Abstract
The regulatory elements governing the process of lymphopoiesis from pluripotential stem cells to mature lymphocytes are not well understood. In this study we found that in bone marrow chimeras made by reconstituting lethally irradiated normal mice with bone marrow taken from genetically B-cell–deficient animals (μMT.B6 → F1) the B-cell compartment is reconstituted with host-derived B cells. Similarly, in animals reconstituted with bone marrow taken from mice with genetic deficiencies in the development of T cells (TCR–/– → F1) or both B and T cells (RAG–/– → F1), the missing lymphocyte lineage(s) was specifically reconstituted from host-derived cells. In all chimeras, all other blood lineages were generated from donor-derived stem cells. Control chimeras (B6 → F1) had only donor-derived hematopoietic cells as expected. The reconstituted, host-derived lymphoid compartments contained normal functional cell populations as determined by the presence of T cells expressing all 16 common TCR Vβ families, and the presence of all antibody isotypes in the serum. Reconstituted TCR–/– → F1 chimeras were also able to mount T-cell proliferative responses to foreign antigens equal to those of control animals. This observation would seem to suggest that during lymphopoietic reconstitution, missing lymphoid lineages can dictate their own reconstitution.
Introduction
It is well established that all 4 blood-borne cell lineages (ie, erythroid, lymphoid, myeloid, and megakaryocytic) are generated from a common progenitor cell called the pluripotential hematopoietic stem cell (PHSC) through a process called hematopoiesis. The PHSC is a rare cell present in the bone marrow at an estimated frequency of 1 × 10—5 to 5 × 10—5 cells. One of the hallmarks of PHSCs is their ability of self-renewal and continuous contribution to hematopoiesis for the entire life span of the host (reviewed in Aguila et al,1 Metcalf,2 Morrison, Uchida, and Weissman,3 and Muller-Sieburg and Deryugina4 ).
The mechanisms governing the differentiation of PHSCs into the different hematopoietic lineages are for the most part unknown. There are 2 prevailing theories of how stem cells “decide” to self-renew or to differentiate. One proposes that the decision is stochastic (random) or self-regulated while the other proposes an instructive mechanism where cytokine levels or cell-to-cell contacts with stromal cells instruct the pluripotential cell toward one pathway or the other (reviewed in Metcalf,2 Morrison et al,3 Morrison et al,5 and Zandstra et al6 ). A variation of the instructive model is that competition by different stem cells to niches in the stromal bone marrow will determine their fate where access to one site would lead to a particular outcome, whereas access to a different site would yield an alternative result.3, 4, 5
Another unclear aspect of hematopoiesis is whether negative and/or positive feedback between the periphery and the PHSC pool plays a role in stem cell development and differentiation. In the case of erythropoiesis it is well established that erythrocyte production is tightly regulated by peripheral factors since in response to hypoxia the kidney secretes erythropoietin which induces marrow-resident progenitors to differentiate into red cells until tissue oxygen levels are restored.7 Similarly, thrombopoiesis is a peripherally regulated process in which the number or mass of circulating platelets determines precursor differentiation.8
In the case of lymphopoiesis, although the peripheral lymphocyte pool size remains remarkably constant during normal conditions, no evidence for feedback regulation between the periphery and precursors has been found. In studies in which peripheral B or T cells were depleted in vivo by antibody treatment, precursor proliferation rate and cellular output did not change in bone marrow or thymus.9, 10, 11 Similarly, selective depletion of CD4+ T cells had no effect on the export of thymic CD8+ or CD4+ cells.11 The lack of feedback regulation by mature peripheral B cells and the central pre-B cell compartment was also reported in studies in which B-cell production was found to be unchanged in irradiated B-cell–deficient (μMT) mice reconstituted with normal B6 bone marrow (BM) diluted at different ratios with B-cell–incompetent BM from μMT mice.12,13 Thymic grafts in neonatally thymectomized mice also were found to export T cells at a constant rate even when the peripheral T-cell pool was far from full.14 The opposite scenario in which an excess of thymic lobes were transplanted into normal mice also failed to demonstrate peripheral regulation of thymic output.9,14, 15, 16 Negative regulation of B-cell production by peripheral B cells has not been detected either. It has been estimated that the BM generates 1-2 × 107 B cells daily in normal mice even when the peripheral B-cell pool is full.17
Taken together, these results point to an absence of a feedback mechanism between peripheral lymphocyte numbers and lymphopoiesis since depleted or excess numbers of peripheral cells do not result in increased or decreased proliferation of early precursors nor do they affect the output of new lymphocytes from the bone marrow or the thymus.
In this study we report the long-term and specific contribution to the lymphopoid lineage by host PHSCs in lethally irradiated mice reconstituted with bone marrow cells taken from genetically deficient mice incapable of generating either B cells, T cells, or both. In all cases, the deficient population(s) was specifically regenerated by host-derived stem cells, whereas other lineages were reconstituted by donor type cells.
Materials and methods
Mice
C57BL/6J μMT (μMT.B6) male and female mice were initially purchased from The Jackson Laboratory (Bar Harbor, ME) and were maintained in a germ-free environment. Mice made T-cell deficient by disruption of the T-cell–receptor beta chain (TCR—/—) and recombination-activating gene knockout mice deficient in both B and T lymphopoiesis (RAG—/—) in the C57BL/6J background were also obtained from The Jackson Laboratory and bred in our facility. CB6F1 animals (BALB/c × C57BL/6J) and C57BL/6J (B6.Thy-1.2) mice were either bred in our facility or purchased from The Jackson Laboratory. C57BL/6J-Igha Thy-1a Gpia (B6.Thy-1.1) congenic mice were purchased from The Jackson Laboratory.
Monoclonal antibodies
Hybridomas J1j and T24 (rat anti–Thy-1.2), T11De (rat anti–Thy-1.1), J11d (rat antimouse HSA), GK1.5 (rat antimouse CD4), 3.168 (rat antimouse CD8), 120.1.2 (antimouse class II, I-Ab), RA3.3 (rat antimouse B220), 34-4-21S (antimouse class I, H-2Dd), were originally obtained from ATCC (Manassas, VA). Antibody purification and labeling with fluorescein isothiocyanate (FITC) or biotin were performed in our laboratory according to standard protocols. Monoclonal phycoerythrin (PE)–labeled antimouse CD19 antibodies as well as anti–H-2Kb and –H-2Kd antibodies were purchased from PharMingen (San Diego, CA).
Bone marrow chimeras
Recipient mice were lethally irradiated (137Cs source, 1.1 Gy/min, Gamma-cell 40; Nordion, Ottowa, ON, Canada) at 2 to 3 months of age. CB6F1 mice were irradiated with 10.5 Gy at least 2 hours before BM transfer. Mice were also treated by intraperitoneal injection of T24 ascites after lethal irradiation to further deplete host T cells. Bone marrow from donor μMT, TCR—/—, RAG—/—, or control C57BL/6J mice were obtained from the femur and tibia bones and depleted of T cells by antibody plus complement lysis, using a mixture of J1j, GK1.5, and 3.168 monoclonal antibodies (mAbs) and guinea pig C (Colorado Serum, Denver, CO). All recipient mice were injected with 2 × 106 to 8 × 106 BM cells and allowed to reconstitute for 2 months. For cotransfer experiments mature T cells were purified from the lymph nodes of B6.Thy-1.1 mice and 20 × 106 T cells mixed with 3 × 106 T-cell–depleted BM from either TCR—/— or B6.Thy-1.2 and injected intravenously into irradiated (9.0 Gy) B6.Thy-1.2 hosts. All chimeras were kept on drinking water containing 100 000 U polymyxin B and 25 mg neomycin sulfate (Pharma-Tek, Huntington, NY) per liter for 4 to 6 weeks before returning to regular water.
Fluorescence activated cell sorting (FACS) analysis
The origin of peripheral lymphocytes and myeloid cells in bone marrow chimeras was determined by flow cytometry. The chimeric status of bone marrow–reconstituted mice was analyzed by 2-color staining of 1 × 106 lymph node and spleen cells with J1j and 34-4-21S or anti–H-2Kb for typing of T cells, and with CD19 or RA3.3 plus 34-4-21S or anti–H-2Kb for typing of B cells. The origin of myeloid cells was analyzed by single-color staining of 1 × 106 thyoglicollate-induced peritoneal cells with anti–H-2Kb or –H-2Kd antibodies. Samples from all mice used in the experiments were analyzed by flow cytometry. The samples were processed in an EPICS Profile Analyzer (Coulter, Hialeah, FL). Peritoneal cavity macrophages were mobilized by thyoglicollate injection intraperitoneally 4 days before collection by a peritoneal cavity lavage with phosphate-buffered saline (PBS).
T-cell proliferation assay
TCR—/— → F1 and control B6 → F1 mice were immunized by injecting 50 μL/footpad of antigen (1 mg/mL keyhole limpet hemocyanin [KLH] or fowl γ-globulin [FGG]) emulsified in complete Freund adjuvant (CFA). After 8 days, draining lymph nodes were removed and single-cell suspensions were prepared by using the frosted ends of 2 glass slides. Total lymph node (LN) cells were plated in 96-well plates at a concentration of 4 × 105 cells/well in T-cell media (RPMI 1640 supplemented with 10% fetal calf serum [FCS] and 5 × 10—5 M 2-mercaptoethanol). Cells were cultured for 90 to 96 hours together with increasing doses of the relevant antigen and with 1 μCi (0.037 MBq) [3H]thymidine during the last 8 to 12 hours. Cells were then harvested and processed for liquid scintillation measurements of radioactivity.
Reverse transcriptase–polymerase chain reaction (RT-PCR)
Whole spleen RNA was extracted from TCR—/— → F1 and control B6 → F1 mice using Trizol (Life Technologies, Grand Island, NY). First-strand complementary DNA (cDNA) was synthesized using 2 μg total RNA, which was primed with oligo dT, then reverse-transcribed using Superscript II reverse transcriptase (Life Technologies) at 42°C for 90 minutes. Duplicate reactions without reverse transcriptase were performed as negative controls (data not shown). Primers for the specific amplification of Vβ families have been previously described.18 The cycling parameters were as follows: 3 minutes at 94°C followed by 35 cycles of 1 minute at 94°C, 2 minutes at 57°C, and 3 minutes at 72°C. Products after amplification were analyzed by agarose gel electrophoresis.
Enzyme-linked immunosorbent assay (ELISA)
Total immunoglobulin M (IgM), IgA, IgG1, IgG2a, and IgG2b antibody levels in sera were determined by ELISA using a standard curve of known concentration of each immunoglobulin (Southern Biotechnology Associates, Birmingham, AL). Then, 96-well plates were coated with 5 μg/mL goat antimouse immunoglobulin at 4°C overnight and blocked with 20% chicken serum for 2 hours at 37°C. Sera from μMT → F1 and B6 → F1 controls were then added in serial dilutions and incubated for 2 hours at 37°C. Alkaline phosphatase–labeled goat antimouse IgM, IgA, IgG1, IgG2a, and IgG2b detection antibodies (Southern Biotechnology Associates) were diluted 1:500 and incubated for 1 hour at 37°C. Color was developed with p-nitrophenyl phosphate substrate and optical density was measured at 405 nm.
Results
Host-derived B cells repopulate irradiation bone marrow chimeras reconstituted with bone marrow from B-cell–deficient (μMT) mice
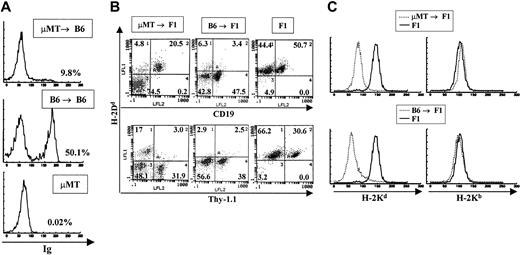
In experiments aimed at studying the function of B cells as antigen-presenting cells, irradiation BM chimeras were made using μMT.B6 (MHC H-2b) mice as BM donors and normal C57BL/6J (H-2b) or CB6F1 ([BALB/c × C57BL/6J]F1, [H-2d × H-2b]) as recipients. Recipient mice were allowed to reconstitute their immune system for 2 months and were subsequently used for the experiments previously described by Rivera et al.19 Prior to use, all chimeras were analyzed by flow cytometry to verify that they were devoid of B cells. To our surprise, all animals analyzed 3 months after transfer had varying numbers of host-derived B cells in their lymph nodes and spleens. A representative FACS profile of a μMT → B6 animal that yielded positive B-cell staining is shown in Figure 1A. Given that μMT.B6 mice have a genetic deficiency that completely abolishes their ability to contribute to B lymphopoiesis, the B cells present in these animals can be derived only from the normal recipients. Since both the donor and the recipient in this case were of the B6 background we could not rule out the contribution of host cells to other hematopoietic lineages. On the other hand, analysis of μMT.B6 → CB6F1 (H-2b → H-2b,d) chimeras by double staining with lineage-specific antibodies and anti–H-2d (expressed only by host cells) antibodies, allows the typing of all hematopoietic lineages in these mice. A representative FACS profile of one such bone marrow chimeric animal is shown in Figure 1B. As is evident from the figure, only the B-cell population was generated from host stem cell precursors. All T cells and myeloid cells were of donor type (negative for H-2d staining, Figure 1B-C). In contrast, in control animals where normal B6 mice were used as bone marrow donors for CB6F1 reconstitution, both B and T cells as well as myeloid cells were of donor (H-2b) origin and no contribution by host cells was observed (Figure 1B-C). It is important to note that the small percentage of cells that stain as donor type B cells represent a nonspecific background generated by bleeding of the red fluorescence into the green mostly by nonlymphoid cells that bind antibody nonspecifically. To determine the origin of the myeloid lineage in these bone marrow chimeras, we mobilized macrophages and granulocytes to the peritoneal cavity by intraperitoneal injection of thyoglicollate. Peritoneal macrophages were always negative for staining with anti–host H-2Kd antibodies and positive for H-2Kb in both μMT and normal B6 → F1 chimeras indicating their donor origin (Figure 1C).
Detection of B-cell reconstitution in μMT → B6 chimeras. (A) Staining of spleen cells from μMT → B6 chimeras with anti-immunoglobulin antibodies 3 months after preparation. A representative staining profile of a B6 → B6 chimera as well as the staining profile of a donor μMT mouse are shown. All samples were stained with polyclonal rat antimouse immunoglobulin antibodies that were labeled with FITC. (B) μMT → F1 chimeras and control B6 → F1 chimeras were prepared as described in “Materials and methods.” Reconstitution of blood lineages was assessed by antibody staining of lymph node and spleen cells with anti-Thy-1 antibodies (biot-J1j) for T-cell lineage identification, PE–anti-CD19 for B-cell lineage identification together with anti–class I antibodies specific for H-2Dd molecules (FITC-34-4-21S). (C) Myeloid lineages were analyzed by staining peritoneal cavity cells with anti–H-2Kb (right panels) or anti–H-2Kd (left panels) antibodies. Representative staining profiles for one mouse from each group (μMT → F1 and B6 → F1, broken lines) are shown. Staining of normal F1 mice with each antibody is depicted by solid lines.
Detection of B-cell reconstitution in μMT → B6 chimeras. (A) Staining of spleen cells from μMT → B6 chimeras with anti-immunoglobulin antibodies 3 months after preparation. A representative staining profile of a B6 → B6 chimera as well as the staining profile of a donor μMT mouse are shown. All samples were stained with polyclonal rat antimouse immunoglobulin antibodies that were labeled with FITC. (B) μMT → F1 chimeras and control B6 → F1 chimeras were prepared as described in “Materials and methods.” Reconstitution of blood lineages was assessed by antibody staining of lymph node and spleen cells with anti-Thy-1 antibodies (biot-J1j) for T-cell lineage identification, PE–anti-CD19 for B-cell lineage identification together with anti–class I antibodies specific for H-2Dd molecules (FITC-34-4-21S). (C) Myeloid lineages were analyzed by staining peritoneal cavity cells with anti–H-2Kb (right panels) or anti–H-2Kd (left panels) antibodies. Representative staining profiles for one mouse from each group (μMT → F1 and B6 → F1, broken lines) are shown. Staining of normal F1 mice with each antibody is depicted by solid lines.
A total of more than 40 chimeric animals reconstituted with B-cell–deficient bone marrow were analyzed and in all cases we observed B-cell reconstitution by host cells in chimeras older than 3 months after preparation.
Reconstitution of T cells by host progenitors in TCR–/– → F1 chimeric mice
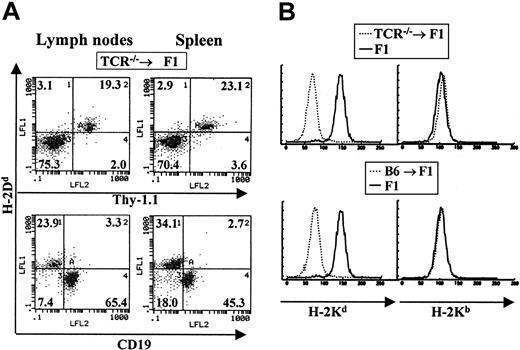
To determine if the observations made for B lymphopoiesis in the experiments described above were also true for T lymphopoiesis, we prepared chimeric animals where the donor bone marrow was derived from animals impaired in T-cell development (TCRβ chain knockout mice arrested at the double-negative stage of T-cell development).20 TCR—/— BM donor mice were also of the C57BL/6J background and normal lethally irradiated CB6F1 animals were used as recipients. Chimeras were allowed to reconstitute their immune system for at least 3 months before analysis. Contribution by host versus donor cells was determined using the same major histocompatibility complex (MHC) class I–specific antibodies described above. In these bone marrow chimeras we observed the specific reconstitution of the T-cell compartment by host cells with no contribution by such host cells to other blood lineages (Figure 2A-B). B cells and myeloid cells were of donor H-2b origin (Figure 2B). A total of 25 TCR—/— → F1 chimeric mice were examined and all were found to be reconstituted with T cells of host origin.
Host cells contribute specifically to the T-cell lineage in TCR–/– → F1 bone marrow chimeras. Bone marrow cells from T-cell–deficient mice were used to reconstitute normal CB6F1 recipients as described in “Materials and methods.” (A) The staining profile of one representative mouse is shown. Reconstitution of blood lineages was assessed starting at 3 months after transfer, by antibody staining of lymph node and spleen cells with anti-Thy-1 antibodies (biot-J1j) for T-cell–lineage identification, PE–anti-CD19 for B-cell–lineage identification together with anti–class I antibodies specific for H-2Dd molecules (FITC-34-4-21S). (B) Myeloid lineages were analyzed by staining peritoneal cavity cells with anti–H-2Kb (right panels) or anti–H-2Kd (left panels) antibodies. Representative staining profiles for one mouse from each group (TCR—/— → F1 and B6 → F1, broken lines) are shown. Staining of normal F1 mice with each antibody is depicted by solid lines.
Host cells contribute specifically to the T-cell lineage in TCR–/– → F1 bone marrow chimeras. Bone marrow cells from T-cell–deficient mice were used to reconstitute normal CB6F1 recipients as described in “Materials and methods.” (A) The staining profile of one representative mouse is shown. Reconstitution of blood lineages was assessed starting at 3 months after transfer, by antibody staining of lymph node and spleen cells with anti-Thy-1 antibodies (biot-J1j) for T-cell–lineage identification, PE–anti-CD19 for B-cell–lineage identification together with anti–class I antibodies specific for H-2Dd molecules (FITC-34-4-21S). (B) Myeloid lineages were analyzed by staining peritoneal cavity cells with anti–H-2Kb (right panels) or anti–H-2Kd (left panels) antibodies. Representative staining profiles for one mouse from each group (TCR—/— → F1 and B6 → F1, broken lines) are shown. Staining of normal F1 mice with each antibody is depicted by solid lines.
Both B- and T-cell lineages are reconstituted by host cells in RAG–/– → F1 chimeras
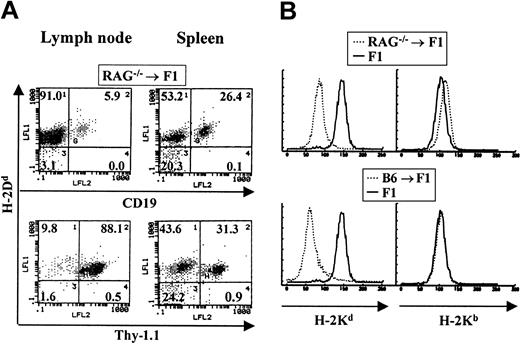
To examine lymphopoiesis in bone marrow chimeras where the donor BM is deficient in both B- and T-cell development, BM from RAG—/— mice of the C57BL/6J background was used to reconstitute normal CB6F1 recipients. RAG—/— mice lack the recombination-activating gene required for the rearrangement of both T-cell and B-cell receptors whose expression on the cell surface is required for both T- and B-lymphocyte maturation. Therefore, bone marrow from these animals cannot support lymphopoiesis. The staining of a representative chimeric animal is shown in Figure 3A. In these RAG—/— chimeric animals both T and B lymphocytes found in spleen and lymph nodes were of host origin. In contrast, all myeloid cells were of donor origin (Figure 3B). No host-derived myeloid cells could be detected. A total of 10 chimeric animals were analyzed and in all cases we observed B- and T-cell reconstitution by host cells.
Host cells reconstitute both T- and B-cell lineages in RAG–/– → F1 bone marrow chimeras. Bone marrow from RAG—/— mice was used to reconstitute CB6F1 mice as described. (A) The staining profile of one representative mouse is shown. Reconstitution of blood lineages was assessed starting at 3 months after transfer, by antibody staining of lymph node and spleen cells with anti–Thy-1 antibodies (biot-J1j) for T-cell–lineage identification, PE–anti-CD19 for B-cell–lineage identification, together with anti–class I antibodies specific for H-2Dd molecules (FITC-34-4-21S). (B) Myeloid lineages were analyzed by staining peritoneal cavity cells with anti–H-2Kb (right panel) or anti–H-2Kd (left panel) antibodies. Representative staining profiles for one mouse from each group (RAG—/— → F1 and B6 → F1, broken lines) are shown. Staining of normal F1 mice with each antibody is depicted by solid lines.
Host cells reconstitute both T- and B-cell lineages in RAG–/– → F1 bone marrow chimeras. Bone marrow from RAG—/— mice was used to reconstitute CB6F1 mice as described. (A) The staining profile of one representative mouse is shown. Reconstitution of blood lineages was assessed starting at 3 months after transfer, by antibody staining of lymph node and spleen cells with anti–Thy-1 antibodies (biot-J1j) for T-cell–lineage identification, PE–anti-CD19 for B-cell–lineage identification, together with anti–class I antibodies specific for H-2Dd molecules (FITC-34-4-21S). (B) Myeloid lineages were analyzed by staining peritoneal cavity cells with anti–H-2Kb (right panel) or anti–H-2Kd (left panel) antibodies. Representative staining profiles for one mouse from each group (RAG—/— → F1 and B6 → F1, broken lines) are shown. Staining of normal F1 mice with each antibody is depicted by solid lines.
Reconstitution of lymphoid compartments is incomplete in bone marrow chimeras
In all 3 types of chimeras described above, the level of reconstitution of host-derived B cells, T cells, or both varied between individual mice. To determine the long-term extent of reconstitution observed in these chimeras, we allowed chimeric mice to reconstitute for a full year before analysis. A summary of the results is presented in Table 1. Data for 9 individual animals of μMT → F1 and TCR—/— → F1 as well as B6 → F1 controls are presented. The mean number of B cells found in μMT → F1 mice was 16.1 ± 7.8 × 106 per spleen, whereas control B6 → F1 mice had 48.6 ± 11.6 × 106 per spleen. In the case of TCR—/— → F1 mice, a mean of 15.0 ± 5.7 × 106 T cells per spleen were found as compared with 25.3 ± 4.4 × 106 T cells found in the spleen of B6 → F1 mice. It should be emphasized that the low percentage of host-derived B cells in the case of TCR—/— → F1 and host-derived T cells in the μMT → F1 mice represent nonspecifically stained cells. A similar number of donor-derived B and T cells are also scored as positive in μMT → F1 and TCR—/— → F1 chimeras, respectively, although such cells cannot be generated by donor BM. Overall, it can be observed that although the reconstitution was variable between individuals, in all mice the missing lineages were reconstituted, and the average reconstitution level was calculated to be approximately 30% of normal for B cells and 50% of normal for T cells (Table 1). The implications of this finding are discussed later.
Assessment of the functionality of the reconstituted lymphocytes
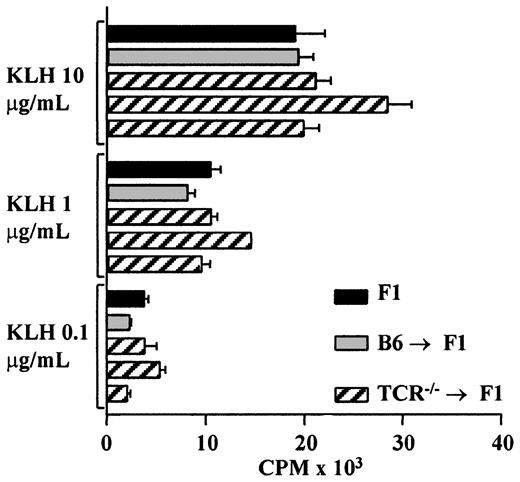
It could be argued that the host-derived lineages seen in all 3 cases described above do not represent normal lineage reconstitution and that the cells are oligoclonal in nature. To determine whether the reconstituted T cells represent a functional population, TCR—/— → F1, control B6 → F1, as well as unmanipulated F1 mice were primed with KLH, and T-cell proliferative responses were analyzed. TCR—/— → F1 chimeric mice had a normal T-cell proliferative response to KLH despite the reduced T-cell numbers found in these animals (Figure 4). T-cell proliferation to FGG and T-cell responses to sheep red blood cells as measured in a helper T-cell assay were also found to be similar in TCR—/— → F1 and control mice (data not shown).
TCR–/– → F1 bone marrow chimeras have normal responses to KLH after host cells contribute to T-cell differentiation. TCR—/— → F1 (▨), B6 → F1 (▦) bone marrow chimeras, and normal CB6F1 mice (▪) were primed with KLH/CFA in the hind footpads; 8 to 10 days later, T-cell recall responses were measured as described in “Materials and methods.” Each bar represents one mouse, black bars represent CB6F1, gray bars represent B6 → F1, and hatched lines represent TCR—/— → F1 mice. Results are representative of 2 independent experiments.
TCR–/– → F1 bone marrow chimeras have normal responses to KLH after host cells contribute to T-cell differentiation. TCR—/— → F1 (▨), B6 → F1 (▦) bone marrow chimeras, and normal CB6F1 mice (▪) were primed with KLH/CFA in the hind footpads; 8 to 10 days later, T-cell recall responses were measured as described in “Materials and methods.” Each bar represents one mouse, black bars represent CB6F1, gray bars represent B6 → F1, and hatched lines represent TCR—/— → F1 mice. Results are representative of 2 independent experiments.
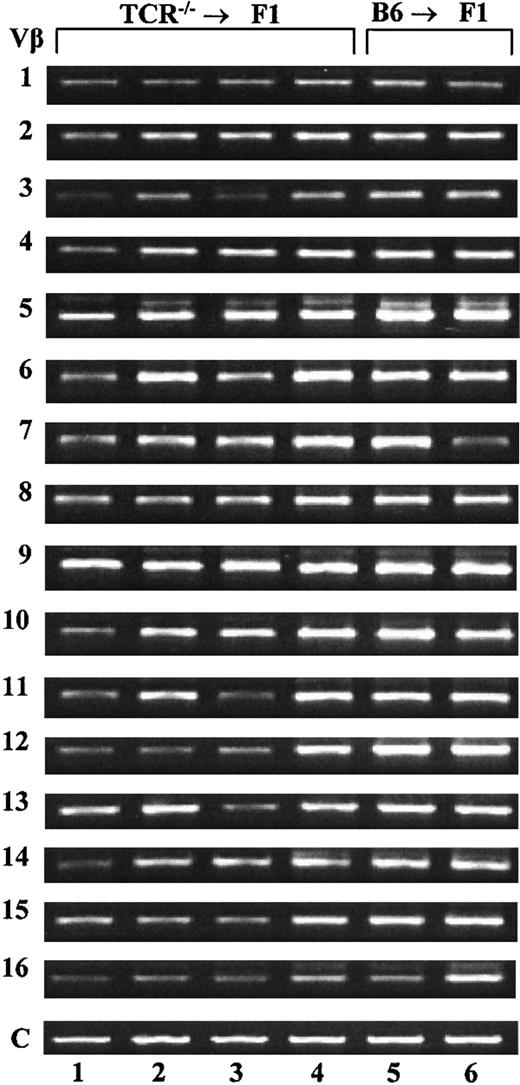
Another possible explanation for the presence of host-derived T cells in TCR—/— → F1 chimeras could be the expansion of a few irradiation-resistant T cells. Actually, several groups have reported that T cells adoptively transferred into T-cell–deficient hosts (reviewed in Gabor et al11 and Agenés et al12 ) can undergo several rounds of proliferation. It has been reported that T cells resulting from peripheral expansion are characterized as predominantly memory cells, positive for CD44 staining and skewed in their T-cell repertoire.21, 22, 23, 24 To rule out peripheral expansion of a residual T-cell population as the mechanism behind the observed reconstitution, we analyzed the expression of 16 different Vβ chains in the spleens of TCR—/— → F1 and control animals by RT-PCR as previously described.18 Representative results of such an analysis are shown in Figure 5 for 4 experimental and 2 control mice. All chimeric mice were found to express all 16 families analyzed, suggesting that the T cells present in these mice are polyclonal and more likely to be the product of de novo T-cell differentiation rather than expansion of a small population of cells. Another characteristic of cells arising from peripheral expansion is the predominance of CD44 expression. Anti-CD44 staining of lymph node cells derived from TCR—/— → F1 chimeric mice revealed the same level and distribution of CD44 expression as LN taken from control animals (data not shown). These results, together with the observation of host-derived B-cell reconstitution in μMT chimeras (no peripheral B-cell expansion has ever been reported), point to lymphoid reconstitution by de novo cell production and against T-cell peripheral expansion.
Host T cells in TCR–/– → F1 bone marrow chimeras represent a polyclonal population. RT-PCR amplification was performed on total RNA extracted from the spleens of TCR—/— → F1 and B6 → F1 chimeras. Lanes 1 to 4 represent results for 4 individual TCR—/— → F1 mice and lanes 5 to 6 are amplification products for 2 individual B6 → F1 mice. Amplification products for Vβ families 1 to 16 are shown. As a control, primers for constant regions of Vβ were used and are denoted as “C.” Results are representative of 2 independent experiments.
Host T cells in TCR–/– → F1 bone marrow chimeras represent a polyclonal population. RT-PCR amplification was performed on total RNA extracted from the spleens of TCR—/— → F1 and B6 → F1 chimeras. Lanes 1 to 4 represent results for 4 individual TCR—/— → F1 mice and lanes 5 to 6 are amplification products for 2 individual B6 → F1 mice. Amplification products for Vβ families 1 to 16 are shown. As a control, primers for constant regions of Vβ were used and are denoted as “C.” Results are representative of 2 independent experiments.
To rule out oligoclonality of the B-cell compartment in chimeric mice with host-derived B cells, the serum immunoglobulin levels of various isotypes was determined by ELISA in μMT → F1 chimeras and compared with control animals. Representative results for 4 individual chimeric and 2 control mice are shown in Figure 6. All tested isotypes were present in all the chimeras, IgM and IgA antibodies were present at similar levels as found in controls, while IgG1, IgG2a, and IgG2b were lower than controls probably due to the reduced number of B cells present in the μMT → F1 chimeras.
μMT → F1 mice have serum antibodies similar to control animals. Serum levels of IgM (A), IgA (B), IgG1 (C), IgG2a (D), and IgG2b (E) antibodies were determined by quantitative isotype-specific ELISA (Southern Biotechnologies). Each bar represents one mouse, black bars (▪) are μMT → F1 and white bars (□) are control chimeras. Results are representative of 2 independent experiments.
μMT → F1 mice have serum antibodies similar to control animals. Serum levels of IgM (A), IgA (B), IgG1 (C), IgG2a (D), and IgG2b (E) antibodies were determined by quantitative isotype-specific ELISA (Southern Biotechnologies). Each bar represents one mouse, black bars (▪) are μMT → F1 and white bars (□) are control chimeras. Results are representative of 2 independent experiments.
Cotransfer of mature T cells does not inhibit the emergence of host-derived T cells in TCR–/– → F1 chimeric mice
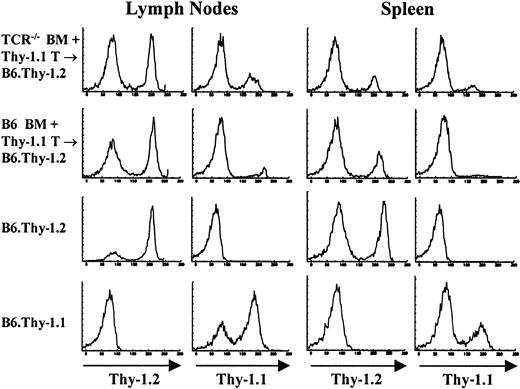
In the chimera model system used throughout these studies, lymphopoiesis occurs in an environment that is devoid of any mature lymphocytes in the periphery of the host animal. It is therefore tempting to speculate that host T cells in these mice develop because there is no negative feedback from peripheral T cells, a situation that does not exist in normal animals. To address this possibility, mice were cotransferred with a large number of mature Thy-1.1 congenic T cells together with the reconstituting bone marrow cells derived from TCR—/— mice. As shown in Figure 7 and Table 2, host-derived T cells developed at the same pace and in the same numbers as in mice reconstituted with BM cells alone. Also, as shown in Table 2, about one third of the cotransferred mature congenic T cells can be found in the spleen of the chimeras reconstituted with BM from TCR—/— mice. Cotransferred congenic cells did not survive as well in the spleens of chimeras reconstituted with BM from control B6 mice, where approximately half the number that survived in TCR—/— → F1 mice were found. More importantly, the number of T cells reconstituted by the host in TCR—/— + Thy-1.1 T → B6 mice was basically identical to the number of T cells found in the spleens of TCR—/— → F1 mice, demonstrating that the presence of some mature peripheral T cells did not inhibit the contribution by the host.
Cotransferred congenic (Thy-1.1) cells do not inhibit the emergence of host-derived (Thy-1.2) cells in TCR–/– → F1 mice. To determine the influence of mature peripheral T cells on T-cell reconstitution by host cells, bone marrow chimeras were prepared in which 20 × 106 Thy-1.1+ T cells were cotransferred with either B6.Thy-1.2 BM or TCR—/—.Thy-1.2 BM into lethally irradiated B6.Thy-1.2 recipients. Staining profiles of lymph node and spleens of one mouse from each group as well as control mice are shown. Samples were stained with FITC-J1j (anti-Thy-1.2) and FITC-T11De (anti-Thy-1.1).
Cotransferred congenic (Thy-1.1) cells do not inhibit the emergence of host-derived (Thy-1.2) cells in TCR–/– → F1 mice. To determine the influence of mature peripheral T cells on T-cell reconstitution by host cells, bone marrow chimeras were prepared in which 20 × 106 Thy-1.1+ T cells were cotransferred with either B6.Thy-1.2 BM or TCR—/—.Thy-1.2 BM into lethally irradiated B6.Thy-1.2 recipients. Staining profiles of lymph node and spleens of one mouse from each group as well as control mice are shown. Samples were stained with FITC-J1j (anti-Thy-1.2) and FITC-T11De (anti-Thy-1.1).
Taken together, these results suggest that host-derived T cells in these mice represent a fully functional compartment and that these reconstituted lymphocytes are likely to be the product of de novo production by surviving host cells.
Discussion
The main and novel finding in this study is that radiation chimeras reconstituted with genetically defective BM lacking the ability to generate part of or the entire lymphoid lineage, will selectively reconstitute the missing compartment from host-derived stem cells, while other lineages are reconstituted from donor-derived cells. This was the case when the donor BM could not reconstitute B cells, T cells, or both. It is not clear how the lack of a certain lymphoid population is sensed by the host and what type of stem cells are responsible for generating the missing population. The reconstituting cells, by definition, fall under the functional group of stem cells but their differentiation stage is unknown. The most obvious candidate would be PHSCs, especially since it is well documented that some PHSCs can survive what is considered a lethal dose of irradiation. This was demonstrated in experiments where very few bone marrow cells (10 000-20 000) or a few hundred highly enriched PHSCs (Thy-1lo, Sca1+, Lin—) were used to reconstitute lethally irradiated hosts.25, 26, 27, 28 It was clearly shown that in both cases, host stem cells survived and progressively contributed to hematopoiesis of all lineages. However, in this study, the host-derived cells were restricted to the population of cells that could not be generated by the donor BM (due to a genetic defect). Selective reconstitution of a particular lineage implies that along the differentiation pathway from PHSCs to mature, lymphoid-committed cells, either the system can sense the lack of a certain cell type and compensate for it, or the lack of competition by donor-derived cells allows for, or facilitates, the reconstitution of the missing lineage by the surviving host stem cells. Such competition (for niche space or resources) could occur at the level of the stem cell or at the level of mature cells.
As for stem cells, according to the accepted dogma that only PHSCs are long-lived and therefore the only population that continuously maintain all hematopoietic lineages, the only competition in the long term could be between host and donor PHSCs. The existence of competition between PHSCs in donor bone marrow and surviving host cells was clearly observed by Soper et al.29 In their experiments, the transfer of 2 × 105 purified precursors into lethally irradiated recipients resulted in the host contributing to 43% of the red blood cell content, and an increase in the number of transferred cells (to 5 × 105) resulted in the “suppression” of host cell expansion so that in those mice the host only contributed to 8% to 14% of the red blood cell content.29 What is surprising in our studies is that at the high number of donor bone marrow cells used, where host cells are completely “suppressed” in the control animals, we still observe the specific contribution by the host to only those lineages that are missing. Moreover, in μMT-derived bone marrow there are normal PHSC and B-cell precursors until the pro-B cell stage and in the TCR—/—-derived bone marrow there are normal PHSC and T-cell precursors until the double-negative stage. If competition at the precursor level is at play, the presence of normal PHSCs in the donor BM should have “outcompeted” the surviving host stem cells, or it would have to be assumed that only the precursors past the genetic block compete but not the ones before the blocked stage. We therefore think that competition among host and donor PHSCs and even higher precursor cells would be an unlikely explanation for the observed selective reconstitution.
As for competition (or lack there of) between mature lymphocytes, we show that coinjections of large numbers of donor-type mature (Thy-1 congenic) T cells together with T-cell–defective donor BM has no effect on the reconstitution pattern, and the missing T-cell compartment is reconstituted by host T cells to the same degree (Figure 7; Table 2). In the case of B cells, the existence of competition or feedback within the mature B-cell population was never demonstrated except between normal and autoreactive B cells where normal B cells can displace the autoreactive ones from follicular niches30,31 and between X-linked immunodeficiency (xid) and normal B cells.32 However, in both of these studies competition was shown to occur between cells in which one population has an advantage over the other.
Another potential explanation for the finding reported in this paper is that some mature lymphocytes have survived lethal irradiation and expanded by homeostatic proliferation. It has been reported that mature T cells adoptively transferred into either T-cell–deficient mice or sublethally irradiated recipients undergo homeostatic proliferation but not when they are transferred into normal recipients (reviewed in Tanchot et al,13 Mackall et al,33 and Surh and Sprent34 ). T cells that undergo such proliferation have been found to up-regulate CD44 expression and to have a skewed repertoire.13,33 Furthermore, homeostatic proliferation has been recently found to be inhibited by the cotransfer of mature T cells.34 To determine whether homeostatic proliferation was responsible for the observed reconstitution, we compared the TCR-Vβ expression between B6 → F1 and TCR—/— → F1 chimeras, analyzed CD44 expression, and determined whether cotransfer of mature congenic T cells would affect our results. As described in “Results,” TCR-Vβ usage was identical between B6 → F1 and TCR—/— → F1 chimeras (Figure 6), as was the percentage of CD44+ T cells (data not shown). Moreover, the cotransfer of congenic mature T cells and TCR—/— BM did not suppress the contribution by the host to the peripheral T-cell pool (Figure 7; Table 2). In fact, similar numbers of T cells were reconstituted in TCR—/— → F1 and TCR—/— + Thy-1.1 T → F1 (Tables 1 and 2). These results strongly argue against homeostatic proliferation as the mechanism responsible for the observed reconstitution. Another argument against expansion is the fact that homeostatic proliferation has been shown to give rise to a very limited number of cells35, 36, 37 while in our system the T-cell pool reached approximately half of its normal size. It should also be noted that all chimeras were injected with 1 mg anti–Thy-1 antibodies 2 to 3 hours after irradiation to completely deplete host T cells prior to bone marrow transfers. In addition, homeostatic proliferation of B cells after transfer into B-cell–deficient mice has not been reported,38,39 and in our system host cells also reconstituted peripheral B cells when the donor BM was unable to do so.
Another interesting point is the fact that the reconstitution of the lymphoid compartment by cells of host origin was never complete. The T-cell compartment in TCR—/— → F1 mice was reconstituted to a level that is approximately 50% that of B6 → F1 mice, while the B-cell compartment in μMT → F1 mice was reconstituted to about one-third the level of the B-cell compartment in B6 → F1 mice, implying that the number of surviving host-derived PHSCs is not enough to sustain a full reconstitution of the missing lymphoid population in the host. This is a surprising finding since it was suggested by several reports that, at least for T cells, even very few stem cells can repopulate the entire thymus and give rise to the entire T-cell population.40 The results presented here suggest that this might not be the case and that a continuous supply by a certain number of stem cells is required to keep the T-cell pool at its normal size. Indeed, for B cells, it has been found that a continuous contribution by the bone marrow is required in order to maintain a normal number of peripheral B cells.41 Furthermore, Agenés et al reported that chimeras reconstituted with a limited number of competent pre-B cells were unable to fully reconstitute peripheral B-cell numbers.12 Strikingly, in this latter study, chimeras reconstituted with 2% or 5% of the normal pre-B cell pool had 14 × 106 and 18 × 106 peripheral B cells, respectively, which is very close to the average of 15 × 106 B cells that we observed in the present study.12 Almeida et al also reported that a limited number of precursors in the thymus resulted in an incomplete peripheral T-cell pool.9 Therefore, it is likely that the incomplete reconstitution that we observed in our system is due to the very limited number of precursors that survived the irradiation.
Another possible explanation is that a radiation-resistant lymphoid precursor cell is directly responsible for the lineage-specific reconstitution observed in this study and, contrary to the prevailing dogma, this cell is long-lived and self-renewing. As mentioned in “Introduction,” in both erythropoiesis and thrombopoiesis where a definite regulation between the periphery and the bone marrow has been shown to exist, the target of such regulation is a lineage-committed precursor cell.7,8 We have also shown in a previous study that, at least for the myeloid compartment, long-lived, lineage-specific stem cells do exist in the spleen and maintain the myeloid lineage for the lifetime of the host.42 Recent evidence for the existence of a common lymphoid precursor34 also raises the possibility of a long-lived committed lymphoid precursor being responsible for the observed reconstitution.
Taken together, the observation that missing lymphoid lineages can be specifically restored by host-derived precursors during hematopoietic reconstitution implies that during hematopoiesis, the lack of a lymphoid subpopulation or the entire lymphoid lineage is somehow monitored and results in the differentiation of host-derived lymphoid precursors into mature lymphocytes.
Prepublished online as Blood First Edition Paper, February 13, 2003; DOI 10.1182/blood-2002-06-1902.
Supported in part by National Institutes of Health grants 5 RO1NS38272 (Y.R.), RO1NS38272, and RO1CA50777 (J.P.D.), National Research Service Award T32AI07403 (C.-C.C.), and an Individual National Service Award, GM19331 (A.R.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors gratefully acknowledge Malvika Kaul and Annmarie Pacchia for constructive comments on the manuscript.