Abstract
The deoxyspergualin derivative LF 15-0195 has demonstrated some efficacy in animal models of autoimmune and graft-versus-host diseases and is currently tested in clinics. The molecular mechanisms of LF 15-0195 immunosuppressive activity remained unknown. We show that exposure to LF 15-0195 sensitizes Jurkat T cells to apoptosis induced by an agonistic anti-CD95 antibody (CH11 clone) and by the cytokine TNF-related apoptosis-inducing ligand. LF 15-0195 does not demonstrate any significant effect on the postmitochondrial activation of caspases, nor does it modify overall expression of CD95, Fas-associated death domain, and procaspase-8. The compound facilitates the recruitment of these molecules to the death-inducing signaling complex (DISC) and enhances caspase-8 and -10 activation, thus increasing cytochrome c and direct IAP binding with low pI (DIABLO)/Smac mitochondrial release. LF 15-0195 also sensitizes Jurkat T cells to CD3-mediated apoptosis, an in vitro model for activation-induced T-cell death (AICD). LF 15-0195–mediated sensitization to AICD was further confirmed in human peripheral T cells exposed to anti-CD3 antibodies, then cultured in the presence of interleukin-2. In these cells, LF 15-0195 increased apoptosis triggered by either anti-CD95 antibodies or CD3 restimulation, whereas no effect was observed on “passive apoptosis.” Finally, in bone marrow recipient mice, LF 15-0195 enhanced allogeneic donor T-cell death, which required a functional CD95 pathway. These results suggest that LF 15-0195 sensitizes T cells to AICD by increasing caspase activation at the DISC level in response to CD95 engagement. This original mechanism, together with LF 15-0195 efficacy in various disease models, makes this compound a promising immunosuppressive drug.
Introduction
15-deoxyspergualin is an antibiotic that possesses both antitumor1 and immunosuppressive2activities. This compound was shown to bind specifically to the constitutively expressed cytosolic heat shock protein (Hsp) Hsc70 as well as the Hsp90 family of proteins and to inhibit their ATPase activity.3-5 Although it has proven effectiveness in the prevention and treatment of transplant rejection, the clinical use of 15-deoxyspergualin has been limited by its toxicity.6Several analogs were obtained recently through organic synthesis.7,8 One of these compounds, designated LF 15-0195, has demonstrated its efficacy in preventing graft-versus-host disease8 and in treating collagen type II–induced arthritis9 10 in mice models. Although LF 15-0195 is now tested in human clinics, the molecular mechanisms of its immunosuppressive activity differ from currently used immunosuppressive agents but remain poorly understood.
Mature peripheral T cells can be deleted by a so-called “passive apoptosis.”11 It occurs in T lymphocytes that are not sufficiently stimulated by growth factors.12,13 T cells are also capable of undergoing a unique form of cell death called activation-induced cell death (AICD).14-17 This cell death results from repeated antigenic stimulation and down-regulates the number of reactive cells in order to terminate the immune response.11 AICD initiation is due largely to coexpression of CD95 (Fas/Apo-1) and CD95 ligand (CD95L).14 Stimulation of long-term activated T cells through the T-cell receptor (TCR)/CD3 complex induces expression of CD95L, which activates the CD95 pathway by binding to the CD95 surface receptor in an autocrine/paracrine pathway. Transduction of the apoptotic signal requires oligomerization of CD95 and the formation of a death-inducing signaling complex (DISC) that involves the adapter molecule Fas-associated death domain (FADD) and procaspase-8.18-22 The critical role of FADD and procaspase-8 for CD95-induced apoptosis has been demonstrated in FADD or caspase-8 knock-out mice in which the CD95 pathway is disabled in T cells.23-26 Next, procaspase-8 is proteolytically activated into caspase-8 that is released from the DISC to the cytoplasm and either directly catalyzes the cleavage of downstream caspases such as caspase-3 (type 1 cells such as T lymphocytes) or cuts the Bcl-2 protein–related Bid to generate a truncated Bid that induces the release of proapoptotic molecules from the mitochondria (type 2 cells such as Jurkat cells).27
AICD was shown also to promote deletion of self-reactive T cells in the periphery. Accordingly, mutations in CD95 induce an autoimmune disease in both mice and humans.28-30 Thus, facilitation of CD95-mediated pathway to cell death may provide a strategy for increasing elimination of self-reactive T cells in the treatment of autoimmune diseases. It was proposed that this approach may provide specificity for autoreactive T cells that have failed to undergo AICD, whereas the normal immune response to foreign antigens should remain less affected.31 For example, several bisindolylmaleimide derivatives were shown to facilitate CD95-mediated T-cell apoptosis and to prevent the development of autoimmune diseases in 2 distinct experimental models in the rat.31
In the present study, we show that LF 15-0195 sensitizes T lymphocytes to AICD by modulating the CD95-mediated cell death pathway. This effect, which was observed in vitro in Jurkat and human peripheral T cells and was confirmed in vivo by studying the death of allogeneic T cells in a mouse model of bone marrow transplantation, may account for the immunosuppressive activity of the molecule.
Materials and methods
Reagents
LF 15-0195, LF 15-0969, and LF 12-0080 (Fournier Laboratories, Dijon, France) were prepared in saline solution. Tumor necrosis factor-α (TNFα) was purchased from PeproTech (Tebu, France), recombinant human TNF-related apoptosis-inducing ligand (TRAIL) from Alexis Biochemicals (Coger, Paris, France), agonistic anti–human CD95 monoclonal antibody (mAb; clone CH11) from Immunotech (Marseille, France), antagonistic anti–human CD95L mAb (clone NOK-1) from BD Biosciences (Heidelberg, Germany), anti-CD3 mAb (clone OKT3) from Janssen-Cilag (Issy-les-Moulineaux, France), and anti–Flag M2 mAb, etoposide (VP16), and cycloheximide from Sigma-Aldrich Laboratories (St Quentin Fallavier, France).
Graft-versus-host disease
Experimental graft-versus-host disease (GVHD) was induced in cyclophosphamide-immunosuppressed B6D2F1 mice by intravenous injection of 2 × 108 viable spleen cells from B6 origin according to a previously described protocol.32 LF derivatives (0.1 μM) dissolved in 0.9% NaCl solution or the vehicle alone were administered intraperitoneally from day 1 to day 10 (day 6 omitted), and survival was followed until day 60. Survival curves were compared by using a log-rank test.
Preparation and stimulation of primary human T cells
Peripheral blood samples were obtained from healthy volunteers with informed consent. T cells were separated by negative selection (R&D Systems Europe, Abingdon, United Kingdom) and cultured in RPMI 1640 medium supplemented with 10% heat-inactivated (vol/vol) fetal bovine serum (FBS), sodium pyruvate, and nonessential amino acids (Gibco BRL, Life Technologies, Cergy-Pontoise, France). For stimulation, resting T cells (day 0; 2 × 106 cells/mL) were activated with plate-coated anti-CD3 Abs (30 μg/mL) for 24 hours, then cultured for 4 days in the presence or not of 30 U/mL interleukin-2 (IL-2) or antagonistic anti-CD95L Ab (NOK-1, 5 μg/mL) or both before restimulation through TcR/CD3 by using plate-coated anti-CD3 Abs (30 μg/mL).
Cell lines
The parental FADD−/− and caspase-8−/− Jurkat T-lymphoma cell lines were kindly provided by John Blenis (Department of Cell Biology, Harvard Medical School, Boston, MA) and cultured in RPMI 1640 medium with glutamax-I supplemented with 10% FBS in a humidified atmosphere of 95% air and 5% CO2 at 37°C and resuspended in fresh medium (0.4 × 106 cells/mL) 24 hours before each treatment.
Identification of apoptosis
DNA fragmentation was identified by ethidium bromide staining of DNA extracted with DNAzol Reagent (Gibco BRL) and separated by agarose gel electrophoresis (1.8%) in Tris [tris(hydroxymethyl)aminomethane]-borate-EDTA (ethylenediaminetetraacetic acid) buffer (pH8.0). Morphological changes were identified by staining the nuclear chromatin with Hoechst 33342 (Sigma-Aldrich) for 15 minutes at 37°C before counting apoptotic cells by fluorescence microscopy.
Western blot analysis
Whole cell lysates were obtained in boiling buffer (1% SDS [sodium dodecyl sulfate], 1 mM Na-Vanadate, 10 mM Tris, pH 7.4) in the presence of protease inhibitors (0.1 mM phenylmethylsulfonyl fluoride [PMSF], 2.5 μg/mL pepstatine, 10 μg/mL aprotinin, 5 μg/mL leupeptin). Viscosity of the samples was reduced by several passages through a 26-gauge needle. Protein concentration was measured by the Bio-Rad DC protein assay kit (Bio-Rad, Ivry sur seine, France). Fifty micrograms of protein were incubated in loading buffer (125 mM tris-HCl, pH 6.8, 10% β-mercapto-ethanol, 4.6% SDS, 20% glycerol, and 0.003% bromophenol blue), separated by sodium dodecyl sulfate–polyacrylamide gel (SDS-PAGE), and electroblotted to polyvinylidene difluoride membrane (Bio-Rad). After blocking nonspecific binding sites overnight by 5% nonfat milk in TPBS (0.1% PBS [phosphate-buffered saline]-Tween 20), the membrane was incubated for 2 hours at room temperature with primary antibody. After 2 washes in TPBS, the membrane was incubated with horseradish peroxidase–conjugated goat anti–mouse or anti–rabbit antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA) for 30 minutes at room temperature, then washed twice in TPBS. Immunoblots were revealed using enhanced chemiluminescence detection kit (Amersham Pharmacia Biotech Europe GmbH, Orsay, France) by autoradiography.
Mouse mAbs used for Western blotting include anti–human FADD (Transduction Labs), anti–c-FLIPL (Euromedex, Mundolsheim, France), anti–caspase-8 (Immunotech, Marseille, France), anti–caspase-10 (MBL International, Watertown, MA), anti–Bcl-2 (Dako A/S, Glostrup, Denmark), anti–cytochrome c (BD Biosciences), anti-Hsc70 (Santa Cruz Biotechnology, Santa Cruz, CA) and anti–mitochondrial Hsp 70 (Affinity Bioreagents, Interchim, France). We also used rabbit polyclonal Abs raised against human poly-(ADP-ribose)polymerase (Roche Molecular Biochemicals, Meylan, France), caspase 3 (BD Biosciences), XIAP (R&D Systems Europe), and Smac/direct IAP binding protein with low pI (DIABLO) (kindly provided by Xiadong Wang, Howard Hughes Medical Institute, Dallas, TX).
Immunoprecipitation
Jurkat cells were treated with 1 μg/mL CD95L (Alexis) in the presence or absence of cross-linking anti–Flag M2 antibody (2 μg/mL; Sigma) for the indicated time (in the negative control, CD95 and the cross-linking antibody were added after lysis). Cells were quickly cooled down by adding 5 volumes of ice-cold PBS, then lysed with 0.2% NP40, 20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 2 mM sodium vanadate, 10% glycerol, and the protease inhibitor cocktail. Cytosolic fractions were precleared with Sepharose 6B for 90 minutes and then incubated with protein G–coupled Sepharose beads for 4 hours. Beads were washed 4 times with the lysis buffer. Proteins were separated on 10% SDS-PAGE and blotted onto nitrocellulose filters. The following antibodies were used for Western blotting: anti-CD95 (Santa Cruz), anti-FADD (Transduction Labs), anti–caspase-8 (MBL, Pharmingen, Heidelberg, Germany), and anti-FLIP Dave-2 (Alexis Biochemical, Illkirch, France).
Caspase activity assays
Cells were homogenized at 4°C for 30 minutes in lysis buffer containing 150 mM NaCL, 50 mM Tris-HCl, pH 8.0, 0.1% SDS, 1% NP40, 0.5% Na desoxycholate, centrifuged (×10 000g for 20 minutes at 4°C), and supernatants collected and frozen at −80°C. Protein concentration was measured by the Bio-Rad DC protein assay kit. Typically, 50 μg protein was used to monitor caspases activities. Caspases -2, -3, -8, and -9 activities were assessed by the cleavage of the fluorometric substrates Z-VDVAD-AFC, AC-DEVD-AMC, Z-IETD-AFC, and Ac-LEHD-AFC, respectively. The assay mixture contained 100 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), pH 7.0, 1 mM EDTA, 0.1% CHAPS (3-[(3-cholamidopropyl)dimethylamonio]-1-propyl sulfonate), 10% glycerol, 20 mM dithiothreitol, and 100 μM fluorogenic peptide substrates. amino-4-trifluoro-methylcoumarin (AFC) and amino-4-trifluoro-methylcoumarin (AMC) released from the substrates were excited at 400 and 380 nm while emissions were measured at 505 and 460 nm, respectively. Enzyme activities were determined as initial velocities expressed as relative intensity per minute per milligram.
Cell fractionation
Cells were suspended in ice-cold buffer A (250 mM sucrose, 20 mM HEPES, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA (ethyleneglycoltetraacetic acid), 1 mM dithiothreitol, 100 μM PMSF, 10 μg/mL leupeptin, 2 μg/mL aprotinin) before passing them through an ice-cold cell homogenizer. Unlysed cells and nuclei were pelleted by two 10-minute, 750g spins while the supernatant was centrifuged at 10 000g for 25 minutes at 4°C. The pellet was resuspended in buffer A (mitochondria fraction). The supernatant was further centrifuged at 100 000g for 1 hour at 4°C. The new supernatant (cytosolic fraction; S100) was frozen at −80°C.
Cell-free system
Nuclei-free, mitochondria-free cytosolic extracts (cell-free extracts) were generated as described.33Briefly, cells (1-2 × 108) were pelleted and washed twice with PBS, pH 7.2, followed by a single wash with 4 mL ice-cold cell extract buffer (CEB) [HEPES 20 mM, KCl 10 mM, MgCl21.5 mM, EDTA 1 mM, EGTA 1 mM, dithiothreitol 1 mM, PMSF 100 μM, aprotinin 2 μg/mL, leupeptine 10 μg/mL (pH 7.4)]. Two volumes of ice-cold CEB were added to one volume of packed cell pellet before transferring the cellular suspension to a 2-mL dounce homogenizer. Cells were allowed to swell under hypotonic conditions for 20 minutes, then disrupted with 30 strokes of a B-type dounce. Lysis was confirmed using trypan blue exclusion test before centrifugation of lysates at × 16 000g for 15 minutes at 4°C. Supernatants (cell-free extracts, 10 μL, 5-10 mg/mL protein) were incubated at 37°C with 5 μM horse heart cytochrome c (Sigma-Aldrich) and 1 mM deoxy adenosine triphosphate (dATP) (Pharmacia, Orsay, France) before studying caspase activities.
Flow cytometry
Jurkat cells (5 × 105) were washed in PBS containing 1% bovine serum albumin (BSA) and 0.1% sodium azide, then incubated with 1/100 anti-CD95 Ab (clone ZB4, Immunotech) or a mouse isotype-matched control Ab (Dako) in a total volume of 100 μL for 1 hour at 4°C, washed, incubated with an anti–mouse fluorescein isothiocyanate (FITC)–labeled Ab (Amersham) for 30 minutes at 4°C, washed again and resuspended in 200 μL 1% paraformaldehyde (Sigma-Aldrich). Samples were analyzed on a FACScalibur (Becton-Dickinson, BD Bioscience) using CellQuest software (Becton Dickinson).
In vivo apoptosis
Pathogen-free male, 5- to 6-week-old C57BL/6 (B6, H-2b), BALB/c (H-2d) (Iffa-Credo, L'Abresle, France), and B6 (lpr/lpr) mice (CDTA CNRS, Orléans, France) were used to perform bone marrow transplantation (BMT) according to previously described procedures.34 Briefly, bone marrow (BM) and splenic T cells were isolated from B6 or B6/lpr mice and suspended in PBS (BioWhittaker, Verviers, Belgium). BALB/c mice, used as recipients, were submitted to total body irradiation (TBI: 8 Gy single dose at 2.7 Gy/min) 16 hours before BMT. These recipients received a single intravenous injection of 107 BM cells in 300 μL PBS, either alone or combined with 3 × 106 T cells. The LF 15-195 immunosuppressive agent (0.625 mg/kg/j) was administrated intraperitoneally every day from day 3. Detection of donor apoptotic cells was performed at day 6 after BMT. Donor H-2 T cells were gated using phycoerythrin-labeled anti–H-2b (AF6-88.5, mouse IgG2a) and APC-conjugated anti-CD3 (145-2C11, hamster IgG) Abs (BD Pharmingen, San Diego, CA). Apoptosis was detected by measuring FITC-conjugated Annexin-V–labeled cells (Immunotech) in this gated population. Analysis was performed on a FACScalibur (BD Immunocytometry Systems, San Jose, CA) using CellQuest software. Percentages of apoptotic cells are expressed in “Results” as mean ± SD of 3 animals per group in 1 representative of 2 experiments.
Results
LF 15-0195 increases CD95-mediated Jurkat cell death
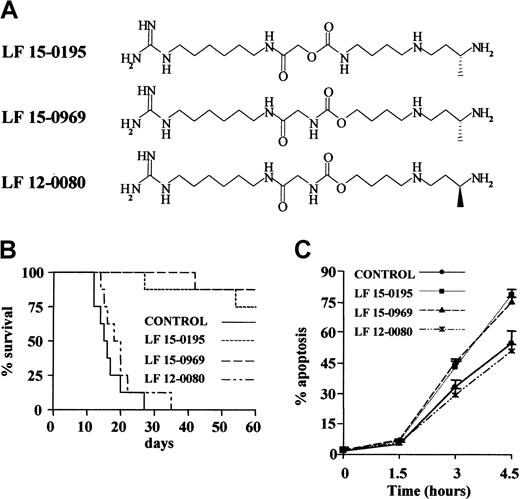
To analyze the influence of LF 15-0195 on AICD, we used the human T leukemia cell line Jurkat.35 Because CD95 engagement had been shown to play a role in AICD initiation,14 we first tested the influence of LF 15-0195 on CD95-mediated Jurkat cell death. LF 15-0195 activity was initially compared to that of 2 other deoxyspergualine derivatives (Figure1A) that demonstrated differential efficacy in a graft-versus-host disease (GVHD) model in mice. Whereas LF 15-0195 and LF 15-0969 significantly improved the survival of mice developing GVHD, LF 12-0080, the LF 15-0969 isomer, did not demonstrate any significant activity in this model (Figure 1B). Jurkat cells were cultured in the presence of either vehicle alone or 0.1 μM deoxyspergualine derivative for 3 days before adding an agonistic anti-CD95 Ab (CH11, 25 ng/mL) to the culture medium. The 2 compounds that increased survival of mice in the GVHD model also sensitized Jurkat cells to CD95-mediated apoptosis, while the third derivative, LF 12-0080, that was ineffective in controlling GVHD did not demonstrate any sensitizing activity (Figure 1C). This observation led us to further explore the influence of LF 15-0195 on CD95-mediated cell death in Jurkat T cells.
LF 15-0195 sensitizes Jurkat T cells to AICD.
(A) Chemical structure of LF 15-0195 and related compounds. (B) Influence of the 3 tested compounds on survival of animals in the graft-versus-host model. (C) Influence of the 3 indicated compounds' pretreatment (0.1 μM for 3 days) on anti-CD95–induced Jurkat cell death (CH11, 25 ng/mL for indicated times). Apoptosis was studied by Hoechst 33342 staining of the nuclear chromatin.
LF 15-0195 sensitizes Jurkat T cells to AICD.
(A) Chemical structure of LF 15-0195 and related compounds. (B) Influence of the 3 tested compounds on survival of animals in the graft-versus-host model. (C) Influence of the 3 indicated compounds' pretreatment (0.1 μM for 3 days) on anti-CD95–induced Jurkat cell death (CH11, 25 ng/mL for indicated times). Apoptosis was studied by Hoechst 33342 staining of the nuclear chromatin.
LF 15-0195 sensitizes Jurkat cells to CD95-mediated caspase activation
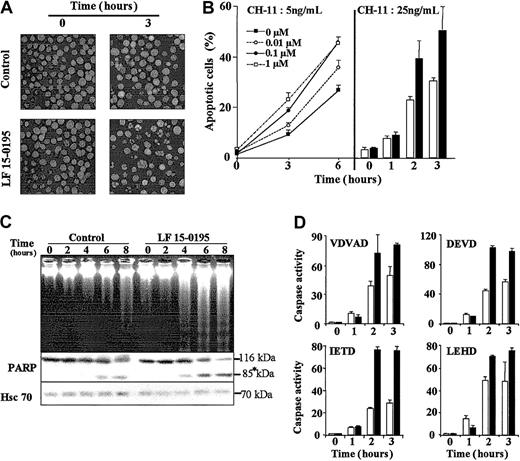
Hoechst 33342 staining of the nuclear chromatin indicated that exposure of Jurkat cells to LF 15-0195 sensitized these cells to apoptosis triggered by various concentrations of CH-11 mAb (Figure2B) and at various time points (Figure 2A-B). Analysis of the dose-dependent effects of LF 15-0195 indicated that 0.1 μM was an optimal concentration for further studies. Higher concentrations such as 1 μM (Figure 2B) and 10 μM (not shown) did not demonstrate significantly higher sensitization to 5 ng/mL CH11 anti-CD95 mAb. The 0.1-μM concentration is about 10-fold lower than plasma levels measured in rats and monkeys receiving an intravenous injection of the studied compound (D. de F., unpublished data, April 2002). LF 15-0195–mediated sensitization to Fas-induced apoptosis was further confirmed by studying internucleosomal DNA fragmentation in agarose gel electrophoresis (Figure 2C) and the cleavage of the 116-kDa poly(ADP-ribose)polymerase into an 85-kDa fragment (Figure 2C). These observations, suggesting increased caspase activation, were confirmed by studying the cleavage of 4 caspase substrates, zVDVAD-AFC, Ac-DEVD-AMC, zIETD-AFC, and Ac-LEHD-AFC. This cleavage was increased in CH11-treated Jurkat cells exposed to LF 15-0195 compared with those exposed to the vehicle alone (Figure 2D). Increased activation of caspase-2, caspase-3, and caspase-8 was further confirmed by Western blotting (data not shown).
Influence of LF 15-0195 on Jurkat T-cell apoptosis triggered by an agonistic anti-CD95 Ab.
(A) Hoechst 33342 staining of the nuclear chromatin of Jurkat T cells exposed to either vehicle (control) or LF 15-0195 (0.1 μM) for 3 days, then treated with CH11 anti-CD95 Ab (25 ng/mL for 3 hours). Original magnifications, × 40. (B) A similar experiment was performed with Jurkat T cells exposed to either indicated concentrations (left panel) or 0.1 μM (right panel) LF 15-0195 for 3 days, then treated with indicated concentration of CH11 Ab for various times. (Right panel: ■ indicates control cells; ▪, LF 15-0195–pretreated cells.) Results are expressed as the means ± SD of 3 independent experiments in triplicate. (C) Top panel: agarose gel electrophoresis of DNA from Jurkat T cells exposed to either vehicle (control) or LF 15-0195 (0.1 μM) for 3 days, then exposed to CH11 mAbs (25 ng/mL) for indicated times. Bottom panel: simultaneous analysis of poly(ADP-ribose)polymerase by Western blotting (loading control: Hsc70). Asterisk indicates the cleavage fragments. (D) Caspase activities were explored by studying the cleavage of indicated peptides at indicated times after the beginning of CH11 Ab treatment (25 ng/mL). ■ indicates control cells; ▪, LF 15-0195–treated cells. One representative of 3 independent experiments is shown (means ± SD).
Influence of LF 15-0195 on Jurkat T-cell apoptosis triggered by an agonistic anti-CD95 Ab.
(A) Hoechst 33342 staining of the nuclear chromatin of Jurkat T cells exposed to either vehicle (control) or LF 15-0195 (0.1 μM) for 3 days, then treated with CH11 anti-CD95 Ab (25 ng/mL for 3 hours). Original magnifications, × 40. (B) A similar experiment was performed with Jurkat T cells exposed to either indicated concentrations (left panel) or 0.1 μM (right panel) LF 15-0195 for 3 days, then treated with indicated concentration of CH11 Ab for various times. (Right panel: ■ indicates control cells; ▪, LF 15-0195–pretreated cells.) Results are expressed as the means ± SD of 3 independent experiments in triplicate. (C) Top panel: agarose gel electrophoresis of DNA from Jurkat T cells exposed to either vehicle (control) or LF 15-0195 (0.1 μM) for 3 days, then exposed to CH11 mAbs (25 ng/mL) for indicated times. Bottom panel: simultaneous analysis of poly(ADP-ribose)polymerase by Western blotting (loading control: Hsc70). Asterisk indicates the cleavage fragments. (D) Caspase activities were explored by studying the cleavage of indicated peptides at indicated times after the beginning of CH11 Ab treatment (25 ng/mL). ■ indicates control cells; ▪, LF 15-0195–treated cells. One representative of 3 independent experiments is shown (means ± SD).
LF 15-0195 does not influence the postmitochondrial pathway to cell death
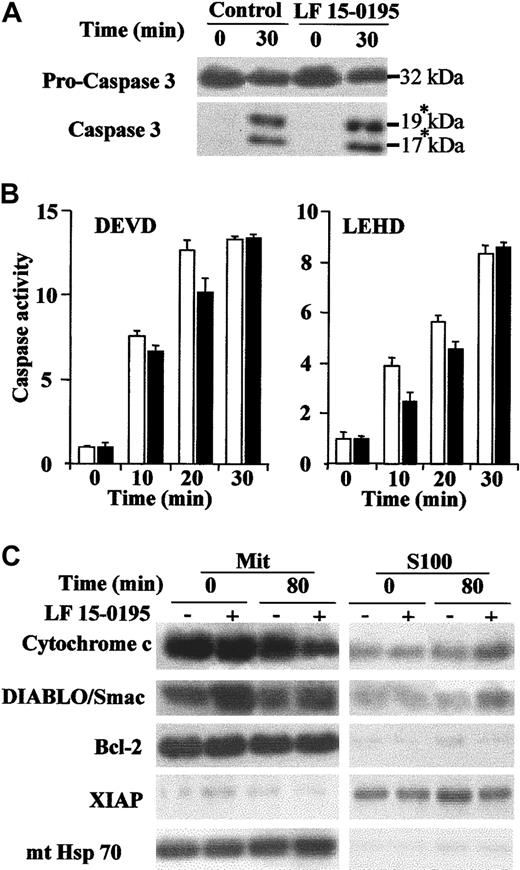
To determine whether exposure to LF 15-0195 could sensitize Jurkat cells to caspase activation, downstream of the mitochondrial events, we used a previously described cell-free system in which caspases are activated by addition of cytochrome c in the presence of dATP.33 Exposure of Jurkat cells to LF 15-0195 for 3 days did not demonstrate any effect on the cleavage of the 32-kDa procaspase-3 into its p19 and p17 active fragments (Figure3A) nor on the cleavage of the 2 studied caspase substrates (Figure 3B) induced by addition of the cytochrome c/dATP combination to cell-free extracts. By contrast, exposure to LF 15-0195 increased the release of cytochrome c and DIABLO/Smac from the mitochondria to the cytosol upon anti-CD95 mAb exposure (Figure 3C). LF 15-0195 had no significant influence on the expression and subcellular localization of Bcl-2, XIAP, and mitochondrial Hsc70 that were used as loading controls (Figure 3C). Finally, exposure to LF 15-0195 for 3 days did not sensitize Jurkat cells to apoptosis induced by the topoisomerase inhibitor etoposide (not shown). Altogether, these data suggest that LF 15-0195–mediated sensitization to CD95-induced apoptosis in Jurkat cells may be specific to the premitochondrial events.
LF 15-0195 does not influence the postmitochondrial pathway to cell death.
(A-B) Cell-free extracts were obtained from Jurkat T cells exposed to either vehicle or LF 15-0195 for 3 days and treated for indicated times with a cytochrome c (5 μM)/dATP (1 mM) combination before studying caspase-3 cleavage by Western blot. The asterisk indicates the cleavage products (A) and caspase activities by using indicated peptide substrates (B). ■ indicates control cells; ▪, LF 15-0195–treated cells. Error bars indicate means ± SD. (C) Expression of cytochrome c, DIABLO/Smac, XIAP, mitochondrial Hsc70 (mt Hsc70), and Bcl-2 was studied by Western blot in the mitochondria and the S100 fractions of Jurkat T cells exposed for 3 days to either vehicle (−) or LF 15-0195 (+; 0.1 μM), then treated with CH11 Ab (25 ng/mL) for 80 minutes.
LF 15-0195 does not influence the postmitochondrial pathway to cell death.
(A-B) Cell-free extracts were obtained from Jurkat T cells exposed to either vehicle or LF 15-0195 for 3 days and treated for indicated times with a cytochrome c (5 μM)/dATP (1 mM) combination before studying caspase-3 cleavage by Western blot. The asterisk indicates the cleavage products (A) and caspase activities by using indicated peptide substrates (B). ■ indicates control cells; ▪, LF 15-0195–treated cells. Error bars indicate means ± SD. (C) Expression of cytochrome c, DIABLO/Smac, XIAP, mitochondrial Hsc70 (mt Hsc70), and Bcl-2 was studied by Western blot in the mitochondria and the S100 fractions of Jurkat T cells exposed for 3 days to either vehicle (−) or LF 15-0195 (+; 0.1 μM), then treated with CH11 Ab (25 ng/mL) for 80 minutes.
LF 15-0195 does not modulate Jurkat T-cell content in DISC components
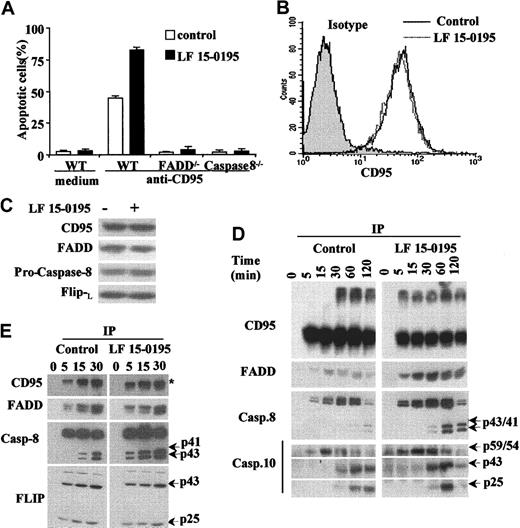
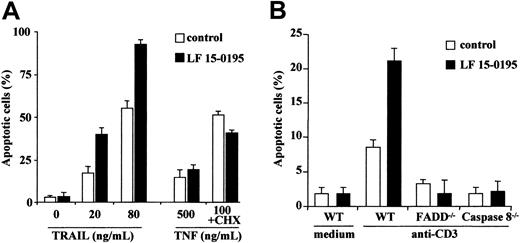
Apoptosis induced by anti-CD95 Abs in parental Jurkat T cells, either left untreated or pretreated with LF 15-0195 for 3 days, could not be observed in Jurkat T-cell derivatives that do not express either the adapter molecule FADD or caspase-8 (Figure4A). By using a flow cytometry method, we observed also that LF 15-0195 had no effect on the expression of CD95 at the surface of Jurkat cells (Figure 4B). Confocal microscopy analysis did not detect the formation of CD95 molecule clusters at the surface of treated cells (not shown). In addition, LF 15-0195 treatment did not significantly modify the cellular level of CD95, FADD, procaspase-8, and c-FLIPL (Figure 4C). By performing immunoprecipitation experiments, we first observed that pretreatment with LF 15-0195 facilitated the formation of CD95 trimers in response to CD95 engagement. LF 15-0195 pretreatment also increased the amount of FADD, procaspase-8, and procaspase-10 recruited at the DISC level (Figure 4D). Active fragments of caspase-8 and caspase-10 were observed to appear more rapidly and intensively in LF 15-0195–treated cells than in control cells (Figure 4D). Increased activation of caspase-8 in the DISC was confirmed in RAJI cells exposed to LF 15-0195 for 3 days (Figure 4E), whereas no change in c-FLIP recruitment to the DISC was identified (Figure 4E).
LF 15-0195 sensitizes Jurkat cells to FADD and caspase-8–dependent apoptosis.
(A) Wild-type, FADD−/−, and caspase-8−/−Jurkat T cells were treated with either vehicle (■) or 0.1 μM LF 15-0195 (▪) for 3 days, then treated with CH11 Ab (25 ng/mL) for 6 hours. Apoptosis was studied by Hoechst 33342 staining of the nuclear chromatin. Data are expressed as means ± SD of 3 independent experiments. (B-C) Jurkat T cells were treated with either vehicle or 0.1 μM LF 15-0195 for 3 days, before studying CD95 expression by flow cytometry (panel B: black line, vehicle; dotted line, LF 15-0195), and CD95, FADD, procaspase-8, and c-FLIPL expression by Western blotting (C). (D) Jurkat T cells were treated with either vehicle or 0.1 μM LF 15-0195 for 3 days before exposure to soluble CD95L (1 μg/mL) for indicated times, then immunoprecipitated with an anti–FLAG M2 Ab (2 μg/mL) followed by Western blot analysis of indicated proteins. (E) The same experiment was performed in RAJI cells. (D-E) The asterisk indicates trimerized CD95, and arrows indicate cleavage fragments.
LF 15-0195 sensitizes Jurkat cells to FADD and caspase-8–dependent apoptosis.
(A) Wild-type, FADD−/−, and caspase-8−/−Jurkat T cells were treated with either vehicle (■) or 0.1 μM LF 15-0195 (▪) for 3 days, then treated with CH11 Ab (25 ng/mL) for 6 hours. Apoptosis was studied by Hoechst 33342 staining of the nuclear chromatin. Data are expressed as means ± SD of 3 independent experiments. (B-C) Jurkat T cells were treated with either vehicle or 0.1 μM LF 15-0195 for 3 days, before studying CD95 expression by flow cytometry (panel B: black line, vehicle; dotted line, LF 15-0195), and CD95, FADD, procaspase-8, and c-FLIPL expression by Western blotting (C). (D) Jurkat T cells were treated with either vehicle or 0.1 μM LF 15-0195 for 3 days before exposure to soluble CD95L (1 μg/mL) for indicated times, then immunoprecipitated with an anti–FLAG M2 Ab (2 μg/mL) followed by Western blot analysis of indicated proteins. (E) The same experiment was performed in RAJI cells. (D-E) The asterisk indicates trimerized CD95, and arrows indicate cleavage fragments.
LF 15-0195 also sensitizes Jurkat T cells to TRAIL and anti-CD3–mediated cell death
We then tested whether exposure to LF 15-0195 for 3 days could sensitize Jurkat T cells to the cytokines TRAIL (20 and 80 ng/mL for 6 hours) and TNFα (500 ng/mL alone or 100 ng/mL in the presence of 0.8 μg/mL cycloheximide for 24 hours). We observed that LF 15-0195 sensitized Jurkat cells to TRAIL-induced cell death, whereas it did not sensitize these cells to TNFα-induced apoptosis (Figure5A). LF 15-0195 also sensitized Jurkat cells to apoptosis triggered by an anti-CD3 Ab that induces cell death through a FADD and caspase-8–dependent pathway (Figure 5B). Altogether, our results indicate that LF 15-0195 specifically sensitizes Jurkat cells to the FADD and caspase-8–mediated cell death pathways triggered by CD95L, TRAIL, and anti-CD3 Abs.
LF 15-0195 sensitizes Jurkat T cells to TRAIL- and anti-CD3–induced cell death.
(A) Jurkat T cells were treated with either vehicle or 0.1 μM LF 15-0195 for 3 days before exposure to indicated concentrations of TRAIL or TNFα (without or with 0.8 μg/mL cycloheximide). Apoptosis was studied by Hoechst 33342 staining of the nuclear chromatin. ■ represents vehicle alone; ▪, LF 15-0195. (B) Wild-type (WT), FADD−/−, and caspase-8−/− Jurkat T cells were treated with either vehicle (■) or 0.1 μM LF 15-0195 (▪) for 3 days, then treated with an anti-CD3 Ab for 24 hours. Apoptosis was studied by Hoechst 33342 staining of the nuclear chromatin.
LF 15-0195 sensitizes Jurkat T cells to TRAIL- and anti-CD3–induced cell death.
(A) Jurkat T cells were treated with either vehicle or 0.1 μM LF 15-0195 for 3 days before exposure to indicated concentrations of TRAIL or TNFα (without or with 0.8 μg/mL cycloheximide). Apoptosis was studied by Hoechst 33342 staining of the nuclear chromatin. ■ represents vehicle alone; ▪, LF 15-0195. (B) Wild-type (WT), FADD−/−, and caspase-8−/− Jurkat T cells were treated with either vehicle (■) or 0.1 μM LF 15-0195 (▪) for 3 days, then treated with an anti-CD3 Ab for 24 hours. Apoptosis was studied by Hoechst 33342 staining of the nuclear chromatin.
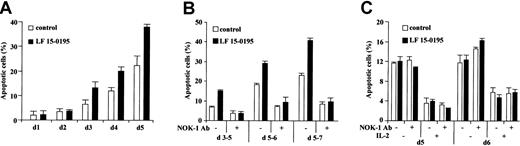
LF 15-0195 sensitizes human peripheral T cells to AICD
To further determine the influence of LF 15-0195 on AICD, we stimulated human peripheral T cells from healthy volunteers for 24 hours with an anti-CD3 mAb, then cultured the cells in the presence of IL-2 for up to 5 days, in the absence or in the presence of LF 15-0195 (0.1 μM, continuous exposure). The ability of CH11 anti-CD95 Ab (25 ng/mL for 6 hours) to trigger apoptosis, as identified by Hoechst 33342 staining of the nuclear chromatin, was evaluated every day. In the absence of LF 15-0195, we observed an apoptotic response to CH11 Ab at day 3 after the beginning of cell treatment, and the ability of CH11 Ab to trigger cell death increased with time to reach ∼ 20% at day 5. In the presence of LF 15-0195, apoptosis in response to CH11 Ab also appeared at day 3. The number of apoptotic cells was significantly increased by LF 15-0195 pretreatment, reaching ∼ 40% at day 5 (Figure 6A). Again, when T cells were restimulated with anti-CD3 Ab for 24 or 48 hours at various times after initial stimulation, the number of apoptotic cells was more important in the presence than in the absence of LF 15-0195 (Figure 6B). This apoptotic response was blocked by an antagonistic anti-CD95L Ab (Nok-1), indicating the role of a CD95L/CD95 interaction in the death process (Figure 6B). In contrast, LF 15-0195 did not demonstrate any influence on passive death of unstimulated or IL-2–stimulated T cells, a process that does not depend on CD95L/CD95 interaction, thus is not influenced by Nok-1 anti-CD95L Ab (Figure 6C).
LF 15-0195 sensitizes human peripheral T cells to AICD.
Peripheral T cells were purified from blood samples from healthy volunteers. (A) Cells were exposed to OKT3 Ab for 24 hours, then cultured in the presence of IL-2 for indicated times. Every day, an aliquot of cells was exposed to CH11 Ab (25 ng/mL) for 6 hours, and apoptosis was determined by Hoechst 33342 staining. (B) Cells were exposed to OKT3 Ab for 24 hours, then cultured in the presence of IL-2, then restimulated with OKT3 Ab for 24 hours at day 5 (d5-6) or 48 hours at days 3 (d3-5) or 5 (d5-7) before studying apoptosis as described in the legend to Figure 1. (C) Cells were exposed to OKT3 Ab for 24 hours, then cultured in the absence or presence of IL-2, in the presence or absence of an anti-CD95L Ab (Nok1, 5 μg/mL) for indicated times. Then, apoptosis was studied as in Figure 1. Results are expressed as means ± SD of triplicate experiments. One representative experiment is shown. ■ represents cells cultured with vehicle alone; ▪, cells cultured in presence of 0.1 μM LF 15-0195.
LF 15-0195 sensitizes human peripheral T cells to AICD.
Peripheral T cells were purified from blood samples from healthy volunteers. (A) Cells were exposed to OKT3 Ab for 24 hours, then cultured in the presence of IL-2 for indicated times. Every day, an aliquot of cells was exposed to CH11 Ab (25 ng/mL) for 6 hours, and apoptosis was determined by Hoechst 33342 staining. (B) Cells were exposed to OKT3 Ab for 24 hours, then cultured in the presence of IL-2, then restimulated with OKT3 Ab for 24 hours at day 5 (d5-6) or 48 hours at days 3 (d3-5) or 5 (d5-7) before studying apoptosis as described in the legend to Figure 1. (C) Cells were exposed to OKT3 Ab for 24 hours, then cultured in the absence or presence of IL-2, in the presence or absence of an anti-CD95L Ab (Nok1, 5 μg/mL) for indicated times. Then, apoptosis was studied as in Figure 1. Results are expressed as means ± SD of triplicate experiments. One representative experiment is shown. ■ represents cells cultured with vehicle alone; ▪, cells cultured in presence of 0.1 μM LF 15-0195.
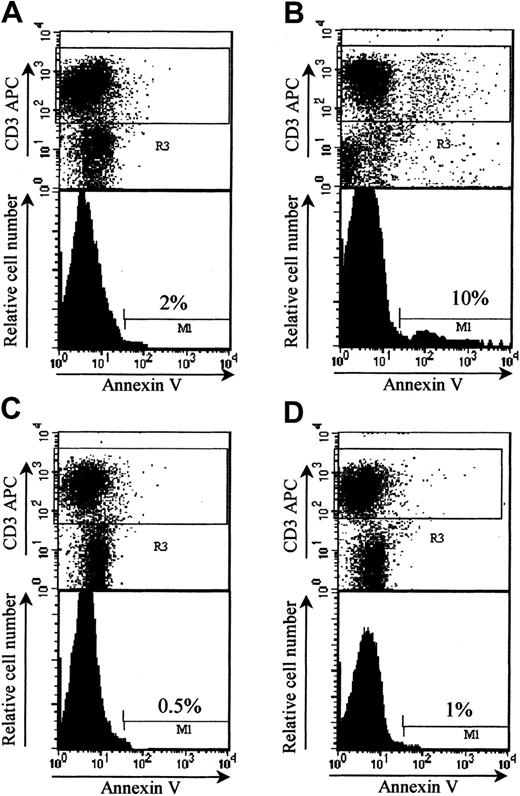
LF 15-0195 protects from GVHD by inducing apoptosis of T donor lymphocytes
CD95 signaling plays an important role in AICD of host-reactive T cells.36 To determine whether LF 15-0195–induced sensitization to AICD identified in vitro could account for increased mice survival in a GVHD model, we performed in vivo experiments. For that purpose, we used donor T cells from wild-type B6 and CD95-deficient B6 mice to trigger GVHD in bone marrow BALB/c mice recipients. The percentage of donor apoptotic T cells was increased when measured in LF 15-0195–treated (9.33% ± 0.58%) compared with nontreated (1.17% ± 0.76%) animals at day 6 after engraftment. This effect was not observed when donor T cells were obtained from Fas-deficient lpr B6 mice (0.67% ± 0.29% both without and with LF, respectively; Figure7). These results indicated that a functional CD95 expression was required for donor T-cell apoptosis observed in LF 15-0195–treated animals at day 6 after engraftment.
LF 15-0195 sensitizes donor T cells to AICD in BMT model.
Irradiated BALB/c mice were engrafted with bone marrow and 3 × 106 splenic T cells from either B6 (A-B) or lprB6 (C-D), then left untreated (A,C) or treated with daily subcutaneous injection of LF 15-0195 from day 3 to day 6 (B,D). Animals were humanely killed at day 6, and apoptosis was measured in by annexin-V staining of CD3+ cells among donor (H2b-gated) splenocytes. Numbers indicate the percentages of apoptotic cells in representative animals from each group.
LF 15-0195 sensitizes donor T cells to AICD in BMT model.
Irradiated BALB/c mice were engrafted with bone marrow and 3 × 106 splenic T cells from either B6 (A-B) or lprB6 (C-D), then left untreated (A,C) or treated with daily subcutaneous injection of LF 15-0195 from day 3 to day 6 (B,D). Animals were humanely killed at day 6, and apoptosis was measured in by annexin-V staining of CD3+ cells among donor (H2b-gated) splenocytes. Numbers indicate the percentages of apoptotic cells in representative animals from each group.
Discussion
The deoxyspergualin derivative LF 15-0195 has demonstrated therapeutic efficacy in animal models of autoimmune diseases,9,10 heart transplantation,37,38 and GVHD.39 However, the molecular mechanisms of its immunosuppressive activity remain poorly understood. In a collagen-induced arthritis model in mice, the LF 15-0195 therapeutic effect was associated with a decreased level of anti–collagen II IgG2a antibody production and cytokine secretion by lymph node infiltrating T cells.9 In the present study, we demonstrate that LF 15-0195 sensitizes T cells to AICD by increasing caspase-8 activation at the DISC level in response to CD95 engagement. This effect may account for the therapeutic activity of the molecule in GVHD given that an increased apoptosis of donor T cells was observed in bone marrow recipient animals when treated with LF 15-0195, an effect that was not observed when donor T cells were CD95 deficient.
CD95 ligand has been shown as an important effector molecule in acute GVHD.40 The ability of LF 15-0195 and 2 related compounds to sensitize Jurkat T cells to CD95-mediated apoptosis was observed to correlate with their therapeutic efficacy in GVHD. Activation of alloreactive donor CD4+ T cells plays a crucial role in the pathogenesis of active GVHD. The fate of alloreactive anti–host T cells after hematopoietic stem cell transplantation includes initial expansion followed by massive in situ AICD.36 The consequences of anti–host T-cell AICD remain controversial. The massive death of allogeneic anti–host T cells was suggested to be coupled with bystander lysis of grafted non–host-reactive T cells, thus abrogating immune reconstitution by donor-derived postthymic T lymphocytes.36 On the other hand, the protective effect of IL-12 toward acute GVHD implies a CD95-dependent mechanism.41 One could speculate that LF 15-0195 prevents the massive expansion of allogeneic anti–host T cells by facilitating AICD, thus preventing both GVHD and the negative consequences of massive host and nonhost T-cell death that follows this expansion.36
Exposure to LF 15-0195 is shown to sensitize Jurkat cells to CD95-mediated, caspase-dependent cell death. To determine whether this sensitization was specific to death receptor pathways, we first analyzed the postmitochondrial events that are triggered by the release of proapoptotic molecules such as cytochrome c42 and Smac/DIABLO43 from the mitochondria to the cytosol. By using a previously described cell-free system,33 we show that LF 15-0195 does not sensitize cytosolic caspases to cytochrome c–mediated activation. On the other hand, LF 15-0195 increased cytochrome c and Smac/DIABLO release from the mitochondria to the cytosol in response to CD95 engagement, which suggested that sensitization to apoptosis occurred at the level or upstream of the mitochondria in these type-2 cells.27 In addition, LF 15-0195 was observed to sensitize Jurkat cells to TRAIL-induced cell death, whereas it had little influence on TNFα-mediated apoptosis and etoposide-induced cell death. Thus, LF 15-0195–mediated sensitization of Jurkat T cells to apoptosis appears to be specific on CD95-L and TRAIL-activated pathways.
CD95 signaling is highly regulated during mature T-cell activation and differentiation.44 Resting naive T cells express little surface CD95 and remain unresponsive to CD95-mediated apoptosis, which may promote T-cell proliferation upon acute antigen exposure. TCR stimulation increases CD95 expression and, after IL-2 treatment for at least 2 days, cycling T cells become sensitive to apoptosis triggered by the TCR through autocrine production of CD95-L and signaling through CD95.14 This increase in sensitivity to CD95-induced apoptosis has been attributed to a decreased expression of c-FLIP that occurs over the first 3 days of T-cell activation.45 To determine the mechanism by which LF 15-0195 could sensitize T cells to CD95-mediated cell death, we studied its influence on the DISC that forms in response to CD95 engagement. We first observed that LF 15-0195 did not significantly influence the cellular expression level of the main components of the DISC (CD95, FADD, procaspase-8) nor did it modulate the expression of CD95 at the surface of Jurkat T cells. LF 15-0195 was observed to facilitate the formation of CD95 trimers in response to the receptor engagement, then to facilitate caspase-8 and caspase-10 recruitment and activation in the DISC. How LF 15-0195 facilitates the formation of the DISC is still a matter of speculation. No significant change in c-FLIPL level could be detected in the complex in response to LF 15-0195 exposure. A potential mechanism is the activation of a sphingomyelinase in plasma membrane rafts, as it was shown recently that CD95-mediated clustering by acid sphingomyelinase–released ceramide was required for death signaling.46 47
Several immunosuppressive compounds have been shown to sensitize activated T cells to apoptosis. The folate antagonist methotrexate selectively deletes activated peripheral blood T cells by a CD95-independent pathway.48 Immunosuppressive drugs such as cyclosporin A, FK 506, or rapamycin that inhibit IL-2 signaling do not directly influence CD95-mediated pathway to death but negatively interfere with AICD in CD4+ T-cell hybridomas, since IL-2 signaling is required for CD95-L expression.49 More recently, bisindolylmaleimide VIII was demonstrated to selectively enhance CD95-dependent apoptosis signaling, although the molecular mechanisms of this effect remain to be identified. Facilitation of CD95-mediated apoptosis of activated autoreactive T cells was suggested to account for bisindolylmaleimide VIII efficacy in 2 animal models of autoimmune diseases.31 Another compound, 2-acetylthiomethyl-4- (4-methylphenyl)-4-oxobutanoic acid (KE-198), currently developed as an antirheumatic drug, was shown to increase AICD without modulating CD95 and CD95-L expression. This effect was related to a decreased expression of the caspase inhibitor XIAP in activated peripheral blood T cells.50 Thus, though various immunomodulators were proposed to interfere with AICD, the ability of LF 15-0195 to sensitize T cells to CD95-mediated cell death by increasing caspase recruitment and activation at the DISC level appears as an original and specific mechanism.
15-deoxyspergualin and several stable analogs, including LF 15-0195, were shown to bind specifically to the constitutive and cognate member of the heat shock protein 70 (Hsp70) family of proteins, Hsc70.3,4,51 We and other have shown that overexpression of some Hsps could interfere with the postmitochondrial pathway of apoptosis.33,52,53 Our study suggests that interaction of LF 15-0195 with Hsc70 does not influence caspase activation by cytochrome c. Hsc70 and Hsp70 also could modulate death receptor–mediated apoptotic pathways; for example, the protective effect of silencer of death domains (SODD) protein toward CD95-dependent anoikis was proposed to depend on the chaperone activity of these Hsps.54 Whether increased activation of caspase-8 and caspase-10 in the DISC involves interaction of LF 15-0195 with Hsc70 requires further investigation.
Experiments studying the influence of LF 15-0195 on donor T-cell apoptosis in a bone marrow transplantation mouse model further indicate that the LF 15-0195 immunosuppressive effect may involve T-cell apoptosis through a CD95-dependent mechanism. Altogether, the present study identifies the ability of LF 15-0195 to sensitize activated T cells to AICD by increasing recruitment and activation of caspase-8 and caspase-10 in the DISC. This original mechanism, together with the previously reported efficacy of LF 15-0195 in autoimmune diseases and GVHD, make this compound a promising immunosuppressive drug.
The authors thank F. Martin and P. Tiberghien for helpful advice, and J. Blenis and X. Wang for providing cell lines and anti-Smac antibody, respectively.
Prepublished online as Blood First Edition Paper, August 29, 2002; DOI 10.1182/blood-2002-02-0603.
Supported by the Ligue Nationale Contre le Cancer (INSERM U517: Equipe labelisée “la ligue”), the ComitéDépartemental de la Ligue contre le Cancer du Doubs (Comitéde Montbéliard), and the Association pour la Recherche sur le Cancer (no. 4508). P.D. was supported by a les Conventions Industrielles de Formation par la Recherche (CIFRE) grant that associates Fournier SA Laboratories and the Conseil Régional de Bourgogne. L.D.-D. is supported by the Fondation pour la Recherche Médicale.
Two authors (D. de F., P.D.) are employed by Fournier SA, whose product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Eric Solary, INSERM U517, IFR 100, Faculties of Medicine and Pharmacy, 7 boulevard Jeanne d'Arc, 21000 Dijon, France; e-mail: esolary@u-bourgogne.fr.