Jak3, a member of the Janus kinase family of cytoplasmic tyrosine kinases, is expressed at low levels in immature hematopoietic cells and its expression is dramatically up-regulated during the terminal differentiation of these cells. To better understand the role of Jak3 in myeloid cell development, we have investigated the role of Jak3 in myeloid cell differentiation using the 32Dcl3 cell system. Our studies show that Jak3 is a primary response gene for granulocyte colony-stimulating factor (G-CSF) and the accumulation of tyrosine phosphorylated Jak3 correlated with cell growth inhibition and terminal granulocytic differentiation in response to G-CSF. Ectopic overexpression of Jak3 in 32Dcl3 cells resulted in an acceleration of the G-CSF–induced differentiation program that was preceded by G1 cell cycle arrest, which was associated with the up-regulation of the cyclin-dependent kinase inhibitor p27Kip1 and down-regulation of Cdk2, Cdk4, Cdk6, and Cyclin E. In addition, ectopic overexpression of Jak3 appears to result in the inactivation of PKB/Akt and Stat3-mediated proliferative pathways in the presence of G-CSF. Similarly, overexpression of Jak3 in primary bone marrow cells resulted in an acceleration of granulocytic differentiation in the presence of granulocyte-macrophage colony-stimulating factor, which was associated with their growth arrest in the G1 phase of the cell cycle. Taken together, these results indicate that Jak3-mediated signals play an important role in myeloid cell differentiation.
Introduction
Hematopoietic cell growth and differentiation is regulated by a network of cytokines that bind to their cognate receptors and mediate intracellular signal transduction events, which result in the modulation of gene expression. Evidence has emerged to indicate that most cytokines transmit their signals via tyrosine kinases termed Jak kinases.1 To date, this family consists of 4 members, Jak1, Jak2, Jak3, and Tyk2. These kinases, either alone or in conjunction with each other, appear to be responsible for the effects mediated by most cytokines and neurokines.1Current models suggest that interaction of cytokines with their receptors induces receptor dimerization, which increases the affinity of the cytoplasmic domain of the receptor for Jak kinases. This interaction results in a ligand-dependent formation of a complex that contains receptors and Jak kinases that have been activated through an event associated with tyrosine phosphorylation. The activated kinases appear to subsequently phosphorylate the receptors as well as downstream cellular substrates.
Jak3 is encoded by a 4.2-kb transcript that codes for a 120-kDa protein. Unlike Jak1, Jak2, and Tyk2, the expression of Jak3 is restricted to lymphoid and myeloid cell lines and to hematopoietic tissues such as the thymus, bone marrow (BM), spleen, and fetal liver.2-9 Jak3-deficient mice exhibit severe defects in lymphoid development and show aberrations in B-cell maturation and T-lymphocyte activation.10-12 Apart from the defects in B- and T-cell development, Jak3-deficient mice also show defects in the development of natural killer (NK) cells. Interestingly, Grossman et al recently demonstrated that Jak3 nullizygous mice display dysregulated myelopoiesis.13 Their results demonstrate that Jak3-deficient mice show evidence of increased immature neutrophil and monocyte counts in peripheral blood smears along with splenomegaly. Specific cell surface marker analysis indicated an expansion of cells of the myeloid lineage and a slowdown of their terminal differentiation. Taken together, these observations indicate that Jak3 has an important role in the development of cells destined for both the myeloid and lymphoid lineages.
Although the importance of Jak3 in development of the lymphoid lineage is well characterized, the role of Jak3 in myeloid cell growth and differentiation has not been fully explored. To this end, we have analyzed the role of Jak3 in myeloid cell differentiation using a myeloblastic cell line, 32Dcl3, which is derived from normal mouse BM and strictly requires interleukin 3 (IL-3) for growth.14-16 When these cells are cultured in IL-3–free medium containing granulocyte colony-stimulating factor (G-CSF), the cells proliferate for 4 to 5 days followed by growth arrest and terminal differentiation of the entire cell population. In this cell line, both proliferation and differentiation events are mediated by G-CSF, because withdrawal of G-CSF from the culture medium at any time during the 10-day period required for granulocytic differentiation leads to cell death. It is known that G-CSF can activate Jak1,17 Jak2,18 and Stat3,19 but it is at present unclear as to how the same cytokine can mediate both proliferation and differentiation programs in the same cell. One of the mechanisms by which this could be achieved is through a switch in the Jak-Stat pathways that dictate cell proliferation and differentiation.
In this paper, we report studies describing the role of Jak3 in G-CSF–mediated terminal differentiation of 32Dcl3 cells. We have previously shown that Jak3 levels are up-regulated during terminal differentiation of cells of different hematopoietic lineages.2 The results presented in this report show that the induction of Jak3 levels on G-CSF stimulus is a direct result of increased levels of transcription and does not require any new protein synthesis. In addition, overexpression of Jak3 in 32Dcl3 cells, using exogenous promoters, results in inhibition of myeloid cell proliferation and an acceleration of the differentiation program in response to G-CSF. This acceleration of the differentiation program was preceded by inhibition of PKB/Akt and Stat3–mediated proliferative pathways and cell cycle arrest of the 32D/Jak3 cells with a G1 DNA content. Furthermore, the cyclin kinase inhibitor p27Kip1 was up-regulated in conjunction with the down-regulation of Cyclin E and cyclin-dependent kinases (Cdks) 2, 4, and 6. Accelerated differentiation was also observed in normal mouse BM cells retrovirally transduced with Jak3 and treated with granulocyte-macrophage colony-stimulating factor (GM-CSF). Taken together, our observations suggest that myeloid cell growth and granulocytic differentiation are regulated by Jak3-mediated signaling pathways.
Materials and methods
Cell culture
The murine IL-3–dependent 32Dcl3 cells as well as 32D/Jak3 cells were maintained in Iscove modified Dulbecco medium (IMDM) supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 10% WEHI3B-conditioned medium as a source of IL-3. For IL-3 withdrawal experiments, cells were washed free of IL-3 and plated at 2 × 105/mL in medium without IL-3 for 4 days and subjected to morphologic analysis via May-Grünwald and Giemsa staining.3 Cell viability was monitored using trypan blue staining. To induce differentiation, cells were washed free of IL-3 and plated in the presence of G-CSF. Aliquots of the cell cultures were cytocentrifuged at selected days after treatment with G-CSF. The progression of granulocytic differentiation was monitored by May-Grünwald and Giemsa staining. The distribution of cells at different stages of granulocyte differentiation was determined by counting at least 100 cells in a field. Multilobed “tear-shaped” nuclei containing cells were scored as granulocytes, which is consistent with previously published literature.14
The eukaryotic expression vector pcDNA3Jak3, in which the expression of Jak3 was under the control of the cytomegalovirus (CMV) promoter, was generated by ligating the insert containing the entire open reading frame of Jak3 into the BamHI site of pcDNA3 (Invitrogen, Carlsbad, CA). The eukaryotic expression vector pOPI3Jak3 was generated by ligating the insert containing the entire open reading frame of Jak3 into the NotI site of pOPI3CAT (Stratagene, La Jolla, CA). The vectors were electroporated into 32Dcl3 cells and single-cell clones were derived as described.3 Two independent 32D/Jak3 single-cell clones representing the 2 expression vectors, no. 1.1 (pOPI3Jak3) and no. 10.3 (pcDNA3Jak3), were selected for further analysis.
BM infection, selection, and expansion
Myeloblast-enriched BM cells were obtained from femurs of sodium caseinate–injected, 6- to 8-week-old wild-type C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME), which consisted primarily of cells of the myeloid lineage (95% ± 4%), with 33% ± 3% myeloid precursors at the myeloblast to promyelocyte stage. Murine stem cell virus (MSCV) retroviral vectors, packaged as helper-free infectious ecotropic retroviruses using Bosc23 packaging cells, were used to infect myeloblast-enriched BM cells. The pMSCV-Jak3/neo retroviral vector was constructed by inserting the entire open reading frame of Jak3 into theEcoRI site of the MSCV-neo plasmid. Sequence analysis was used to confirm the correct orientation of Jak3. Briefly, 10 μg pMSCV-neo and pMSCV-Jak3/neo was transfected into Bosc23 cells using the calcium phosphate–DNA precipitation method and after 48 hours the cells were treated with mitomycin C (10 μg/mL) for 3 hours. After repeated washings the cells were refed and BM was added (5 × 106 cells) in the presence of polybrene (8 μg/mL), 10% IL-3 (WEHI-3B–conditioned medium), and recombinant rat stem cell factor (SCF, 200 ng/mL; Amgen, Thousand Oaks, CA). The BM was cocultivated for 48 to 72 hours, and then seeded in methylcellulose (Stem Cell Technologies, Vancouver, BC, Canada) with or without G418 (650 μg/mL) to permit colony selection. G418-resistant colonies were transferred to liquid culture and induced to differentiate with murine recombinant GM-CSF (100 ng/mL; Amgen).
Flow cytometric analysis of Gr-1 expression
BM-neo or BM-Jak3/neo cells (1 × 106cells/sample) were harvested by centrifugation, washed in phosphate-buffered saline (PBS) with 1% FCS, and stained with phycoerythrin (PE)–conjugated anti–Gr-1 antibody (Pharmingen, San Diego, CA) on ice for 30 minutes. Background fluorescence was quantitated by the use of an isotype-matched (rat IgG2b), PE-conjugated antibody (Pharmingen). After washing twice, the cells were resuspended in 0.4 mL PBS and subjected to flow cytometric analysis using a Becton Dickinson FACScan system (Franklin Lakes, NJ).
Cycloheximide assays
32Dcl3 cells were treated with medium alone or with medium containing G-CSF in the presence or absence of cycloheximide (10 μg/mL). Total cellular RNA was extracted at 0, 2, 4, 6, 8, and 24 hours following cytokine treatment and Northern blot analyses were carried out as described.2
Actinomycin D assays
32Dcl3 cells were maintained in IL-3–free medium for 3 hours, after which the cells were divided into 3 batches. One batch was incubated in the presence of G-CSF and another in the presence of IL-3. The third batch was incubated in medium lacking IL-3 or G-CSF. Twelve hours later, actinomycin D (10 μg/mL) was added to each aliquot to prevent transcription. Cells were removed from each aliquot at 0, 2, 4, and 6 hours after actinomycin D addition and total RNA was extracted for Northern blot analysis.
Nuclear run-on transcription assays
Cells (2 × 107) were washed twice with ice-cold PBS and then lysed on ice with lysis buffer (0.5% NP-40; 10 mM Tris [tris(hydroxymethyl)aminomethane], pH 7.4, 10 mM NaCl, 3 mM MgCl2). The nuclear pellet was obtained by centrifugation at 2000 rpm at 4°C. Nuclear run-on transcription was performed at 37°C for 45 minutes in a final volume of 75 μL in reaction buffer containing 10% glycerol, 10 mM Tris, pH 8.0, 5 mM MgCl2,25 mM MnCl2, 150 mM KCl, 5 mM dithiothreitol (DTT), 1 mM nucleoside triphosphates (adenosine triphosphate, guanosine triphosphate, cytosine triphosphate), and 150 μCi (5.55 MBq)32P-rUTP. Termination of transcription was accomplished by the addition of 1 mL RNAzol reagent (Biotecx Laboratories, Houston, TX) followed by ethanol precipitation of the labeled RNA. The precipitated RNA was dissolved in 200 μL 100 mM Tris-HCl, pH 7.5, followed by addition of 200 μL 3.5 mM MgCl2, 1 mM DTT, 100 mM CaCl2, and 1.5 U RNase-free DNase I, and incubated at 30°C for 10 minutes. This was followed by phenol extraction (2 times) and ethanol precipitation of the RNA. Nytran membranes containing 5 μg linearized plasmid specific for full-length murine Jak3 or glucose-3-phosphate dehydrogenase reduced (G3PDH) were hybridized with 2 × 106 cpm nuclear run-on products in 1 mL hybridization solution (3 times standard sodium citrate [SSC], 5 times Denhardt solution, 1 mM sodium pyrophosphate, 20 mM sodium phosphate, pH 7.0, 1% sodium dodecyl sulfate [SDS], 100 μg/mL yeast tRNA, and 50% formamide) at 42°C for 36 hours. Hybridization strips were then washed twice in 2 times SSC (0.3 M NaCl and 0.03 M sodium citrate) at room temperature for 10 minutes each, washed once in 2 times SSC containing 10 μg/mL Rnase A at 37°C for 30 minutes followed by 2 washes in 0.2 times SSC containing 0.1% SDS at 42°C for 10 minutes each and finally one wash in 0.1 times SSC and 0.1% SDS at 42°C for 10 minutes. Strips were then exposed either to x-ray film at −80°C or Fuji illuminating screens at room temperature. When the Fuji screens were used, quantitation was performed using the Fuji Phosphorimager (Edison, NJ).
Immunoprecipitation and Western blot analysis
Immunoprecipitation experiments were carried out as described earlier using cell lysates.3 The antisera used were normal rabbit serum preimmune (PI), and anti-Jak3 (Upstate Biotechnology [UBI], Waltham, MA). The immunoprecipitates were separated on an 8% SDS–polyacrylamide gel electrophoresis (SDS-PAGE) and the proteins were transferred to a nitrocellulose membrane. The level of Jak3 protein was determined by probing the membrane with an anti-Jak3 antibody and visualized by an enhanced chemiluminescence (ECL) system (Amersham, Piscataway, NJ) and autoradiography. The blot was stripped and the presence of tyrosine phosphorylated proteins was detected by probing with the antiphosphotyrosine antibody, 4G10 (UBI, lower panel). For immunoblotting, an antiphosphotyrosine antibody was used at 1 μg/mL, and the anti-Jak3 serum was used at a dilution of 1:1000. Analysis of cell cycle–associated proteins was performed with 50 μg total cellular lysates as described above. Antibodies to p27Kip1, Stat3, pStat3, pStat5, c-myb, Cyclin E, Cdk2, Cdk4, and Cdk6 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Akt and pAkt antibodies were from New England Biolabs, (Beverly, MA).
Fluorescence-activated cell sorting analysis
For analysis of DNA content, cells were washed in PBS containing 1% fetal calf serum (FCS) and fixed by adding cold ethanol to a final concentration of 80%. Cells were stained by adding one-tenth volume of a 38-mM solution of propidium iodide and were analyzed by flow cytometry using an Epics Elite system (Coulter Electronics, Hialeah, FL).
Results
Jak3 is a primary response gene for G-CSF
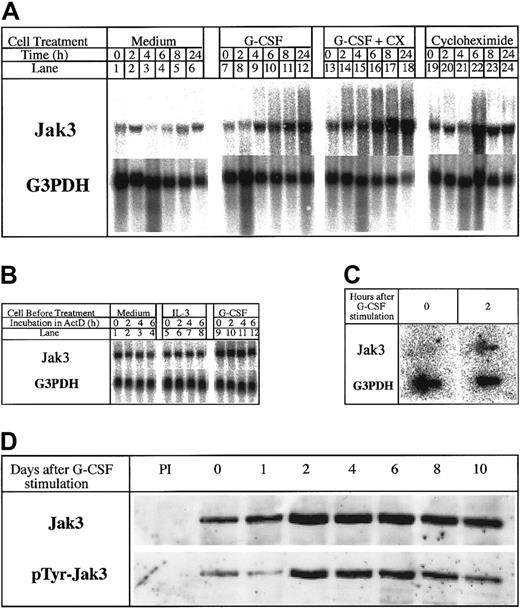
We and others had earlier reported the increased expression of Jak3 in response to a variety of cytokines in myeloid and lymphoid cells.2-9 Jak3 is also up-regulated during the terminal differentiation of 32Dcl3 cells in response to G-CSF.2Because primary response genes link the extracellular activation signals initiated by cytokines to the expression of genes encoding proteins that dictate stimulus-specific functional responses,20-23 we were interested to know whether the up-regulation of Jak3 constituted a primary response to G-CSF stimulation and whether the accumulation of the Jak3 in G-CSF–stimulated 32Dcl3 cells signals the onset of terminal differentiation. To determine whether Jak3 is a primary response gene for G-CSF, we performed Northern blot analysis of total RNA isolated from 32Dcl3 cells stimulated with G-CSF in the presence and absence of cycloheximide for various periods of time ranging from 0 to 24 hours. The results presented in Figure1A show that Jak3 RNA expression was up-regulated within 4 hours after stimulation with G-CSF and that these levels increased by 8- to 10-fold by 24 hours (Figure 1A, lanes 7-12). Cycloheximide failed to inhibit the increase in the levels of Jak3 mRNA and, in fact, superinduction of the message was observed in the presence of G-CSF and cycloheximide (Figure 1A, lanes 13-18). These results indicate that the induction of Jak3 mRNA synthesis by G-CSF does not require new protein synthesis and the observed accumulation of Jak3 mRNA might result either from stabilization of Jak3 mRNA or the induction of new RNA synthesis as a direct consequence of G-CSF interaction with its receptor.
Jak3 is a primary response gene for G-CSF.
(A) 32Dcl3 cells were treated with medium alone (lanes 1-6) and medium containing G-CSF in the absence or presence of cycloheximide (10 μg/mL; lanes 7-18). Total cellular RNA was extracted at 0, 2, 4, 6, 8, and 24 hours following cytokine treatment and 20 μg RNA was used for Northern analysis. The level of expression of Jak3 was determined by hybridization to a 32P-labeled Jak3 probe (upper panel). To control for the amount of RNA loaded, the membrane was stripped and reprobed with a 32P-labeled G3PDH probe (lower panel). (B) Cells were plated in IL-3–free medium (lanes 1-4) or in medium containing IL-3 (lanes 5-8) or G-CSF (lanes 9-12) for 12 hours. Actinomycin D was then added to the cultures and incubation was further continued for the indicated hours. RNA (20 μg) from each sample was subjected to Northern blot analysis. (C) 32Dcl3 cells were stimulated with G-CSF for 2 hours. Cells were pelleted and the labeled RNA was subjected to nuclear run on assays as described in “Materials and methods.” (D) Expression of Jak3 protein in G-CSF–stimulated 32Dcl3 cells. Cells were harvested at the indicated time (days) after exposure to G-CSF and 1.2 mg of the cell lysates was used for immunoprecipitation. The level of Jak3 protein was determined by probing the membrane with an anti-Jak3 antibody (upper panel) and presence of tyrosine phosphorylated proteins was detected by probing with the antiphosphotyrosine antibody, 4G10 (UBI, lower panel).
Jak3 is a primary response gene for G-CSF.
(A) 32Dcl3 cells were treated with medium alone (lanes 1-6) and medium containing G-CSF in the absence or presence of cycloheximide (10 μg/mL; lanes 7-18). Total cellular RNA was extracted at 0, 2, 4, 6, 8, and 24 hours following cytokine treatment and 20 μg RNA was used for Northern analysis. The level of expression of Jak3 was determined by hybridization to a 32P-labeled Jak3 probe (upper panel). To control for the amount of RNA loaded, the membrane was stripped and reprobed with a 32P-labeled G3PDH probe (lower panel). (B) Cells were plated in IL-3–free medium (lanes 1-4) or in medium containing IL-3 (lanes 5-8) or G-CSF (lanes 9-12) for 12 hours. Actinomycin D was then added to the cultures and incubation was further continued for the indicated hours. RNA (20 μg) from each sample was subjected to Northern blot analysis. (C) 32Dcl3 cells were stimulated with G-CSF for 2 hours. Cells were pelleted and the labeled RNA was subjected to nuclear run on assays as described in “Materials and methods.” (D) Expression of Jak3 protein in G-CSF–stimulated 32Dcl3 cells. Cells were harvested at the indicated time (days) after exposure to G-CSF and 1.2 mg of the cell lysates was used for immunoprecipitation. The level of Jak3 protein was determined by probing the membrane with an anti-Jak3 antibody (upper panel) and presence of tyrosine phosphorylated proteins was detected by probing with the antiphosphotyrosine antibody, 4G10 (UBI, lower panel).
To determine whether the observed accumulation of Jak3 mRNA is due to its stabilization, we conducted actinomycin D chase experiments using an actinomycin D concentration of 10 μg/mL. At this concentration of actinomycin D, 32Dcl3 cells remained viable for 6 to 8 hours but did not synthesize any RNA as shown by the absence of labeled uridine incorporation.21 After washing 32Dcl3 cells in IL-3–free medium, we divided the cells into 3 aliquots, one of which was incubated in a medium without any cytokines; the second batch of cells was incubated in a medium containing IL-3; and the third batch was incubated in a medium containing G-CSF. Actinomycin D was then added to the cultures and aliquots of cells were removed at 0, 2, 4, and 6 hours and used for RNA extraction and Northern blot analysis. Results presented in Figure 1B show that Jak3 mRNA has a long half-life in unstimulated (Figure 1B, lanes 1-4) and IL-3 (Figure 1B, lanes 5-8) or G-CSF–stimulated cells, showing minimal decrease in message level over 6 hours in the presence of actinomycin D. No alteration in Jak3 mRNA half-life was apparent after G-CSF exposure, even though one could readily observe an increase in the levels of Jak3 mRNA. We could not extend the incubation beyond a 6-hour period due to a rapid decrease in the viability of cells beyond this time point, due to the toxic effects of actinomycin D. Therefore, we conclude that the observed up-regulation of Jak3 in 32Dcl3 cells in response to G-CSF addition is not due to stabilization of mRNA.
To determine whether the observed up-regulation of Jak3 mRNA in response to G-CSF was due to a direct increase in transcription, we performed nuclear run-on transcription assays using control 32Dcl3 cells and 32Dcl3 cells incubated in the presence of G-CSF (for 2 hours). Following a 2-hour incubation in the presence or absence of G-CSF, nuclei were prepared and incubated in transcription buffer containing 32P-rUTP for 45 minutes to label newly synthesized RNA. Following the termination of transcription, labeled RNA was extracted and used for hybridization with Jak3 or G3PDH (control) DNA immobilized on a Nytran filter. Results from this experiment show that very low levels of Jak3 transcripts were made in unstimulated 32Dcl3 cells, but a 10-fold increase in the levels of Jak3 transcripts could be seen within 2 hours after G-CSF stimulation (Figure 1C). On the other hand, the levels of G3PDH RNA were identical in unstimulated and stimulated cells, suggesting that the increased levels of Jak3 mRNA seen following G-CSF stimulation are indeed due to the direct increase in the levels of transcription of Jak3. Taken together, these results show that Jak3 is a primary response gene for G-CSF.
Our observation that Jak3 is a primary response gene for G-CSF prompted us to study the protein profile and tyrosine phosphorylation status of Jak3 in G-CSF–stimulated 32Dcl3 cells for 10 days using anti-Jak3 antibodies. Results of this experiment, shown in Figure 1D, indicate that whereas low levels of Jak3 protein are observed in unstimulated 32Dcl3 cells, Jak3 protein expression is up-regulated in response to the G-CSF stimulus, which coincides with the levels of Jak3 mRNA seen in these cells.2 Maximal levels of Jak3 protein were observed between day 2 and day 6 following the addition of G-CSF and these high levels of protein were maintained until day 10 (Figure 1D, upper panel). Accumulation of tyrosine phosphorylated Jak3 protein was also observed between day 2 and day 6 when 32Dcl3 cells begin to enter the differentiation program (Figure1D, lower panel). These observations suggested that accumulation of phosphorylated Jak3 coincides with the onset of the differentiation program in 32Dcl3 cells treated with G-CSF.
Effect of ectopic overexpression of Jak3 on myeloid cell proliferation and differentiation
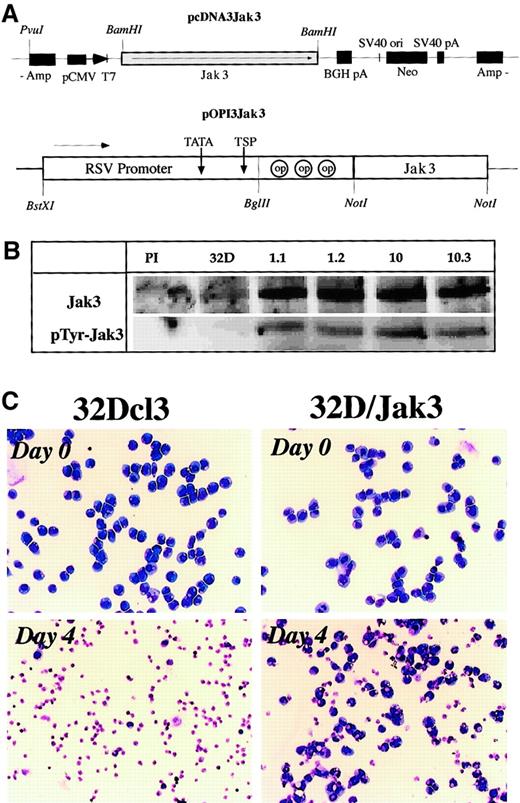
To study the role of the Jak3 pathway in myeloid differentiation, we generated 2 Jak3 expression vectors (Figure2A) where the Jak3 cDNA was placed under the control of either the CMV promoter (pcDNA3Jak3) or the Rous-sarcoma virus–long terminal repeat (RSV-LTR) promoter (pOPI3Jak3). This DNA was electroporated into 32Dcl3 cells and 10 drug-resistant single-cell clones for each expression vector were analyzed for the expression of Jak3 by Northern blot and Western blot analyses. All of the clones analyzed were found to express high levels of Jak3 compared to parental control 32Dcl3 cells (Figure 2B). Interestingly, 32D/Jak3 cells contained high levels of the tyrosine phosphorylated form of Jak3 even in the absence of G-CSF stimulation. These observations are consistent with earlier studies where constitutive phosphorylation of Jak3 was observed when this protein was overexpressed in mammalian cells.24 Two independent single-cell clones derived from the 2 expression vectors, no. 1.1 (pOPI3Jak3) and no. 10.3 (pcDNA3Jak3) were selected for further analysis. All of the studies described below were performed with these 2 single-cell clones (which were designated as 32D/Jak3 cell lines 1.1 and 10.3) and identical results were obtained with both clones.
Generation of 32D/Jak3 cells.
(A) Expression vectors used in the generation of 32D/Jak3 cells. Two independent 32D/Jak3 single-cell clones representing the 2 expression vectors, no. 10.3 (pcDNA3Jak3) and no. 1.1 (pOPI3Jak3) were selected for further analysis. (B) Overexpression of Jak3 in 32D/Jak3 cells. Parental 32Dcl3 and 32D/Jak3 (clone nos. 1.1, 1.2, 10, 10.3) cell lysate was used for immunoprecipitation with either normal rabbit serum (PI) or an anti-Jak3 antibody. The immunoprecipitates were immunoblotted with anti-Jak3 (upper panel) and 4G10 (lower panel) antibodies. (C) Increased cell survival in 32D/Jak3 cells under conditions of IL-3 deprivation. Cells were washed free of IL-3 and plated in medium without IL-3 for 4 days and subjected to morphologic analysis on staining with May-Grünwald-Giemsa staining. Original magnification × 10.
Generation of 32D/Jak3 cells.
(A) Expression vectors used in the generation of 32D/Jak3 cells. Two independent 32D/Jak3 single-cell clones representing the 2 expression vectors, no. 10.3 (pcDNA3Jak3) and no. 1.1 (pOPI3Jak3) were selected for further analysis. (B) Overexpression of Jak3 in 32D/Jak3 cells. Parental 32Dcl3 and 32D/Jak3 (clone nos. 1.1, 1.2, 10, 10.3) cell lysate was used for immunoprecipitation with either normal rabbit serum (PI) or an anti-Jak3 antibody. The immunoprecipitates were immunoblotted with anti-Jak3 (upper panel) and 4G10 (lower panel) antibodies. (C) Increased cell survival in 32D/Jak3 cells under conditions of IL-3 deprivation. Cells were washed free of IL-3 and plated in medium without IL-3 for 4 days and subjected to morphologic analysis on staining with May-Grünwald-Giemsa staining. Original magnification × 10.
To analyze the effects of Jak3 overexpression on IL-3 dependence we conducted IL-3 withdrawal experiments. To this end, 32Dcl3 and 32D/Jak3 cells were cultured in complete medium with or without IL-3 for 4 days and were analyzed for cell viability using May-Grünwald and Giemsa staining. 32Dcl3 cells (which are strictly dependent on the presence of IL-3 for survival) underwent apoptosis and by 48 hours less than 5% viable cells could be observed. In contrast, IL-3–deprived 32D/Jak3 cells sustained the IL-3 withdrawal better and approximately 50% of 32D/Jak3 cells retained viability after a period of 48 hours of culture in the absence of IL-3. Moreover, approximately 25% to 30% of 32D/Jak3 cells retained viability after 96 hours of culturing in the absence of IL-3, indicative of a Jak3-mediated survival stimulus in 32D/Jak3 cells. Morphologic analysis revealed that the majority of the 32D/Jak3 cells that survived the IL-3 withdrawal were immature myeloid cells, indicating that overexpression of Jak3 alone was insufficient to induce granulocytic differentiation (Figure 2C).
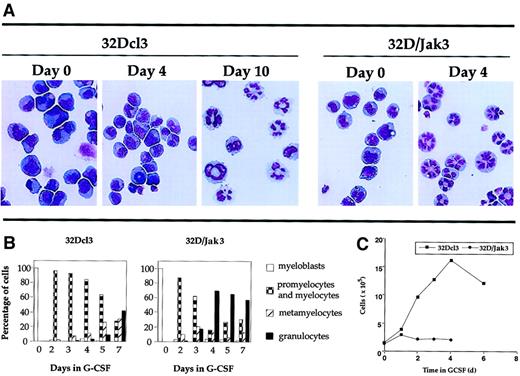
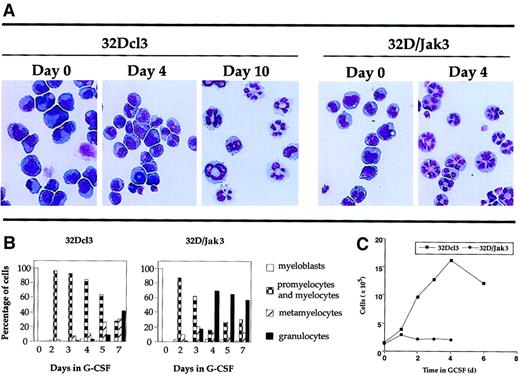
A more dramatic effect was seen when these cells were incubated in medium containing G-CSF. Analysis of the granulocytic differentiation of parental 32Dcl3 and 32D/Jak3 cells was monitored by May-Grünwald and Giemsa staining. The results of this analysis are shown in Figure 3A and are represented in a graphic form in Figure 3B. These results indicate that 32D/Jak3 cells, when incubated in G-CSF, differentiate into morphologically normal metamyelocytes and granulocytes in 3 to 4 days, a process that otherwise requires 10 days for the parental cell line. These results demonstrate that ectopic overexpression of Jak3 in 32Dcl3 cells induces growth arrest and accelerates the differentiation program along the granulocytic pathway in the presence of G-CSF. A comparison of the growth profiles of 32D/Jak3 cells and the parental 32Dcl3 cells is shown in Figure 3C. In this experiment, 32Dcl3 cells or 32D/Jak3 cells were washed free of IL-3 and plated at a density of 1 × 105/mL in the presence of medium containing G-CSF. Aliquots of cells were removed at various intervals after G-CSF treatment and counted after staining with trypan blue. 32D/Jak3 cells ceased to proliferate in the presence of G-CSF and it appeared that only cells that progressed beyond the G1 phase of the cell cycle (and thus committed to divide) completed their division cycle and failed to enter into a new round of cell division cycle. This observation was in contrast to that seen in control 32Dcl3 cells, which continue to proliferate until day 5 or day 6 after G-CSF stimulation. These data suggest that 32D/Jak3 cells undergo immediate growth arrest on G-CSF treatment.
Acceleration of differentiation in G-CSF–stimulated 32D/Jak3 cells.
(A) 32Dcl3 cells or 32D/Jak3 no. 10.3 cells were washed free of IL-3 and plated in the presence of G-CSF. Aliquots of the cell cultures were cytocentrifuged at the indicated days after treatment with G-CSF. The progression of granulocytic differentiation was monitored by May-Grünwald and Giemsa staining. Original magnification × 20. (B) Aliquots of the cell cultures were cytocentrifuged at the indicated days after treatment with G-CSF. The distribution of cells at different stages of granulocyte differentiation was determined by counting at least 100 cells in a field. (C) Cessation of proliferation followed by acceleration of granulocytic differentiation of 32D/Jak3 cells in response to G-CSF. 32Dcl3 cells or 32D/Jak3 no. 10.3 cells were washed free of IL-3 and plated in the presence of G-CSF. Cell viability was monitored using trypan blue staining.
Acceleration of differentiation in G-CSF–stimulated 32D/Jak3 cells.
(A) 32Dcl3 cells or 32D/Jak3 no. 10.3 cells were washed free of IL-3 and plated in the presence of G-CSF. Aliquots of the cell cultures were cytocentrifuged at the indicated days after treatment with G-CSF. The progression of granulocytic differentiation was monitored by May-Grünwald and Giemsa staining. Original magnification × 20. (B) Aliquots of the cell cultures were cytocentrifuged at the indicated days after treatment with G-CSF. The distribution of cells at different stages of granulocyte differentiation was determined by counting at least 100 cells in a field. (C) Cessation of proliferation followed by acceleration of granulocytic differentiation of 32D/Jak3 cells in response to G-CSF. 32Dcl3 cells or 32D/Jak3 no. 10.3 cells were washed free of IL-3 and plated in the presence of G-CSF. Cell viability was monitored using trypan blue staining.
32D/Jak3 cells undergo cell cycle arrest in the G1phase immediately after G-CSF stimulation
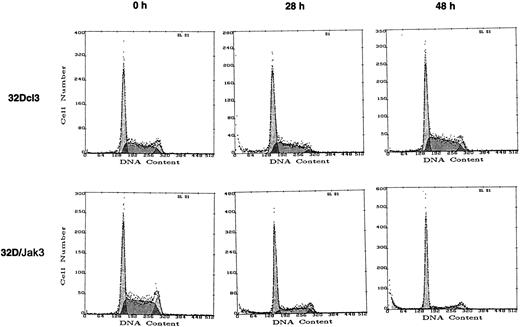
To understand the molecular basis for the observed growth arrest and accelerated differentiation of 32D/Jak3 cells in the presence of G-CSF, we performed cell cycle analysis with normal and 32D/Jak3 cells following the addition of G-CSF. Control 32Dcl3 cells and 32D/Jak3 cells were incubated for 0, 6, 12, 24, 30, and 48 hours in G-CSF and the cells were analyzed by FACS after propidium iodide staining25 to determine the proportion of cells in the G1, S, and G2/M phases of the cell cycle. The cell cycle distribution of 32Dcl3 and 32D/Jak3 cells growing in the presence of IL-3 was indistinguishable (data not shown). In normal 32Dcl3 cells, no accumulation at any phase of the cell cycle was apparent in 48 hours following the addition of G-CSF. However, G-CSF stimulation of 32D/Jak3 cells resulted in an accumulation of cells with a G1 DNA content as early as 24 hours (Figure4 and Table1) and by 48 hours, 80% of cells were found to contain a G1 DNA content. These observations indicated that the acceleration of granulocytic differentiation of Jak3 overexpressing 32Dcl3 cells in response to the G-CSF stimulus was preceded by cell cycle arrest in the G1 phase of the cell cycle. These results strongly suggest that accumulation of Jak3 in myeloid cells mediates a G1 arrest in response to G-CSF stimulation.
32D/Jak3 cells undergo G1 arrest within 24 hours after stimulation with G-CSF.
32Dcl3 cells or 32D/Jak3 no. 10.3 cells were washed free of IL-3 and plated at a density of 1 × 105/mL in the presence of G-CSF. Aliquots of cell were removed at the indicated time (hours) after G-CSF treatment and subjected to FACS analysis.
32D/Jak3 cells undergo G1 arrest within 24 hours after stimulation with G-CSF.
32Dcl3 cells or 32D/Jak3 no. 10.3 cells were washed free of IL-3 and plated at a density of 1 × 105/mL in the presence of G-CSF. Aliquots of cell were removed at the indicated time (hours) after G-CSF treatment and subjected to FACS analysis.
Down-regulation of phosphorylated forms of Akt/PKB and Stat3 proteins in G-CSF–stimulated 32D/Jak3 cells
To understand the molecular mechanisms associated with the Jak3-induced G1 arrest in 32D/Jak3 cells, we performed a detailed analysis of proteins that determine the proliferation capacity and cell cycle progression of myeloid cells. Myeloid cell proliferation in response to cytokines such as IL-3 and G-CSF is regulated by the PI3K/Akt and Stat3 pathways.26 To determine the role of the PI3K/Akt and Stat3 pathways in determining the fate of 32D/Jak3 cells in response to G-CSF, we have examined the levels of phosphorylated forms of Akt and Stat3 proteins in normal 32Dcl3 and 32D/Jak3 cells. G-CSF stimulation of parental 32Dcl3 cells leads to a robust activation of both Stat3 and Akt/PKB proteins via phosphorylation. Within 12 hours of G-CSF stimulation of 32Dcl3 cells, we observed rapid induction of phospho-Stat3 and phospho-Akt proteins. Levels of phospho-Stat3 accumulated as 32Dcl3 cells underwent initial rounds of proliferation and the levels of phospho-Stat3 decreased thereafter coinciding with the growth arrest of cells (Figure5A). In contrast, we observed no up-regulation of phospho-Stat3 protein levels in G-CSF–stimulated 32D/Jak3 cells despite the presence of high levels of Stat3 protein indicative of a block to Stat3 activation in these cells in response to G-CSF. No down-regulation of other myeloid proliferation specific genes, phospho-Stat5 or c-myb, was seen indicating that the observed effects were specific for Stat3 (Figure 5B-C). Similarly, we observed decreased levels of phospho-Akt/PKB protein levels in G-CSF–stimulated 32D/Jak3 cells in comparison to parental 32Dcl3 cells stimulated with G-CSF, indicative of an absence of the Akt pathway activation (Figure 5D). Taken together, these data suggest that the block in myeloid proliferation observed in 32D/Jak3 cells in response to G-CSF may be, at least in part, mediated by inactivation of the Stat3 and PI3K/Akt pathways.
Akt and Stat3 pathways are inactivated in 32D/Jak3 cells stimulated by G-CSF.
32Dcl3 and 32D/Jak3 cells were washed free of IL-3 and plated in IMDM in the presence of G-CSF. Cells were harvested at the indicated time (days) after exposure to G-CSF and lysed in lysis buffer containing 0.5% Triton X-100. Then, 50 μg of the total cell lysates was used for immunoblotting with (A) phospho-Stat3 and Stat3, (B) phospho-Stat5, (C) c-myb, and (D) phospho-Akt and Akt.
Akt and Stat3 pathways are inactivated in 32D/Jak3 cells stimulated by G-CSF.
32Dcl3 and 32D/Jak3 cells were washed free of IL-3 and plated in IMDM in the presence of G-CSF. Cells were harvested at the indicated time (days) after exposure to G-CSF and lysed in lysis buffer containing 0.5% Triton X-100. Then, 50 μg of the total cell lysates was used for immunoblotting with (A) phospho-Stat3 and Stat3, (B) phospho-Stat5, (C) c-myb, and (D) phospho-Akt and Akt.
p27Kip1 protein levels are up-regulated in G1-arrested 32D/Jak3 cells
The cell cycle is regulated by extracellular and intracellular signals that eventually target cyclin/Cdk complexes.27,28These complexes are regulated at various levels, including expression and phosphorylation of subunits and binding to a family of Cdk inhibitors termed CKIs. Accumulating evidence indicates that some of these inhibitors respond to differentiation signals. Consistent with this theme, p21Cip1 is induced during the differentiation of muscle and several hematopoietic cells.29-33 It also appears that other CKIs, especially p27Kip1 and p57Kip2, may be similarly regulated during differentiation in a cell type–dependent manner.34-36 Our observation that Jak3 overexpression leads to growth arrest with a G1DNA content followed by acceleration of granulocytic differentiation warranted an analysis of components of the cell cycle machinery. We hypothesized that G1 cyclin/Cdk holoenzymes could be downstream targets of the G-CSF signals mediated by Jak3. In such a scenario, a Jak3-mediated imbalance between CKI and cyclin/Cdk holoenzymes could explain the G1 arrest and cell cycle exit followed by the acceleration of terminal differentiation.
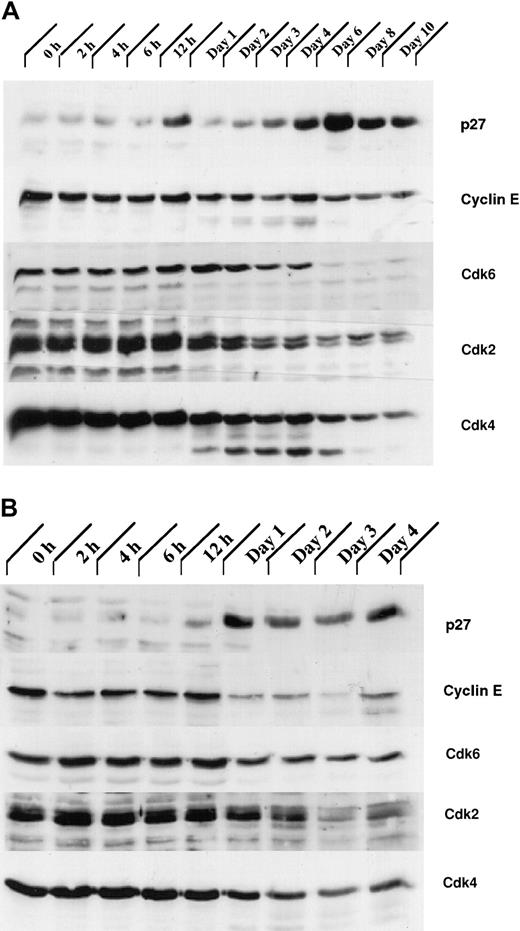
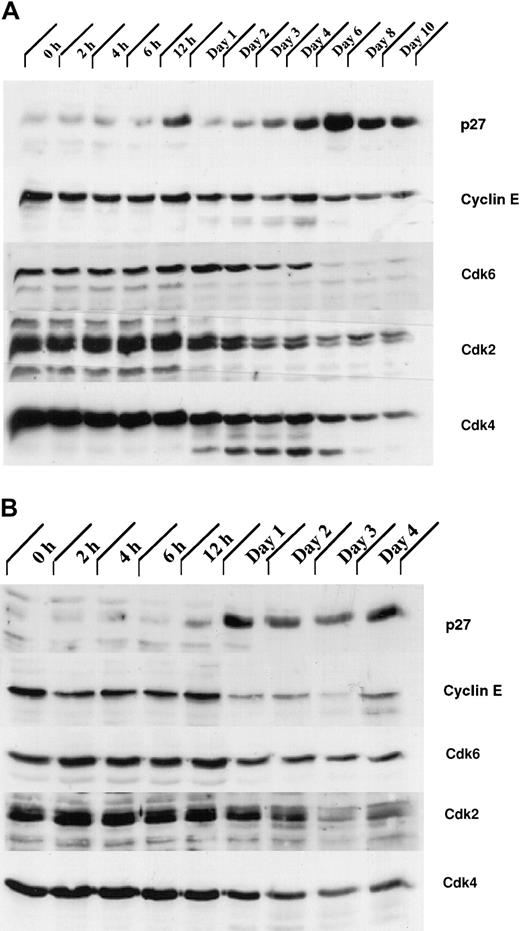
Our analysis of the expression patterns of several CKIs revealed that the expression of one of them, p27Kip1, was up-regulated during granulocytic differentiation. Total cellular proteins from G-CSF– stimulated control 32Dcl3 and 32D/Jak3 cells were subjected to immunoblotting with antibodies against p27Kip1. Expression of p27Kip1 was induced between day 4 and day 6 in normal 32Dcl3 cells, a period that coincides with the growth arrest of these cells (Figure 6A). Interestingly, induction of p27Kip1 in 32D/Jak3 cells was observed between 12 and 24 hours after G-CSF stimulation with levels of p27Kip1 peaking as early as 24 hours, a time point that is much earlier than the parental cell line (Figure 6B). These observations suggest that high-level expression of the Jak3 protein is required to induce p27Kip1 accumulation (Figures 1 and 6A). The highest expression of p27Kip1, on day 1 in 32D/Jak3 cells, correlated well with the time at which these cells undergo growth arrest with a G1 DNA content (Figure 4 and Table 1). These observations therefore implicate p27Kip1 as the CKI that mediates the G1 arrest in 32D/Jak3 cells.
The expression of proteins involved in regulation of the cell cycle is altered in 32D/Jak3 cells stimulated by G-CSF.
(A) 32Dcl3 and (B) 32D/Jak3 cells were washed free of IL-3 and plated in IMDM in the presence of G-CSF. Cells were harvested at the indicated time (days) after exposure to G-CSF and lysed in lysis buffer containing 0.5% Triton X-100. Then, 50 μg of the total cell lysate was used for immunoblotting with p27Kip1, Cyclin E, Cdk6, Cdk2, and Cdk4 antibodies. Immunoblots were visualized by ECL (Amersham) followed by autoradiography.
The expression of proteins involved in regulation of the cell cycle is altered in 32D/Jak3 cells stimulated by G-CSF.
(A) 32Dcl3 and (B) 32D/Jak3 cells were washed free of IL-3 and plated in IMDM in the presence of G-CSF. Cells were harvested at the indicated time (days) after exposure to G-CSF and lysed in lysis buffer containing 0.5% Triton X-100. Then, 50 μg of the total cell lysate was used for immunoblotting with p27Kip1, Cyclin E, Cdk6, Cdk2, and Cdk4 antibodies. Immunoblots were visualized by ECL (Amersham) followed by autoradiography.
Levels of Cyclin E and Cdks 2, 4, and 6 are down-regulated in G1-arrested 32D/Jak3 cells
Initially, p27Kip1 was identified as an inhibitor of the Cyclin E/Cdk2 complex in transforming growth factor β (TGFβ)–inhibited or contact-inhibited Mv1Lu mink cells.37,38 In Mv1Lu cells, p27Kip1, cooperating with the Ink4 CKI p15, appears to contribute to TGF-β–mediated G1 arrest by binding to the Cyclin E-Cdk2 complex and inhibiting its kinase activity.39 We therefore analyzed the expression of Cyclin E and Cdks 2, 4, and 6 in 32Dcl3 and 32D/Jak3 cells. We observed a rapid decrease in the expression of the Cdks 2, 4, and 6 following the onset of terminal differentiation in 32Dcl3 cells (Figure 6A). Also, in normal 32Dcl3 cells treated with G-CSF, high levels of Cyclin E were observed until day 6, which decreased thereafter (Figure 6A). In contrast, Cyclin E levels were dramatically down-regulated in 32D/Jak3 cells between the 12- and 24-hour time points, coinciding with the accumulation of p27Kip1 in these cells (Figure 6B). In addition, a decrease in the expression of the Cdk6 was seen in 32D/Jak3 cells by 24 hours and a similar decrease in the expression of Cdk2 and Cdk4 was observed by 48 hours. Taken together, these results suggest that the G1 arrest and the cell cycle exit in the Jak3 overexpressing 32Dcl3 cells is due to the rapid up-regulation of p27Kip1 and down-regulation of Cyclin E as well as the Cdks 2, 4, and 6.
Ectopic expression of Jak3 in normal BM cells induced to differentiate in response to GM-CSF promotes cell cycle arrest and accelerates granulocytic differentiation
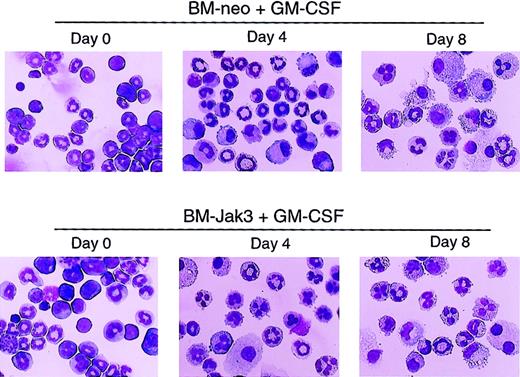
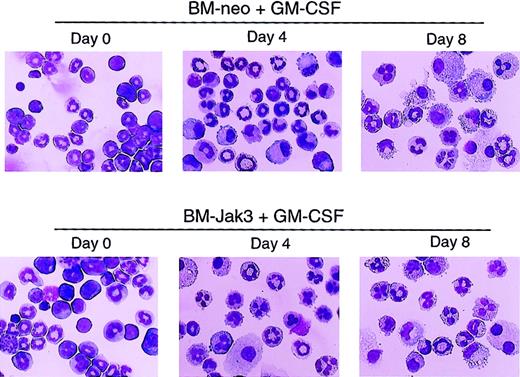
To assess the effect of deregulated Jak3 expression on primary myeloid cells derived from normal mouse BM, myeloblast-enriched BM cells from wild-type (C57BL/6) mice were prepared and infected with retroviral vectors pMSCV-Jak3/neo and pMSCV-neo, as described in “Materials and methods.” Briefly, infected cells were seeded in methylcellulose in the presence of G418 to allow for the selection of G418-resistant colonies. Several colonies from each infection were expanded, transferred to liquid culture, and induced for differentiation with either G-CSF or GM-CSF. At the indicated time points, the cultures were analyzed for cell morphology, cell cycle progression, and cell surface expression of Gr-1, an indicator of granulocytic differentiation.40 As with 32Dcl3 cells treated with G-CSF, ectopic expression of Jak3 in BM cells induced for differentiation with GM-CSF promoted cell cycle arrest and accelerated the differentiation program relative to identically treated control BM-neo cells. This was evident from the mature cell morphology (Figure7), significantly increased Gr-1 expression (Figure 8), and accumulation of BM-Jak3 cells in the G0/G1 phase of the cell cycle by day 4 following induction with GM-CSF (Figure9). In contrast, it took BM-neo cells twice as long (8 days) to achieve a comparable state of growth arrest and differentiation after treatment with GM-CSF. We made similar observations in G-CSF–stimulated BM-Jak3 and BM-neo cells, but the phenotype of accelerated cell cycle arrest and early increase in Gr-1 expression was more readily observed in GM-CSF–stimulated cells. This is not surprising, because there is a much shorter window of time in which to observe accelerated differentiation in G-CSF since G-CSF–stimulated mouse bone marrow cells terminally differentiate in 4 days, whereas GM-CSF–stimulated mouse bone marrow cells terminally differentiate in 8 to 10 days.
Acceleration of morphologic differentiation in GM-CSF–stimulated BM-Jak3 cells.
Myeloblast-enriched BM cells from wild-type (C57/BL6) mice were infected with the retroviral vectors pMSCV-Jak3/neo or pMSCV-neo. Cells were seeded in methylcellulose and selected in the presence of G418. Several colonies from each infection were expanded, transferred to liquid culture, and induced for differentiation with GM-CSF. Cells were cytocentrifuged at the indicated time points and progression of differentiation was monitored by May-Grünwald and Giemsa staining. Original magnification × 20.
Acceleration of morphologic differentiation in GM-CSF–stimulated BM-Jak3 cells.
Myeloblast-enriched BM cells from wild-type (C57/BL6) mice were infected with the retroviral vectors pMSCV-Jak3/neo or pMSCV-neo. Cells were seeded in methylcellulose and selected in the presence of G418. Several colonies from each infection were expanded, transferred to liquid culture, and induced for differentiation with GM-CSF. Cells were cytocentrifuged at the indicated time points and progression of differentiation was monitored by May-Grünwald and Giemsa staining. Original magnification × 20.
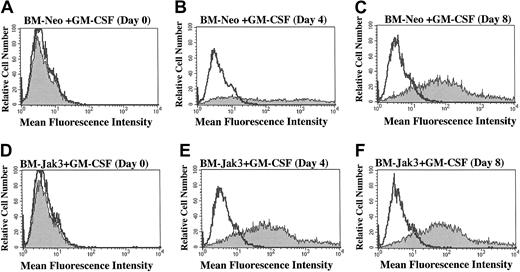
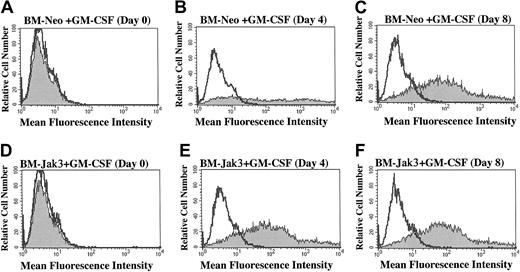
BM-Jak3 cells display accelerated induction of Gr-1 expression following stimulation with GM-CSF.
Myeloblast-enriched BM cells from wild-type (C57/BL6) mice were infected with the retroviral vectors pMSCV-neo (A-C) or pMSCV-Jak3/neo (D-F). Cells were seeded in methylcellulose and selected in the presence of G418. Several colonies from each infection were expanded, transferred to liquid culture, and induced for differentiation with GM-CSF. At the indicated time points, cells were harvested by centrifugation, washed in PBS with 1% FCS, and stained with PE-conjugated anti–Gr-1 antibody. Following staining, the cells were subjected to flow cytometric analysis to determine degree of Gr-1 expression. Gr-1 expression is absent in day 0 BM-Neo (A) and BM-Jak3 (D) cells. In contrast, increased Gr-1 expression is observed in day 4 BM-Jak3 cells (E) compared to BM-Neo cells (B). Similar expression levels are observed in day 8 BM-Neo (C) or BM-Jak3 (F) cells.
BM-Jak3 cells display accelerated induction of Gr-1 expression following stimulation with GM-CSF.
Myeloblast-enriched BM cells from wild-type (C57/BL6) mice were infected with the retroviral vectors pMSCV-neo (A-C) or pMSCV-Jak3/neo (D-F). Cells were seeded in methylcellulose and selected in the presence of G418. Several colonies from each infection were expanded, transferred to liquid culture, and induced for differentiation with GM-CSF. At the indicated time points, cells were harvested by centrifugation, washed in PBS with 1% FCS, and stained with PE-conjugated anti–Gr-1 antibody. Following staining, the cells were subjected to flow cytometric analysis to determine degree of Gr-1 expression. Gr-1 expression is absent in day 0 BM-Neo (A) and BM-Jak3 (D) cells. In contrast, increased Gr-1 expression is observed in day 4 BM-Jak3 cells (E) compared to BM-Neo cells (B). Similar expression levels are observed in day 8 BM-Neo (C) or BM-Jak3 (F) cells.
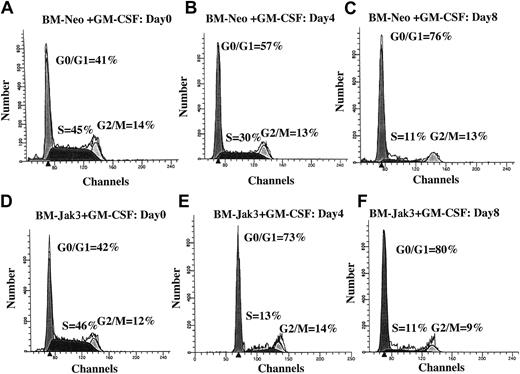
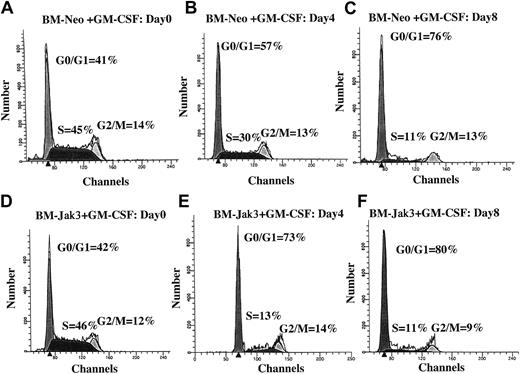
Normal mouse BM cells retrovirally infected with Jak3 (BM-Jak3 cells) undergo accelerated G1 arrest following stimulation with GM-CSF.
Myeloblast-enriched BM cells from wild- type (C57/BL6) mice were infected with the retroviral vectors pMSCV-neo (A-C) or pMSCV-Jak3/neo (D-F). Cells were seeded in methylcellulose and selected in the presence of G418. Several colonies from each infection were expanded, transferred to liquid culture, and induced for differentiation with GM-CSF. At the indicated time points, aliquots of the cells were removed for FACS analysis. The cell cycle distribution of day 0 BM-Neo (A) and BM-Jak3 (D) cells is undistinguishable. In contrast to day 4 BM-Neo cells that showed no accumulation in G1 phase (B), BM-Jak3 cells (E) accumulated in G1 phase. By day 8, both BM-Neo (C) and BM-Jak3 (F) cells showed equivalent degree of G1 arrest.
Normal mouse BM cells retrovirally infected with Jak3 (BM-Jak3 cells) undergo accelerated G1 arrest following stimulation with GM-CSF.
Myeloblast-enriched BM cells from wild- type (C57/BL6) mice were infected with the retroviral vectors pMSCV-neo (A-C) or pMSCV-Jak3/neo (D-F). Cells were seeded in methylcellulose and selected in the presence of G418. Several colonies from each infection were expanded, transferred to liquid culture, and induced for differentiation with GM-CSF. At the indicated time points, aliquots of the cells were removed for FACS analysis. The cell cycle distribution of day 0 BM-Neo (A) and BM-Jak3 (D) cells is undistinguishable. In contrast to day 4 BM-Neo cells that showed no accumulation in G1 phase (B), BM-Jak3 cells (E) accumulated in G1 phase. By day 8, both BM-Neo (C) and BM-Jak3 (F) cells showed equivalent degree of G1 arrest.
Discussion
It is now well established that myeloid precursor cells, when exposed to G-CSF or GM-CSF, proliferate for a brief period of time (2-6 days) followed by growth arrest and terminal differentiation. Interestingly, both the proliferative and growth arrest/differentiation phases of this process seem to require G-CSF or GM-CSF because withdrawal of these cytokines at any stage of this process leads to cell death. The mechanisms by which a cytokine such as G-CSF can induce both the initial rounds of proliferation and the subsequent growth arrest that is observed during the course of myeloid differentiation are not yet clear. We had earlier made the observation that Jak3 protein expression is elevated following stimulation with G-CSF during the granulocytic differentiation of the murine myeloid cell line 32Dcl3.2 In this report, we present evidence to show that Jak3 plays an important role in signal transduction pathways that are associated with myeloid cell differentiation, in addition to the well-characterized role of Jak3 in lymphoid development.
The results presented here show that Jak3 is a primary response gene to G-CSF because the induction of Jak3 mRNA does not require new protein synthesis and the half-life of Jak3 mRNA is not altered on G-CSF stimulation. It is already known that interaction of G-CSF receptor with G-CSF results in the activation of Jak-1 and Jak-2. In addition to the activation of these Jaks, this interaction also leads to the phosphorylation of Stat3.1 41 On phosphorylation, Stat3 along with other associated Stats translocates to the nucleus and initiates transcription of its target genes, which in turn bring about the phenotypic effects induced by this cytokine. It is therefore possible that Jak3 is induced by a group of Stats (such as Stat3), which are activated as a result of G-CSF interaction with its receptor. If this were to be true, this could be the first example of a Jak kinase that is regulated by a Jak/Stat signaling pathway distinct from its own.
Our results also show that Jak3 protein levels in 32Dcl3 cells reach a peak between days 4 and 6, following stimulation with GCSF, a time point that coincides with the termination of proliferation and induction of growth arrest in these cells. Interestingly, this accumulation of Jak3 also appears to result in increased phosphorylation of Jak3 on tyrosine. These results suggest that induction of growth arrest in cells treated with G-CSF may be associated with the accumulation of phosphorylated Jak3. To test this hypothesis, we ectopically expressed Jak3 in 32Dcl3 cells and isolated several cell lines (32D/Jak3), which express high levels of Jak3, comparable to that seen at day 5 following G-CSF treatment of vector-transfected or parental 32Dcl3 cells. Interestingly, the accumulation of Jak3 is associated with its phosphorylation, an observation reported earlier in other cell systems.24
When 32D/Jak3 cells were incubated in the presence of G-CSF, they failed to proliferate and immediately entered into G1arrest, which was accompanied by inactivation of the PI3K/Akt and Stat3 pathways. Strikingly, 32D/Jak3 cells differentiate into granulocytes within 3 to 4 days, whereas this process required 10 days for the parental or vector-transfected cell lines. These results demonstrate that ectopic overexpression of Jak3 promotes growth arrest in the G1 phase of the cell cycle and accelerates granulocytic differentiation in response to G-CSF.
Further analysis of parental 32Dcl3 and Jak3/32D cells for the expression of various Cdk inhibitors revealed that the expression of p27Kip1 was induced between day 4 and day 6 in normal 32Dcl3 cells, a period that coincides with inhibition of proliferation and growth arrest. Most interestingly, induction of p27Kip1in 32D/Jak3 cells was observed between 12 and 24 hours following G-CSF stimulation, a time point that is much earlier compared to the parental cell line, which also correlated well with the time during which these cells undergo growth arrest with G1 DNA content. These observations suggest that high level expression of Jak3 protein results in the accumulation of p27Kip1. Because p27Kip1is a known inhibitor of Cdk2/Cyclin E complexes, it is possible that the Jak3-mediated up-regulation of p27Kip1 results in a block in cell cycle progression at the G1/S border.
The observation that the 32D/Jak3 cells continue to proliferate in the presence of IL-3 and require G-CSF to undergo growth arrest and enter into the differentiation program indicates that Jak3 alone is not sufficient to induce these events, and therefore may act upstream of or in a distinct pathway from a mediator such as C/EBPα (CCAAT/enhancer binding protein–alpha), which can induce differentiation of 32Dcl3 cells in the absence of G-CSF.42 It is likely that G-CSF induces, in addition to Jak3, the expression of other genes that are essential for growth arrest and differentiation. In addition, our observation that overexpression of Jak3 allows 32D/Jak3 cells to better sustain IL-3 deprivation is indicative of the role of Jak3 in the regulation of survival pathways. These results taken together suggest that Jak3 serves as a molecular switch between proliferation and differentiation signals mediated by the G-CSF receptor.
It has been previously shown that Stat3 activation by G-CSF is required for normal granulocytic differentiation.43,44 Our recent studies suggest that Jak3 transcription is regulated in part by Stat3 (J.K.M. et al, unpublished results, May 2000), which provides a molecular basis for this observation.43,44 Since our results show that overexpression of Jak3 leads to inactivation of Stat3 and up-regulation of p27Kip1, we conclude that Jak3-mediated up-regulation of p27Kip1 does not require Stat3. Consistent with our results, mice with a targeted mutation in the G-CSF receptor have yielded insight into the importance of a role for Stat3 in normal G-CSF–dependent proliferation and granulocytic differentiation.43 Inactivation of Stat3 (via a truncated G-CSF receptor that cannot activate Stat3 or through a dominant-negative form of Stat3) impairs myeloid proliferation in response to G-CSF and also impairs the capacity of the myeloid progenitors to undergo G-CSF–induced differentiation. Overexpression of Stat3 rescued the defects and allowed normal G-CSF–induced proliferation and differentiation. Therefore, it appears that Stat3 is important for transmitting G-CSF–induced proliferation and differentiation signals and one of its target genes appears to be Jak3.
It has also been reported by de Koning et al that up-regulation of p27Kip1 was blocked by dominant-negative Stat3, suggesting that Stat3 controls myeloid differentiation via up-regulation of p27Kip1.44 In contrast, our results presented in this report show that the up-regulation of p27Kip1 is independent of Stat3 activation. These differences may be due to the distinctly different in vitro cell system used in the study reported by de Koning and colleagues.44 A clonal variant of 32D cells 32D.C8.6 was used by de Koning and coworkers, which differs from the 32Dcl3 cells used in our studies. In their studies, de Koning and coworkers used ectopic expression vectors to overexpress the G-CSF receptor in 32D.C8.6 cells. Our studies (data not shown) indicate that one of the molecular events associated with G-CSF–induced granulocytic differentiation is the down-regulation of G-CSF receptor at terminal stages of differentiation, which does not occur in clonal variants where the G-CSF receptor expression is under the control of exogenous promoters. Our studies also show that there is a high degree of variability in the G-CSF responsiveness of different 32D cell clonal variants, where the levels of G-CSF receptor expressed plays a critical role. It is not known if the 32D.C8.6 cells used in the study by de Koning et al44 have endogenous expression of G-CSF receptor and whether the G-CSF receptor expression is down-regulated during later stages of terminal differentiation. The 32Dcl3 cells used in our study have a normal G-CSF receptor expression and in such cells it appears that expression of Jak3, rather than activation of Stat3, is necessary for p27Kip1 up-regulation. However, these results can be reconciled based on our unpublished observation (May 2000) that Stat3 is required for Jak3 activation and so it can be expected that a dominant-negative mutant of Stat3 would block Jak3 expression that will also result in a block to the up-regulation of p27Kip1.
To address the issue whether this observation made using 32Dcl3 cell system is relevant to normal BM cells, we also overexpressed Jak3 in primary BM cells from wild-type (C57/BL6) mice using a retroviral transduction system and studied the effect of Jak3 overexpression on G-CSF– or GM-CSF–induced terminal differentiation of primary BM cells. Following incubation of primary BM cells with G-CSF and GM-CSF, we conducted cell cycle analysis, assessed cell morphology, and analyzed cell surface expression of Gr-1, a marker for granulocytic differentiation. As was observed with 32Dcl3 cells, BM-Jak3 cells stimulated by GM-CSF accumulated in the G0/G1phase of the cell cycle by day 4 to a degree not seen in vector-transduced BM cells (BM-neo cells) until day 8. Strikingly, BM-Jak3 cells entered and completed the terminal differentiation process within 3 to 4 days, whereas this process required 8 days for the neo-transfected BM cells. BM-neo cells took twice as long to achieve the level of Gr-1 expression that was observed in BM-Jak3 cells after 4 days of treatment with GM-CSF. These observations are consistent with the cell morphology, which shows that the population of BM-Jak3 cells contains vastly increased numbers of mature granulocytes by day 4 following GM-CSF treatment when compared with BM-neo cells. We made similar observations in G-CSF–stimulated BM-Jak3 cells, but the phenotype of accelerated cell cycle arrest and early increase in Gr-1 expression was more readily observed in GM-CSF–stimulated cells. This is not surprising, because vector transfected mouse BM cells undergo terminal granulocytic differentiation in 8 to 10 days when treated with GM-CSF but terminally differentiate in 4 days when treated with G-CSF. This resulted in a much shorter window of time in which to observe an acceleration of granulocytic differentiation in the presence of G-CSF. The observation that accelerated growth arrest and differentiation of mouse BM cells overexpressing Jak3 can occur in GM-CSF suggests a definitive role for Jak3 in development along the monocytic lineage as well. Although lymphoid development is impaired in mice deficient in Jak145 or Jak3,10-12 and erythroid development is lost in Jak2-deficient mice,46the loss of a single Jak family member has not been implicated in failed myelopoiesis. Our observation that Jak3 overexpression can accelerate granulopoiesis in primary mouse BM cells suggests that the lack of a block in myelopoiesis in Jak3 knockout mice is more likely due to the ability of another Jak family member to compensate for the loss of Jak3. However, the observation that Jak3 can accelerate differentiation and growth arrest in primary mouse BM cells suggests that Jak3 has an intrinsic role in myelopoiesis and integrates the processes of proliferation, growth arrest, and terminal differentiation in myeloid cells. Further studies of the role of Jak3 in the myeloid compartment will be necessary to delineate whether Jak3 works alone or in concert with other Jak or Stat family members to promote terminal differentiation of myeloid cells.
Supported by grants CA68239 and ES09225 from the National Institutes of Health (E.P.R.). The flow cytometry studies were supported by grant CA88261.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
E. Premkumar Reddy, Department of Biochemistry and Fels Institute for Cancer Research and Molecular Biology, Temple University School of Medicine, 3307 N Broad St, Philadelphia, PA 19140; e-mail: reddy@unix.temple.edu.