CD4 and CD8 in one tube (except for thymus), this small subset of fewer than 5% of T cells added not only to the CD4+ but also to the CD8+ T cells. This might have resulted in a slight overestimation of the CD8+ T-cell compartment. To estimate the contribution of the lymphocytes in the organs investigated to the total lymphocyte pool, peripheral blood lymphocytes (PBLs) were isolated from 45 mL blood of 3 uninfected animals 1 day prior to necropsy. After counting and labeling with carboxyfluorescein diacetate succinimidyl ester (CFSE), these cells were reperfused into the same animals. The proportion of CFSE-positive cells among cells isolated from different organs 20 hours later was determined by flow cytometry, and the absolute number of labeled cells was calculated.
Statistical analysis
All data are expressed as mean ± SEM. The proportion and absolute numbers of lymphocyte subsets from uninfected and SIV-infected animals was compared using the Mann-Whitney U test. Correlation between different parameters was assessed using the Spearman rank correlation coefficient. The significance level was set at P ≤ .05.
Results
In this study, we have included a total of 43 juvenile rhesus monkeys. Eleven uninfected monkeys with a mean weight of 3821 ± 175 g were used as controls. Of the 32 SIV-infected monkeys, 12 (mean weight, 4138 ± 222 g) had to be humanely killed due to signs of AIDS (Table 1) between 14 and 67 weeks after infection (wpi) (mean, 38 ± 6 wpi). Twenty animals (mean weight, 4453 ± 173 g) were killed at predetermined time points between 12 and 78 wpi (mean, 38 ± 5 wpi) without signs of AIDS. CD4+ cell counts in blood were decreased from 932 ± 72 cells per microliter in uninfected animals to 584 ± 71 in asymptomatic monkeys and to 503 ± 137 in animals with AIDS. Cell-associated viral load increased from 606 ± 221 infectious cells per 106 PBMCs in asymptomatic monkeys to 3349 ± 1452 in monkeys with AIDS.
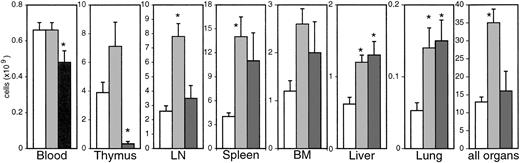
We have first determined the absolute numbers of lymphocytes in several organs (Figure 1). In uninfected monkeys, the spleen represented the largest lymphoid organ. The sum of 6 lymph node (LN) regions investigated resulted in a similar number of lymphocytes. As nonlymphoid organs, we assessed liver, lung, BM, and the brain (1.8 × 106 lymphocytes). The absolute numbers obtained for all organs except lung, where we have isolated fewer cells, are comparable to those found in humans23 and other animal species. From these organs a total of 1.2 × 1010lymphocytes was calculated, which, according to previous estimates for humans,23 represented at least 50% of the total lymphocyte pool. Age, weight, and sex did not correlate with total lymphocyte numbers. To determine the total yield with a different method, we counted ex vivo carboxyfluorescein diacetate succinimidyl ester (CFSE)–labeled peripheral blood lymphocytes in the different organs 20 hours after reperfusion. CFSE-positive cells accounted for 0.1% to 0.3% of lymphocytes in the various organs. Taken together, more than 50% of the reperfused cells were found again in all 3 animals. The remaining cells may have been lost during purification but more likely reside within compartments not investigated with this technique (for example, gut and other LN regions). Because we have assessed more than 50% of the total lymphocyte pool in both lymphoid and nonlymphoid compartments, our results can be viewed as representative for the whole body. Additionally, we have quantified lamina propria lymphocytes (LPLs) in 5 uninfected animals, which added up to approximately 5.1 × 108 ± 2.2 × 108 lymphocytes at an estimated total gut weight of 200 g. Other organs, such as heart (about 2 × 106 lymphocytes), kidney (about 2 × 107), submandibular gland (about 1 × 106), and tonsils (3.7 × 107 ± 6.6 × 106), which we have investigated in some animals, did not contribute significantly to the total number of lymphocytes.
Absolute numbers of lymphocytes in different organs.
Bars represent uninfected (white bars) and SIV-infected macaques with (dark gray bars) or without (light gray bars) signs of AIDS. *Significantly different compared with uninfected monkeys (P ≤ .05).
Absolute numbers of lymphocytes in different organs.
Bars represent uninfected (white bars) and SIV-infected macaques with (dark gray bars) or without (light gray bars) signs of AIDS. *Significantly different compared with uninfected monkeys (P ≤ .05).
After infection, the absolute numbers of lymphocytes increased during the asymptomatic phase in all organs except blood, resulting in a 3-fold overall increase in the sum of the organs investigated (Figure1). In animals with AIDS, absolute numbers of lymphocytes further increased in most nonlymphoid organs (liver, lung, brain) and declined in LNs, spleen, and bone marrow compared with asymptomatic animals but remained increased compared with uninfected monkeys. In blood, the lymphocyte counts were reduced in animals with AIDS compared with preinfection levels. The most dramatic changes in lymphocyte numbers were observed in thymus. In 8 of 11 animals with AIDS, thymocyte numbers were reduced by more than one order of magnitude.
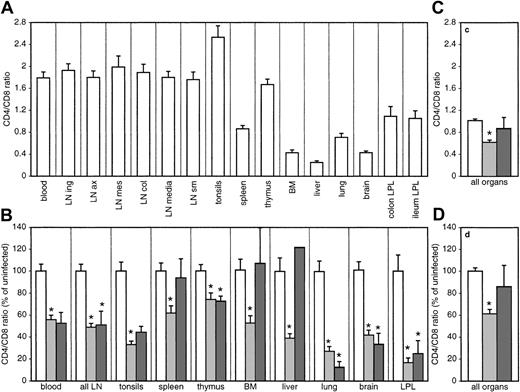
The normal composition of lymphocytes varies between different organs. Accordingly, the CD4/CD8 ratios in uninfected animals ranged from 2.5 in tonsils to less than 0.5 in liver, bone marrow, brain, and LPLs (Figure 2A). For the sum of all major compartments investigated, the mean CD4/CD8 ratio was 1.01 ± 0.03. The CD4/CD8 ratios declined after infection, as expected, in all organs investigated (Figure 2B). However, this decrease differed between the organs, ranging from only 25% in thymus (of mature CD3high-expressing CD4 or CD8 single-positive thymocytes) to more than 70% in nonlymphoid organs such as the lung and the gut of asymptomatic animals (Figure 2B). In animals with AIDS, the CD4/CD8 ratios further decreased in most organs. Two of these monkeys (nos. 1962, 9340) showed exceptionally high CD4/CD8 ratios, a fact that has been often reported for monkeys with a rapid progressor phenotype,30 resulting in a large variation of the CD4/CD8 ratio of animals with AIDS.
CD4/CD8 ratios in different organs.
CD4/CD8 ratio for the different organs (A) and for the sum of all organs investigated (C) was determined by flow cytometry. Infection-induced changes of the CD4/CD8 ratio relative to the mean of uninfected macaques are shown in panel B for the different organs and in panel D for the sum of all organs. Bars represent uninfected (white bars) and SIV-infected macaques with (dark gray bars) or without (light gray bars) signs of AIDS. In thymus, only CD3high-expressing, CD4, CD8 single-positive, mature thymocytes were included. *Significantly different compared with uninfected monkeys (P ≤ .05). In panel B, the error bar for the liver of animals with AIDS was omitted because of the high variation in this group.
CD4/CD8 ratios in different organs.
CD4/CD8 ratio for the different organs (A) and for the sum of all organs investigated (C) was determined by flow cytometry. Infection-induced changes of the CD4/CD8 ratio relative to the mean of uninfected macaques are shown in panel B for the different organs and in panel D for the sum of all organs. Bars represent uninfected (white bars) and SIV-infected macaques with (dark gray bars) or without (light gray bars) signs of AIDS. In thymus, only CD3high-expressing, CD4, CD8 single-positive, mature thymocytes were included. *Significantly different compared with uninfected monkeys (P ≤ .05). In panel B, the error bar for the liver of animals with AIDS was omitted because of the high variation in this group.
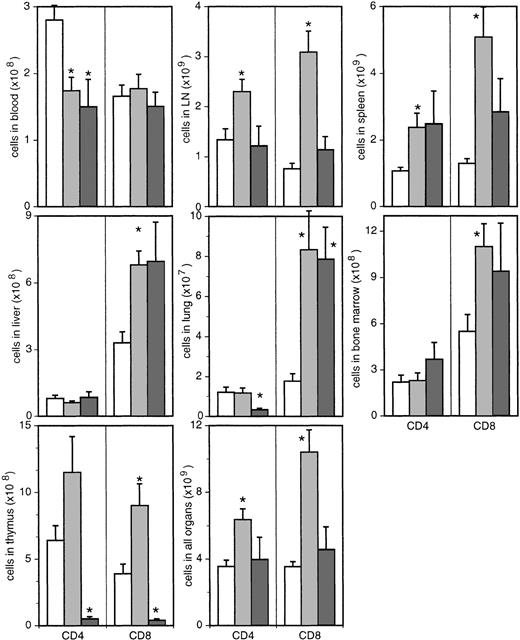
Despite the reduced proportions of CD4+ T cells in all organs of asymptomatic animals, the absolute numbers of CD4+ T cells were decreased only in blood (Figure3). Moreover, in thymus, lymph nodes, and spleen, the absolute numbers of CD4+ T cells were increased compared with uninfected monkeys. Even in nonlymphoid organs, which exhibited the most dramatic decrease in the proportion of CD4+ T cells, this population did not decline in absolute numbers (Figure 3). In the sum of all organs investigated, CD4+ T cells increased significantly from 3.5 × 109 ± 3.9 × 108 in uninfected monkeys to 6.6 × 109 ± 6.3 × 108 cells in the asymptomatic phase (P ≤ .005). Compared with uninfected controls, animals with AIDS exhibited reduced absolute numbers of CD4+ T cells in blood, thymus, and lung but similar levels of CD4+ T cells in all other organs (Figure3). Overall, the numbers of CD4+ T cells (4.7 × 109 ± 1.6 × 109) of animals with AIDS were not different from those in uninfected monkeys. For ethical reasons, animals were killed with the first signs of immunodeficiency. For this reason and especially in the light of a complete loss of thymocytes in these animals, it seems plausible that total CD4 counts would have dropped further.
Absolute numbers of CD4+ and CD8+ T cells in different organs.
Bars represent uninfected (white bars) and SIV-infected macaques with (dark gray bars) or without (light gray bars) signs of AIDS. In thymus, only CD3high-expressing, CD4, CD8 single-positive, mature thymocytes were included. *Significantly different compared with uninfected monkeys (P ≤ .05).
Absolute numbers of CD4+ and CD8+ T cells in different organs.
Bars represent uninfected (white bars) and SIV-infected macaques with (dark gray bars) or without (light gray bars) signs of AIDS. In thymus, only CD3high-expressing, CD4, CD8 single-positive, mature thymocytes were included. *Significantly different compared with uninfected monkeys (P ≤ .05).
Absolute numbers of CD8+ T cells were significantly increased in all organs except blood (Figure 3), resulting in a total 3-fold increase from 3.5 × 109 ± 3.1 × 108 in uninfected animals to 1.15 × 1010 ± 1.4 × 109 in asymptomatic monkeys (P ≤ .0001). In animals with AIDS, mature CD8 single-positive thymocytes were almost completely absent, whereas in most other organs the absolute numbers of cytotoxic T cells were still increased compared with uninfected monkeys (Figure 3). In the sum of all organs, the number of CD8+ T cells (5.2 × 109 ± 1.8 × 109) was not significantly higher than in uninfected monkeys.
Both augmented replenishment of the peripheral T-cell pool from thymic and extrathymic sources or increased peripheral proliferation could explain the observed increase in total T-cell numbers. Unfortunately, thymic output is not accessible to direct measurement. Methods to detect recent thymic emigrants by T-cell receptor excision circles (TRECs) may not properly reflect thymic function, because peripheral proliferation has a greater influence on TREC levels than thymic output.31 Our observation of increased numbers of mature thymocytes is consistent with previous findings of increased levels of cell proliferation in the thymus of SIV-infected macaques32 and abundant thymic tissue in HIV patients33 and shows that thymic function is preserved, if not increased, during the asymptomatic phase of HIV and SIV infection.
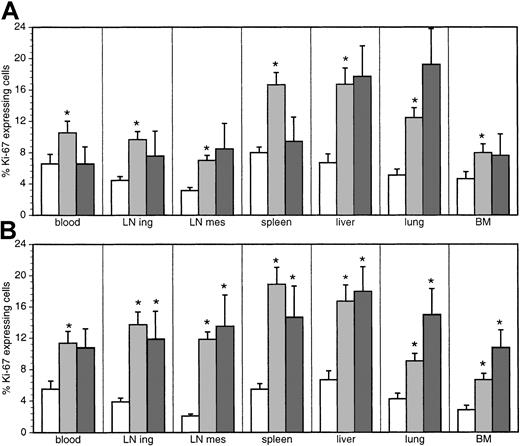
As to the proliferation of peripheral T cells, we determined the expression of Ki-67, a nuclear antigen expressed from G1 to M phase, in cells from blood, inguinal LNs, spleen, liver, lung, and BM of 10 uninfected, 16 asymptomatic, and 10 diseased monkeys. The other LN regions and organs were investigated in fewer animals. Thymus was not included in the analysis because expression of Ki-67 was limited mainly to CD3−- to CD3low-expressing, CD4, CD8 double-positive, immature thymocytes. It was therefore not regarded as part of the peripheral turnover. The fractional proliferation rate (ie, percentage of Ki-67+ cells) of both CD4+ and CD8+ T cells was significantly increased in the asymptomatic phase of the infection in all organs investigated (Figure4).
Proportion of Ki-67–expressing cells.
The percentage of cells expressing Ki-67 among CD4+ (A) and CD8+ (B) T cells in uninfected (white bars) and SIV-infected macaques with (dark gray bars) or without (light gray bars) signs of AIDS was determined by flow cytometry. *Significantly different compared with uninfected monkeys (P ≤ .05).
Proportion of Ki-67–expressing cells.
The percentage of cells expressing Ki-67 among CD4+ (A) and CD8+ (B) T cells in uninfected (white bars) and SIV-infected macaques with (dark gray bars) or without (light gray bars) signs of AIDS was determined by flow cytometry. *Significantly different compared with uninfected monkeys (P ≤ .05).
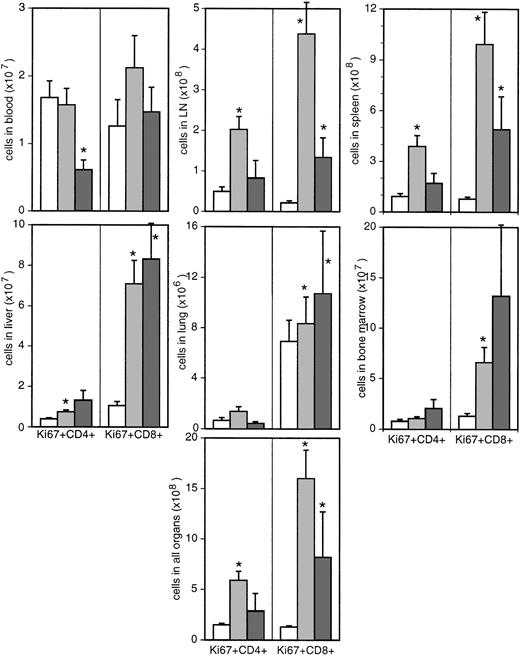
The average fractional proliferation rate for all organs increased from 4.48% ± 0.39% to 9.14% ± 0.97% for CD4+ T cells and from 3.92% ± 0.43% to 12.98% ± 1.36% for CD8+T cells. This fact, combined with the increased absolute counts of the 2 T-cell subsets, led to a 4-fold increase of the total number of Ki-67–expressing CD4+ T cells from 1.5 × 108 ± 1.5 × 107 in uninfected monkeys to 5.9 × 108 ± 8.7 × 107 in asymptomatic monkeys (P ≤ .0001; Figure5) and a more than 10-fold increase of proliferating CD8+ T cells from 1.3 × 108 ± 1.3 × 107 to 1.6 × 109 ± 2.8 × 108 (Figure 5). Thus, assuming that the duration of Ki-67 expression during the cell cycle is not influenced by the infection, the total turnover of CD4+ and CD8+ T cells is increased by a factor of 4 and 12, respectively, during the asymptomatic phase of the infection. In animals with AIDS, neither the proportion nor the absolute numbers (2.9 × 108 ± 1.7 × 108) of Ki-67+ T-helper cells were significantly different from uninfected monkeys. In contrast, Ki-67 expression on CD8+ T cells remained increased in all organs of diseased monkeys (Figures 4-5).
Absolute numbers of Ki-67–expressing cells in different organs.
Bars represent uninfected (white bars) and SIV-infected macaques with (dark gray bars) or without (light gray bars) signs of AIDS. *Significantly different compared with uninfected monkeys (P ≤ .05).
Absolute numbers of Ki-67–expressing cells in different organs.
Bars represent uninfected (white bars) and SIV-infected macaques with (dark gray bars) or without (light gray bars) signs of AIDS. *Significantly different compared with uninfected monkeys (P ≤ .05).
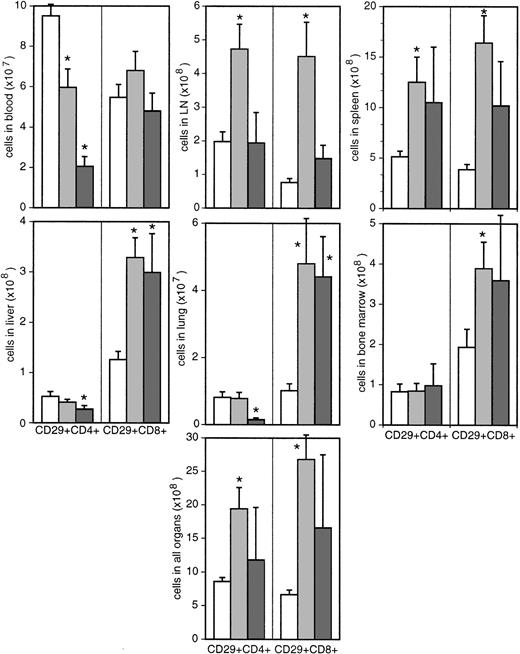
CD4+ T cells can be functionally and phenotypically delineated into naive and memory/effector subsets. We have used the expression level of CD29 to differentiate between these 2 subsets. CD29high-expressing memory cells show higher proliferation rates than CD29low-expressing naive cells29and are preferentially infected by HIV and SIV.34 35 The proportion of memory cells differed between the various organs from less than 20% in LNs to more than 50% in nonlymphoid organs such as liver and lung (Figure 6).
Proportion of memory T cells.
The percentage of memory cells, defined by high expression of CD29, among CD4+ (A) and CD8+ (B) T cells in uninfected (white bars) and SIV-infected macaques with (dark gray bars) or without (light gray bars) signs of AIDS was determined by flow cytometry. *Significantly different compared with uninfected monkeys (P ≤ .05).
Proportion of memory T cells.
The percentage of memory cells, defined by high expression of CD29, among CD4+ (A) and CD8+ (B) T cells in uninfected (white bars) and SIV-infected macaques with (dark gray bars) or without (light gray bars) signs of AIDS was determined by flow cytometry. *Significantly different compared with uninfected monkeys (P ≤ .05).
In asymptomatic animals, the percentage of memory cells remained essentially unchanged, whereas it was reduced among CD4+ T cells in animals with AIDS. These results show that the presumably higher depletion of memory cells by the infection is balanced during the asymptomatic stage either by proliferation within this subset or conversion of naive cells into this cell type. Later in course of the infection, the increasing drain on memory CD4 cells could not be compensated, because proliferation rates were reduced, resulting in a strong decrease in absolute numbers of memory cells in 4 of 5 animals with AIDS (Figure 7).
Absolute numbers of CD29high T cells in different organs.
Bars represent uninfected (white bars) and SIV-infected macaques with (dark gray bars) or without (light gray bars) signs of AIDS. *Significantly different compared with uninfected monkeys (P ≤ .05).
Absolute numbers of CD29high T cells in different organs.
Bars represent uninfected (white bars) and SIV-infected macaques with (dark gray bars) or without (light gray bars) signs of AIDS. *Significantly different compared with uninfected monkeys (P ≤ .05).
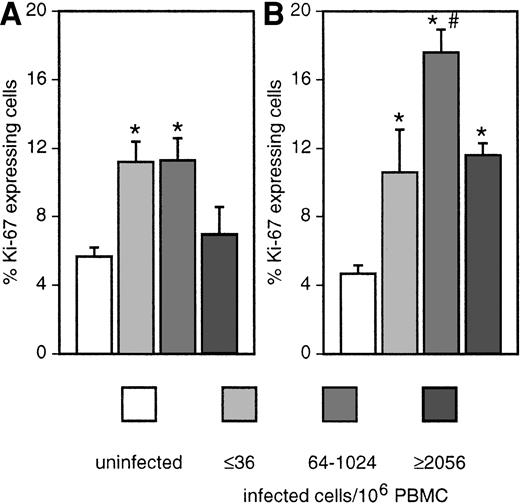
To determine the effect of viral replication on T-cell turnover, we stratified the animals according to the cell-associated viral load (Figure 8). The fractional proliferation rate of CD4+ T cells was increased only in animals with low to medium viral load. In animals with high viral load, the proliferation rate was comparable to uninfected animals. Ki-67 expression of CD8+ T cells increased with virus titers and reached a maximum in monkeys with a medium viral load. In animals with high viral load, the proliferation rate of this subset decreased again but was still higher than in uninfected macaques. According to these findings, it seems that 2 forces are acting in opposite directions. Increased viral replication leads to increased immune activation and proliferation but also results in increased death of CD4+ T cells. At high viral load, a low to absent T-helper cell response might also influence the proliferative capacity of CD8+ T cells.
Correlation of fractional proliferation rate with viral load.
Proportion of Ki-67–expressing cells among CD4+ (A) and CD8+ (B) T cells in the sum of all organs investigated (see Figure 1) except thymus of uninfected (white bars) and SIV-infected macaques, stratified according to the cell-associated viral load in blood. *Significantly different compared with uninfected monkeys (P ≤ .05). #Significantly different compared with other infected groups (P ≤ .05).
Correlation of fractional proliferation rate with viral load.
Proportion of Ki-67–expressing cells among CD4+ (A) and CD8+ (B) T cells in the sum of all organs investigated (see Figure 1) except thymus of uninfected (white bars) and SIV-infected macaques, stratified according to the cell-associated viral load in blood. *Significantly different compared with uninfected monkeys (P ≤ .05). #Significantly different compared with other infected groups (P ≤ .05).
In uninfected individuals, the production rate (thymic output plus peripheral proliferation) and death rate of T cells is balanced, and the net increment of the total T-cell pool is zero. Given the increased number of thymocytes and the increased proliferation rate of peripheral T cells, the production rate was increased in our cohort of animals. Because the absolute numbers of CD4+ and CD8+ T cells were increased, the production rate must be higher than the death rate. We have used the difference in the absolute cell numbers between uninfected and asymptomatic animals to determine the net increment rate. According to the formula it = Ninf/Nuninf, where i is the increment rate, t the duration of infection, and N represents the total number of cells of an individual infected animal and the mean of all uninfected animals, respectively, we calculated a mean increment rate of 0.0024 ± 0.0007 per day for CD4+ T cells and 0.0055 ± 0.0010 for CD8+ T cells in asymptomatic monkeys. These numbers are by far lower than the infection-induced increases in the proliferation rates as measured by Ki-67 expression (%Ki-67+ cells in infected minus %Ki-67+cells in uninfected animals: 0.0466 and 0.0906 for CD4+ and CD8+ T cells, respectively). Therefore, the death rate must be increased by the infection for both subsets as well, assuming that the span of time of Ki-67 expression during cell cycle and the thymic output is not drastically changed.
Discussion
In this work, we determined for the first time absolute numbers of several lymphocyte subsets in solid organs of SIV-infected macaques. Because we have sampled about 50% of the total lymphocyte pool,23 our data provide a solid base for estimates of immunodeficiency virus–induced changes in total T-cell pool and turnover. Our results confirm previous reports of increased fractional proliferation rates of both CD4+ and CD8+T-cell subsets after HIV and SIV infections in blood and LNs8-13,15,17,18 and extend this observation to other organs. Moreover, we could show that the absolute proliferation rate is increased as well and that the pattern of distribution between different organs is altered. Although circumstantial evidence of an altered distribution of CD4+ T cells between the different organs existed previously,15,24 26 our findings provide the first quantitative description of this effect.
The most prominent result of our study, however, is an expanded size of the total CD4+ T-cell pool upon SIV infection. This was completely unexpected in the light of decreased absolute numbers in blood and reduced proportions in all organs. Enlargement of LNs and spleen is frequently found in asymptomatic HIV patients.36,37 In addition, it has been reported that CD4+ cell numbers in tonsils were not decreased in asymptomatic patients.6 These facts, however, have not been taken into account for calculations of the absolute numbers of lymphocytes.13 25
Several hypotheses have been put forward to explain the reduced numbers of CD4+ T cells in blood during HIV and SIV infection. In the low turnover model, it has been postulated that the infection interferes with the replenishment of CD4+ T cells.19,22 However, similar to our results, most studies have found increased fractional proliferation rates of CD4+T cells in blood of HIV-infected patients and SIV-infected macaques.10,11,14,15 In some studies, low CD4 counts in blood outweighed increased proliferation rates, resulting in a reduced total turnover for this T-cell subset,12,13,25 interpreted as evidence for the low turnover model. Our finding of an increased proliferation rate in about half of the CD4+ T-cell pool unambiguously shows that absolute CD4+ T-cell turnover is increased in the asymptomatic phase of immunodeficiency virus infection. At first glance, this would support the high turnover model. According to this model, initially brought up to explain the sharp rise in CD4 counts after initiation of antiretroviral therapy,8,9 the infection leads to increased death rates. As a result, CD4+ T-cell proliferation is augmented in a homeostatic effort of the immune system to maintain total CD4+ T-cell counts. However, according to this scenario, the immune system overshot the mark of just replacing lost CD4+ T cells in the group of asymptomatic animals of our study. Rather than by homeostatic mechanisms, the increased size of the CD4+ T-cell pool could be explained by chronic immune activation38,39 stimulated by the ongoing viral replication. The increased death rates for both subsets would then possibly be the result of a homeostatic mechanism to resolve the increased numbers of T cells. Additional support for the idea that the increased activation and proliferation of T-helper cells in HIV infection is caused by antigenic stimulation is provided by very recent studies.14,15,18,40,41 Because immune activation induces trapping of T cells in lymphoid organs42 and differentially influences the distribution of CD4+ and CD8+ T cells, this model could also explain the altered distribution of lymphocytes found in the SIV-infected macaques.24
The mechanisms for this global activation of T-cell subsets as well as of NK cells and B cells (data not shown and Rosenzweig et al11) are less clear. Both direct stimulation of virus-specific cells or indirect activation of bystander cells could contribute to this phenomenon. If antigenic stimulation is indeed the driving force behind the expansion of CD4+ T cells, a high number of virus-specific T-helper cells should be present. However, a T-helper cell response, as measured by in vitro antigen-specific proliferation, is absent in most HIV patients.43HIV-specific blood T cells may be terminally differentiated effector cells no longer able to proliferate.44 Indeed, HIV-specific T-helper cells with effector function have been detected in blood at all stages of the infection with appropriate techniques.45 Possibly, proliferation of virus-specific cells occurs in lymphoid organs, which account for most of the increase in total T-cell numbers.
Alternatively, some low level of antigen-specific proliferation could be amplified by unspecific stimulation of bystander cells as described for other viral infections.46Interestingly, rapidly progressing animals, which do not mount a virus-specific immune response, also do not show increased activation and proliferation of CD4+ T cells in blood.29Therefore, direct effects of the viral replication alone on the increased T-cell turnover such as viral antigens that drive cytokine production and CD4+ T-cell proliferation seem less likely.
According to our data, the total number of CD4+ and CD8+ T cells does not expand as fast (doubling time 290 days and 130 days, respectively) as the increase in Ki-67 staining would suggest. Most likely the death rates were increased as well in the animals either by activation-induced cell death or by homeostatic mechanisms. In any case, the differences between increased proliferation rates and actual increment rates are similar for CD4+ and CD8+ T cells. This indicates that the same regulatory mechanisms are acting on both subsets. However, direct or immune-mediated killing of infected cells may additionally contribute to death of CD4+ T cells. Such effects could be responsible for the different pattern of correlation between viral load and proliferation rate of CD4+ and CD8+ T cells (Figure 8). In summary, the increased death rates found in HIV-infected patients and SIV-infected animals10-12,18 are the result of increased proliferation, rather than the cause, as was initially suggested.8,9 17
Our results show a biphasic course of T-cell turnover in immunodeficiency virus infection. An expansion of the total T-cell pool during the asymptomatic phase is followed by a decrease of T-cell numbers. What mechanisms upset the balance, ultimately causing immunodeficiency and death? A possible clue is provided by the almost complete loss of thymocytes in all animals with AIDS despite relative normal absolute CD4 counts in other organs. Because these monkeys were killed with the first signs of immunodeficiency, we think that this reflects the picture at the transition between the asymptomatic and the symptomatic stage of the infection. Possibly, either the chronic activation of the immune system or the unrestricted viral replication interferes with the regenerative capacity of the thymus or bone marrow precursors.1
We thank S. Czub, T. Kerkau, C. Hahn, B. Hofmann, F.-J. Kaup, N. Stolte, and E. Kuhn for help in the preparation of organs and cells, and J. Westermann, N. Stilianakis, and S. Coley for critical reading of the manuscript. We are indebted to F. Kirchhoff and U. Sauermann for providing us with material from animals used in their experiments.
Prepublished online as Blood First Edition Paper, October 10, 2002; DOI 10.1182/blood-2002-06-1644.
Supported by the Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie, Germany (BMBF 01 KI 9762/5, 01 KI 0211).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
S. Sopper, Institut für Virologie und Immunbiologie, Julius- Maximilians-Universität, Versbacherstr 7, D-97078 Würzburg, Germany; e-mail:sopper@vim.uni-wuerzburg.de.