We read with interest the article by Kantarjian et al.1 With the advent of allogeneic matched sibling stem cell transplantation (allo-SCT), it is clear that long-term disease-free survival can be achieved in patients with chronic phase (CP) chronic myelogenous leukemia (CML).2 Measurement of BCR/ABL transcript numbers has enhanced the accuracy of assessment of posttransplantation disease activity.3 Failure to detect transcripts or detection of low numbers is associated with prolonged disease-free survival, and this has become the “gold standard” following allo-SCT. In spite of the success of allo-SCT relapse will occur in a small but steadily increasing number of patients over time.4 The use of donor lymphocyte transfusions (DLTs) induces a remission in a substantial number of patients but has been associated with fatal aplasia and severe graft-versus-host disease (GvHD) and relies upon the availability of the original donor.5 6
Kantarjian et al have demonstrated a complete cytogenetic response to imatinib mesylate in patients relapsing after allograft and failing DLTs.1 But a cytogenetic responder may still harbor significant levels of BCR-ABL transcripts. We describe 3 patients who had a molecular response to imatinib mesylate after relapse of CML following allograft, in first CP. One patient relapsed, with clonal evolution, 10 years following matched sibling bone marrow transplantation (BMT; his donor had died from a myocardial infarct 5 years after the transplantion). He had a complete cytogenetic response to imatinib mesylate, 600 mg/d after 3 months, and was a complete donor chimera by polymerase chain reaction (PCR) of short tandem repeats (STRs; sensitivity 10−4).7 He remains in complete cytogenetic remission one year later on imatinib mesylate (400 mg/d). The second patient had a cytogenetic relapse, t(9:22), 5 years following a sibling allograft uncomplicated by GvHD. He had a complete cytogenetic response to imatinib mesylate (400 mg/d) at 3 months and was a donor chimera. He remains in complete cytogenetic remission at 21 months. The third patient had a sibling allograft in July 1998. He had a cytogenetic t(9;22) relapse 6 months later and was a mixed chimera. He received DLTs from the original donor in March and June 2000 without response. He received a nonmyeloablative SCT in February 2001 from the original donor, which was followed by a transient response. He had a complete cytogenetic response at 6 months to imatinib mesylate (400 mg/d). No patient had granulocytopenia or GvHD.
BCR/ABL transcripts were measured in a serial fashion in all patients using real-time quantitative PCR (RQ-PCR) using TaqMan probes (sensitivity 10−6). Standard curves were generated following application of a dilution series of the bcr-abl–expressing plasmids pNC210 (a gift of Nick Cross, University of Southampton) for the b3a2 assay and pb2a2 (generated in house) for the b2a2 assay. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was also tested in serial samples to allow quantitative assessment of BCR-ABL/GAPDH ratios.
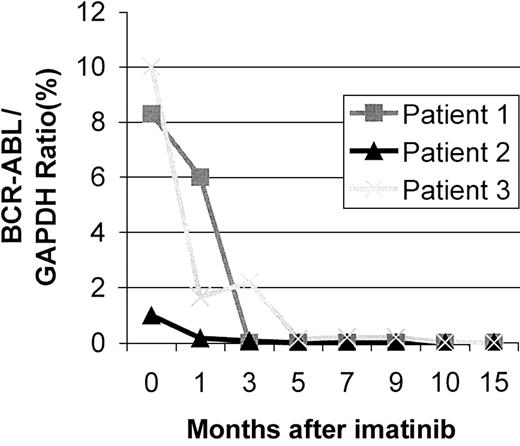
The results are shown in Figure 1. Imatinib mesylate was associated with a molecular remission in 2 patients who were treated for relapsed CML 10 and 5 years following BMT for CP CML, and in 1 patient, who failed allografting, DLTs, and a nonmyeloablative SCT, Bcr/Abl transcripts were almost undetectable. There was no evidence of toxicity in this small group of patients. O'Dwyer et al8 have demonstrated that clonal evolution per se does not impair the response to imatinib mesylate, which concurs with our experience with our first patient. Serial monitoring using both chimerism analysis and RQ-PCR provides evidence that imatinib mesylate can induce molecular remissions in patients who relapse following allo-SCT for CML.
BCR-ABL/GAPDH ratios in serial analysis of imatinib-treated relapsed CML transplantation patients.
The BCR-ABL/GAPDH ratio is expressed as a percentage following RQ-PCR analysis of reverse transcribed cDNA samples. Both patients 1 and 3 were 100% Ph-positive at time 0, whereas patient 2 exhibited 40% Ph-positivity. Both patients 1 and 2 achieved complete BCR-ABL negativity, whereas patient 3 exhibited extremely low BCR-ABL/GAPDH ratio (1 × 10−5) at 14 months.
BCR-ABL/GAPDH ratios in serial analysis of imatinib-treated relapsed CML transplantation patients.
The BCR-ABL/GAPDH ratio is expressed as a percentage following RQ-PCR analysis of reverse transcribed cDNA samples. Both patients 1 and 3 were 100% Ph-positive at time 0, whereas patient 2 exhibited 40% Ph-positivity. Both patients 1 and 2 achieved complete BCR-ABL negativity, whereas patient 3 exhibited extremely low BCR-ABL/GAPDH ratio (1 × 10−5) at 14 months.
Funded by the Health Research Board of Ireland and the Higher Education Authority Program for Research in Third Level Institutions 2