Previous studies demonstrated that Kit activation confers radioprotection. However, the mechanism by which Kit signaling interferes with cellular response to ionizing radiation (IR) has not been firmly established. Based on the role of the sphingomyelin (SM) cycle apoptotic pathway in IR-induced apoptosis, we hypothesized that one of the Kit signaling components might inhibit IR-induced ceramide production or ceramide-induced apoptosis. Results show that, in both Ba/F3 and 32D murine cell lines transfected with wild-type c-kit, stem cell factor (SCF) stimulation resulted in a significant reduction of IR-induced apoptosis and cytotoxicity, whereas DNA repair remained unaffected. Moreover, SCF stimulation inhibited IR-induced neutral sphingomyelinase (N-SMase) stimulation and ceramide production. The SCF inhibitory effect on SM cycle was not influenced by wortmannin, a phosphoinositide-3 kinase (PI3K) inhibitor. The SCF protective effect was maintained in 32D-KitYF719 cells in which the PI3K/Akt signaling pathway is abolished due to mutation in Kit docking site for PI3K. In contrast, phospholipase C γ (PLCγ) inhibition by U73122 totally restored IR-induced N-SMase stimulation, ceramide production, and apoptosis in Kit-activated cells. Moreover, SCF did not protect 32D-KitYF728 cells (lacking a functional docking site for PLCγ1), from IR-induced SM cycle. Finally, SCF-induced radioprotection of human CD34+ bone marrow cells was also inhibited by U73122. Altogether, these results suggest that SCF radioprotection is due to PLCγ1-dependent negative regulation of IR-induced N-SMase stimulation. Beyond the scope of Kit-expressing cells, it suggests that PLCγ1 status could greatly influence the post-DNA damage cellular response to IR, and perhaps, to other genotoxic agents.
Introduction
Ionizing radiation (IR) cytotoxicity is generally thought to be the result of a DNA double-strand break (dsb) caused by either direct interaction of IR with DNA or indirect action via the production of free radicals following radiolysis of water.1 Following DNA damage, several modalities of cellular response have been described including reversible cell cycle alterations, reproductive (or mitotic) cell death, and apoptosis. It has been described that apoptosis, a rapid and irreversible process, is generally correlated with a high radiosensitivity profile suggesting that post-DNA damage response plays an important role in IR cytotoxicity. For this reason, IR-induced apoptosis signaling has received a great deal of attention. In previous studies, it has been proposed that IR, as some other genotoxic agents, induces apoptosis by stimulating the sphingomyelin (SM) cycle. This signaling cascade consists of SM hydrolysis through the activation of a sphingomyelinase (SMase) with concomitant generation of ceramide, which can mediate this apoptotic process.2-8 Both acidic and neutral SMase (N-SMase) have been implicated in IR-induced ceramide production and apoptosis.3,4 Indeed, acid SMase has been implicated in IR-induced cell death in endothelial cells, oocytes, and embryonic fibroblasts9-11; N-SMase has been implicated mainly in leukemic cell models,12,13 indicating cell-type specificity for the different ceramide-producing pathways. The fact that the sensitivity to IR could be restored in the asmase(−/−) mouse embryonic fibroblasts by administration of natural ceramide provides unequivocal evidence for the requirement of ceramide in IR-induced apoptotic death.10
Other studies have demonstrated that the SM-ceramide apoptosis pathway is efficiently regulated by different parameters that can interfere either with ceramide production or ceramide-induced apoptosis. Among these regulators, protein kinase C (PKC) appears to play an important role. Indeed, it has been shown that phorbol ester (TPA)– or diacylglycerol (DAG)-induced PKC stimulation both resulted in inhibition of ceramide-induced apoptosis.14 Moreover, it has been described that PKC contributes to basal N-SMase enzymatic activity regulation,15 and that TPA- or phosphatidylserine-driven PKC stimulation resulted in inhibition of SMase activation, SM hydrolysis, and ceramide generation induced by IR or by antileukemic drugs.2 16 Therefore, it is conceivable that any internal or external signals resulting in PKC stimulation may significantly affect IR-induced cytotoxicity by interfering with upstream or downstream (or both) ceramide generation. This represents an attractive mechanism to explain the radioprotective effect of growth factors or cytokines that, through activation of their cognate receptors and subsequent downstream signaling pathways, may stimulate PKC.
Stem cell factor (SCF), a hematopoietic growth factor, was found to protect animals against IR.17,18 The influence of Kit signaling on the cellular response to IR is also illustrated by the hypersensitivity to IR of Steel and White Spotting mice, which are deficient for SCF or its cognate receptor, the tyrosine kinase c-kit product (Kit), respectively. Activation of Kit by SCF promotes Kit dimerization, autophosphorylation, and transphosphorylation of Kit at specific tyrosine residues that can serve as docking sites for src-homology-2 (SH2) domain-containing signaling molecules. Among these, phosphoinositide-3 kinase (PI3K) and phospholipase C γ (PLCγ) were found critical for Kit signaling propagation.19-23 PLCγ induces phosphatidylinositol (PI) 4,5-biphosphate hydrolysis and production of DAG and inositol 1,4,5-triphosphate (IP3). These 2 messengers induce PKC activation and intracellular Ca++ release, respectively.24PI3K-lipid products (PI3-phosphates) have also been described as PKC activators.25 Moreover, it has been reported that some PI3K-lipid products may also activate PLCγ, suggesting that PLCγ and PI3K signaling pathways may interact.26 Therefore, it is reasonable to speculate that Kit activation may alter the apoptotic response to IR by interfering with the SM-ceramide pathway through a PKC-dependent mechanism mediated by either PLCγ or PI3K stimulation.
Such a hypothesis may have important clinical implications. Indeed, new insights into the SCF radioprotective effect may help to define optimal conditions for its clinical use to protect hematopoiesis against IR-induced hematoxicity after extended field radiotherapy. Moreover, better understanding of the mechanism by which Kit signaling interferes with IR cytotoxicity might offer new approaches for improving radiotherapy efficiency in Kit-activated tumor cells. This could be a major concern in the treatment of small-cell lung carcinomas and breast cancers that display a SCF/Kit autocrine loop,27 28 or in systemic mastocytosis and gastrointestinal stroma tumor (GIST), which usually harbor active Kit variants.
The aim of this study was to evaluate the influence of Kit activation by SCF on IR-induced apoptosis and cytotoxicity and to investigate whether Kit signaling may interfere with IR-activated SM cycle through PI3K or PLCγ signaling pathway.
Materials and methods
Drugs and reagents
RPMI 1640, penicillin, streptomycin, and fetal calf serum (FCS) were obtained from Gibco BRL (Cergy Pontoise, France). Murine SCF was obtained from R & D Systems (Abigdon, United Kingdom). Silica gel 60 thin-layer chromatography plates were from Merck (Darmstadt, Germany). U73122 and U73343 were purchased from Calbiochem (San Diego, CA). Wortmannin was supplied by Sigma Chemical (St Louis, MO). Cell permeant ceramide (C6) was provided by Alexis (Coger, France). Rabbit anti-PLCγ1 polyclonal antibody (no. 1249) and rabbit anti–Bcl-2 polyclonal antibody were from Santa Cruz, TEBU (Le Perray en Yveline, France). Mouse antiphosphotyrosine monoclonal antibody (PY-20) was obtained from Transduction Laboratories (Pantin, France). Mouse antiphospho-Akt antibody was provided by Biosource (Clinisciences, Montrouge, France). [9,10 (n)-3H] palmitic acid and enhanced chemiluminescence detection system were from Amersham (Les Ulis, France). [Choline-methyl-14C] SMase was obtained from Dupont NEN (Les Ulis, France).
Cell culture
Ba/F3 a murine, interleukin 3 (IL-3)–dependent, Kit− pro-B lymphoid cell line was transfected with LXSN-Kit retroviral expression vector. 32D is a myelomonocytic IL-3–dependent, Kit− myelomonocytic cell line. 32D cells were obtained from Dr Joel Greenberger (Pittsburgh, PA) and transfected with retroviral vectors expressing murine wild-type c-kit (32D-Kit) or mutants KitYF728 and KitYF719 as described.29 Both cell types were cultured in RPMI 1640 supplemented with 10% FCS, 2 mM glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% of conditioned media from WEHI cells containing IL-3. Human bone marrow CD34+ cells were purchased from Poietics Human Stem Cell Systems (Biowhittaker, Verviers, Belgium).
DNA dsb analysis
Cells were radiolabeled by preincubation for 48 hours with 0.5 μCi/mL (1.8 × 104 Bq/mL) of methyl [3H]thymidine. Medium was replaced by fresh medium for 2 hours, then cells were then treated with SCF (200 ng/mL) for 30 minutes. Then, 0.5 × 106 control and irradiated cells were embedded in 0.7% low-melting agarose (BRL, Bethesda, MD) plugs. These were incubated overnight at 48°C in 0.2 M EDTA containing 10 mg/mL proteinase K. The agarose plugs were washed 3 times for 1 hour in 0.2 M EDTA and stored at 4°C prior to analysis. The samples were analyzed on a 1% agarose gel in a × 0.5 TBE buffer (45 mM Tris-borate, 1 mM EDTA, pH 8). The buffer was cooled by continuously recirculating water. Electrophoresis voltage was increased as follows: 18 V (36 hours), 45 V (7 hours), 90 V (1.5 hours), and 270 V (0.5 hours). The gel was stained with ethidium bromide (0.5 μg/mL) and visualized under UV illumination. Intact and fragmented DNA were separated and radioactivity of the 2 fractions was counted by liquid scintillation. Results were expressed as the percentage of fragmented DNA radioactivity/fragmented DNA radioactivity plus intact DNA radioactivity.
N-SMase activity assay
Activity of SMase was assayed as previously described using [choline-methyl-14C] SMase (120000 dpm/assay) as substrate.30
Metabolic cell labeling and ceramide quantitation
Total cellular ceramide quantitation was performed by labeling cells to isotopic equilibrium with 1 μCi/mL (3.7 × 104Bq/mL) of [9, 10-3H] palmitic acid (53.0 Ci/mmol or 1.96 × 1012 Bq/mmol, Amersham) for 48 hours in complete medium as described previously.30 Cells were then washed and resuspended in serum-free medium for kinetic experiments. Lipids were extracted and resolved by thin-layer chromatography. Ceramide was scraped and quantitated by liquid scintillation spectrometry.
Irradiation
Irradiations were performed using a 60Co source (1.25 MeV; Theratron, General Electric, Toronto, ON, Canada) at a dose rate of 1 Gy/min.
DAPI staining
Changes in nuclear chromatin were evaluated by fluorescence microscopy by DAPI staining.31
Clonogenic assay
Cells were seeded in 96-well flat-bottom plates at the concentration of 10 Ba/F3 cells/well and cultured in medium in the presence of 200 ng/mL SCF. The optimal SCF concentration was obtained from dose-response curves (where 50% proliferation was observed at ∼50 ng/mL SCF). After 7 days, colonies (minimum size of 50 cells) formed in each well were scored under an inverted microscope. For untreated sample, cloning efficiency of untreated cells was about 50%. To measure IR-induced cytotoxicity, cells were irradiated at 0 to 10 Gy, then seeded into microplates. The surviving fraction was calculated at each dose level as the percentage of survival colonies compared with the nonirradiated sample. Best fit survival curve was then calculated by computer using the linear quadratic model.32 33
Measurement of phosphatidylserine externalization by annexin V binding
Human CD34+ bone marrow cells (10 × 103) were pelleted and resuspended in Hepes buffer (10 mM Hepes-NaOH, pH 7.4, 150 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2). These were then incubated for 5 minutes with 1 μg/mL annexin V–fluorescein isothiocyanate (FITC; Bender Medsystem, Vienna, Austria), and 10 μg/mL propidium iodide followed by flow cytometry on a FACScan (Becton Dickinson, Paris, France).34
Statistical analysis
The Student t test was used to test for statistical significance.
Results
Influence of SCF stimulation on radiation-induced clonogenic cell death and apoptosis
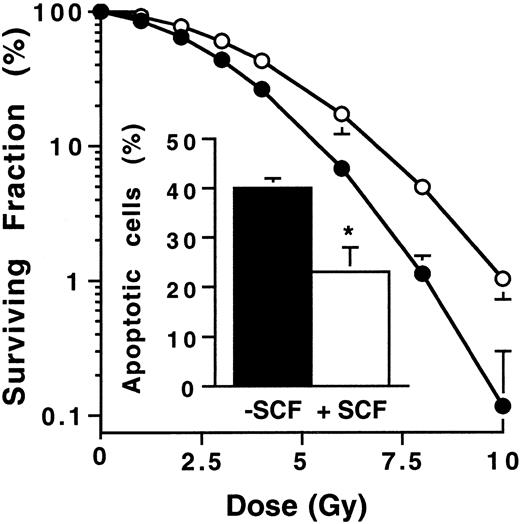
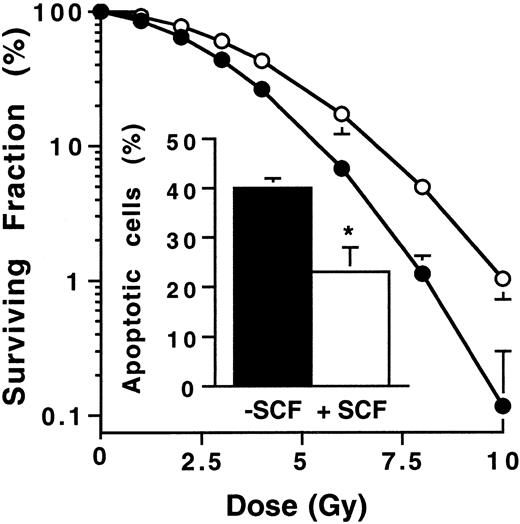
Ba/F3-Kit cells were stimulated or not with SCF (200 ng/mL for 30 minutes), irradiated with increasing IR doses, and then assayed for their capacity to form colonies in liquid medium. Clonogenic efficiency was about 50% without differences whether or not cells were stimulated by SCF (data not shown). IR induced a dose-dependent inhibition of clonogenicity in both unstimulated and stimulated Ba/F3-Kit cells. However, SCF stimulation resulted in a significant reduction of IR-induced cytotoxicity as assessed by D0 determination (dose required for 37% cytoreduction) with D0 value of 4.38 Gy and 3.35 Gy for stimulated and unstimulated Ba/F3-Kit, respectively (P < .05). We also investigated the influence of SCF stimulation on IR-induced apoptosis. Based on D0 value, the dose of 4 Gy was used. We found that SCF-induced Kit activation resulted in an approximate 2-fold reduction in IR-induced apoptosis as measured by DAPI staining (Figure1, insert). Altogether, these results demonstrate that SCF confers significant radioprotection in Ba/F3-Kit cells. For this reason, we investigated the possible influence of SCF stimulation on DNA repair in irradiated Ba/F3-Kit cells.
Role of SCF pretreatment on irradiated Ba/F3-Kit cell clonogenicity.
Cells were pretreated (○) or not (●) with SCF (200 ng/mL for 30 minutes) and then seeded in flat-bottom 96-wells plates at a density of 10 cells/well. Cells were irradiated at a dose ranging between 0 and 10 Gy. Colonies were counted after 1 week of culture. Results are the mean ± SD of 3 independent experiments. The insert shows the effect of SCF on apoptosis of irradiated Ba/F3-Kit cells. SCF-stimulated or unstimulated Ba/F3-Kit cells were irradiated at 4 Gy and morphology was examined by fluorescence microscopy after DAPI staining at 24 hours. Results are the mean ± SD of 3 independent experiments (*P < .05).
Role of SCF pretreatment on irradiated Ba/F3-Kit cell clonogenicity.
Cells were pretreated (○) or not (●) with SCF (200 ng/mL for 30 minutes) and then seeded in flat-bottom 96-wells plates at a density of 10 cells/well. Cells were irradiated at a dose ranging between 0 and 10 Gy. Colonies were counted after 1 week of culture. Results are the mean ± SD of 3 independent experiments. The insert shows the effect of SCF on apoptosis of irradiated Ba/F3-Kit cells. SCF-stimulated or unstimulated Ba/F3-Kit cells were irradiated at 4 Gy and morphology was examined by fluorescence microscopy after DAPI staining at 24 hours. Results are the mean ± SD of 3 independent experiments (*P < .05).
Influence of SCF stimulation on DNA repair
DNA dsbs are believed to be responsible for IR-induced lethality. Therefore, we investigated whether SCF stimulation could influence either postirradiation dsb levels or DNA repair kinetics. Stimulated or unstimulated Ba/F3-Kit cells were irradiated (4 Gy) and dsbs were measured by high-voltage electrophoresis over a 6-hour period. We observed that SCF stimulation did not influence immediate post-IR damage or DNA repair kinetics (Figure 2). This result suggested that Kit signaling influenced postdamage cellular response rather than IR-induced lesions. For this reason, we hypothesized that SCF stimulation could interfere with IR-activated SM cycle apoptosis signaling. In a first series of experiments, we evaluated the influence of SCF-mediated Kit activation on IR-induced ceramide production.
Effect of SCF on DNA fragmentation of irradiated Ba/F3-Kit cells.
Cells were labeled with 0.5 μCi/mL [3H]thymidine (1.8 × 104 Bq/mL) for 48 hours. Cells were then washed, treated (○) or not (●) with SCF (200 ng/mL for 30 minutes), and irradiated at 4 Gy. At indicated times, cells were embedded in agarose plugs and digested and the DNA was separated by high-voltage electrophoresis. Fragmented and unfragmented DNA-associated radioactivities were quantitated. The results are expressed as percentage of fragmented over total DNA-associated radioactivity. Results are the mean ± SD of 3 independent experiments.
Effect of SCF on DNA fragmentation of irradiated Ba/F3-Kit cells.
Cells were labeled with 0.5 μCi/mL [3H]thymidine (1.8 × 104 Bq/mL) for 48 hours. Cells were then washed, treated (○) or not (●) with SCF (200 ng/mL for 30 minutes), and irradiated at 4 Gy. At indicated times, cells were embedded in agarose plugs and digested and the DNA was separated by high-voltage electrophoresis. Fragmented and unfragmented DNA-associated radioactivities were quantitated. The results are expressed as percentage of fragmented over total DNA-associated radioactivity. Results are the mean ± SD of 3 independent experiments.
Influence of SCF stimulation on IR-induced ceramide production and SMase activation
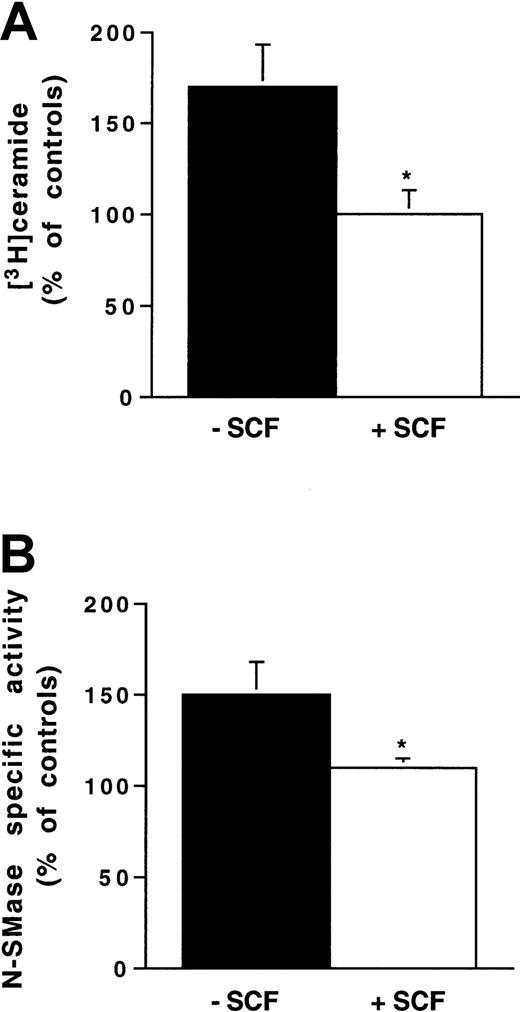
Stimulated or unstimulated Ba/F3-Kit cells were irradiated (4 Gy) and, based on previous kinetics studies,4 intracellular ceramide concentration was monitored over a 30-minute period. As shown in Figure 3, IR induced a time-dependent ceramide accumulation with a maximum 75% increase at 10 to 12 minutes in unstimulated Ba/F3-Kit cells. In contrast, IR induced no ceramide accumulation in SCF-stimulated cells. This result suggested that Kit signaling inhibited SMase-mediated SM hydrolysis and subsequent ceramide generation. In fact, whereas IR induced a time-dependent N-SMase stimulation more than 50% at 10 to 12 minutes in unstimulated Ba/F3-Kit cells, SCF stimulation resulted in abrogation of IR-induced N-SMase stimulation (Figure 4). However, we were unable to detect any stimulation of acidic SMase up to 30 minutes after IR (data not shown). Moreover, we found that SCF stimulation had no influence on the toxicity of cell-permeant C6-ceramide used in a dose range between 10 and 50 μM (data not shown). These results suggested that SCF protection was essentially due to inhibition of IR-induced N-SMase stimulation. To confirm these results in another cellular model, we investigated the influence of SCF stimulation on IR-induced N-SMase activation and ceramide production in 32D-Kit cells. As shown in Figure 5, IR significantly boosted N-SMase activity and enhanced intracellular ceramide concentration at a similar magnitude to those measured in Ba/F3-Kit cells, whereas these effects were not observed in SCF-stimulated 32D-Kit cells. Among different previously identified Kit signaling components, we first examined the possible role of the PI3K/Akt pathway on SCF inhibitory effect in both Ba/F3-Kit and 32D-Kit cells.
Effect of SCF on ceramide generation in irradiated Ba/F3-Kit cells.
Cells were prelabeled with [3H]palmitate for 48 hours. Cells were then washed, treated (○) or not (●) with SCF (200 ng/mL for 30 minutes), and irradiated at 4 Gy. Following incubation during the indicated times, aliquots (3 × 106 cells/mL) were collected and lipids were extracted. Labeled ceramide was resolved by thin-layer chromatography and identified as described in “Materials and methods.” Results are the mean ± SD of 3 independent experiments (*P < .05).
Effect of SCF on ceramide generation in irradiated Ba/F3-Kit cells.
Cells were prelabeled with [3H]palmitate for 48 hours. Cells were then washed, treated (○) or not (●) with SCF (200 ng/mL for 30 minutes), and irradiated at 4 Gy. Following incubation during the indicated times, aliquots (3 × 106 cells/mL) were collected and lipids were extracted. Labeled ceramide was resolved by thin-layer chromatography and identified as described in “Materials and methods.” Results are the mean ± SD of 3 independent experiments (*P < .05).
Effect of SCF on N-SMase activity in irradiated Ba/F3-Kit cells.
Cells were treated or not with SCF (200 ng/mL for 30 minutes) and irradiated at 4 Gy. Following incubation during the indicated times (as shown in the insert), aliquots (3 × 106 cells/mL) were collected and enzyme assay was performed as described in “Materials and methods” using [choline-methyl-14C]-SM (60.000 dpm/assay) as substrate. Results are shown at maximum N-SMase stimulation (10-15 minutes after irradiation) and are the mean ± SD of 3 independent experiments (*P < .05). The insert shows N-SMase activity of SCF-treated (○) or untreated (●) Ba/F3-Kit irradiated at 4 Gy that was monitored during a 20-minute period. Results are representative of 3 independent experiments.
Effect of SCF on N-SMase activity in irradiated Ba/F3-Kit cells.
Cells were treated or not with SCF (200 ng/mL for 30 minutes) and irradiated at 4 Gy. Following incubation during the indicated times (as shown in the insert), aliquots (3 × 106 cells/mL) were collected and enzyme assay was performed as described in “Materials and methods” using [choline-methyl-14C]-SM (60.000 dpm/assay) as substrate. Results are shown at maximum N-SMase stimulation (10-15 minutes after irradiation) and are the mean ± SD of 3 independent experiments (*P < .05). The insert shows N-SMase activity of SCF-treated (○) or untreated (●) Ba/F3-Kit irradiated at 4 Gy that was monitored during a 20-minute period. Results are representative of 3 independent experiments.
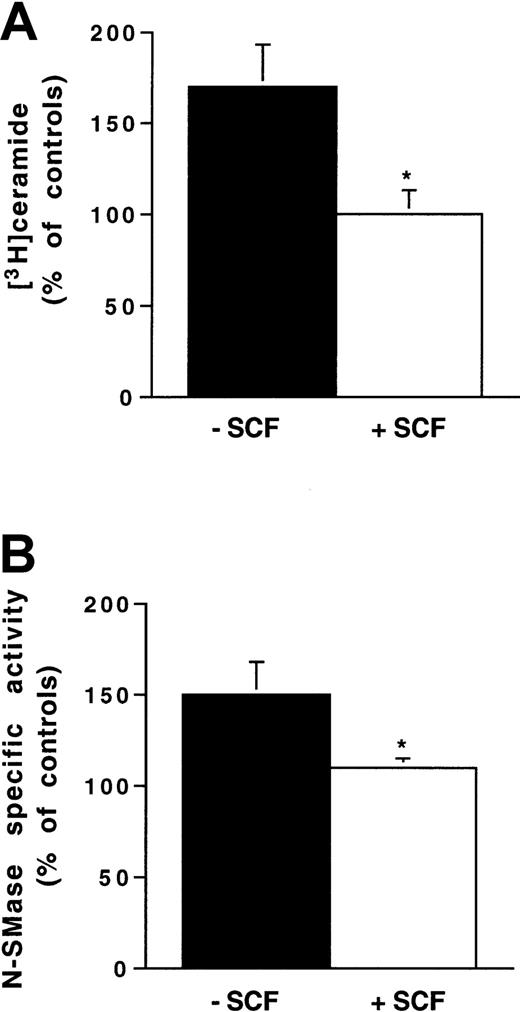
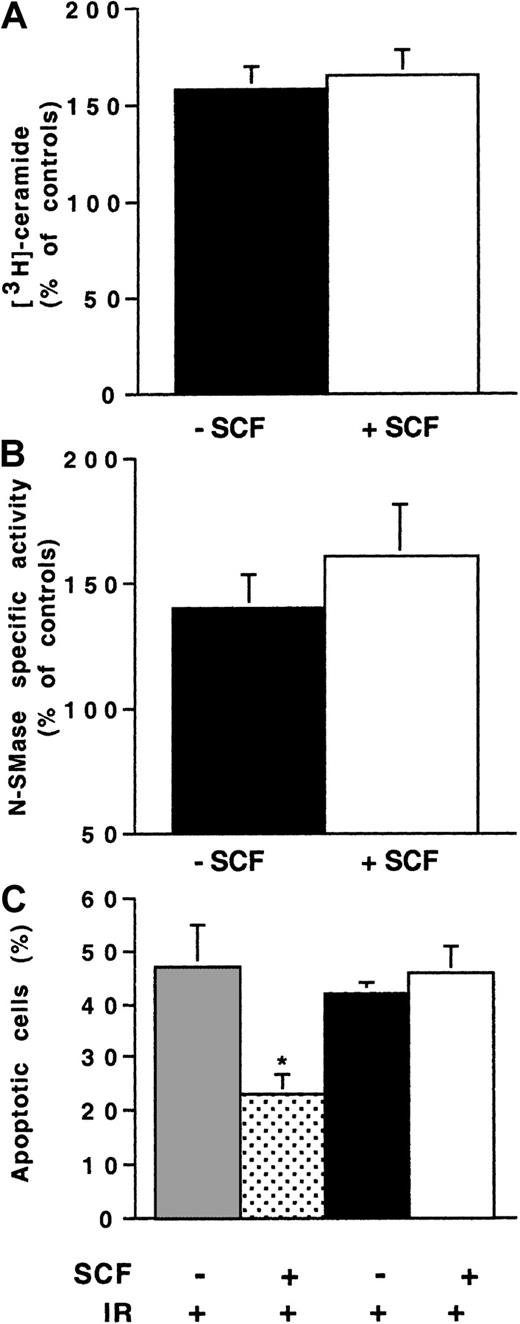
Influence of SCF stimulation on ionizing radiation-induced N-SMase stimulation and ceramide generation in 32D-Kit cells.
Cells were pretreated or not with SCF (200 ng/mL for 30 minutes) and irradiated with 4 Gy. N-SMase activity and ceramide levels were performed as described previously. Results are shown at maximum ceramide production (A) and N-SMase stimulation (B) (10-15 minutes) and are the mean ± SD of 3 independent experiments (*P < .05).
Influence of SCF stimulation on ionizing radiation-induced N-SMase stimulation and ceramide generation in 32D-Kit cells.
Cells were pretreated or not with SCF (200 ng/mL for 30 minutes) and irradiated with 4 Gy. N-SMase activity and ceramide levels were performed as described previously. Results are shown at maximum ceramide production (A) and N-SMase stimulation (B) (10-15 minutes) and are the mean ± SD of 3 independent experiments (*P < .05).
Influence of PI3K pathway on IR-induced N-SMase stimulation
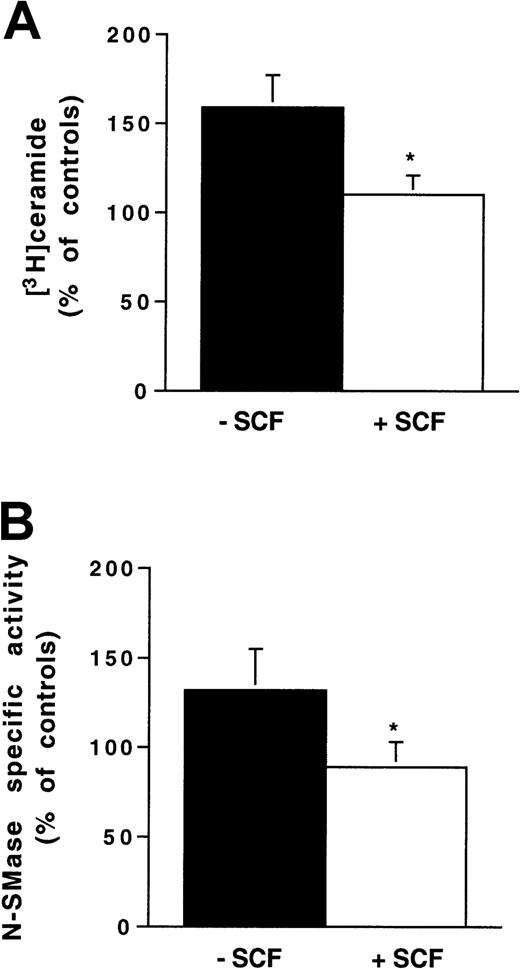
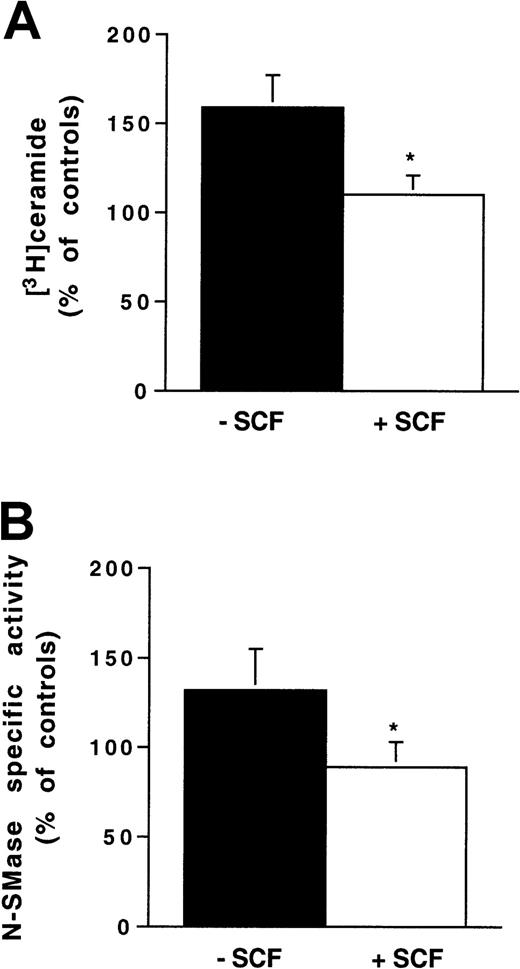
In preliminary experiments performed with antiphospho-Akt antibody, we confirmed that, in both Ba/F3-Kit cells and 32D-Kit cells, SCF stimulation resulted in PI3K-dependent Akt phosphorylation, which peaked at 10 minutes after stimulation and was totally inhibited by pretreatment with wortmannin (25 nM, for 1 hour), a PI3K inhibitor (data not shown). However, pretreatment with wortmannin did not abrogate the inhibitory effect of SCF on IR-induced N-SMase stimulation (data not shown). These results suggested that the PI3K/pathway was not involved in N-SMase inhibition. To confirm this hypothesis, we performed a similar experiment with 32D-KitYF719 cells. These cells express a c-kit point mutant that prevents the recruitment of the p85 regulatory subunit of PI3K without interfering with SCF-induced Kit phosphorylation.29 As shown in Figure6, in 32D-KitYF719 cells, the SCF inhibitory effect on IR-induced ceramide generation and N-SMase stimulation was maintained. Altogether, these results confirmed that, among Kit signaling components, the PI3K/Akt pathway played little, if any, role in Kit-induced negative regulation of IR-activated SM-ceramide apoptosis pathway. For this reason, we investigated the possible role of Kit-induced PLCγ1 activation in the SCF protective effect.
Influence of SCF stimulation on ionizing radiation-induced N-SMase stimulation and ceramide generation in 32D-KitYF719 cells.
Cells were pretreated or not with SCF (200 ng/mL for 30 minutes) and irradiated at 4 Gy. N-SMase activity and ceramide levels were performed as previously described. Results are shown at maximum ceramide production (A) and N-SMase stimulation (B; 10-15 minutes) and are the mean ± SD of 3 independent experiments (*P < .05).
Influence of SCF stimulation on ionizing radiation-induced N-SMase stimulation and ceramide generation in 32D-KitYF719 cells.
Cells were pretreated or not with SCF (200 ng/mL for 30 minutes) and irradiated at 4 Gy. N-SMase activity and ceramide levels were performed as previously described. Results are shown at maximum ceramide production (A) and N-SMase stimulation (B; 10-15 minutes) and are the mean ± SD of 3 independent experiments (*P < .05).
Influence of PLCγ1 on IR-induced ceramide production, N-SMase stimulation, and apoptosis
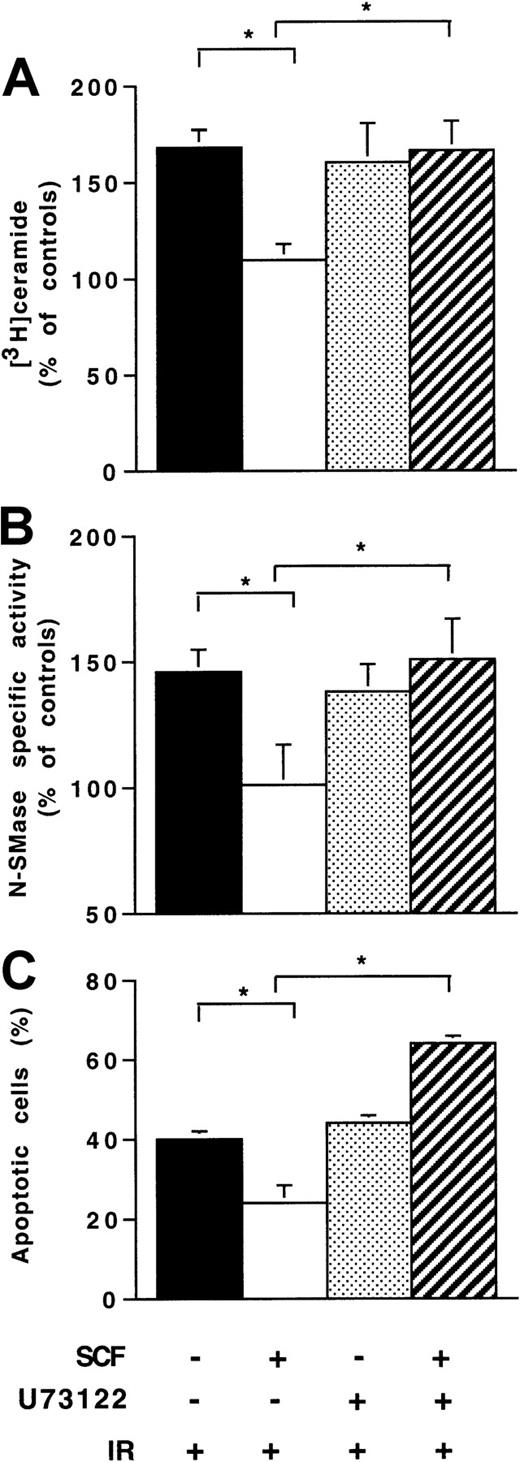
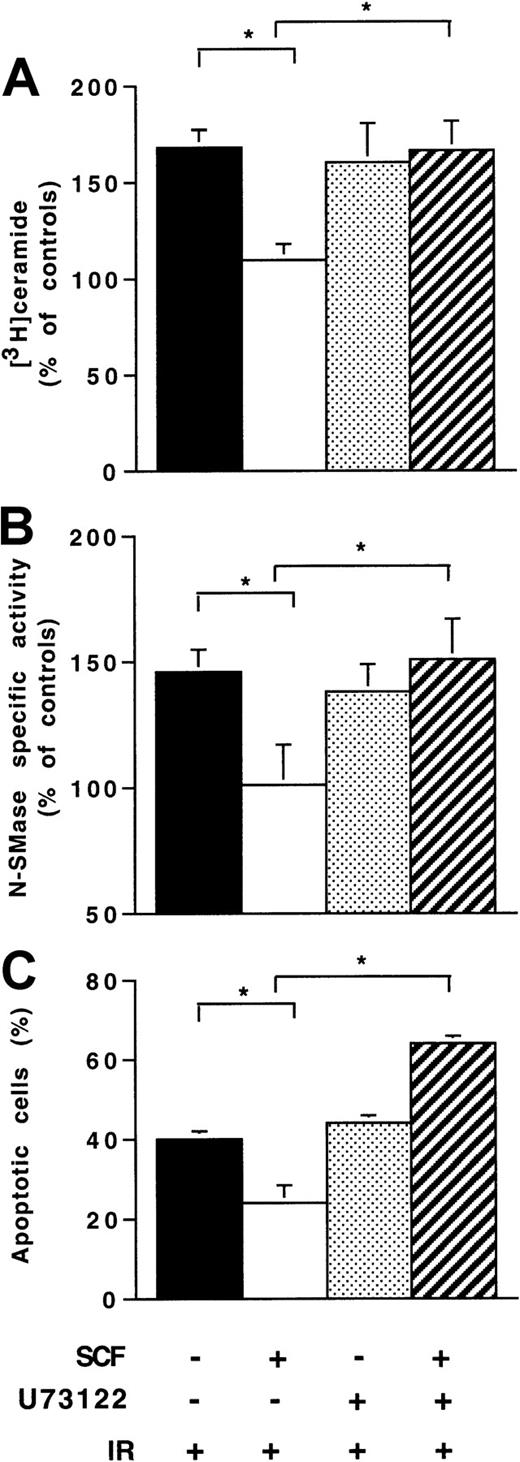
In preliminary experiments conducted in Ba/F3-Kit cells, we found that SCF stimulation resulted in PLCγ1 tyrosine phosphorylation within 2 to 5 minutes as revealed by immunoprecipitation with anti-PLCγ1 and immunoblotting with antiphosphotyrosine (data not shown). Moreover, SCF stimulated Ca++ mobilization, which was inhibited by pretreatment with U73122 (1 μM, for 1 hour), a PLCγ inhibitor (data not shown). Pretreatment with U73122 abrogated the inhibitory effect of SCF on IR-induced ceramide production (Figure7A) and N-SMase (Figure 7B), suggesting that PLCγ activation played an important role in the SCF protective effect in Ba/F3-Kit cells. In fact, in these cells, treatment with U73122 abrogated SCF-induced inhibition of the IR apoptotic effect (Figure 7C).
Influence of U73122 on SCF inhibitory effect on ionizing radiation-induced N-SMase stimulation, ceramide generation, and apoptosis.
Ba/F3-Kit cells were pretreated with U73122 alone (1 μM; 1 hour) or with SCF alone (200 ng/mL; 30 minutes) or with U73122 and SCF, then irradiated at 4 Gy. Untreated cells are also indicated. Results are shown at maximum ceramide production (A), N-SMase stimulation (B; 10-15 minutes), and apoptosis evaluated by DAPI staining (24 hours; C). Values are the mean ± SD of 3 independent experiments (*P < .05).
Influence of U73122 on SCF inhibitory effect on ionizing radiation-induced N-SMase stimulation, ceramide generation, and apoptosis.
Ba/F3-Kit cells were pretreated with U73122 alone (1 μM; 1 hour) or with SCF alone (200 ng/mL; 30 minutes) or with U73122 and SCF, then irradiated at 4 Gy. Untreated cells are also indicated. Results are shown at maximum ceramide production (A), N-SMase stimulation (B; 10-15 minutes), and apoptosis evaluated by DAPI staining (24 hours; C). Values are the mean ± SD of 3 independent experiments (*P < .05).
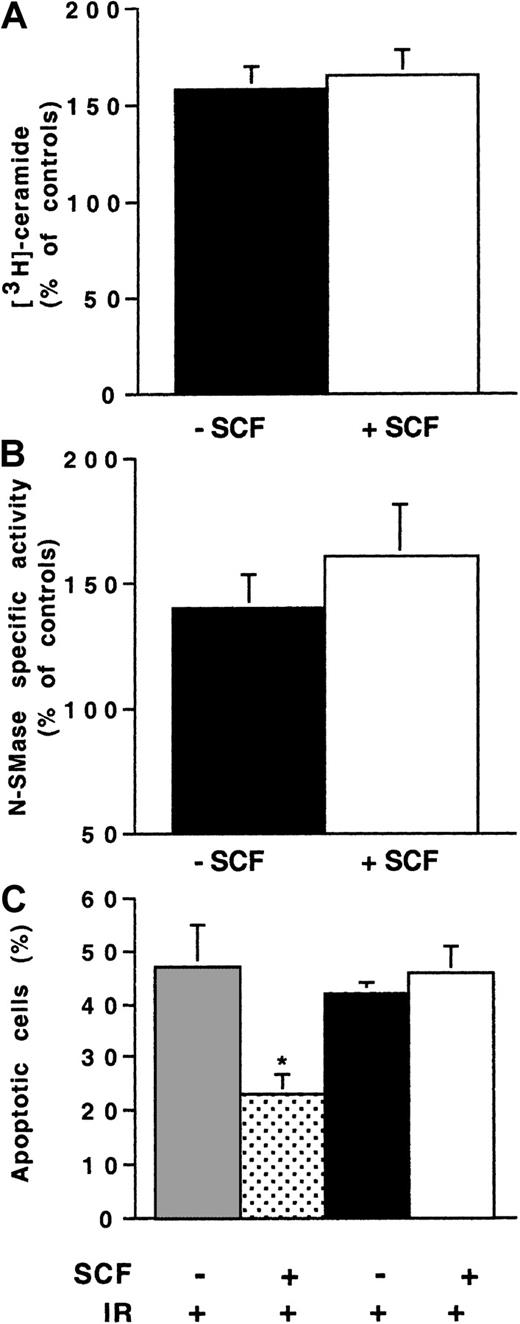
To further confirm the role of PLCγ1 in N-SMase regulation, we used 32D-KitYF728 cells. These variants display a mutation in Kit intracellular domain, which prevents the recruitment of PLCγ1 without interfering with PI3K stimulation.35 In these cells, SCF stimulation influenced neither ceramide production (Figure8A) nor N-SMase activation (Figure 8B) induced by IR. Moreover, the SCF-mediated protective effect against IR observed in 32D-Kit was not observed in 32D-KitYF728 cells (Figure 8C). Altogether, these results confirmed that PLCγ1 activation played a crucial role in the SCF-induced inhibition of N-SMase and IR-induced apoptosis.
Influence of SCF stimulation on irradiated-induced N-SMase stimulation and ceramide generation in 32D-KitYF728 cells.
32D-KitYF728 cells were pretreated or not with SCF (200 ng/mL for 30 minutes) and irradiated at 4 Gy. N-SMase activity and ceramide production were measured as previously described. Results are shown at maximum ceramide production (A), N-SMase stimulation (B; 10-15 minutes). (C) Apoptosis evaluated by DAPI staining at 24 hours of 32D-Kit (░, ) and 32D-KitYF728 cells (▪, ■) irradiated at 4 Gy. Values are the mean ± standard deviation of 3 independent experiments (*P < .05).
) and 32D-KitYF728 cells (▪, ■) irradiated at 4 Gy. Values are the mean ± standard deviation of 3 independent experiments (*P < .05).
Influence of SCF stimulation on irradiated-induced N-SMase stimulation and ceramide generation in 32D-KitYF728 cells.
32D-KitYF728 cells were pretreated or not with SCF (200 ng/mL for 30 minutes) and irradiated at 4 Gy. N-SMase activity and ceramide production were measured as previously described. Results are shown at maximum ceramide production (A), N-SMase stimulation (B; 10-15 minutes). (C) Apoptosis evaluated by DAPI staining at 24 hours of 32D-Kit (░, ) and 32D-KitYF728 cells (▪, ■) irradiated at 4 Gy. Values are the mean ± standard deviation of 3 independent experiments (*P < .05).
) and 32D-KitYF728 cells (▪, ■) irradiated at 4 Gy. Values are the mean ± standard deviation of 3 independent experiments (*P < .05).
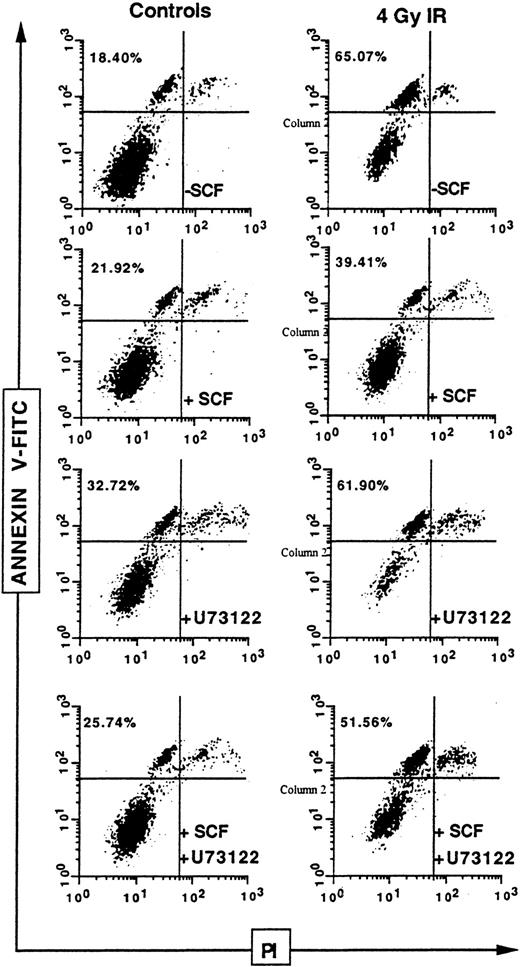
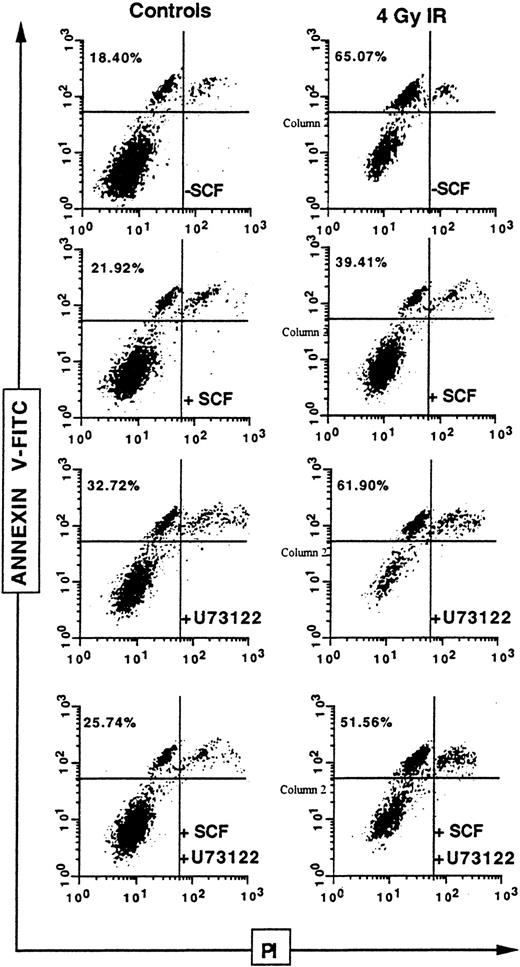
Finally, to evaluate the physiologic relevance of SCF-mediated protection against IR, we examined phosphatidylserine externalization that generally precedes the nuclear changes that define apoptosis,34 on human CD34+ bone marrow cells (Figure 9). The CD34+ cells, gated on the propidium iodine–negative population, presented a significant increase in annexin V binding 24 hours after 4 Gy IR, increasing from a basal level of 18.40% to 65.07%. However, when these cells, which express wild-type c-Kit, were stimulated with SCF, annexin V binding decreased to 39.41%. This result clearly demonstrates that SCF-induced radioprotection can be observed on human primary cells. Furthermore, although the PLCγ inhibitor U73122 presented no significant effect on annexin V binding of irradiated CD34+ cells (61.90%), it almost totally blocked the protective effect of SCF, which increased from 39.41% to 51.56%. This confirmed that PLCγ activation is implicated in SCF radioprotection.
Influence of U73122 on SCF inhibitory effect on ionizing radiation-induced apoptosis on primary CD34+ cells.
Human CD34+ bone marrow cells were pretreated or not with U73122 alone (1 μM; 1 hour) or with SCF alone (200 ng/mL; 30 minutes) or with U73122 and SCF, then irradiated at 4 Gy. Phosphatidylserine externalization, as assessed by the binding of annexin V–FITC on the propidium iodide–negative population, was evaluated after 24 hours.
Influence of U73122 on SCF inhibitory effect on ionizing radiation-induced apoptosis on primary CD34+ cells.
Human CD34+ bone marrow cells were pretreated or not with U73122 alone (1 μM; 1 hour) or with SCF alone (200 ng/mL; 30 minutes) or with U73122 and SCF, then irradiated at 4 Gy. Phosphatidylserine externalization, as assessed by the binding of annexin V–FITC on the propidium iodide–negative population, was evaluated after 24 hours.
Discussion
Previous studies have shown that SCF suppressed apoptosis induced by γ-irradiation in vitro and protected mice and dogs from radiation-induced hematopoietic and intestinal cell death in vivo.17,18,36-38 Our study confirms in 2 different cellular models these findings and shows that SCF stimulation has no influence on DNA repair kinetics, suggesting that SCF acts by interfering with postdamage cellular response. This result is in agreement with a recent study showing that SCF protects human cord blood CD34+ against IR without influencing DNA repair capacity as assessed by the comet assay.39 In a recent study, it has been documented that ligand-induced activation of hepatocyte growth factor receptor, another tyrosine kinase receptor (RTK), exerts its radioprotective effect by stimulating DNA repair through a PI3K/Akt-dependent signaling pathway. These findings suggest that, although RTK families share common downstream signaling pathways, dsb repair capacity can be differently regulated.40Therefore, we hypothesized that Kit signaling interfered with postdamage response and, for example, inhibited IR-triggered apoptotic signals.
Different mechanisms of Kit-mediated suppression of apoptosis have been reported, depending on the cellular model and stress conditions. For example, it has been suggested that the protective effect of SCF on growth factor deprivation-induced apoptosis of natural killer cells could be related to SCF-induced Bcl-2 overexpression.41 For this reason, and based on the influence of Bcl-2 on IR-induced apoptosis, we have investigated the possible influence of SCF-induced Kit activation on Bcl-2 expression in Ba/F3-Kit cells. However, we found that Bcl-2 expression remained unchanged on SCF stimulation over a period of 24 hours (data not shown). In fact, the role of Bcl-2 in SCF-induced apoptosis inhibition remains controversial and, for example, could not be confirmed in mast cells.36 Other studies have identified the PI3K/Akt pathway as a critical component of Kit signaling in the protective effect of SCF against serum withdrawal–induced apoptosis.42,43 This mechanism has been also proposed for Kit-mediated radioprotection in mast cells44 and in epithelial cells.40,45 However, more recent studies have minimized the role of Kit/PI3K signaling in hematopoietic cells.46 In fact, we found that PI3K plays little, if any, role in the SCF radioprotective effect in Ba/F3-Kit and 32D-Kit cells. Therefore, although it remains possible that the PI3K/Akt signaling pathway contributes to SCF radioprotective effect in other cells, our study provides strong evidence that PLCγ can play a more important role.
Based on previous studies that demonstrated the role of the SM cycle in the IR-induced apoptosis, we hypothesized that PLCγ-dependent Kit signaling interfered with either ceramide production or ceramide-induced apoptosis. However, we found that SCF stimulation had no influence on apoptosis induced by exogenous cell-permeant ceramide suggesting that Kit signaling acted at the level of SMase. In fact, we show here that Kit activation results in a PLCγ-dependent SMase inhibition. It is important to note that, according to our previous studies with a human myeloid cell line, we found that IR stimulates neutral but not acidic SMase.12 The mechanism by which PLCγ stimulation inhibited N-SMase stimulation has not yet been elucidated. However, it is reasonable to speculate that PLCγ-mediated IP hydrolysis and subsequent DAG release result in PKC stimulation which, in turn, blocks N-SMase activation. In fact, the contribution of DAG-dependent PKC regulation in N-SMase activity and ceramide production has been already established.47 However, the PKC isoforms involved in N-SMase regulation have not been characterized. The presumed role of DAG suggests involvement of classical or novel, but not atypical, PKC isoforms.
Our study identified PLCγ activity as an important parameter for IR-induced apoptosis and cytotoxicity, including in human CD34+ bone marrow cells. Although it has been extensively documented that PLCγ greatly contributes to mitogenesis and cellular transformation, its role in apoptosis regulation has been much less investigated. However, recent studies suggest that this enzyme is an important regulator of cell survival. For example, it has already been documented that caspase-mediated cleavage of PLCγ1 is required for apoptosis induced by drugs and tumor necrosis factor α, whereas PLCγ tyrosine phosphorylation confers significant protection against these inducers.48 Moreover, PLCγ confers significant protection against apoptosis induced by UV-C and H2O2.49,50 These results suggest that the level of PLCγ activation or expression can greatly influence apoptosis induced by genotoxic agents. This may have important clinical implications beyond the scope of Kit-expressing cells. Indeed, PLCγ can be activated by a large variety of lipid messengers, receptor and nonreceptor tyrosine kinase, and oncogenic products such as Ras.51-53 Therefore, PLCγ activation could severely limit IR-induced cytotoxicity and more generally the cytotoxicity of antitumor agents that have been found to activate the SM cycle such as UV radiation,54 and anticancer drugs including anthracyclines,30 cytosine arabinoside,55 and mitoxantrone.47
To conclude, our results fit with a model in which Kit signaling interferes with IR-activated SM cycle and apoptosis through a PLCγ- and PKC-dependent mechanism. The fact that PLCγ inhibition results in radiosensitization of Kit-activated cells offers a promising approach for future pharmacologic manipulation to improve the efficiency of radiotherapy in Kit-activated tumor cells such as mastocytosis or GIST.
Supported by l'Association pour la Recherche sur le Cancer grant 5897 (to J.-P.J.), La Ligue (to P.D.), and La Faculté de Médecine Toulouse-Rangueil (to G.L). S.M. is a recipient of an Association pour la Recherche sur le Cancer Fellowship.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jean-Pierre Jaffrézou, INSERM E9910, Institut Claudius Régaud, 20 rue du Pont St Pierre, 31052 Toulouse, France; e-mail: jaffrezou@icr.fnclcc.fr.

![Fig. 2. Effect of SCF on DNA fragmentation of irradiated Ba/F3-Kit cells. / Cells were labeled with 0.5 μCi/mL [3H]thymidine (1.8 × 104 Bq/mL) for 48 hours. Cells were then washed, treated (○) or not (●) with SCF (200 ng/mL for 30 minutes), and irradiated at 4 Gy. At indicated times, cells were embedded in agarose plugs and digested and the DNA was separated by high-voltage electrophoresis. Fragmented and unfragmented DNA-associated radioactivities were quantitated. The results are expressed as percentage of fragmented over total DNA-associated radioactivity. Results are the mean ± SD of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/4/10.1182_blood.v100.4.1294.h81602001294_1294_1301/6/m_h81622982002.jpeg?Expires=1767702397&Signature=obVqP32I~RV5N~UknIx5X3Y1uy~kVsy5YQ5CsSuxQucneyrWqTUdEzQsbpAQPspdheVpltFomG2UCenUcF5ZlSLeMQ3jNIqQZ9NqCiULg9jm0inSyZwoSBPkAYfQllHjytWuZKxVbVEGIwC8EQsGeRJEkOm-UvDl~Tso75eH9iD14I9uejD78xIepFjXAFpVLbIwswqBob-tCOiND0IQ7-Y8EF5oVxLSA6kR4OJlU1YdYogGkBriniLWZdJgAXM3jmn2ElA4KQ49c2W95G1w7ML8qLpllRsBY4YSZBFMO~kwwsdPznxVfNMxZAlkRVHIYYp4LZo5cXcCQiGAY7~ukg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Effect of SCF on ceramide generation in irradiated Ba/F3-Kit cells. / Cells were prelabeled with [3H]palmitate for 48 hours. Cells were then washed, treated (○) or not (●) with SCF (200 ng/mL for 30 minutes), and irradiated at 4 Gy. Following incubation during the indicated times, aliquots (3 × 106 cells/mL) were collected and lipids were extracted. Labeled ceramide was resolved by thin-layer chromatography and identified as described in “Materials and methods.” Results are the mean ± SD of 3 independent experiments (*P < .05).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/4/10.1182_blood.v100.4.1294.h81602001294_1294_1301/6/m_h81622982003.jpeg?Expires=1767702397&Signature=AjOdywJJz75gi4QJGCjKv-H0yGoJkchK6P1PpAXJxlGcGi4aoBJyTqHUtgZtt~pz-m6b7dXA74NIhWxlAoJ1Vs9eocdBuFkrSP1NL4snGTN-1QxaCHzUAis-PPVcHbDi0GdnNR6not3cERsr~kunOeDaufakV-xXL70ClGC~VEl1DEt0eTelw3mhx1tlalCWNi0hEIpTZEgxK9NIUL9qP4LuFTntxdxXAfARm7b-QNsG4LpohdMT6o890nyR4Heo1INvrvuvvdxqfnQpK5advlFupyNEZOdXu~wUuNVEBTN82W~Uw4s1ngXuHUqw6cRNXlGeXwKO-ShFRC~sJ9HaSQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Effect of SCF on N-SMase activity in irradiated Ba/F3-Kit cells. / Cells were treated or not with SCF (200 ng/mL for 30 minutes) and irradiated at 4 Gy. Following incubation during the indicated times (as shown in the insert), aliquots (3 × 106 cells/mL) were collected and enzyme assay was performed as described in “Materials and methods” using [choline-methyl-14C]-SM (60.000 dpm/assay) as substrate. Results are shown at maximum N-SMase stimulation (10-15 minutes after irradiation) and are the mean ± SD of 3 independent experiments (*P < .05). The insert shows N-SMase activity of SCF-treated (○) or untreated (●) Ba/F3-Kit irradiated at 4 Gy that was monitored during a 20-minute period. Results are representative of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/4/10.1182_blood.v100.4.1294.h81602001294_1294_1301/6/m_h81622982004.jpeg?Expires=1767702397&Signature=hVOLp9dbIDBMWwyFhUDLpNGVZs6SiZmbTZLMTfpg47FMpdLmlUZVNxx5XDUjg4lO4iRw9hkJuk8dkZfAt12BfvBC-z8JXxugHl0lf300VJklLbmBvgXcSnzqpximL7wRvRkAsee4WXR1PSmZ5UoweZ7BuzWkaJXwINsXXJJPBhfg4pu2aOOBcheYFxLWM3FHMIx7dMBPFSnfhz5rGixZBHsqqIpu5tgWg4lYlEFr6bVb3n1D75ZKBw6j4mCorlcghDgWUk-99OO9f39lsAZUg-yTC2Ll84JhGPb-Gnmz-ZGY2dfQ9KJqlkDoiG3WPRd8dBMme0y6a60X~4DIcnMNxg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 2. Effect of SCF on DNA fragmentation of irradiated Ba/F3-Kit cells. / Cells were labeled with 0.5 μCi/mL [3H]thymidine (1.8 × 104 Bq/mL) for 48 hours. Cells were then washed, treated (○) or not (●) with SCF (200 ng/mL for 30 minutes), and irradiated at 4 Gy. At indicated times, cells were embedded in agarose plugs and digested and the DNA was separated by high-voltage electrophoresis. Fragmented and unfragmented DNA-associated radioactivities were quantitated. The results are expressed as percentage of fragmented over total DNA-associated radioactivity. Results are the mean ± SD of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/4/10.1182_blood.v100.4.1294.h81602001294_1294_1301/6/m_h81622982002.jpeg?Expires=1768804595&Signature=b0C-lTTJp7UpKNrtbvdmLSmItDo21xtNNJJQ70KzWDaGn9WWqvsWf64UNFLOda-w2Rzo1kuJxJYuhStOl3Am9V3yQMVVjoDi3dzYfcyo~ay4~Tb5gYaJev6iIrMmoK9kHokdFHQym-4rzK9ACJeuty01S25Gv-hszcxZPMQA1oc8JcC7QaxN3KKcOPseOAGo0LZdEZOyJrz2f0-kshkOYSVoe571cs2rwNpVg11PFLdHZGVwVluifxHLmQB5V79tgAcEG3ifgEg6VBtwLLpjrTX9jnVe2jyLROZ4tuUc7bHSLpOGSwHOLyf4b6aXNPFHes3RwvYJi-MyD-0mgvuJAg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Effect of SCF on ceramide generation in irradiated Ba/F3-Kit cells. / Cells were prelabeled with [3H]palmitate for 48 hours. Cells were then washed, treated (○) or not (●) with SCF (200 ng/mL for 30 minutes), and irradiated at 4 Gy. Following incubation during the indicated times, aliquots (3 × 106 cells/mL) were collected and lipids were extracted. Labeled ceramide was resolved by thin-layer chromatography and identified as described in “Materials and methods.” Results are the mean ± SD of 3 independent experiments (*P < .05).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/4/10.1182_blood.v100.4.1294.h81602001294_1294_1301/6/m_h81622982003.jpeg?Expires=1768804595&Signature=QYiG5h3ZNeBqBY2qdAibBb8qZRiGs3Jr4xC5EG-2t5ETJeDjOTbvaYfKhuAC9rE5fEhgOTcBaKx8AQE3M548OOtye9u6puofbARt11LO4sP8EE0KfwWvjwyvUGUiJrcD6GU1NJMfAUl~ETGHMEtnznoC7wy6VLGhrAsrbU53-ZrT7bGd22VmhaNKlB5wHrRCwpadhXU6acikG-ub8Mgbp-hH~G6694bJOV9Cw76DeyGl6IpvnMxD7uFLoUs4mPVJwTXCEump7AH82-tyuN5-VLG-nEywhgMr65rQNZbQkLz8rNA7goU6thce60qoWrwg4sbc4E8TWTBWxIMBpVL0zg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Effect of SCF on N-SMase activity in irradiated Ba/F3-Kit cells. / Cells were treated or not with SCF (200 ng/mL for 30 minutes) and irradiated at 4 Gy. Following incubation during the indicated times (as shown in the insert), aliquots (3 × 106 cells/mL) were collected and enzyme assay was performed as described in “Materials and methods” using [choline-methyl-14C]-SM (60.000 dpm/assay) as substrate. Results are shown at maximum N-SMase stimulation (10-15 minutes after irradiation) and are the mean ± SD of 3 independent experiments (*P < .05). The insert shows N-SMase activity of SCF-treated (○) or untreated (●) Ba/F3-Kit irradiated at 4 Gy that was monitored during a 20-minute period. Results are representative of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/4/10.1182_blood.v100.4.1294.h81602001294_1294_1301/6/m_h81622982004.jpeg?Expires=1768804595&Signature=M~u6~fi6bOmU7yMHKloFaAsQ9-d1928eR2dzmFvCMp8Hhi1JyNe8rpYw0MJOQdZ4ublbBPyGLsze4oHuOs3St-mxGgfxdtOgTnxSvekpGRh89WrlLiahZ2LZN9TDGJ0an8eqbaixfAAFrL4A37sgAvr1S8cCYUK8IyvwMR8DYNnDz3O2uO4hxNDJiYKmgmExkSP31OkJdFHtUWng8ekecCZ-3T79Xziy1iA6-Qvx4-5gsjzlvqm-5PxGTtBdi6oqbDwcx7Qo7jvBJ1fKzyKt1o7Air~ydaIfaj19NPMOEuGbOCyTKO5cPPIIrLXBbIRvnPsWT2te~hA8y20gAGVV9g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
 ) and 32D-KitYF728 cells (▪, ■) irradiated at 4 Gy. Values are the mean ± standard deviation of 3 independent experiments (*P < .05).
) and 32D-KitYF728 cells (▪, ■) irradiated at 4 Gy. Values are the mean ± standard deviation of 3 independent experiments (*P < .05).