Abstract
Hepatocyte growth factor (HGF) is a mesenchyme-derived pleiotropic growth factor and a powerful stimulator of angiogenesis, which acts on cells by binding to the c-met receptor. The exact role of the endogenous HGF/c-met system in one or more steps of the angiogenic process is not completely understood. To contribute to this question we used immunocytochemical analysis, Western blotting, and reverse transcription–polymerase chain reaction to study the expression of c-met in endothelial cells cultured in different growth conditions. We found that c-met is not colocalized with vascular endothelial (VE)–cadherin in cell-cell junctions. c-met and VE-cadherin were shown to be inversely regulated by cell density, at both the protein and the mRNA levels. We established that c-met is up-regulated during the in vitro recapitulation of several steps of angiogenesis. The c-met expression was increased shortly after switching to angiogenic growth conditions and remained high during the very first steps of angiogenesis, including cell migration, and cell proliferation. The endothelial cells in which the expression of c-met was up-regulated were more responsive to HGF and exhibited a higher rate of morphogenesis. Moreover, the antibody directed against the extracellular domain of the c-met inhibited angiogenesis in vitro. Our results suggest that c-met is a marker of angiogenic phenotype for endothelial cells and represents an attractive target for the development of new antiangiogenic therapies.
Introduction
Angiogenesis, the process by which new blood vessels are formed by splitting or sprouting from pre-existing ones, occurs in several physiologic and pathologic situations. Angiogenesis-associated disorders include tumor growth and metastasis, ischemic disease, diabetes, rheumatoid arthritis, atherosclerosis, inflammatory processes, and infectious processes.1 Therefore, the modulation of angiogenesis, either by promoting therapeutic angiogenesis or by preventing pathologic angiogenesis, is an exciting prospect for modern medicine.
Angiogenesis is a complex physiologic process consisting of several distinct steps. It begins with the local degradation of the basement membrane surrounding capillaries. The endothelial cells (ECs) then migrate toward angiogenic stimuli and proliferate. Finally, the ECs undergo morphogenesis, leading to the formation of a 3-dimensional capillary network. It has long been recognized that the activation of angiogenesis is associated with a number of distinct phenotypic changes in ECs that enable them to enter an angiogenic cascade.2
The acquisition of angiogenic phenotype depends on the local balance between positive and negative angiogenic regulators.3 Several proangiogenic factors have been identified so far, among them the hepatocyte growth factor (HGF),4 also known as scatter factor (SF).5 The angiogenic activity of HGF is mediated primarily through its direct actions on ECs,6,7 by binding to its receptor, a transmembrane tyrosine kinase encoded by the MET proto-oncogene (c-met).8,9 HGF can also stimulate angiogenesis indirectly by inducing other EC mitogens from non-EC populations.10,11 In a rabbit hind limb model of ischemia, angiographic, physiologic, and histologic findings indicated that HGF stimulated angiogenesis even more efficiently than vascular endothelial growth factor (VEGF).10
Most of the data concerning HGF angiogenic activity were obtained from studies on the effects of exogenously administrated HGF. In the physiologic situation, the role of endogenous HGF/c-met system in one or more steps of angiogenic process remains unclear. To contribute to this question we analyzed the expression of the HGF receptor in human umbilical vein endothelial cells (HUVECs) grown in different culture conditions, recapitulating in vitro some steps of angiogenesis. We focused on c-met because previous investigations failed to demonstrate the production of HGF by endothelial cells.12 Moreover, it is generally accepted that HGF is a mesenchyme-derived factor that acts predominantly on neighboring epithelial and endothelial cells.13
It has been shown earlier that c-met is selectively targeted to the basolateral plasma membrane domain of epithelial cells14 where it is colocalized with E-cadherin at cell-cell adhesion sites.15 The disruption of these sites is accompanied by endocytosis of both E-cadherin and c-met, suggesting that c-met is involved in the regulation of junctional dynamics. To determine whether similar cross-talk occurs between c-met and junctional components of ECs, we compared the expression patterns of c-met with those of vascular endothelial (VE)–cadherin (cadherin-5), an endothelium-specific member of the cadherin family.16
Materials and methods
Antibodies and reagents
All cell culture reagents were purchased from Life Technologies (Rockville, MD) unless otherwise specified. Normal human AB male serum (NHS) and fetal bovine serum (FBS) were obtained from Biowest (Cholet, France). Rabbit polyclonal anti–c-met IgG C-28 raised against a peptide mapping at the carboxy terminus of c-met, blocking peptide for IgG C-28, and rabbit polyclonal IgG H-190 raised against a recombinant protein mapping within the extracellular domain of c-met were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). A mouse monoclonal antibody against VE-cadherin, clone TEA 1/31, was purchased from Immunotech (Marseille, France). A fluorescein isothiocyanate (FITC)-conjugated antibody against rabbit IgG, a tetrarhodamine isothiocyanate (TRITC)–conjugated antibody against mouse IgG, a peroxidase-conjugated antibody against rabbit IgG, and a control rabbit IgG were all purchased from Sigma (St Louis, MO). A peroxidase-conjugated antibody against mouse IgG was purchased from Jackson Immunoresearch Laboratories (West Grove, PA). Recombinant human HGF, HGF R/Fc chimera (c-met extracellular domain fused to human IgG1 Fc), and platelet-derived growth factor (PDGF) BB were purchased fromR&D Systems (Minneapolis, MN). The BCA protein assay reagent was purchased from Pierce (Rockford, IL) and the cell proliferation reagent WST-1 was purchased from Roche Diagnostics (Mannheim, Germany). A mouse monoclonal antiactin antibody, nitrocellulose Hybond C Extra membrane, enhanced chemiluminescence (ECL) plus Western blotting detection reagent, and [methyl3H]thymidine (74 GBq/mmol) were all purchased from Amersham Biosciences (Buckinghamshire, United Kingdom).
Cells
HUVECs were isolated by collagenase (Roche Diagnostics) digestion.17 Cells were routinely grown in flasks coated with 0.2% gelatin in M199 medium containing 2 mM glutamine, 50 U/mL penicillin, 50 μg/mL streptomycin, 2.5 μg/mL amphotericin B, 7.5% NHS, and 7.5% FBS (regular medium). HUVECs from the second or third passage were used. The human colorectal carcinoma cells DLD-1 were obtained from the American Type Culture Collection (Manassas, VA) and were cultured in RPMI supplemented with 10% FBS. Human aortic smooth muscle cells (hASMCs) and the corresponding growth medium (SmGM-2) were purchased from Clonetics (BioWhittaker, Emerainville, France). Normal human dermal fibroblasts (NHDFs) and fibroblast growth medium (FGM) were purchased from Promocell (Heidelberg, Germany).
“In gel” 3-dimensional collagen culture
Type I rat-tail collagen was purchased from BD Biosciences (Bedford, MA) and gels were formed according to the manufacturer's recommendations. HUVECs (passage 2) were added to a final concentration of 6 × 105 cells/mL in 0.5 mg/mL collagen solution. One milliliter of this suspension was poured into a 35-mm culture dish, and the gels were allowed to form at 37°C for 40 minutes. The gels were then overlaid with 1 mL regular medium and incubated at 37°C in a humidified atmosphere containing 5% CO2. After 24 hours in these conditions, more than 95% of the HUVECs were organized into capillary-like structures. Digital images were captured using phase contrast optics and a Kappa CF11DSP charge-coupled device camera (Kappa Opto-electronics, Gleichen, Germany).
When indicated, 20 μg/mL anti–c-met antibody H-190 or 20 μg/mL control rabbit IgG were added to the overlaying medium. In the control experiments, the H-190 antibody was depleted of specific activity by incubation for 1 hour at room temperature with an affinity adsorbent containing immobilized c-met (c-met–Sepharose). The affinity adsorbent was prepared by covalent coupling HGF R/Fc chimera to NHS-activated Sepharose 4 (Amersham), according to the manufacturer's recommendations.
Wound healing assay
HUVECs were grown to confluence in 35-mm dishes coated with gelatin. A linear wound was then made by scratching the monolayer with a plastic tip. The wounded monolayers were washed 3 times with regular medium and incubated in fresh regular medium. At the indicated times, cells were fixed and processed for immunofluorescence staining.
Immunofluorescence analysis
HUVECs were plated in 35-mm dishes at 6 × 104 cells/cm2 (high density) or at 6 × 103 cells/cm2 (low density) and grown for 48 hours. Confluent, sparse, and 3-D collagen cultures were treated accordingly. Cells were washed 3 times with PBS and fixed in acetone/methanol (1:1) at –20°C for 5 minutes. The collagen gels were then dried with Whatman grade 1 paper disks (Maidstone, United Kingdom). The cells were subsequently incubated with polyclonal anti–c-met antibody C-28 (1:100) for 1 hour at room temperature, followed by anti–VE-cadherin antibody (1:100) for 1 hour at room temperature, and finally with a mixture of FITC-conjugated antirabbit IgG antibody (1:100) and TRITC-conjugated antimouse IgG antibody (1:200) for 40 minutes at room temperature. The negative controls consisted of cultures that were incubated only with the secondary antibodies. In the other control experiments, the anti–c-met antibody C-28 was preincubated with the blocking peptide (1:10; wt/wt) for 1 hour at room temperature before being added to the cells. Cells were observed by use of a Nikon (Yokohama, Japan) Eclipse E600 fluorescence microscope and images were recorded with the Perfect Image 5.1 software (Clara Vision, Orsay, France).
Western blot analysis
Monolayer HUVEC cultures were treated with 5 mM EDTA (ethylenediaminetetraacetic acid) in PBS for 3 minutes at room temperature; cells were scraped and collected by centrifugation. Collagen gels with embedded HUVECs were incubated with 0.5 U/mL collagenase for 3 minutes at 37°C. The collagen matrix was dispersed by shaking vigorously, and the gel homogenates were diluted 10-fold with PBS. Cells were recovered by centrifugation, washed 3 times with PBS, and counted. Cell pellets were lysed in 0.5 mL lysis buffer (10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], pH 7.4, 135 mM NaCl, 10% glycerol, 1% Nonidet P-40, 1 mM phenylmethylsulfonyl fluoride, and 2 mM NaF) and the lysates were clarified by centrifugation at 17 000g for 10 minutes. The protein concentration of the supernatants was determined by use of the BCA protein assay reagent. Samples were resolved by 8% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions and transferred onto nitrocellulose Hybond C Extra membranes. Blots were probed with the first antibody (anti–c-met C-28, 1:800, or anti–VE-cadherin, 1:1000) overnight at 4°C, followed by the peroxidase-conjugated antirabbit, or antimouse secondary antibody for 1 hour at room temperature. Membranes were developed with the ECL plus Western blotting detection reagent and scanned using a Hewlett Packard ScanJet ADF laser densitometer.
RT-PCR analysis
Total cellular RNA was isolated using the RNAXEL reagent (Eurobio, Les Ulis, France) according to the manufacturer's instructions. An aliquot of 800 ng was reverse transcribed using the First Strand cDNA Synthesis Kit for reverse transcription–polymerase chain reaction (RT-PCR) and a random hexamer primer (Roche Dignostics). One microliter of the cDNA product, as well as 1 μL of the cDNA that had been diluted 1:5 and 1:25, were amplified with Taq DNA polymerase (Roche Diagnostics) for 40 cycles with an annealing temperature of 55°C and an extension time of 1 minute. The primers used for PCR were: c-met forward 5′-ACAGTGGCATGTCAACATCGCT-3′, and c-met reverse 5′-GCTCGGTAGTCTACAGATTC-3′ (expected product 656 bp)18 ; VE-cadherin forward 5′-GACTGACCATCATGCCCTCT-3′, and VE-cadherin reverse 5′-GAACATCTGCCCCTTCTCAG-3′ (expected product 400 bp); β-actin forward 5′-TTGTAACCAACTGGGACGATATGG-3′, and β-actin reverse 5′-GATCTTGATCTTCATGGTGCTAGG-3′ (expected product 764 bp).19
Proliferation assays
Confluent and sparse HUVEC cultures isolated from the same umbilical cord were treated simultaneously. Cells that had been starved for 24 hours in serum-free medium were harvested by trypsinization and their proliferation was assessed either by use of a [3H]thymidine incorporation assay or by use of the cell proliferation reagent WST-1.
[3H]Thymidine incorporation assay. Cells were plated in 24-well plates at a density of 2 × 104 cells/well in serum-free medium. Different concentrations of HGF were added to the cells and they were incubated for a further 24 hours. [3H]Thymidine (0.037 MBq/well) was added for the last 24 hours of incubation. Cells were washed 3 times with PBS and treated with ice-cold 10% (wt/vol) trichloracetic acid for 30 minutes. The resulting precipitates were solubilized with 0.3 N NaOH (0.5 mL/well) and incorporated radioactivity was measured in a Beckman Coulter (Fullerton, CA) LS-6500 multipurpose scintillation counter.
WST-1 assay. Cells were plated in 96-well plates, HUVECs at a density 8 × 103 cells/well, DLD-1 at a density of 103 cells/well, and NHDFs and hASMCs at a density of 2 × 103 cells/well. HGF (25 ng/mL), PDFG BB (10 ng/mL), or FBS (10%) was added to the cells, either alone or together with anti–c-met H-190 IgG or control rabbit IgG. The cells were then incubated for a further 72 hours or 96 hours. The cell proliferation reagent WST-1 (10 μL) was added for the last 4 hours of incubation and the absorbance at 450 nm was measured using an automated microplate reader Elx808 (Bio-Tek Instruments, Winooski, VT).
Statistics
Results are expressed as mean ± SD. Statistical significance was evaluated by ANOVA followed by Bonferroni/Dunn analysis for more than 2 means. Two-way ANOVA was used to test for interaction.
Results
Effect of cell density on c-met and VE-cadherin protein levels in monolayer HUVEC cultures
We used double-staining experiments followed by immunofluorescence microscopy to demonstrate that in proliferating sparse HUVECs the c-met and VE-cadherin immunostaining patterns are different from those observed in growth-arrested confluent HUVECs (Figure 1A). Thus, strong immunolabeling with the VE-cadherin–specific antibody was seen in confluent HUVECs at cell-cell contact sites. The immunostaining corresponding to c-met was hardly detectable; only the perinuclear area was very faintly labeled. In contrast, no immunostaining corresponding to VE-cadherin was seen on sparse HUVEC cultures, obtained from the same cells seeded at a lower density (Figure 1A). Conversely, strong immunostaining was seen in sparse HUVECs with the anti–c-met antibody. c-met was distributed rather diffusely over the whole cell surface and did not cluster at precise membrane compartments. No staining was observed when cultures were treated with the anti–c-met antibody that had been preincubated with the blocking peptide, or when the cells were treated with the secondary antibody alone (data not shown).
c-met and VE-cadherin protein levels vary in cultured HUVECs in relation to cell density. (A) HUVECs grown as a confluent monolayer or as a sparse monolayer were fixed and double-stained with anti–c-met C-28, and with anti–VE-cadherin antibodies, followed by immunofluorescent-labeled secondary antibodies. Original magnification for all images in panel A, × 400. In parallel, 5 μg protein extracted from confluent (c) or sparse (s) HUVECs was resolved by 8% SDS-PAGE and analyzed by Western blotting. Blots probed with anti–c-met C-28 (B), or with anti–VE-cadherin antibody (C) are shown, along with the corresponding scanning densitometry results. Arrows indicate the positions of the unprocessed 170-kDa c-met precursor and the 140-kDa c-met β-chain. In panels B-C, relative density is reported in arbitrary units. (D) Filters were stripped and reprobed with an antiactin antibody. Data are representative of 6 independent experiments.
c-met and VE-cadherin protein levels vary in cultured HUVECs in relation to cell density. (A) HUVECs grown as a confluent monolayer or as a sparse monolayer were fixed and double-stained with anti–c-met C-28, and with anti–VE-cadherin antibodies, followed by immunofluorescent-labeled secondary antibodies. Original magnification for all images in panel A, × 400. In parallel, 5 μg protein extracted from confluent (c) or sparse (s) HUVECs was resolved by 8% SDS-PAGE and analyzed by Western blotting. Blots probed with anti–c-met C-28 (B), or with anti–VE-cadherin antibody (C) are shown, along with the corresponding scanning densitometry results. Arrows indicate the positions of the unprocessed 170-kDa c-met precursor and the 140-kDa c-met β-chain. In panels B-C, relative density is reported in arbitrary units. (D) Filters were stripped and reprobed with an antiactin antibody. Data are representative of 6 independent experiments.
To confirm that c-met and VE-cadherin are inversely regulated by cell density, we used Western blotting to analyze the amounts of these proteins present in confluent and sparse HUVECs. The results of these experiments (Figure 1B-C) confirmed our immunofluorescence observations. Scanning densitometry showed that between 50% and 70% more c-met protein was present in extracts from sparse HUVECs than in extracts from confluent HUVECs (Figure 1B). In contrast, between 60% and 80% less VE-cadherin protein could be detected in sparse HUVECs than in confluent HUVECs (Figure 1C).
In Western blots obtained with HUVEC extracts, the c-met protein presented a characteristic 2-band pattern, with the upper band corresponding to an unprocessed 170-kDa precursor and the lower band corresponding to the 140-kDa β-chain (Figure 1B). Furthermore, several bands of between 40 and 62 kDa were revealed with the anti–c-met C-28 antibody. These bands were shorter c-met fragments, as demonstrated by experiments in which the antibody was neutralized with a blocking peptide. They most likely were generated by limited proteolysis of the intact c-met protein during experimental manipulation. In fact, the amount of these fragments in the HUVEC extracts increased when collagenase (containing some contaminating enzyme activities) was used to recover the cells. Furthermore, these fragments could be produced from the full-length c-met protein by mild collagenase treatment of EDTA-recovered HUVECs, which generally contain very little of the low-molecular-weight c-met species (data not shown).
Immunofluorescence analysis of c-met and VE-cadherin expression in wound healing assay
An in vitro wound healing assay (ie, the ability to fill in artificial gaps created in cell monolayers) requires both cell growth and cell migration.20 We used an in vitro wound healing assay to investigate whether the HGF receptor was expressed differently in a normal and a repairing endothelium. The surface of a confluent HUVEC monolayer was scratched and we followed its healing during incubation in regular medium. Figure 2 shows an area in which HUVECs were engaged in the process of repair. The expression of c-met and VE-cadherin in this area was monitored by use of double-staining immunofluorescence analysis. During the first 12 hours after scratching, single HUVECs detached from the border of the confluent monolayer and moved into the damaged area (Figure 2). These single cells were not stained with anti–VE-cadherin antibody but were stained with the anti–c-met antibody. The neighboring undamaged region was not labeled with anti–c-met antibody. Interestingly, VE-cadherin immunolabeling of this region was also drastically reduced. More cells in the moving edge were labeled with anti–c-met antibody after 12 more hours of incubation when most of the cells were engaged in the healing process. These moving cells were still not labeled with the anti–VE-cadherin antibody, whereas VE-cadherin immunostaining was reinforced in the adjacent confluent monolayer. After 48 hours of incubation, when migrating cells had completely filled the free space, c-met immunolabeling disappeared and VE-cadherin immunolabeling was restored to a level comparable to that in the undamaged confluent monolayer. Thus, c-met expression is up-regulated during the repair of the endothelium.
c-met and VE-cadherin are inversely regulated at the healing edge in wound assay. Confluent HUVEC monolayers were damaged by scratching a linear wound and incubated in regular medium. At the indicated times cultures were fixed and processed for double-staining immunofluorescence analysis of c-met and VE-cadherin expression at the border of the wounded area. Data are representative of 3 experiments. Images in far left column are brightfield images. For all images in Figure 2: original magnification, × 400.
c-met and VE-cadherin are inversely regulated at the healing edge in wound assay. Confluent HUVEC monolayers were damaged by scratching a linear wound and incubated in regular medium. At the indicated times cultures were fixed and processed for double-staining immunofluorescence analysis of c-met and VE-cadherin expression at the border of the wounded area. Data are representative of 3 experiments. Images in far left column are brightfield images. For all images in Figure 2: original magnification, × 400.
Time-course analysis of c-met and VE-cadherin expression in HUVECs cultured in 3-dimensional collagen gel
When grown in 3-dimensional collagen gel (model known as “angiogenesis in vitro”), HUVECs rapidly underwent morphogenesis, forming a network of capillary-like tubes.21 We used double-staining immunofluorescence analysis and Western blotting to determine whether the expression of c-met and VE-cadherin was modulated at different stages of HUVEC tubulogenesis (Figure 3). Figure 3A documents the appearance of the HUVECs, as well as their immunostaining with the anti–c-met and the anti–VE-cadherin antibody at 0, 4, 8, and 24 hours in 3-D collagen gel. As early as 4 hours, the changes in cell morphology and sprouts could be observed in most cells. Some of the cell were already aligned and had formed short structures. At 4 hours the immunolabelling for c-met was drastically increased, compared with the initial culture. Western blot analysis detected about 4 times more c-met protein after 4 hours than at 0 hours (Figure 3B). After 8 hours, the level of c-met protein had declined back to the level of c-met in the initial culture. At this time, most of the HUVECs were involved in tubulogenesis, and the VE-cadherin protein was accumulating (Figure 3C). After 24 hours, when an interconnected network of tubelike structures was formed, the VE-cadherin protein accumulated further, whereas c-met was barely detectable (Figures 3C and B, respectively). Thus, c-met expression was up-regulated during the earlier steps of angiogenesis in vitro.
c-met expression is up-regulated during the earlier steps of angiogenesis in vitro. (A) c-met and VE-cadherin immunostaining patterns in HUVECs cultured in a 3-dimensional collagen gel for 0, 4, 8, and 24 hours. At the indicated times, the cells were recovered from the parallel collagen cultures, and processed for Western blot analysis. Images in far left column are phase micrographs. For all images in panel A: original magnification, × 400. Blots probed with anti–c-met (B) or with anti–VE-cadherin antibodies (C) are shown, along with the corresponding scanning densitometry results. In panels B-C, relative density is reported in arbitrary units. (D) Filters were stripped and reprobed with an antiactin antibody. Data are representative of 5 independent experiments.
c-met expression is up-regulated during the earlier steps of angiogenesis in vitro. (A) c-met and VE-cadherin immunostaining patterns in HUVECs cultured in a 3-dimensional collagen gel for 0, 4, 8, and 24 hours. At the indicated times, the cells were recovered from the parallel collagen cultures, and processed for Western blot analysis. Images in far left column are phase micrographs. For all images in panel A: original magnification, × 400. Blots probed with anti–c-met (B) or with anti–VE-cadherin antibodies (C) are shown, along with the corresponding scanning densitometry results. In panels B-C, relative density is reported in arbitrary units. (D) Filters were stripped and reprobed with an antiactin antibody. Data are representative of 5 independent experiments.
Analysis of c-met and VE-cadherin mRNA in HUVECs grown in different culture conditions
We used a semiquantitative analysis by RT-PCR to determine whether the culture condition–dependent differences in the c-met and VE-cadherin protein levels are accompanied by differences in the corresponding mRNA levels. We used serial dilutions of the reverse transcriptase reaction as a template for PCR (Figure 4). In our experimental conditions the quantity of amplified product decreased in relation to template concentration. This indicated that the PCR yield reflected the changes in the abundance of the corresponding mRNA. Therefore, these conditions were used for semiquantitative evaluation of the relative levels of 3 mRNAs: c-met, VE-cadherin, and β-actin.
RT-PCR analysis of c-met, VE-cadherin, and β-actin mRNA in HUVECs grown in different culture conditions. Total RNA extracted from confluent and sparse HUVECs and from the HUVECs grown in 3-dimensional collagen gel was reverse transcribed with AMV reverse transcriptase and amplified using PCR, as described in “Materials and methods.” Amplified products were analyzed on a 1.5% agarose gel after staining with ethidium bromide. Figures on the top indicate the dilution of the single-stranded cDNA template used for the PCR. Results are representative of 4 independent experiments.
RT-PCR analysis of c-met, VE-cadherin, and β-actin mRNA in HUVECs grown in different culture conditions. Total RNA extracted from confluent and sparse HUVECs and from the HUVECs grown in 3-dimensional collagen gel was reverse transcribed with AMV reverse transcriptase and amplified using PCR, as described in “Materials and methods.” Amplified products were analyzed on a 1.5% agarose gel after staining with ethidium bromide. Figures on the top indicate the dilution of the single-stranded cDNA template used for the PCR. Results are representative of 4 independent experiments.
We could hardly detect c-met mRNA in confluent HUVECs. However, the amount of c-met mRNA increased dramatically when the cells were switched to low-density conditions or during the formation of capillary-like structures in the 3-D collagen gel (Figure 4). In contrast, high levels of VE-cadherin mRNA were detected in confluent HUVECs, whereas substantially lower levels were observed in sparse conditions. The amounts of VE-cadherin mRNA increased when HUVECs were cultured in a 3-D collagen gel.
For comparison, we observed no changes in the amount of β-actin mRNA in any of the conditions (Figure 4).
Effect of cell density on the mitogenic response of HUVECs to HGF
To determine the functional consequence of the density-dependent changes in c-met expression, we examined the effect of HGF on the proliferation of HUVECs harvested from confluent monolayers and from a duplicate culture of the same cells that had been replated at a lower density after the last feeding (Figure 5). [3H]Thymidine incorporation experiments showed that HGF stimulated the proliferation in a dose-dependent manner in both confluent and sparse HUVECs. Moreover, the treatment with HGF led to an expected increased stimulation of DNA synthesis in sparse HUVECs, compared to that in confluent HUVECs. The difference between the 2 growth conditions was greatest at the lowest HGF concentration (1 ng/mL). In these conditions, the effect of HGF was nearly 30-fold greater in sparse cells than in confluent cells (42% and 1.4% stimulation, respectively). For comparison, the effect was only 2.6-fold higher in sparse HUVECs than in confluent HUVECs when cells were treated with 25 ng/mL HGF (227% and 86% stimulation, respectively).
Sparse HUVECs are more responsive to HGF than confluent HUVECs. HUVECs isolated from the same cord and grown as confluent or sparse monolayers were harvested and replated at the same density. They were then treated overnight with increasing concentrations of HGF: white bars, 1 ng/mL; gray bars, 5 ng/mL; and black bars, 25 ng/mL. The mitogenic response was assessed by measuring [3H]thymidine incorporation and expressed as a percentage of stimulation, calculated as follows: 100% × (cpmexperiment – cpmcontrol)/cpmcontrol, where the cpm values are the means from triplicate cultures. The 100% point was the background [3H]thymidine incorporation (2 × 104 cpm) in the absence of HGF. The responsiveness of HUVECs to HGF is dependent significantly on the state of confluence of the cells (2-way ANOVA; P < .0001). Data are representative of 3 independent experiments. Error bars represent SDs.
Sparse HUVECs are more responsive to HGF than confluent HUVECs. HUVECs isolated from the same cord and grown as confluent or sparse monolayers were harvested and replated at the same density. They were then treated overnight with increasing concentrations of HGF: white bars, 1 ng/mL; gray bars, 5 ng/mL; and black bars, 25 ng/mL. The mitogenic response was assessed by measuring [3H]thymidine incorporation and expressed as a percentage of stimulation, calculated as follows: 100% × (cpmexperiment – cpmcontrol)/cpmcontrol, where the cpm values are the means from triplicate cultures. The 100% point was the background [3H]thymidine incorporation (2 × 104 cpm) in the absence of HGF. The responsiveness of HUVECs to HGF is dependent significantly on the state of confluence of the cells (2-way ANOVA; P < .0001). Data are representative of 3 independent experiments. Error bars represent SDs.
Effect of cell density on the rate of the HUVEC morphogenesis
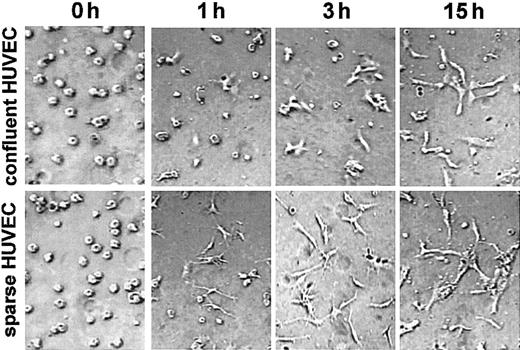
Because de novo c-met synthesis seems to be required for angiogenesis in vitro, the ECs that produce more c-met may undergo morphogenesis more readily than cells with a low c-met content. To test this hypothesis we analyzed the time course of angiogenesis in 3-dimensional collagen cultures obtained with confluent and sparse HUVECs. Indeed, as shown in Figure 6 the sparse HUVECs were characterized by the highest level of activity in morphogenesis. Just 1 hour after seeding in the 3-dimensional collagen matrix, virtually all of the sparse HUVECs had formed sprouts, whereas most of the confluent HUVECs were still round. After 3 hours, small sprouts could be seen in several confluent cells, whereas some short structures, formed by cells aligned end-to-end, were observed in the culture of sparse HUVECs. After 15 hours, the difference between these 2 types of culture was less great, probably due to the burst of c-met synthesis following the change of growth conditions.
Sparse HUVECs undergo morphogenesis more rapidly in 3-dimensional collagen culture. HUVECs isolated from the same cord and grown as confluent or sparse monolayers were trypsinized and seeded in a 3-dimensional collagen gel. Photographs were taken at 0, 1, 3, and 15 hours using phase contrast optics. Data are representative of 3 experiments. For all images in Figure 6: original magnification, × 200.
Sparse HUVECs undergo morphogenesis more rapidly in 3-dimensional collagen culture. HUVECs isolated from the same cord and grown as confluent or sparse monolayers were trypsinized and seeded in a 3-dimensional collagen gel. Photographs were taken at 0, 1, 3, and 15 hours using phase contrast optics. Data are representative of 3 experiments. For all images in Figure 6: original magnification, × 200.
Effect of anti–c-met antibody on angiogenesis in vitro
To investigate the specific involvement of the c-met receptor in angiogenesis, we carried out blocking experiments using the commercially available H-190 antibody directed against the extracellular domain of c-met. We have first analyzed whether this antibody inhibited the stimulatory activity of HGF, the action of which is strongly dependent on specific interaction with the c-met receptor. Figure 7A represents a dose-dependent effect of this antibody on proliferation of HUVECs induced by 25 ng/mL HGF. The incubation of both confluent and sparse HUVECs with increasing concentrations of H-190 antibody induced a progressive decrease in the number of metabolically active cells. This decrease was significant from the lowest H-190 concentration (0.5 μg/mL). At 4 μg/mL, the H-190 antibody completely blocked the proliferation of HUVECs induced by HGF. A control rabbit IgG, added at the same concentrations as H-190 IgG, did not inhibit the proliferation of HUVECs (data not shown). Neither the H-190 antibody nor the control rabbit IgG affected the basal HUVEC metabolic activity when cultured in the absence of HGF (data not shown).
Blocking anti–c-met antibody specifically inhibits the HGF-induced proliferation of HUVECs and HUVEC tubulogenesis in vitro. (A) Confluent (▵) or sparse (▴) HUVECs were treated for 96 hours with 25 ng/mL HGF in the presence of increasing concentrations of the anti–c-met H-190 antibody. Following incubation with the cell proliferation reagent WST-1, metabolically active cells were quantified by measuring the absorbance at 450 nm. White bar (□) indicates unstimulated control for confluent HUVECs; black bar (▪), unstimulated control for sparse HUVECs. Results are the mean values from triplicate cultures. (B) NHDFs were stimulated with 10 ng/mL PDGF BB, and hASMCs and DLD-1 cells were stimulated with 10% FBS, in the presence or absence of increasing concentrations of anti–c-met H-190 antibody. After 72 hours, proliferation was assessed by use of the cell proliferation reagent WST-1. Results are the mean values from triplicate cultures. Data are representative of 2 or more experiments. (C) HUVECs were cultured in 3-dimensional collagen in the presence of 20 μg/mL of either control rabbit IgG (left) or H-190 IgG (right). After 24 hours, only the cells in the control culture were reorganized into cords and tubes. In the presence of the H-190 antibody, the formation of cord network was blocked. Data are representative of 3 independent experiments. For all images in panel C: original magnification, × 200.
Blocking anti–c-met antibody specifically inhibits the HGF-induced proliferation of HUVECs and HUVEC tubulogenesis in vitro. (A) Confluent (▵) or sparse (▴) HUVECs were treated for 96 hours with 25 ng/mL HGF in the presence of increasing concentrations of the anti–c-met H-190 antibody. Following incubation with the cell proliferation reagent WST-1, metabolically active cells were quantified by measuring the absorbance at 450 nm. White bar (□) indicates unstimulated control for confluent HUVECs; black bar (▪), unstimulated control for sparse HUVECs. Results are the mean values from triplicate cultures. (B) NHDFs were stimulated with 10 ng/mL PDGF BB, and hASMCs and DLD-1 cells were stimulated with 10% FBS, in the presence or absence of increasing concentrations of anti–c-met H-190 antibody. After 72 hours, proliferation was assessed by use of the cell proliferation reagent WST-1. Results are the mean values from triplicate cultures. Data are representative of 2 or more experiments. (C) HUVECs were cultured in 3-dimensional collagen in the presence of 20 μg/mL of either control rabbit IgG (left) or H-190 IgG (right). After 24 hours, only the cells in the control culture were reorganized into cords and tubes. In the presence of the H-190 antibody, the formation of cord network was blocked. Data are representative of 3 independent experiments. For all images in panel C: original magnification, × 200.
We further tested the effect of the H-190 antibody on the proliferation of other cells. In the preliminary experiments we showed that HGF was unable to stimulate the proliferation of NHDFs or hASMCs. In contrast, the NHDFs and hASMCs responded to PDGF BB and serum (Figure 7B), whereas PDGF BB had no effect on the proliferation of HUVECs (data not shown). The DLD-1 tumor cells did not respond to any of the growth factors tested in this study but could be stimulated with 10% FBS (Figure 7B). These cells were also able to grow for several days in the absence of growth factors or serum. The H-190 antibody had no effect on the proliferation of NHDFs and hASMCs induced with PDGF BB or FBS, or on the growth of DLD-1 cells in the presence of 10% FBS (Figure 7B). Thus, the effect of H-190 antibody was specific for the HGF/c-met–dependent stimulation of ECs.
Finally, we investigated the effect of the H-190 antibody on the tubulogenesis of HUVECs in 3-dimensional collagen gel. In this assay the cells were kept in the presence of serum (see “Materials and methods”). As shown in Figure 7C, the anti–c-met H-190 antibody blocked cord formation, whereas in the presence of the control rabbit IgG, capillary-like structures were formed normally. To establish the specificity of this effect of H-190 antibody, we preincubated it with c-met covalently linked to Sepharose 4. This treatment reduced the specific anti–c-met titer by more than 10-fold, as determined by standard enzyme-linked immunosorbent assay (data not shown). The H-190 antibody preadsorbed with c-met–Sepharose no longer inhibited the HUVEC proliferation induced with HGF, or angiogenesis in vitro. In contrast, the same antibody preadsorbed with Sepharose with covalently attached bovine serum albumin preserved its blocking activity (data not shown). Thus, the in vitro antiangiogenic effect of the H-190 antibody was specifically dependent on the c-met binding activity.
Discussion
HGF is a pleiotropic cytokine that acts on a wide array of target cell types, including epithelial, endothelial, neuronal, and hematopoietic cells.13 HGF is thought to be a key regulator of invasive growth, a complex physiologic process that depends on cellular events such as proliferation, survival, migration, and differentiation into tubular-like structures.22 Angiogenesis is a specialized case of invasive growth. Accordingly, HGF was shown to be a potent angiogenic stimulator. To determine the role of the endogenous HGF/c-met system in angiogenesis, we analyzed the expression patterns and the functional consequences of blocking of the c-met receptor in cultured HUVECs.
We found that c-met was hardly detectable in quiescent confluent ECs. c-met was not essential and was down-regulated in ECs that had differentiated into capillary-like structures in a 3-dimensional collagen gel. In contrast, c-met was up-regulated in activated ECs during the in vitro recapitulation of some steps of angiogenesis. Therefore, c-met is a marker of the angiogenic phenotype of ECs.
The way in which c-met is regulated in ECs is reminiscent of that reported for other cell types. Thus, c-met is under the control of cell contacts in cultured epithelial cells.23 In physiologic conditions, c-met is barely detectable in most tissues and organs. Its expression is dramatically increased in response to injury and is specifically implicated in the repair and regeneration of epithelium in the kidney,24 skin,25 small intestine,26 and lung.27 c-met also contributes to the activated phenotype of some mesenchymal cell types, such as fibroblasts25 and muscle satellite cells.28 In activated monocytes, c-met is a factor defining invasiveness.29
Our results indicate that the up-regulation of c-met in ECs is essential to promote the earlier steps of angiogenesis, during which the cells change shape, penetrate the extracellular matrix, migrate, and proliferate. The induction of c-met in ECs under angiogenic conditions may render them more sensitive to HGF, an invasive growth regulator. Indeed, we showed that sparse HUVECs in which the expression of c-met had been up-regulated, proliferated faster in response to HGF, and underwent morphogenesis more rapidly in 3-D collagen gel. The addition of an anti–c-met H-190 antibody prevented angiogenesis in vitro. These experiments clearly demonstrate the importance of c-met in angiogenesis.
The H-190 antibody was raised against a recombinant protein mapping within the extracellular domain of c-met. The extracellular portion of the c-met has a complex modular structure and possesses several putative protein-binding domains.30 These domains include a Sema domain (found in semaphorins, a large family of secreted or transmembrane proteins that mediate guidance events during neural development31 ), a PSI domain (found in plexins, semaphorins, and integrins), and 4 IPT repeats (for immunoglobulin-like fold shared by plexins and transcription factors).32 No data are currently available on the role of these domains in the functioning of c-met. Several mechanisms may be involved in the inhibition of c-met by the H-190 antibody. Thus, the H-190 antibody may (1) block the HGF/c-met interaction; (2) generate steric hindrance thus preventing the dimerization of 2 c-met molecules (a process that appears to be critical for the activation of c-met 33 ); and (3) prevent the formation of supramolecular complexes between c-met and some other proteins. For example, the interaction with certain integrin subclasses is essential for the c-met activity in some cell types.34 Additional structure-function analyses of the c-met protein could provide further insights into the mechanism of the inhibitory action of the H-190 antibody.
Our in vitro observations are consistent with the results of several in vivo studies that showed that HGF is mobilized during angiogenesis. In this way, increased HGF levels contribute to the angiogenesis associated with several human cancers,35-37 and with some other pathologic situations, such as myocardial ischemia,38 the healing of hepatic wounds,39 diabetes,40 diabetic retinopathy,41 and arthritis and osteoarthritis.42 Taken together, these results highlight the importance of the HGF/c-met system in vascular remodeling.
Our data suggest a negative biologic link between VE-cadherin and c-met. Indeed, c-met is colocalized with E-cadherin in epithelial cells15 ; however, it is not colocalized with VE-cadherin in endothelial junctions. VE-cadherin and c-met are inversely regulated by cell density. Interestingly, HGF, a potent angiogenic factor, inhibits the expression of VE-cadherin in HUVECs.43 Conversely, we found that the HUVEC response to HGF is strongly dependent on the state of confluence. Similarly, it has been reported earlier that confluent ECs are less responsive to growth factors.44 More recently, the VEGF-dependent tyrosine phosphorylation of VEGF receptor 2 was shown to be reduced in confluent ECs.45 The underlying mechanisms appear to involve VE-cadherin, but they probably differ from those involved in c-met regulation, because the level of the VEGF receptor 2 protein is not density dependent.
In conclusion, we have demonstrated that angiogenic stimulation leads to the induction of the c-met receptor in ECs. Unlike VE-cadherin, which is required mostly during the last steps of angiogenesis, the expression of the HGF receptor is required during the earlier steps. Our results indicate that the endogenous HGF/c-met system is activated during physiologic angiogenesis and suggest that HGF and its receptor are very attractive targets for the development of new proangiogenic or antiangiogenic therapies.
Prepublished online as Blood First Edition Paper, February 20, 2003; DOI 10.1182/blood-2002-06-1731.
Supported by grants from the Ligue Nationale Contre le Cancer, from the Association pour la Recherche sur le Cancer (G.T.), and from the Association Franco-Chinoise pour la Recherche Scientifique and Technique (S.D.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are very grateful to the maternity departments of the Lariboisière (Paris) and Jean Rostand (Ivry-sur-Seine) hospitals for providing umbilical cord specimens. We thank Claire Bal dit Sollier for help with the statistical analysis.





![Figure 5. Sparse HUVECs are more responsive to HGF than confluent HUVECs. HUVECs isolated from the same cord and grown as confluent or sparse monolayers were harvested and replated at the same density. They were then treated overnight with increasing concentrations of HGF: white bars, 1 ng/mL; gray bars, 5 ng/mL; and black bars, 25 ng/mL. The mitogenic response was assessed by measuring [3H]thymidine incorporation and expressed as a percentage of stimulation, calculated as follows: 100% × (cpmexperiment – cpmcontrol)/cpmcontrol, where the cpm values are the means from triplicate cultures. The 100% point was the background [3H]thymidine incorporation (2 × 104 cpm) in the absence of HGF. The responsiveness of HUVECs to HGF is dependent significantly on the state of confluence of the cells (2-way ANOVA; P < .0001). Data are representative of 3 independent experiments. Error bars represent SDs.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/12/10.1182_blood-2002-06-1731/6/m_h81234470005.jpeg?Expires=1767730326&Signature=MAL3ayogacfNUbjK6yTyBcsiAIKF613o1cv68b-A-jjzRjW99nIx5viOCRdkT5QOffy98AxuSwgFEke~eAPGGFfTrwqi~8AvJ7JplT7ob8cznirLPqhk2kP28DCZ0qNnu3qE3fTEOZwfE5qRtLdu9sBqmxuNE2hD-4QpGevRRkZDQ~9FVl6DZkMq1diNDn3T7uuTDYNCv8L0xowXGrQqGQ7zK0xLQDcTn2oh~Rkl97K6cPPhqRz3YsJZ5f0cfZdXnEyHrawY9puqIXyprQKSQaa0Y9CP9L8a~DuQZbOpVDbRa30bmZPeea6PnDMtw0MJY~PdYka~txTxpCpukgm~~A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)