Abstract
Interactions of CD47 and RhAG and the Rh proteins are visualized between one another and with the cytoskeleton of intact erythrocytes. In a first study, CD47 is labeled with a phycoerythrin (PhE)– tagged antibody, which generates discrete spots that reflect induced clusters of CD47. Rh and RhAG colocalize with each other and to these induced clusters, whereas Band 3 and glycophorin C remain more homogeneously dispersed on the cell periphery. In a second study, red cells are aspirated into a micropipette, and immunofluorescent maps of the surface gradients that develop for CD47 and RhAG determine cytoskeletal connectivity. CD47 and RhAG gradients on normal red cells prove to be nearly identical and also appear intermediate to those found for the fluid bilayer and network-linked glycophorin C. Similar gradients are obtained for CD47 on Rhnull cells, suggesting that linkage of CD47 to the spectrin-actin skeleton is independent of Rh or RhAG and is not affected by CD47's reduced surface expression on these cells. The results show that CD47 colocalizes with Rh and RhAG but is fractionally attached to the red cell membrane skeleton independent of these and other major integral membrane proteins involved in cytoskeletal attachment. The results imply a homogeneous base distribution of CD47, restrained by cytoskeleton linkages, plus a smaller fraction of CD47, which is able to diffuse in the membrane.
Introduction
CD47, also known as IAP (integrin associated protein) or OA3, is a ubiquitously expressed 5-span transmembrane protein with a single extracellular immunoglobulin domain and a short, multiform intracellular tail.1 CD47 in nonerythroid cells appears to be involved in a variety of functions ranging from adhesion to signal transduction. In association with αvβ3 integrins, CD47 mediates leukocyte activation and chemotaxis2 as well as thrombospondin interactions with endothelium.3 Interactions of CD47 with the tyrosine kinase signal regulatory protein (SIRP)–α result in multinucleation of macrophages4 and cellular signaling in neuronal cells.5 CD47 also has been hypothesized to signal apoptosis in T cells.6
On mature erythrocytes, which lack integrins, CD47 appears to mediate cell-cell interactions with SIRP-α of splenic macrophages. This association is thought to inhibit a phosphorylation cascade that blocks phagocytosis and prevents erythrocyte clearance from the circulation.7 CD47 interacts with soluble thrombospondin and, in sickle erythrocytes, appears to generate an intracellular signal that ultimately increases sickle cell adhesiveness, perhaps contributing to vaso-occlusive events.8 Whether additional erythrocyte components contribute to these varied observations is not yet clear and motivates a better understanding of CD47's associations within the erythrocyte membrane.
Among various cell types, 4 isoforms of CD47 have been found: the isoforms differ only in their cytoplasmic tails, which range in length from 20 to 40 amino acids. The hematopoietic form of CD47 found in bone marrow (form 2) contains 20 amino acids in its cytoplasmic tail.9 In many nonerythroid cell lines, attachment of CD47 to the cytoskeleton has been shown to occur via PLIC (Proteins Linking IAP to Cytoskeleton) linkage to intermediate filaments.10However, neither intermediate filaments nor PLIC proteins are detectable in erythrocytes (E. Brown, written personal communication, March 2001).
Association of CD47 on erythrocytes with proteins of the Rh membrane complex is suggested by the observation that Rhnullerythrocytes, which lack Rh and Rh-associated glycoprotein (RhAG), express significantly less CD47.11 A physical or functional association between CD47 and the Rh complex has not otherwise been directly demonstrated.
The major attachment sites between the erythrocyte spectrin-actin cytoskeleton and the lipid bilayer are widely understood to be glycophorin C and Band 3.12 However, additional attachment sites are suggested by the observation that targeted deletion of Band 3 does not result in loss of membrane assembly.13 A loss of cytoskeletal attachment through Rh, RhAG, or associated proteins (eg, CD47) might explain the altered morphology of Rhnullcells.14 Indeed, previous studies employing statistical analysis of the distribution of Rh antigens on the erythrocyte membrane15 and resistance to Triton X-100 extraction16 17 strongly suggest that Rh is connected to the cytoskeleton.
The present study was undertaken to visualize the interactions of CD47 with Rh, RhAG, and the cytoskeletally attached proteins Band 3 and glycophorin C and to measure cytoskeletal connectivity of CD47 and RhAG. In a first study, we show colocalization of Rh and RhAG, but not Band 3 and glycophorin C, to clusters of CD47 induced by a cross-linking antibody moiety on the surface of erythrocytes. In a second study, we use micropipette aspiration of cells labeled with fluorescent antibody to show that a partial yet significant fraction of both CD47 and RhAG is linked to the cytoskeleton. However, CD47 cytoskeletal attachment in Rhnull cells, which lack Rh and RhAG and have reduced CD47, is comparable to normal erythrocytes, strongly suggesting that linkage of CD47 to the cytoskeleton can occur without Rh and RhAG.
We conclude that CD47 colocalizes with Rh and RhAG but is fractionally attached to the red cell membrane skeleton independent of Rh, RhAG, Band 3, or glycophorin C. Recent studies of protein 4.2–deficient cells indeed suggest a connection between CD47 and/or Rh and this cytoskeletal protein.18
Materials and methods
Erythrocytes
Erythrocytes were collected at room temperature in phosphate buffered saline (PBS) (Sigma-Aldrich, St Louis, MO) and washed twice with PBS. Rh-negative (ce/ce) and Rh-positive (DcE/DcE) cells were used for most experiments. Glycerol frozen control cells and Rhnull cells were thawed in PBS at 37°C and washed twice with PBS. Rhnull cells showed no labeling with antibodies to RhAG or Rh (c and E).
Antibodies
The following mouse monoclonal antibodies were used: R-phycoerythrin (PhE)–labeled BRIC126 (IgG2b; IBGRL, Bristol, United Kingdom) and 6H9 (IgG1) anti-CD4719; 2D10 (IgG1) anti-RhAG20; BRIC6 (IgG3) anti–Band 3 (IBGRL); and fluorescein isothiocyanate (FITC)–labeled BRIC10 anti–glycophorin C (IgG1; IBGRL). Rh was detected using a human polyclonal serum containing anti-c and anti-E. Binding of unlabeled mouse antibody was detected with FITC-labeled Fab fragment of goat, antimouse (Jackson Laboratories, West Grove, PA), or tetramethylrhodamine-5(and 6)–isothiocyanate (TRITC)–labeled goat, antimouse (Sigma-Aldrich) as indicated, and human antibody binding was detected with FITC-labeled anti–human IgG Fab (Cappel, Aurora, OH). The secondary antibodies were tested to ensure no nonspecific binding to human erythrocytes. For colabeling experiments requiring the use of labeled antimouse with the PhE-BRIC126, the primary and secondary antibodies were followed by the addition of PhE-BRIC126. This stepwise sequence was used to avoid artifactual colocalization due to possible binding of the antimouse antibody to the murine PhE-BRIC126. Both positive and negative colocalization data were obtained, validating the use of this approach.
For fluorescence imaged microdeformation (FIMD) experiments, unlabeled BRIC126 was converted to Fab fragments (ImmunoPure Fab Preparation Kit, Pierce, Rockford, IL) and fluorescently tagged with TRITC (FluoReporter Tetramethylrhodamine Protein Labeling Kit, Molecular Probes, Eugene, OR) per manufacturer's instructions. All labeling was done at room temperature. Dilution curves were generated for the binding of each antibody to red blood cells. Antibody concentrations below the apparent KD of cell binding were used to ensure that the fluorescent signal was not overly saturated.
Membrane labeling
The lipid membrane was labeled by incubation with the fluorescent lipid FL-PE (N-(fluoroscein-5-thiocarbamoyl)-1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine triethylammonium salt, Molecular Probes). For colocalization experiments the actin cytoskeleton was labeled with rhodamine-tagged phalloidin (Sigma-Aldrich) or Alexa 488–tagged phalloidin (Molecular Probes) incorporated into erythrocytes reversibly permeabilized by cold and low osmotic concentration and resealed with KCl and MgCl2 at 37°C.21
Fluorescence-imaged microdeformation
Analysis of colocalization
Images were acquired on a Nikon TE300 inverted microscope with a 60 × (oil, 1.4 NA) objective using a liquid nitrogen cooled CCD camera (Roper Scientific, Trenton, NJ). Image acquisition and analyses were performed with Image Pro software (Media Cybernetics, Silver Spring, MD). Images of osmotically swollen cells were taken at the equatorial position of the cell, with one image per fluorophore. For cluster analyses, images were changed from gray scale to black and white to accentuate the areas of localized intensity. The area of peripheral pixels of positive intensity was counted in each image. The images were then mathematically overlaid such that common pixels were also counted, and the coincident intensity index was determined as the common pixels divided by the total pixels less the common. At least 15 cells were used for each analysis. Statistical significance was assessed with a Student t test.
Results
Nano-scale colocalization of CD47 and Rh
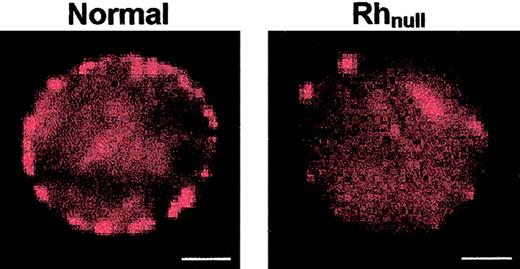
CD47 labeling of erythrocytes at subsaturating concentrations of BRIC126 conjugated with R-phycoerythrin (PhE-BRIC126) leads to discrete surface spots (Figure 1). At the concentrations of PhE-BRIC126 used for these experiments, the average cluster size on normal red cells appears to be 520 ± 130 nm in diameter spaced by 1017 ± 248 nm. Reduced expression of CD47 on Rhnull cells is reflected in a reduction in overall fluorescence intensity as well as a decreased number of PhE-BRIC126 spots. It is true that individual PhE-BRIC126 conjugates are sufficiently large and fluorescent to be visualized when simply adsorbed to a coverslip in the absence of any cells. These intense spots thus could be providing visual tags of individual CD47 molecules. However, the large size (240 kDa) of the PhE probe also provides multiple sites for conjugation to primary antibodies. It is likely, as explained in “Discussion,” that the resultant spots represent cross-link–induced clusters of PhE antibody and CD47. Also sequestered into these clusters are membrane components that are physically associated with the cross-linked antigen. Colabeling and fluorescence colocalization of other membrane components with the PhE-BRIC126 can, therefore, visibly indicate surface association. Examples of antibody labeling and visual colocalization are shown in Figure2, and quantitative analyses of the overlap images for all membrane components tested are shown in Figure3.
Equatorial sections of normal and Rhnullerythrocytes labeled for CD47 with phycoerythrin-tagged monoclonal antibody BRIC126 (PhE-BRIC126).
Spots on the membrane of these osmotically sphered cells are the result of both the size and the intensity of the antibody probe and the production of clusters of cross-linked CD47. Rhnull cells labeled with the same concentration of probe clearly exhibit a lower overall fluorescence intensity as well as a decrease in the number of clusters on the cell. The bar in each image represents 2 μm.
Equatorial sections of normal and Rhnullerythrocytes labeled for CD47 with phycoerythrin-tagged monoclonal antibody BRIC126 (PhE-BRIC126).
Spots on the membrane of these osmotically sphered cells are the result of both the size and the intensity of the antibody probe and the production of clusters of cross-linked CD47. Rhnull cells labeled with the same concentration of probe clearly exhibit a lower overall fluorescence intensity as well as a decrease in the number of clusters on the cell. The bar in each image represents 2 μm.
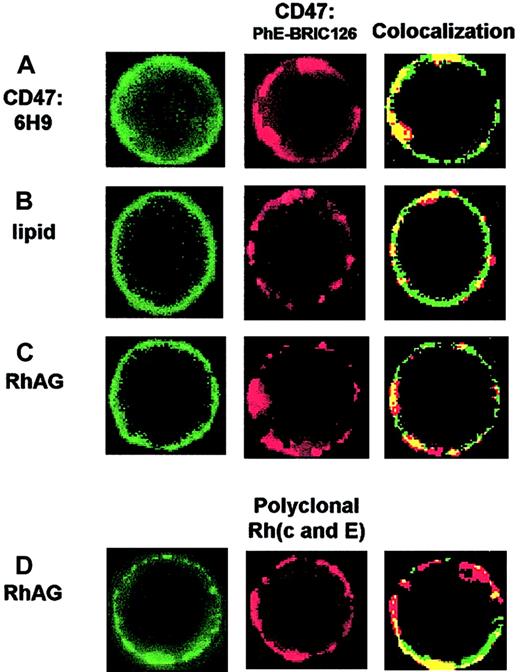
Colocalization of membrane components to clusters of PhE-BRIC126–labeled CD47 or to polyclonal-labeled Rh.
(A) Positive colocalization: double labeling of CD47 with a noncompeting monoclonal antibody, 6H9. Higher intensity spots of 6H9 (green) generally coincide with the clusters of CD47 produced by PhE-BRIC126 (red). The overlap image shows yellow colocalized regions. (B) Negative colocalization: uniform labeling of the lipid bilayer with fluorescein-phosphatidylethanolamine (FL-PE). Similar images were obtained with antibody labeling of Band 3 as well as glycophorin C, as quantified in Figure 3. (C) Yellow spots in the colocalization image indicate significant overlap of antibody-labeled RhAG (green) with PhE-BRIC126 (red). (D) A polyclonal antibody against Rh(c and E) also generates clusters to which RhAG colocalizes.
Colocalization of membrane components to clusters of PhE-BRIC126–labeled CD47 or to polyclonal-labeled Rh.
(A) Positive colocalization: double labeling of CD47 with a noncompeting monoclonal antibody, 6H9. Higher intensity spots of 6H9 (green) generally coincide with the clusters of CD47 produced by PhE-BRIC126 (red). The overlap image shows yellow colocalized regions. (B) Negative colocalization: uniform labeling of the lipid bilayer with fluorescein-phosphatidylethanolamine (FL-PE). Similar images were obtained with antibody labeling of Band 3 as well as glycophorin C, as quantified in Figure 3. (C) Yellow spots in the colocalization image indicate significant overlap of antibody-labeled RhAG (green) with PhE-BRIC126 (red). (D) A polyclonal antibody against Rh(c and E) also generates clusters to which RhAG colocalizes.
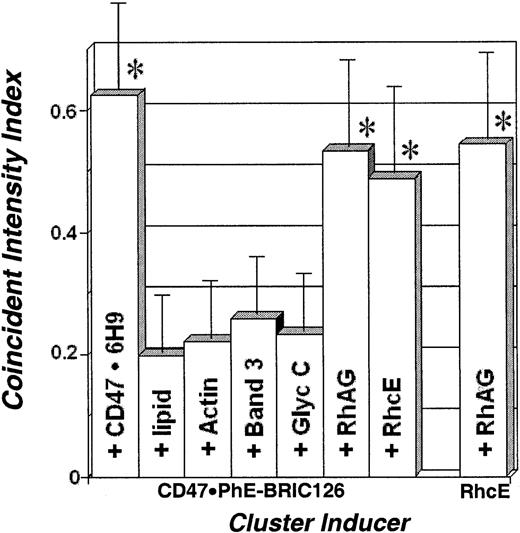
Assessment of colocalization with induced clusters.
The coincident intensity index is the area of coincident intensity (shown in yellow in Figure 2) normalized by the area of noncoincident intensities (red + green). The average ± standard deviation for at least 15 cells is plotted for each inducer. The maximum apparent range of this index is established from double labeling of CD47 with the noncompeting antibody 6H9 and cluster-inducing PhE-BRIC-126. The minimum apparent range of this index is established from labeling CD47 with PhE-BRIC-126 together with the lipid bilayer. Cytoskeletal F-actin, Band 3, and glycophorin C do not appear to be colocalized with CD47. In comparison, both RhAG and RhcE appear to colocalize in these clusters with CD47. Additionally, RhAG is colocalized to clusters induced by polyclonal anti-Rh(c and E).
Assessment of colocalization with induced clusters.
The coincident intensity index is the area of coincident intensity (shown in yellow in Figure 2) normalized by the area of noncoincident intensities (red + green). The average ± standard deviation for at least 15 cells is plotted for each inducer. The maximum apparent range of this index is established from double labeling of CD47 with the noncompeting antibody 6H9 and cluster-inducing PhE-BRIC-126. The minimum apparent range of this index is established from labeling CD47 with PhE-BRIC-126 together with the lipid bilayer. Cytoskeletal F-actin, Band 3, and glycophorin C do not appear to be colocalized with CD47. In comparison, both RhAG and RhcE appear to colocalize in these clusters with CD47. Additionally, RhAG is colocalized to clusters induced by polyclonal anti-Rh(c and E).
Double labeling of CD47 with the monoclonal antibody 6H9 and the noncompeting PhE-BRIC126 shows a punctate pattern of green-6H9 coincident with the spots of red-PhE-BRIC126 (Figure 2A). In addition to these intense spots being in the same regions of the cell when shown separately, the degree of colocalization is emphasized by the yellow-overlap image. In contrast, labeling of the membrane with the green lipid probe FL-PE and the red-PhE-BRIC126 shows a uniform green fluorescence at the periphery in the overlap image (Figure 2B). The lack of intense spots with this lipid probe in the presence of the cluster-inducing PhE-BRIC126 is consistent with the idea that constraints on proteins have little to no effect on the bilayer. The double labeling of CD47 is used to establish an upper bound for the coincident intensity index, and lipid with CD47 provides a lower bound for the index (Figure 3). This coincident intensity index allows comparison for colocalization of various membrane components with a cluster inducer. At least 15 cells were examined for each case.
Samples showing positive colocalization (denoted with an asterisk) are statistically similar to the positive control, double-labeled CD47 (P > .15) and statistically different than the negative control, lipid with CD47 (P < .0007). Conversely, samples showing no colocalization are statistically similar to the negative control (P > .08) and statistically different from the positive control (P < .0003).
PhE-BRIC126–labeled CD47 with phalloidin-labeled cytoskeletal F-actin results in no significant coincidence. Band 3 and glycophorin C, thought to be the major cytoskeletally connected proteins in the red cell membrane, do not show fluorescence colocalization to CD47. However, RhAG appears coincident to the CD47 clusters, suggesting that RhAG and CD47 colocalize and associate on the cell surface (Figures 2C,3). RhcE also colocalizes with CD47 (Figure 3).
Polyclonal antibodies directed against Rh(c and E) also exhibit intense localized fluorescence. This could be due to cross-linking of RhcE molecules. Colocalization of RhAG with cross-linked RhcE provides additional evidence of their membrane surface association (Figure 2D). Colocalization of both RhcE and RhAG to one another and to CD47 is quantitatively similar to the positive control (Figure 3).
Microscale views of CD47 and RhAG connectivity to the spectrin-actin skeleton
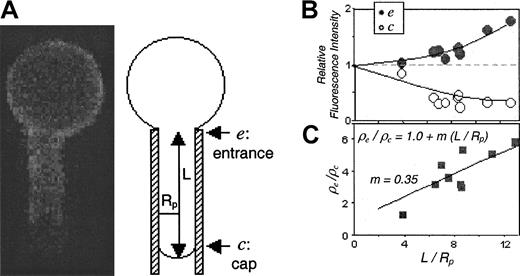
Fluorescent labeling of almost any red cell component—bilayer, cytoskeleton, or integral membrane protein—appears homogeneous on a normal discocyte. Fluorescence-imaged microdeformation (FIMD)22 segregates structurally diverse membrane components on a micron scale. The method involves first fluorescently labeling a membrane component on intact cells or ghosts and then aspirating an individual cell or ghost into a micropipette of 1-2 μm diameter (Figure 4A). Analysis of the resulting fluorescent image focuses on the aspirated projection of the cell, which exhibits one of 3 responses or a combination thereof: (1) The lipid bilayer displays a completely uniform fluorescent intensity over the entire projection length and is consistent with a fluid membrane (Figure 5C). (2) The spectrin-actin network and connected proteins, such as glycophorin C, resist the aspiration and appear concentrated at the entrance of the pipette and diminished at the cap (Figure 5D). This is consistent with an overall elastic resistance to deformation. (3) Mobile integral membrane proteins such as GPI-linked CD59 are sterically excluded from the entrance and produce a brightly fluorescent cap with an intensity profile that increases toward the cap.21 Intermediate responses between 1 and 2 are indicative of proteins with a significant, but not complete, attached fraction.22Regardless of response (1, 2, 3, or mixed), the fluorescence intensity on the minimally strained section of cell outside of the micropipette provides a means of normalizing the intensity of the projection to give a relative density, ρ.
Fluorescence imaged microdeformation (FIMD) analysis of RhAG on a normal erythrocyte.
(A) Image of a micropipette-aspirated erythrocyte with fluorescently labeled RhAG. The schematic illustrates an aspirated cell of projection length L in a pipette of radius Rp. The entrance, e, and cap, c, regions are identified. (B) Relative fluorescence intensities or densities averaged across the entrance and cap for 9 cell projections of various lengths. See “Materials and methods” for normalization method. (C) Ratio of entrance-to-cap densities as a function of projection length. This plot (with linear fit forced to interceptρe/ρc = 1) illustrates the increasing component gradients that are characteristic of considerable cytoskeletal attachment.21 For typical bilayer probes, the slope, m, has a value close to 0.0, whereas freely diffusing proteins such as GPI-linked CD59 give definitively negative slopes.
Fluorescence imaged microdeformation (FIMD) analysis of RhAG on a normal erythrocyte.
(A) Image of a micropipette-aspirated erythrocyte with fluorescently labeled RhAG. The schematic illustrates an aspirated cell of projection length L in a pipette of radius Rp. The entrance, e, and cap, c, regions are identified. (B) Relative fluorescence intensities or densities averaged across the entrance and cap for 9 cell projections of various lengths. See “Materials and methods” for normalization method. (C) Ratio of entrance-to-cap densities as a function of projection length. This plot (with linear fit forced to interceptρe/ρc = 1) illustrates the increasing component gradients that are characteristic of considerable cytoskeletal attachment.21 For typical bilayer probes, the slope, m, has a value close to 0.0, whereas freely diffusing proteins such as GPI-linked CD59 give definitively negative slopes.
Fluorescence images of CD47, lipid, and glycophorin C on normal and Rhnull-aspirated erythrocytes.
(A) FIMD of CD47-labeled cells (using TRITC-BRIC126 Fab) shows gradient from entrance to cap, indicative of considerable cytoskeletal attachment. (B) FIMD of CD47-labeled Rhnull cells (using TRITC-BRIC126 Fab) shows similar cytoskeletal attachment. (C) FIMD of lipid bilayer–labeled Rhnull cells illustrates the absence of any significant gradient, consistent with a homogeneous fluid bilayer. (D) FIMD of glycophorin C–labeled Rhnull cells illustrates the strong entrance to cap gradient, consistent with a cytoskeletally linked protein.
Fluorescence images of CD47, lipid, and glycophorin C on normal and Rhnull-aspirated erythrocytes.
(A) FIMD of CD47-labeled cells (using TRITC-BRIC126 Fab) shows gradient from entrance to cap, indicative of considerable cytoskeletal attachment. (B) FIMD of CD47-labeled Rhnull cells (using TRITC-BRIC126 Fab) shows similar cytoskeletal attachment. (C) FIMD of lipid bilayer–labeled Rhnull cells illustrates the absence of any significant gradient, consistent with a homogeneous fluid bilayer. (D) FIMD of glycophorin C–labeled Rhnull cells illustrates the strong entrance to cap gradient, consistent with a cytoskeletally linked protein.
Quantitative analyses of relative densities at both the entrance (ρe) of the pipette and the cap (ρc) provide deeper insights into connectivity and mobility. When plotted as a function of the relative projection length (L/Rp) aspirated into the micropipette, a progressive increase in entrance density and decrease in cap density are observed (Figure 4B); such results are typical of cytoskeletally linked populations of protein. More simply, the ratioρe/ρc plotted versusL/Rp yields a positive slope (Figure 4C). This indicates a significant level of cytoskeletal attachment since similar plots for phospholipid show near-zero slope and proteins such as CD59 produce a negative slope.21 FIMD of RhAG (Figure 4) and CD47 (Figure 5A) visually show similar fluorescent gradients along the aspirated projection. Both gradients exhibit a higher fluorescence at the entrance than the cap, closer in form to the response of a spectrin-actin network (2) than that of either lipid (1) or freely diffusible membrane proteins (3). CD47 on Rhnull cells, shown to have no RhAG or Rh and reduced expression of CD47 by immunolabeling, exhibit a very similar FIMD gradient to CD47 on normal erythrocytes (Figure 5B). In comparison, FIMD analyses of glycophorin C generally show a 2- to 3-fold steeper slope than CD47 or RhAG (Figure5D). Normal red cells show similar projections for lipid and glycophorin C as Rhnull cells. The slopes of the fluorescent gradients of the projections can be used to analytically determine the fractional connectivity of proteins to the underlying cytoskeleton (Figure 6).
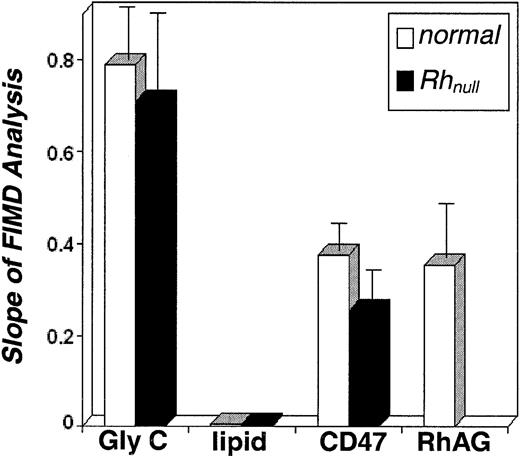
Gradient slopes,
ρe/ρc versusL/Rp, from FIMD analysis (Figure 4C) of both normal and Rhnull cells. Slopes of FIMD analysis are indicative of cytoskeletal attachment. Results for CD47 and RhAG suggest a cytoskeletal attachment similar to one another and intermediate to glycophorin C and the lipid bilayer. CD47 retains this attachment in the absence of the Rh proteins in Rhnullcells. The connectivity, f*, of CD47 and RhAG can be determined from these FIMD slopes (Table 1).
Gradient slopes,
ρe/ρc versusL/Rp, from FIMD analysis (Figure 4C) of both normal and Rhnull cells. Slopes of FIMD analysis are indicative of cytoskeletal attachment. Results for CD47 and RhAG suggest a cytoskeletal attachment similar to one another and intermediate to glycophorin C and the lipid bilayer. CD47 retains this attachment in the absence of the Rh proteins in Rhnullcells. The connectivity, f*, of CD47 and RhAG can be determined from these FIMD slopes (Table 1).
Connectivity of CD47 and RhAG from FIMD
Membrane proteins are rarely completely attached or freely mobile; most exhibit a fractional attachment or connectivity, f*, to the underlying cytoskeleton. The slopes of the fluorescent projection gradients provided by FIMD allow a quantitative measure of this connectivity for the fluorescently labeled protein of interest (Figure6). Proteins with a high mobile fraction or low connectivity have negative projection gradients closer in form to those of freely diffusible CD59. Proteins that have a high degree of connectivity to the underlying cytoskeleton show fluorescent projection gradients similar to cytoskeleton-attached glycophorin C. FIMD analysis of glycophorin C suggests an f* near 100%,21which agrees with f* of about 90% by Triton X-100 extraction.23 Fluorescence recovery after photobleaching (FRAP), which examines diffusive processes over longer time scales, also shows that the majority of glycophorin C is cytoskeletally immobilized, indicative of agreement between the various measures.24
Using glycophorin C and CD5921 for calibration of the networklike gradients and fully mobile components, one can estimate the connectivity of RhAG and CD47 (Table 1).
Fractional connectivity of various membrane components
| Protein . | Erythrocyte type . | |
|---|---|---|
| Normal . | Rhnull . | |
| Glycophorin C | 100% | 88% |
| CD47 | 62% | 58% |
| RhAG | 60% | NP |
| CD59 | 0% | ND |
| Protein . | Erythrocyte type . | |
|---|---|---|
| Normal . | Rhnull . | |
| Glycophorin C | 100% | 88% |
| CD47 | 62% | 58% |
| RhAG | 60% | NP |
| CD59 | 0% | ND |
Connectivity of various membrane components as described byf
=(m − mCD59)/(mGlyC − mCD59),where m is the slope of the FIMD projection, as given in Figure 6.
NP indicates not possible; ND, not done.
Discussion
Colocalization to induced clusters of CD47 and Rh
Phycoerythrin (PhE) molecules have been used for tracking the diffusion of single proteins because of their high intensity.25 The large size of the PhE probe also provides sites for binding of multiple immunoglobulin molecules and a mechanism for cross-linking of CD47 on the cell surface. Particle tracking of individual PhE-BRIC126 spots on the erythrocyte membrane indicates a diffusion constant several orders of magnitude below that of lipid (data not shown); this diffusivity even appears lower than that of proteins with significant immobile fractions such as glycophorin C. This apparent immobility of PhE-BRIC126–labeled CD47 is inconsistent with the FIMD data, which indicates a minor yet significant mobile fraction, unless CD47 is being cross-linked.
Immobilization of surface proteins has been shown by others to occur with the addition of cross-linking antibodies to red cells.24,26 CD47 has been shown on other cells to be clustered as recognized by the antibody CDw148, but this clustering does not occur in erythrocytes.27 Here, cross-linking of the mobile fraction of CD47 to the immobile fraction would tend to nucleate localized concentrations of CD47. Further evidence of this is shown by the lack of colocalization of the actin cytoskeleton with PhE-BRIC126, indicating no deformation of the underlying network. Clusters of CD47 on Rhnull cells also imply a mobile and immobile fraction, despite a significant reduction in overall number of CD47 proteins. Molecules physically associated with CD47, such as Rh and RhAG, would tend to be displaced into these induced clusters. CD47 is shown to colocalize with RhAG as well as with RhcE. Since the size of the clusters being analyzed is close to the limit of optical resolution, it cannot be shown that colocalized surface proteins are directly bound to one another. However, the active movement of RhAG and the Rh proteins with the clustering of CD47 shows that these proteins are physically associated in some way on the red cell membrane. The previous observation of reduced levels of CD47 on Rhnullerythrocytes11 is consistent with these results, but here membrane interactions on normal red cells are shown.
By the same method, the colocalization of RhAG to RhcE clusters is highly suggestive of an Rh protein complex. This is consistent with previous, indirect results showing that Rh proteins coimmunoprecipitate with RhAG and exist as heterotetramers in the erythrocyte membrane.28 One significant advantage of the fluorescence colocalization method used here over coimmunoprecipitation, in addition to being more visually direct, is the minimal disruption of intact cells.
Band 3 is known to have a significant immobile fraction and has been suggested to be a component of the Rh complex since K562 erythroleukemia cells transfected with Band 3 have increased levels of detectable RhD and RhcE by flow cytometry.29 However, unlike Rh or RhAG, Band 3 and glycophorin C are neither stoichiometrically coincident with CD47 nor are they displaced into the induced clusters. This suggests that there is little interaction of Band 3 or glycophorin C with CD47 on the surface of the erythrocyte. Band 3 and glycophorin C have been thought to represent the majority of cytoskeletal attachment sites of the red cell membrane.30The fact that CD47 does not colocalize with either Band 3 or glycophorin C together with the results of the FIMD studies showing cytoskeletal linkage of CD47 and RhAG suggests that the CD47-Rh complex must be cytoskeletally linked either directly or via a novel set of associations. This appears consistent with recent data on cells lacking Band 3, in which RhAG and CD47 are both resistant to Triton X-100 extraction.16 Protein 4.2–deficient human red cells recently have been shown to have a 75% reduction in CD47 compared to normal red cells, implicating the cytoskeletal protein 4.2 in the network association of CD47.18
Fractional connectivity of RhAG and CD47 to the spectrin-actin cytoskeleton
FIMD results for RhAG indicate significant (about 60%) cytoskeletal attachment. This association is either direct or via an associated complex, confirming data from nearest-neighbor statistical analysis of immunoelectron micrographs15 and Triton X-100 extractions.16 17 Triton X-100 results can be affected by the considerable hydrophobicity of the membrane-spanning proteins involved. Nonetheless, the approaches here on the intact membrane also suggest that Rh proteins interact, directly or indirectly, with the erythrocyte spectrin-actin cytoskeleton.
The FIMD results for CD47 on normal cells also indicate significant (about 60%) cytoskeletal attachment, suggesting that RhAG and CD47 may be associated. This result supports the fluorescent colocalization data. The connectivity of CD47 is not significantly reduced in Rhnull cells, which contain neither RhAG nor RhcE. This suggests that CD47 itself mediates a cytoskeletal connection of the Rh complex.
The fact that CD47 has significant cytoskeletal attachment could suggest a novel contribution to cytoskeletal assembly. CD47 has been shown to be present throughout the entire erythrocyte maturation process in cultured progenitor cells.31 Rhnullcells lack Rh protein and have a reduced level of CD47 and are often stomatocytic or spherocytic,14 as are human 4.2–deficient red cells.18 Band 3–deficient cells also are spherocytes, and glycophorin C–deficient cells are elliptocytes. In contrast, no abnormalities in cell morphology are reported for deficiencies in abundant transmembrane proteins that are not network linked.32 Since erythrocytes with deficient cytoskeletal linkage sites have altered morphology and since the results here suggest that CD47 is also a site for cytoskeletal linkage, we tentatively conclude that CD47 could have a role in cell morphology. Rhnull cells have about 60% cytoskeletally attached CD47, but the level of CD47 is severely reduced, and the overall number of cytoskeletal connections via CD47 is therefore reduced. Rh proteins may contribute as well, but the cytoskeletal connection of CD47 in Rhnull cells, despite its reduced expression level, indicates that CD47 connects independently to the red cell cytoskeleton.
As introduced, erythrocyte CD47 has a putative role in binding macrophage SIRP-α and signaling “self.”7 The fractional connectivity (about 60%) found here might be critical in this signaling process. A substantial degree of cytoskeletal connection ensures that an immobile fraction of CD47 remains equally distributed around the red cell at all times. Moreover, model systems show that freely diffusible receptors tend to accumulate in zones of adhesive contact.33 34 CD47's mobile fraction (about 40%) could likewise be recruited to contact sites with macrophages. Such reinforcement is emulated here in the antibody cross-linking of mobile to immobile proteins. We speculate that this accumulation could reinforce and amplify some critical signal of “self” (Figure 7).
Schematic of network-linked plus mobile CD47 molecules as they might function in signaling red cell “self” to macrophages.7
Not shown are the many Rh proteins (and other components), so that the picture is most relevant perhaps to Rhnull red cells. The network-linked CD47 ensures a uniform, if sparse, distribution of this putative “marker of self,” while the mobile fraction of CD47 allows for affinity-driven clustering that reinforces the signal to SIRP-α on the macrophage.
Schematic of network-linked plus mobile CD47 molecules as they might function in signaling red cell “self” to macrophages.7
Not shown are the many Rh proteins (and other components), so that the picture is most relevant perhaps to Rhnull red cells. The network-linked CD47 ensures a uniform, if sparse, distribution of this putative “marker of self,” while the mobile fraction of CD47 allows for affinity-driven clustering that reinforces the signal to SIRP-α on the macrophage.
Summary
Using the intensity and discrete separation of the PhE-BRIC126 and polyclonal Rh(c and E) antibodies binding on the cell surface, it is possible to visually confirm the presence of an Rh protein complex and the colocalization of CD47 to the Rh protein complex. FIMD data suggest that a fraction of these complexes are anchored to the cytoskeleton. The attachment appears to be largely independent of glycophorin C and Band 3, suggesting novel attachment by a protein of the Rh complex. Finally, FIMD analysis of CD47 on Rhnull red cells shows essentially no change in cytoskeletal attachment, suggesting that the Rh proteins are not necessary for this novel attachment.
The authors thank A. E. von dem Borne, Netherlands Red Cross, Amsterdam, for the monoclonal antibody 2D10; M. Telen, Duke University, Durham, NC, for the monoclonal antibody 6H9; and M. E. Reid, New York Blood Center, for the polyclonal anti-c and anti-E antibodies. The authors also gratefully acknowledge important discussions with Mohandas Narla, New York Blood Center.
Prepublished online as Blood First Edition Paper, September 26, 2002; DOI 10.1182/blood-2002-04-1187.
Supported by an NIHR01 grant and a Whitaker Graduate Fellowship.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Dennis E. Discher, School Engineering and Applied Science, University of Pennsylvania, Towne 112, 220 S 33rd St, Philadelphia, PA 19104; e-mail:discher@seas.upenn.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal