Diamond-Blackfan anemia (DBA) is a congenital bone marrow failure syndrome characterized by a specific deficiency in erythroid progenitors. Forty percent of the patients are blood transfusion–dependent. Recent reports show that the ribosomal protein S19 (RPS19) gene is mutated in 25% of all patients with DBA. We constructed oncoretroviral vectors containing theRPS19 gene to develop gene therapy for RPS19-deficient DBA. These vectors were used to introduce the RPS19 gene into CD34+ bone marrow (BM) cells from 4 patients with DBA withRPS19 gene mutations. Overexpression of theRPS19 transgene increased the number of erythroid colonies by almost 3-fold. High expression levels of the RPS19transgene improved erythroid colony-forming ability substantially whereas low expression levels had no effect. Overexpression of RPS19 had no detrimental effect on granulocyte-macrophage colony formation. Therefore, these findings suggest that gene therapy for RPS19-deficient patients with DBA using viral vectors that express the RPS19gene is feasible.
Introduction
Diamond-Blackfan anemia (DBA) is a congenital or early-onset pure red cell aplasia/hypoplasia. The disease is characterized by a moderate-to-severe aregenerative anemia and erythroblastopenia in an otherwise normocellular bone marrow.1,2 Typically, the disorder may present with severe chronic normochromic, macrocytic anemia, and reticulocytopenia.3,4 In approximately 30% to 40% of patients, there are associated physical malformations, including prenatal or postnatal growth retardation, hand and thumb malformations, and congenital heart defects.5,6 Most of the reported cases of DBA are sporadic but 10% to 25% have a positive family history.7,8 Seventy percent of patients respond initially to corticosteroid treatment,2,9 but 40% become transfusion-dependent.9 Allogeneic bone marrow transplantation has been shown to be an effective cure for the disease, which demonstrates that the cause of the disease is intrinsic to the bone marrow.10-12 However, the mainstay of therapy for transfusion-dependent patients is frequent blood transfusions, which lead to iron overload. As a consequence, hemosiderosis is a major cause of death among transfusion-dependent patients with DBA. In vitro hematopoietic progenitor culture studies indicate that DBA results from an intrinsic defect in erythroid progenitors, erythroid burst-forming units (BFU-Es) and erythroid colony-forming units (CFU-Es),5,13 and not from a defect in the bone marrow microenvironment,14 which is consistent with successful marrow transplantation as a treatment for DBA.
Several studies have ruled out a number of candidate genes for DBA, including those encoding interleukin 9, the erythropoietin receptor, stem cell factor (SCF) and its receptor, c-kit.3,15-18Recently, a balanced translocation (X;19) was identified in a patient with DBA and the translocation breakpoint was shown to disrupt the ribosomal protein S19 (RPS19) gene.8,19,20Subsequent analysis of the RPS19 gene revealed mutations in a subset of patients with DBA. Heterozygous mutations in theRPS19 gene have to date been found in approximately 25% of patients with DBA and families with a history of DBA, including nonsense, frameshift, splice site, and missense mutations.19,21 22
It is not known why a gene that is expressed at relatively high levels in all tissues can be responsible for the specific erythroid progenitor cell deficiency. All patients with RPS19mutations are believed to have one normal allele and a suggested molecular mechanism behind the disease is haploid insufficiency (allelic exclusion and genomic imprinting have not been excluded). We set out to investigate whether forced expression of the RPS19gene can improve erythroid colony formation in RPS19-deficient patients with DBA through transduction of the RPS19 gene into hematopoietic progenitor cells using oncoretroviral vectors. We found that overexpression of RPS19 improves erythroid development in RPS19-deficient patients with DBA. Noteworthy, the higher the expression level of the RPS19 transgene in erythroid progenitors from these patients, the better the correction of the erythroid defect. These findings indicate that gene replacement therapy for RPS19-deficient DBA may be feasible using vectors that overexpress the RPS19 gene.
Materials and methods
Bone marrow CD34+ cells
Bone marrow samples were collected from 4 patients with DBA and healthy volunteers after informed consent using a protocol approved by Lund University Hospital Ethics Committee. Mononuclear cell isolation was performed using a Lymphoprep density gradient (Nycomed, Oslo, Norway) and CD34+ cell enrichment was performed using Midi MACS LS+ separation columns and isolation kit (Miltenyi Biotec, Auburn, CA). CD34+ cell purity was over 80% (fluorescence-activated cell-sorter [FACS] analysis using anti–CD34 antibody HPCA-2 PE; Becton Dickinson Immunocytometry Systems, San José, CA). CD34+ cells were used fresh or stored in liquid nitrogen in aliquots of 1 × 106cells in 1 mL of freezing medium (Gibco BRL, Cleveland, OH) prior to use.
Oncoretroviral vectors
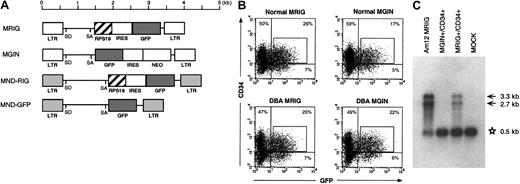
The oncoretrovirus vector MRIG was derived from MSCV, an oncoretrovirus vector based on the murine stem cell virus generously provided by Robert G. Hawley.23 The 427bp cDNA of humanRPS19 was inserted in front of an internal ribosomal entry site (IRES) from the encephalomyocaditis virus followed by the gene for enhanced green fluorescent protein (GFP). The oncoretrovirus vector MND-RIG was derived from MND-MFG, an oncoretrovirus vector24 and the RPS19 gene was inserted in front of an IRES-GFP cassette as in MRIG. The control vector MND-GFP contains the GFP gene. The retroviral vector MGIN has been described previously.25
Oncoretroviral producer cell lines
The Phoenix Eco packaging cell line (generously provided by Garry P. Nolan, Stanford University School of Medicine, Stanford, CA) was cultured in Dulbecco modified Eagle medium (DMEM) with 10% fetal calf serum (FCS) and penicillin/streptomycin and transfected with the different vectors using CaPO4 under standard conditions. Supernatants were harvested, supplemented with protamine sulfate (Sigma Chemicals, St Louis, MO) at a final concentration of 4 μg/mL, and used for repeated transduction of GP+ envAM12 cells.26 The MRIG clone with the highest titer was used but the other 3 producer cell lines were used as polyclonal populations. The titers of the vectors (at dilutions 1/20-1/1000) were assessed by transduction of HeLa cells and subsequent determination of proportion of GFP+ cells by FACS analysis. The viral titers were calculated from the dilution of supernatant yielding approximately 10% GFP+ cells and were as follows: MRIG, 2.0 × 106 infectious U/mL; MGIN, 2.3 × 106 infectious U/mL; MND-RIG, 1.2 × 106 infectious U/mL; and MND-GFP, 1.1 × 106 infectious U/mL.
Transduction of CD34+ cells
Frozen bone marrow CD34+ cells were thawed in DNase I (Roche Diagnotics GmbH, Mannheim, Germany) containing medium to avoid clumping of cells. Prestimulation of CD34+ cells was performed under serum-free conditions in X-Vivo15 medium (Bio Whittaker, Walkersville, MD) supplemented with 1% bovine serum albumin (BSA; Stem Cell Technologies, Vancouver, BC, Canada), 2 mM L-glutamine (Gibco BRL), 10−4 mM, 0.22 μm filtered 2-mercapto-ethanol (Sigma Chemicals), 100 IU/mL penicillin, 100 μg/mL streptomycin (Gibco BRL), cytokines IL-3 at 20 ng/mL (a generous gift from Amgen, Thousand Oaks, CA), IL-6 at 50 ng/mL (a generous gift from Novartis, Basel, Switzerland), and SCF at 100 ng/mL (a generous gift from Amgen). After 24 hours the CD34+cells were transferred to 24-well plates, precoated with the human fibronectin fragment CH-296 (Retronectin; Takara Shuzo, Otsu, Japan), blocked with 2% BSA in phosphate buffered saline (PBS) for 30 minutes at room temperature, and preloaded with viral supernatant for 60 minutes at 37°C. Subsequently, the cells were added to the wells in serum-free medium and with cytokines as above and cells were harvested from the plates on day 4.
Flow cytometric analysis and cell sorting
Transduced cells were stained for 30 minutes with the anti–CD34 antibody HPCA-2-PE (Becton Dickinson) and then sorted using a FACS Vantage cell sorter (Becton Dickinson). Single cells were sorted on the basis of their expression of GFP and CD34.
Colony-forming unit cell (CFU-C) assay
Freshly isolated bone marrow CD34+ cells (10 000 cells) were plated in triplicate in 35-mm dishes in 1.1 mL of H4230 methlcellulose (Stem Cell Technologies). Since erythroid colonies in DBA are often small and poorly hemoglobinized, 5 U/mL erythropoietin (Epo; Janssen-Cilag, Sollentuna, Sweden) were added to the methylcellulose to assay erythroid colony formation. For macrophage and granulocyte-macrophage colony formation, 100 U/mL IL-3 and 200 U/mL GM-CSF (a gift from Novartis) were used in the methylcellulose cultures. Cells (1000 cells) that had been transduced in the presence of IL-3, IL-6, and SCF were sorted for GFP expression and plated in methylcellulose with either 5 U/mL Epo alone or 5 U/mL Epo plus 100 ng/mL SCF for erythroid colony formation, and with 100 U/mL IL-3 + 200 U/mL GM-CSF for macrophage and granulocyte-macrophage colony formation. The cells were cultured at 37°C in a humidified atmosphere with 5% CO2. CFU-Es were counted on day 7, and BFU-Es and macrophage– and granulocyte-macrophage–colony-forming units (CFU-M/GMs) were counted on day 14. Colonies were always counted in a blinded fashion to avoid unconscious bias in evaluating the effect of the experimental vector compared with the control vector.
Northern blot analysis
Total RNA was isolated from 5 × 106 to 1 × 107 cells using the RNeasy kit as suggested by the manufacturer (Qiagen, Chatsworth, CA). RNA from each sample was loaded on a 1% agarose gel, transferred onto Hybond N+ membrane (Amersham Pharmacia, Buckinghamshire, United Kingdom) and hybridized using a full-length RPS19 probe labeled with [32P]dCTP using a random priming kit (Amersham Pharmacia). Filters were washed and exposed to Kodak x-ray film. The expression level of theRPS19 transgene mRNA was estimated by BAS 5000 Phosphoimager (Fuji Photo Film, Kanagawa, Japan).
Quantitative RT-PCR Analysis
Total RNA was isolated from 105 CD34+cells or 106 mononuclear cells as described above and cDNA was reverse transcribed using SupersciptII (Gibco BRL) according to manufacturer's instructions. The expression level of RPS19 was analyzed by quantitative reverse transcriptase–polymerase chain reaction (Q–RT-PCR) using a LightCycler instrument (Roche Diagnostics GmbH) with LightCycler Software version 5.32. The cDNA was used for Q-PCR using SybrGreenI (Sigma-Aldrich) for detection of PCR products. cDNA (2 μL) was used in a 15 μL final volume reaction containing 1 U Platinum Taq DNA Polymerase (Invitrogen, Paisley, United Kingdom), 1x buffer (provided with the enzyme), 0.8 mM dNTP, 3 mM MgCl2, 0.5 mg/mL BSA, 5% dimethyl sulfoxide (DMSO), 0.5 μm RPS19 forward (5′-GCC TGG AGT TAC TGT AAA AGA CG-3′), 0.5 μm RPS19 reverse (5′-CCC ATA GAT CTT GGT CAT GGA GC-3′) and 1:20 000 dilution of SybrGreenI. The LightCycler was programmed to run an initial denaturation step at 94°C for 3 minutes followed by 40 cycles of denaturation (94°C, 5 seconds), annealing (60°C, 5 seconds), and extension (72°C, 10 seconds), measuring the build-up of PCR products at the end of the extension step of each cycle. The RPS19 values were normalized against human β-actin (huAktEx4F: 5′-CCA TTG GCA ATG AGC GGT T – 3′, huAktEx6R: 5′-GCG CTC AGG AGG AGC AA-3′), using the same program as above except for changing the annealing temperature to 55°C.
Statistical analysis
All colony counts were done by 2 independent persons and in a blinded fashion. To evaluate the statistical significance of the difference in hematopoietic colony frequencies from patients and controls generated by fresh (uncultured) bone marrow cells, the Wilcoxon rank sum test (2-sided test) was used to calculate theP value. For hematopoietic colony frequencies generated by transduced sorted cells, the Wilcoxon rank sum test (2-sided test) was also used to compare colony frequencies between cells (transduced and sorted for GFP) from each patient that were treated by the control vector (GFP alone) and the therapeutic vector (RPS19 andGFP genes). The null hypothesis of no treatment effect was tested in each patient by comparing the erythroid colony number generated by the “untreated” CD34+ cells transduced with the control vector and the “treated” CD34+ cells transduced with the vector containing the RPS19 andGFP genes.
Results
CD34+ BM cells from RPS19-deficient patients generate few erythroid colonies
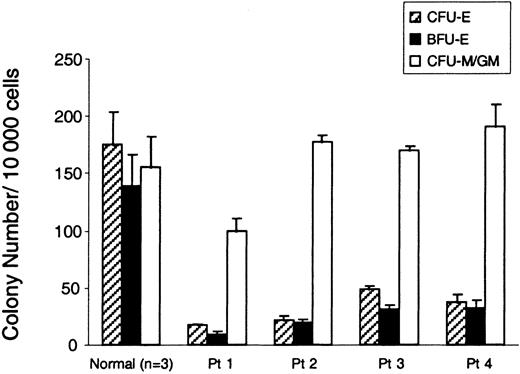
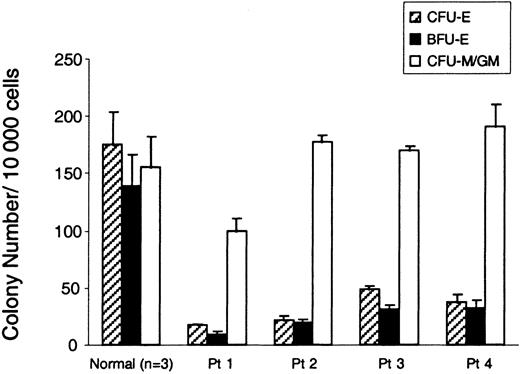
Several investigators have reported that the number of erythroid progenitor colonies derived from BM cells of patients with DBA is severely reduced.5,13 We asked whether erythroid colony formation was reduced in the subgroup of patients with DBA who harbor RPS19 deficiency. There were 4 patients who were studied, all of whom have been reported on previously.7,19 21 The clinical features and the RPS19 gene mutations of the patients are briefly presented in Table1. Patient 1 has a deletion spanning the complete RPS19 gene; patient 2 has a translocation [t(X;19)(q21;q13)] with a breakpoint in intron 3; patient 3 has a missense mutation in exon 3; and patient 4 has a [TT157-158AA, 160 ins CT] mutation which predicts a truncated form of the RPS19. Patients 1, 2, and 3 are severely affected and transfusion-dependent. Patient 4 was in spontaneous remission without anemia at the time of this study. From these 4 patients, we harvested bone marrow samples, selected CD34+ cells, and determined the number of hematopoietic colonies in methylcellulose cultures. The frequencies of BFU-E and CFU-E colonies from all the patients with DBA were severely reduced (6.4%-27.6% of normal, P < .04) when these erythroid colonies were grown in erythropoietin alone (Figure1). In contrast, the number of CFU-M/GM colonies was normal in all 4 patients. It's noteworthy that patient 4, who is not transfusion-dependent, exhibited a severe reduction in erythroid colony-forming ability.
Severe reduction of erythroid colonies in RPS19-deficient patients with DBA.
The bar graph shows the number of erythroid and myeloid progenitor colonies from bone marrow cells of RPS19-deficient patients with DBA and a healthy individual. CD34+ cells (10 000) from patients with DBA (Patients 1-4) and normal healthy donors were plated in methylcellulose in the presence of erythropoietin (CFU-Es and BFU-Es) or IL-3 and GM-CSF (CFU-M/GM). CFU-Es (hatched bars) were scored on day 7. The number of BFU-E (black bars) and CFU-M/GM (white bars) colonies were scored on day 14. The mean ± SEM for 3 experiments is shown. The frequency of erythroid colonies is significantly reduced in patients versus contols (P < .04, Wilcoxon rank sum test) but no difference was found with regard to CFU-M/GM colonies. One experiment with 3 independent plates is shown.
Severe reduction of erythroid colonies in RPS19-deficient patients with DBA.
The bar graph shows the number of erythroid and myeloid progenitor colonies from bone marrow cells of RPS19-deficient patients with DBA and a healthy individual. CD34+ cells (10 000) from patients with DBA (Patients 1-4) and normal healthy donors were plated in methylcellulose in the presence of erythropoietin (CFU-Es and BFU-Es) or IL-3 and GM-CSF (CFU-M/GM). CFU-Es (hatched bars) were scored on day 7. The number of BFU-E (black bars) and CFU-M/GM (white bars) colonies were scored on day 14. The mean ± SEM for 3 experiments is shown. The frequency of erythroid colonies is significantly reduced in patients versus contols (P < .04, Wilcoxon rank sum test) but no difference was found with regard to CFU-M/GM colonies. One experiment with 3 independent plates is shown.
Expression of the RPS19 gene in hematopoietic cells from RPS19-deficient patients with DBA
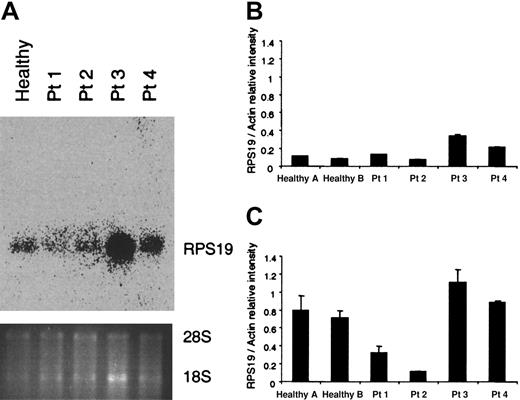
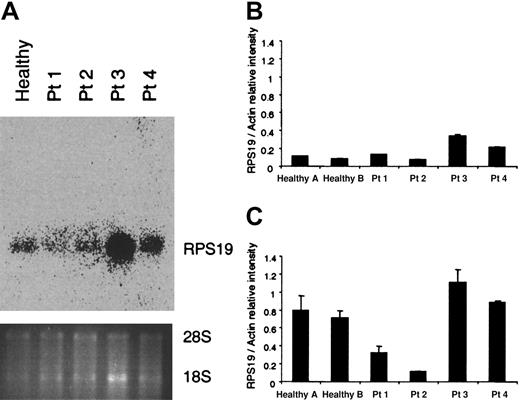
Since all RPS19-deficient patients are assumed to have one normal and one mutant RPS19 allele, we wanted to determine the RPS19 RNA levels in hematopoietic cells. RNA was extracted from mononuclear cells of the 4 patients tested and a healthy individual and examined by northern blot analysis (Figure 2A). All patients exhibited the normal-size transcript whereas abnormal-size transcripts could not be detected with a full-length RPS19 probe. Patient 2, who has a translocation, does not express any abnormal RNA species generated by the translocated chromosome which is detectable by northern blot analysis. Patients 1 and 2, who have a deletion and a translocation, respectively, had lower levels of the RNA than patients 3 and 4, who have point mutations and a small insertion (patient 4 only). Quantification of the RNA done by the northern blot analysis (Figure 2A) was confirmed by quantitative RT-PCR (Figure 2B). In order to quantify the endogenous RPS19 transcripts in CD34+cells, quantitative RT-PCR was performed by comparing the RPS19 RNA levels with those of actin in each sample (Figure 2C). Interestingly, patients 1 and 2 had similar RNA levels in mononuclear cells as the healthy individuals tested. Noteworthy, the RPS19 levels were proportionally higher in CD34+ cells but in contrast to what is seen in mononuclear cells, the patients with the deletion or translocation (patients 1 and 2) had lower levels of RPS19 transcripts than the control samples. Patients 3 and 4 had levels of RPS19 RNA in CD34+ cells similar to those of healthy individuals, probably because the mutated transcript was also detected (Figure 2C). These results indicate that there are higher levels of RNA in the hematopoietic progenitor population than in mature cells, when the RPS19 transcripts are compared with the levels of actin RNA.
RNA analysis in mononuclear and CD34+ cells from RPS19-deficient patients.
(A) RNA was isolated from mononuclear cells of a healthy individual and 4 patients (Patients 1-4, Table 1) with RPS19 mutations and examined by northern blot analysis using a full-length RPS19 probe. No abnormal transcripts were detected. (B, C) RNA from mononuclear cells (B) and CD34+ cells (C) was analyzed by quantitative RT-PCR using a LightCycler to amplify RPS19 and actin transcripts. The figures show the signal intensity ratio between the RPS19 and actin transcripts. The Q-RT-PCR experiments in B and C represent the average of 2 experiments.
RNA analysis in mononuclear and CD34+ cells from RPS19-deficient patients.
(A) RNA was isolated from mononuclear cells of a healthy individual and 4 patients (Patients 1-4, Table 1) with RPS19 mutations and examined by northern blot analysis using a full-length RPS19 probe. No abnormal transcripts were detected. (B, C) RNA from mononuclear cells (B) and CD34+ cells (C) was analyzed by quantitative RT-PCR using a LightCycler to amplify RPS19 and actin transcripts. The figures show the signal intensity ratio between the RPS19 and actin transcripts. The Q-RT-PCR experiments in B and C represent the average of 2 experiments.
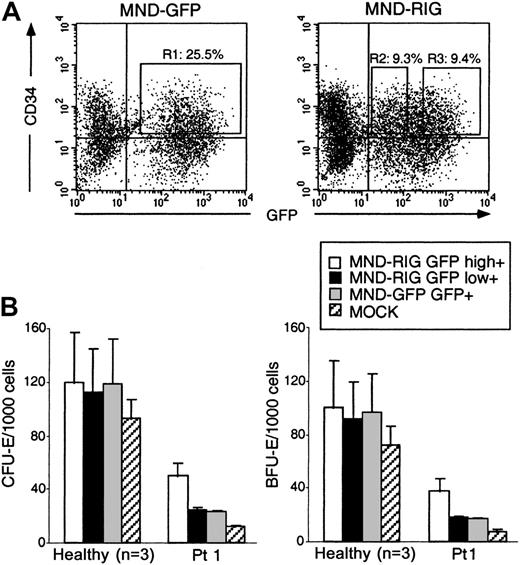
The RPS19 transgene is expressed in transduced CD34+BM cells from patients with DBA
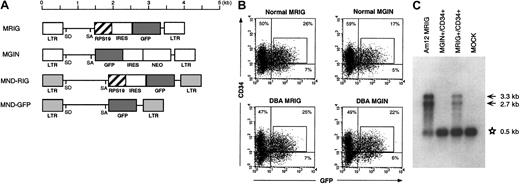
In DBA, there is a deficiency in erythroid development in all patients and other developmental abnormalities in a subset of patients. Since RPS19 is produced in all tissues at relatively high levels, it is not obvious why mutations in the RPS19 gene cause the tissue-specific characteristics of DBA. We therefore asked whether we could improve erythroid development in vitro by overexpression of the RPS19 cDNA in hematopoietic progenitors from patients with DBA. For this purpose we designed oncoretroviral vectors MRIG and MND-RIG containing the RPS19 gene driven by the viral LTR followed by an IRES and the GFP gene to allow FACS sorting of transduced cells (Figure 3A). The control vectors contain the GFP gene without theRPS19 gene. CD34+ cells from healthy individuals and patients with DBA were prestimulated and transduced by the MRIG vector or the MGIN control vector. Approximately 25% of the transduced cells from patients and healthy individuals were GFP+ and CD34+ (Figure 3B). Northern blot analysis was performed to estimate the RPS19 mRNA level generated by the MRIG vector. CD34+ cells transduced with the MRIG and control vectors were sorted by flow cytometry and the total RNA from each fraction was extracted. The MRIG vector generated 2 mRNA species (unspliced genomic vector RNA and the spliced form) seen in both the vector-producing GP+ envAm12 cells and the transduced CD34+cells. MRIG-transduced cells contained RPS19 transgene expression levels that were approximately 20% of endogenous expression levels in normal CD34+ cells (Figure 3C).
RPS19 transgene expression in transduced CD34+ cells from patients with DBA.
(A) The retroviral vector MRIG consists of the MSCV retrovirus backbone containing the RPS19 cDNA followed by an IRES from the encepharomyocarditis virus and the GFP gene. To obtain higher transgene expression, we constructed the MND-RIG vector containing the same RPS19-IRES-GFP casette. The MND-RIG vector was derived from the MND-MFG vector which contains the myeloproliferative sarcoma virus LTR without the negative control region and the region between the donor and acceptor splice sites is derived from the MFG vector.24 SD and SA represent the splice donor and splice acceptor sites, respectively. MGIN and MND-GFP are respective control vectors containing the GFP gene without the RPS19 cDNA. (B) Flow cytometric analysis of transduced CD34+ cells. The figure shows a representative experiment demonstrating MRIG and MGIN transduction of CD34+ BM cells from an RPS19-deficient patient with DBA and a healthy individual. The CD34+ cells were exposed to a cocktail of growth factors during the transduction. The CD34+ fractions of the bone marrow cells from a healthy donor and a patient with DBA were transduced with the oncoretroviral vectors MRIG or MGIN. The percentage numbers show the ratios of cells in each of the quadrants. The GFP+ and CD34+cells (box) were sorted by FACS vantage. The controls used to set the gates showed that mock transduced cells were 0.27% CD34+/GFP+ and 0.05% were CD34−/GFP+. (C) Northern blot analysis of the transduced cells. One microgram of total RNA from GFP+ and CD34+ cells were electrophoresed in 1% agarose, transferred onto a nylon membrane, and hybridized with radioactively labeled DNA fragment specific for RPS19. The MRIG GP+envAm12-producing cell line was used as a positive control. The asterisk indicates the endogenous expression of RPS19 (0.5 kb). Arrows indicate the vector generated RPS19 (3.3 kb, unspliced and 2.7 kb, spliced). The mock sample represents mock-transduced CD34+ cells that were not sorted on the FACS.
RPS19 transgene expression in transduced CD34+ cells from patients with DBA.
(A) The retroviral vector MRIG consists of the MSCV retrovirus backbone containing the RPS19 cDNA followed by an IRES from the encepharomyocarditis virus and the GFP gene. To obtain higher transgene expression, we constructed the MND-RIG vector containing the same RPS19-IRES-GFP casette. The MND-RIG vector was derived from the MND-MFG vector which contains the myeloproliferative sarcoma virus LTR without the negative control region and the region between the donor and acceptor splice sites is derived from the MFG vector.24 SD and SA represent the splice donor and splice acceptor sites, respectively. MGIN and MND-GFP are respective control vectors containing the GFP gene without the RPS19 cDNA. (B) Flow cytometric analysis of transduced CD34+ cells. The figure shows a representative experiment demonstrating MRIG and MGIN transduction of CD34+ BM cells from an RPS19-deficient patient with DBA and a healthy individual. The CD34+ cells were exposed to a cocktail of growth factors during the transduction. The CD34+ fractions of the bone marrow cells from a healthy donor and a patient with DBA were transduced with the oncoretroviral vectors MRIG or MGIN. The percentage numbers show the ratios of cells in each of the quadrants. The GFP+ and CD34+cells (box) were sorted by FACS vantage. The controls used to set the gates showed that mock transduced cells were 0.27% CD34+/GFP+ and 0.05% were CD34−/GFP+. (C) Northern blot analysis of the transduced cells. One microgram of total RNA from GFP+ and CD34+ cells were electrophoresed in 1% agarose, transferred onto a nylon membrane, and hybridized with radioactively labeled DNA fragment specific for RPS19. The MRIG GP+envAm12-producing cell line was used as a positive control. The asterisk indicates the endogenous expression of RPS19 (0.5 kb). Arrows indicate the vector generated RPS19 (3.3 kb, unspliced and 2.7 kb, spliced). The mock sample represents mock-transduced CD34+ cells that were not sorted on the FACS.
RPS19 transgene expression in CD34+RPS19-deficient DBA cells improves erythroid colony formation
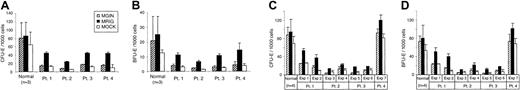
To investigate whether expression of the RPS19transgene rescues the erythropoiesis of RPS19-deficient patients with DBA, bone marrow CD34+ cells from patients with DBA and healthy individuals were transduced by the MRIG and control vectors. Transduced cells sorted for GFP and CD34 expression were plated in methylcellulose in the presence of 5 U/mL of erythropoietin alone. The numbers of CFU-E and BFU-E colonies were scored on days 7 and 14, respectively. As shown in Figure 4A-B, the number of erythroid colonies from MRIG-transduced CD34+cells was compared with that from control vector–transduced, GFP+ sorted cells or mock-transduced cells that had not been sorted. As expected, the transduced sorted cells created green colonies with the MRIG and control vector. Neither MRIG nor control vector transduction of normal CD34+ BM cells affected the number of erythroid colonies. In contrast, overexpression of RPS19 in CD34+ BM cells from patients with DBA consistently increased the erythroid colony formation. In all patients, there was a close to 3-fold increase in the number of CFU-E and BFU-E colonies following gene transfer. The difference in the frequency of erythroid colonies generated by the MRIG-transduced and sorted CD34+cells and control vector–treated cells was statistically significant for each patient (P < .03, Wilcoxon rank sum test). In all patients, the MRIG-transduced samples generated a 3-fold increase in erythroid colony formation, from approximately 18% to approximately 55% of normal in patients 1, 3, and 4, but patient 2 had a lower baseline value (10% of normal with control vector) but the increase was also approximately 3-fold (approximately 30% of normal with MRIG). Since a previous report27 has demonstrated that the frequency of erythroid colony formation can be increased in patients with DBA when the progenitors are grown in SCF and erythropoietin, compared with erythropoietin alone, we decided to ask whether the forced expression of RPS19 would further improve erythroid colony formation in the presence of both cytokines. Using these 2 cytokines, the number of erythroid colonies before gene transfer varied from one patient to another (Figure 4C-D). Just as in the experiment with Epo alone, the MRIG vector generated close to a 3-fold increase in the frequency of erythroid colonies from the 3 transfusion-dependent patients compared to the control vector (P < .005, Wilcoxon rank sum test). Noteworthy, patient 1, who has a complete deletion of the RPS19 gene, had a higher number of erythroid colonies before gene transfer than the 2 other transfusion-dependent patients. Overexpression of RPS19 in patient 1 increased the number of erythroid progenitors up to 40% to 60% of normal. Interestingly, in transfusion-independent patient 4, whose mutation leads to a truncation of the RPS19 molecule, the number of erythroid colonies becomes normalized when transduced with the control vector and/or cultured (mock transduced) with SCF and erythropoietin (Figure 4C-D). However, when the methylcellulose contained erythropoietin alone, colony formation was approximately 20% of normal for cells transduced with the control vector and increased to approximately 60% of normal following overexpression of RPS19 (Figure 4A-B). Interestingly, overexpression of RPS19 increased the baseline erythroid colony formation (control vector) by 3-fold (or almost 3-fold) in every experiment irrespective of whether Epo alone or Epo plus SCF were used in the methylcellulose cultures. Added together, the findings demonstrate that overexpression of RPS19 in CD34+ cells improves erythroid colony formation significantly in all patients investigated. Despite this clear increase in erythroid colony formation the colonies generated by the RPS19 vector were not increased in size or degree of hemoglobinization. The erythroid colonies in transfusion-independent patient 4 were also smaller and less hemoglobinized than seen in healthy individuals, regardless of whether these were treated with the RPS19 vector or the control vector.
Overexpression of RPS19 in CD34+ DBA cells improves erythroid colony formation.
CD34+ BM cells from patients with DBA (Patients 1-4) andnormal healthy donors were transduced with the oncoretroviral vectors MRIG or MGIN. Since the cells were grown with cytokines during prestimulation and transduction, the colony frequency is different than presented for the fresh cells in Figure 1. (A, B) A quantity of 1000 transduced, sorted progenitor cells (CD34+/GFP+ cells) from 4 patients (Patients 1-4, Table 1) was grown in methylcellulose as described in “Materials and methods” with erythropoietin as the only growth factor present. CFU-Es (A) were scored on day 7 and BFU-Es (B) on day 14. We analyzed 4 to 6 plates for each experiment to derive the mean and SEM. Hatched bars indicate the number of colonies from MGIN-transduced cells. Black bars indicate the number of colonies from MRIG-transduced cells. White bars indicate the number of colonies from mock-transduced, unsorted cells. Colony frequencies generated by the cells transduced with the control vector and the MRIG vector were significantly different for each patient (P < .03) using the Wilcoxon rank sum test. (C, D) A quantity of 1000 transduced progenitor cells (GFP+ and CD34+ cells) was grown in methylcellulose in the presence of erythropoietin and SCF. CFU-E (C) and BFU-E (D) colonies were scored on day 7 and day 14, respectively. We analyzed 3 plates for each experiment to derive the mean and SEM. Colony frequencies generated by the MRIG vector–transduced cells were significantly higher when compared with those generated by the control vector in patients 1-3 (P < .005, Wilcoxon rank sum test), but no difference was seen in patient 4.
Overexpression of RPS19 in CD34+ DBA cells improves erythroid colony formation.
CD34+ BM cells from patients with DBA (Patients 1-4) andnormal healthy donors were transduced with the oncoretroviral vectors MRIG or MGIN. Since the cells were grown with cytokines during prestimulation and transduction, the colony frequency is different than presented for the fresh cells in Figure 1. (A, B) A quantity of 1000 transduced, sorted progenitor cells (CD34+/GFP+ cells) from 4 patients (Patients 1-4, Table 1) was grown in methylcellulose as described in “Materials and methods” with erythropoietin as the only growth factor present. CFU-Es (A) were scored on day 7 and BFU-Es (B) on day 14. We analyzed 4 to 6 plates for each experiment to derive the mean and SEM. Hatched bars indicate the number of colonies from MGIN-transduced cells. Black bars indicate the number of colonies from MRIG-transduced cells. White bars indicate the number of colonies from mock-transduced, unsorted cells. Colony frequencies generated by the cells transduced with the control vector and the MRIG vector were significantly different for each patient (P < .03) using the Wilcoxon rank sum test. (C, D) A quantity of 1000 transduced progenitor cells (GFP+ and CD34+ cells) was grown in methylcellulose in the presence of erythropoietin and SCF. CFU-E (C) and BFU-E (D) colonies were scored on day 7 and day 14, respectively. We analyzed 3 plates for each experiment to derive the mean and SEM. Colony frequencies generated by the MRIG vector–transduced cells were significantly higher when compared with those generated by the control vector in patients 1-3 (P < .005, Wilcoxon rank sum test), but no difference was seen in patient 4.
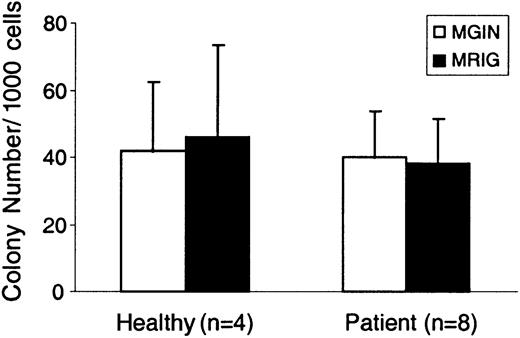
Overexpression of RPS19 has no detrimental effects on the development of CFU-M/GM colonies
Since it is possible that overexpression of RPS19 might have a detrimental effect on the development of hematopoietic lineages outside the erythroid compartment, we asked whether colony formation by CFU-M and CFU-GM progenitors was affected by forced expression of RPS19. As shown in Figure 5, no difference in CFU-M/GM colony formation could be detected when progeny cells from MGIN- and MRIG-transduced CD34+, GFP sorted cells were analyzed and compared. No difference in the size or morphology of these colonies was detected. Therefore, overexpression of RPS19 does not seem to negatively affect the development of macrophage- or granulocyte-macrophage colonies.
Normal growth of CFU-M/GM colonies despite overexpression of RPS19.
CD34+ cells from DBA patients 1-4 (2 experiments each, n = 8) and healthy individuals (n = 4) were transduced with the MRIG and MGIN vectors, sorted for GFP+ cells, and plated in methylcellulose. On day 14, the number of CFU-M and CFU-GM colonies was evaluated. The CFU-M and CFU-GM colony data were pooled together and are shown as CFU-M/GM colonies. The bar graphs show the mean ± SEM. No significant difference in colony formation was detected between the MRIG-transduced and the control vector–transduced cells.
Normal growth of CFU-M/GM colonies despite overexpression of RPS19.
CD34+ cells from DBA patients 1-4 (2 experiments each, n = 8) and healthy individuals (n = 4) were transduced with the MRIG and MGIN vectors, sorted for GFP+ cells, and plated in methylcellulose. On day 14, the number of CFU-M and CFU-GM colonies was evaluated. The CFU-M and CFU-GM colony data were pooled together and are shown as CFU-M/GM colonies. The bar graphs show the mean ± SEM. No significant difference in colony formation was detected between the MRIG-transduced and the control vector–transduced cells.
CD34+ DBA cells generate more erythroid colonies when RPS19 transgene expression is high
Since the transduced CD34+ cells are multiclonal and the expression level of the RPS19 and GFPtransgenes is variable in each cell (Figure 3B) depending to some extent on the integration site of the vector, we asked whether transduced CD34+ cells expressing high levels of theRPS19 transgene were able to generate more erythroid colonies than cells expressing low levels. Since the vectors containing the RPS19 gene generate a bicistronic mRNA that encodes both RPS19 and GFP, transduced cells expressing high levels of GFP from the bicistronic mRNA are expected to express high levels of the RPS19transgene. CD34+ cells from patient 1 were transduced with MND-RIG (used here because this vector expresses slightly higher levels of the transgenes than MRIG), and sorted for GFP positivity (all GFP+ cells) or divided into populations expressing relatively low levels of GFP (GFPlow) and relatively high levels of GFP (GFPhigh) as shown in Figure6. The mean fluorescensce intensity (MFI) of the GFPhigh fraction was 789.0 whereas the MFI of the GFPlow fraction was approximately 10 times lower (62.2). The GFP+ cells and the subfractions producing high and low levels of GFP were plated in methylcellulose, and the number of erythroid colonies from each fraction was determined. In CD34+ BM cells from healthy individuals, the number of erythroid colonies from the GFPhigh fraction was similar to that of the GFPlow fraction. In MND-RIG–transduced CD34+ BM cells from patient 1, the GFPhighfraction increased the colony-forming ability to 40% of normal whereas the GFPlow cells had the same erythroid colony-forming ability as the sorted cells transduced with the control vector (Figure6). These findings suggest that gene replacement therapy of RPS19-deficient DBA may be feasible provided that high levels ofRPS19 transgene expression are generated in hematopoietic progenitors.
High RPS19 transgene expression increases erythroid colony formation.
Transduction of bone marrow cells from DBA patient 1 using the MND-RIG vector and the MND-GFP control vector. (A) Flow cytometric analysis of transduced CD34+ cells. The transduced GFP+ and CD34+ cells (squared fractions) were sorted by FACSVantage. R1: MND-GFP–transduced CD34+/GFP+ fraction. R2: MND-RIG–transduced CD34+/GFPlow fraction. R3: MND-RIG–transduced CD34+/ GFPhighfraction. (B) A quantity of 1000 transduced progenitor cells (GFP+ and CD34+ cells) were embedded in methylcellulose in the presence of erythropoietin and SCF. CFU-E and BFU-E colonies were scored on day 7 and day 14, respectively. Open bars indicate the number of colonies from MND-RIG–transduced GFPhigh cells. Black bars indicate the number of colonies from MND-RIG–transduced GFPlow cells. Gray bars indicate the number of colonies from MND-GFP–transduced GFP+ cells. Hatched bars indicate the number of colonies from the mock-transduced, unsorted fraction. One experiment was performed and 3 plates were counted in a blinded fashion. The mean ± SEM are shown.
High RPS19 transgene expression increases erythroid colony formation.
Transduction of bone marrow cells from DBA patient 1 using the MND-RIG vector and the MND-GFP control vector. (A) Flow cytometric analysis of transduced CD34+ cells. The transduced GFP+ and CD34+ cells (squared fractions) were sorted by FACSVantage. R1: MND-GFP–transduced CD34+/GFP+ fraction. R2: MND-RIG–transduced CD34+/GFPlow fraction. R3: MND-RIG–transduced CD34+/ GFPhighfraction. (B) A quantity of 1000 transduced progenitor cells (GFP+ and CD34+ cells) were embedded in methylcellulose in the presence of erythropoietin and SCF. CFU-E and BFU-E colonies were scored on day 7 and day 14, respectively. Open bars indicate the number of colonies from MND-RIG–transduced GFPhigh cells. Black bars indicate the number of colonies from MND-RIG–transduced GFPlow cells. Gray bars indicate the number of colonies from MND-GFP–transduced GFP+ cells. Hatched bars indicate the number of colonies from the mock-transduced, unsorted fraction. One experiment was performed and 3 plates were counted in a blinded fashion. The mean ± SEM are shown.
Discussion
Since it is not understood how mutations in the RPS19gene can prevent normal erythroid development, it was important to investigate whether improved erythroid development ensues upon overexpression of RPS19 in primary hematopoietic progenitors from RPS19-deficient patients. This study demonstrates that erythroid colony formation in patients with RPS19-deficient DBA can be substantially improved when the RPS19 cDNA is expressed following gene transfer to CD34+ hematopoietic progenitors. The number of erythroid colonies increased by approximately 3-fold in every experiment performed using CD34+ cells from 3 transfusion-dependent, RPS19-deficient patients with DBA and these findings were statistically significant for each patient tested. Similarly, the colony formation increased 3-fold in the transfusion-independent patient when Epo alone was used (the colony frequency was almost normalized in Epo plus SCF withoutRPS19 gene transfer in this patient). It is noteworthy that higher levels of RPS19 expression led to markedly increased erythroid colony formation in vitro whereas low RPS19 transgene expression had no significant effect. Since overexpression of RPS19 did not seem to cause abnormal myeloid development in vitro, these data collectively suggest that the development of gene replacement therapy for RPS19-deficient DBA is feasible in the 3 transfusion-dependent patients tested here. However, erythroid colony formation is variable from patient to patient and the effects of RPS19 gene transfer on colony formation have to be performed in each transfusion-dependent patient to test whether gene transfer improves erythroid colony formation. Therefore, while we have shown that enforced expression of RPS19 significantly improves erythroid colony formation in the 4 patients tested, our findings from this small study do not prove that enforced expression of RPS19 can improve erythroid development in RPS19-deficient patients who have not been tested here.
The levels of RPS19 mRNA were analyzed in mononulear and CD34+ cells from all 4 patients. It was noteworthy that the relative levels of RPS19 (compared to actin) are 3- to 5-fold higher in CD34+ cells compared with mononuclear cells from healthy individuals and patients with small insertions/point mutations that are not likely to generate unstable RPS19 RNA mutant molecules. These findings are consistent with a recent report demonstrating down-regulation of murine RPS19 expression upon erythroid differentiation of murine splenic erythroblasts.28 This suggests that relatively high levels of RPS19 may be required in hematopoietic progenitors to allow effective early erythroid development. The findings presented in Figure 6 would also support this notion since improved erythroid colony formation was only seen in MRIG-transduced GFP+ progenitors that were expressing high levels of GFP and therefore also high levels of the RPS19 from the transgene since both genes are encoded by a bicistronic mRNA. It is therefore likely that vectors that can express RPS19 to higher levels than the vectors used here may be able to increase the erythroid colony-forming ability even further, perhaps to normal levels. The findings presented here demonstrate an effect on early erythroid development, that is, the number of BFU-Es generated by more primitive myeloid progenitors is increased. Since we have shown that RPS19 RNA levels are considerably higher in the CD34+ population than in more mature mononuclear cells, it is tempting to speculate that it is at the earlier stages of hematopoiesis where high levels of RPS19 are needed for development of erythroid progenitors. We did not observe increased colony size or improved hemoglobinization of colonies upon forced expression of RPS19. It is of interest to note that the RPS19-deficient patient who is not dependent on transfusions had almost normal erythroid colony formation in the presence of SCF and Epo, but the colonies were smaller and less hemoglobinized than normal, indicating improved proliferative reponse of early progenitors without much improvement in the later stages of erythroid development. Further studies are required to determine whether high level expression of theRPS19 transgene in progenitor cells with down-regulation upon erythroid maturation of the transgene is essential to achieve optimal hemolobinization in vitro and whether such down-regulation is a condition for developing effective gene therapy for RPS19-deficient DBA.
It is of interest that the 4 different patients analyzed have different baseline values for erythroid colony formation. When the RPS19gene was overexpressed in CD34+ cells from the transfusion-dependent patients tested here, the erythroid colony-forming ability was increased by a factor of approximately 3 in every situation tested (except transfusion-independent patient 4, using SCF plus Epo where the erythroid colony formation is practically normal with the control vectors). Therefore, the baseline erythroid colony-forming frequency following RPS19 gene transfer is dependent on the basline frequency using the vectors that are tested here. Some of this variability may be related to experimental variation although these differences between the patients may also be related to the type of mutation (genotype) in the RPS19 gene. For example, some mutations may allow some RPS19 protein activity while other mutations may be detrimental and cause dominant-negative effects that counteract the function of the protein generated by the normal endogenous allele and the normal gene introduced by gene transfer. Similarly, modifier genes that affect the function of RPS19 or genes other than RPS19 that affect the efficiency of erythroid colony formation may vary between individual patients. Therefore, it is important to test the effect of RPS19 gene transfer in each patient to determine whether it is possible to improve erythroid colony formation.
Several studies have indicated that erythropoietin signaling is disturbed in DBA,5,13,29 which we confirm here for patients with DBA that are RPS19 deficient. Erythroid colony formation is poor in the presence of erythropoietin alone but there is significant increase in the number and size of BFU-E colonies from DBA progenitors upon addition of SCF together with erythropoietin.27,30 These findings may be clarified by mechanistic studies that have shown cooperation between the c-kit and erythropoietin receptors. SCF stimulation of c-kit is essential for the maintenance of the erythropoietin receptor and Stat5 production, and this in turn results in significantly enhanced Bcl-xL induction and survival of erythroid progenitors in response to erythropoietin.31 Consequently, several possible candidate genes that have a role in erythroid development have been investigated in patients with DBA. These efforts have failed to detect mutations in the genes for the erythropoietin receptor, c-kit, and SCF.3,16,17 The downstream signaling molecules from the erythropoietin receptor, Jak2 and Stat5, would be possible candidates in patients with DBA with normal RPS19. The Jak2 and Stat5a/5b knockouts have a selective defect in definitive fetal erythropoiesis and Stat5 deficiency has been shown to cause apoptosis in erythroid progenitors.32-34 While none of these regulatory genes of erythropoiesis have been shown to represent a primary gene defect in DBA, some or many of these genes or their products may be dysregulated or dysfunctional as a consequence of the primary genetic mutation (for example the mutated RPS19) and this may in turn explain the erythroid specificity of the disease phenotype.
It is not clear why mutations in RPS19 cause a phenotype that is dominated by a specific defect in erythroid development. However, recent evidence suggests that a number of ribosomal proteins have extraribosomal functions including regulation of cell proliferation.35 For example, the L22 ribosomal protein gene is disrupted by a chromosomal translocation in patients with some forms of leukemia.36 The S4 ribosomal protein has been implicated in the pathogenesis of Turner syndrome due to haploinsufficiency of S4,37,38 and inhibition of expression of the ribosomal protein S3a induces apoptosis.39 It is possible that haploinsufficiency ofRPS19 leads to apoptosis of erythroid progenitors and that some RPS19 mutations may exacerbate the haploinsufficiency through a dominant-negative mechanism that interferes with the function of the RPS19 protein that is produced by the normal allele. Further studies are required to reveal the molecular mechanism of RPS19-deficient DBA. Identification of RPS19 target molecules will be particularly important to clarify the mechanism of the erythroid deficiency.
Our findings demonstrate the feasibility of developing gene therapy for RPS19-deficient DBA in the 3 patients tested here. However, it is not clear whether a gene transfer strategy to treat this disorder will require expression vectors that can completely correct the erythroid deficiency in vitro. It is conceivable that a combination ofRPS19 gene replacement therapy and growth factor treatment (erythropoietin, SCF) may be essential to obtain a clinical benefit. It is interesting that some patients with DBA have neutropenia and/or thrombocytopenia in addition to the erythroid deficiency. Pancytopenia and generalized BM hypoplasia have been reported in many patients and long-term BM culture studies revealed hematopoietic defects in several lineages.4 It is therefore possible that the development of gene therapy for RPS19-deficient DBA will require expression of theRPS19 transgene in primitive progenitors that later develop into erythroid progenitors. Recent preliminary findings from our laboratory indicate that there is increased apoptosis and reduced proliferative capacity in primitive CD34+/CD38− progenitors from patients with DBA, supporting the notion that high levels of RPS19 may be required to achieve optimal development of early erythroid progenitors from a common myeloid or erythroid-megakaryocyte progenitor.40Alternatively, and less likely, it may only be essential to express the transgene in erythroid cells to receive full therapeutic benefit. The latter would be desirable, since it will avoid potential complications from the overexpression of the transgene in nonerythroid hematopoietic lineages. Recently, very effective methods have been developed to transduce SCID repopulating cells using lentiviral vectors.41-44 Lentiviral vector design for high-level expression has recently been reported45 and this includes tissue-specific expression.46 Therefore it seems feasible to develop gene therapy for RPS19-deficient DBA using vectors that can transduce human hematopoietic stem cells with high efficiency and express the RPS19 transgene at levels that will be required to achieve clinical benefit.
The authors would like to thank the patients and their families for their support, and Drs. Albert Bekássy and Göran Elinder for their invaluable help. We would also like to thank Dr Roland Perfekt and Dr Peter Nilsson-Ehle for help with statistical analysis, and our colleagues in the Department of Molecular Medicine and Gene Therapy and the Department of Stem Cell Biology, Lund University Hospital, for fruitful discussions.
Supported by The Swedish Medical Research Council, The Swedish Gene Therapy Program, The Swedish Cancer Foundation, The Diamond-Blackfan Anemia Foundation, USA, and Clinical Research Support (ALF) from Lund University Hospital.
A portion of the results presented in this paper were presented at The American Society of Hematology meeting in San Francisco, December, 2000.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Stefan Karlsson, Molecular Medicine and Gene Therapy, BMC A12, 221 84, Lund, Sweden; e-mail:stefan.karlsson@molmed.lu.se.