Two alternatively spliced stem cell factor (SCF) transcripts encode protein products, which differ in the duration of membrane presentation. One form, soluble SCF (S-SCF) gets rapidly processed to yield predominantly secreted protein. The other form, membrane-associated SCF (MA-SCF) lacks the primary proteolytic cleavage site but is cleaved slowly from an alternate site, and thus represents a more stable membrane form of SCF. Mutants of SCF that lack the expression of MA-SCF (Steel-dickie) or possess a defect in its presentation (Steel17H) manifest deficiencies in erythroid cell development. In this study, we have compared the consequence(s) of activating Kit, the receptor for SCF by MA-SCF with S-SCF, and an obligate membrane-restricted (MR) form of SCF (MR-SCF) on erythroid cell survival, proliferation, cell cycle progression, and the activation of p38 and ERK MAP kinase pathways. Activation of Kit by MR-SCF was associated with a significantly lower incidence of apoptosis and cell death in erythroid cells compared to either other isoform. MR- or MA-SCF–induced stimulation of erythroid cells resulted in similar and significantly greater proliferation and cell cycle progression compared to soluble SCF. The increase in proliferation and cell cycle progression via MA- or MR-SCF stimulation correlated with sustained and enhanced activation of p38 and ERK MAP kinase pathways. In addition, MR- or MA-SCF–induced proliferation was more sensitive to the inhibitory effects of ERK inhibitor compared to S-SCF–induced proliferation. In contrast, soluble SCF-induced proliferation was more sensitive to the inhibitory effects of p38 inhibitor compared with MR- or MA-SCF. These results suggest that different isoforms of SCF may use different biochemical pathways in stimulation of survival and/or proliferation of erythroid cells.
Introduction
Mice mutant at either the Steel(Sl) or dominant White spotting(W) loci display similar phenotypic abnormalities, including defects in hematopoiesis, gametogenesis, and melanogenesis.1 The receptor tyrosine kinase Kit and the ligand of Kit, stem cell factor (SCF), are encoded at the Wand Sl loci, respectively.2Slencodes a membrane protein with extracellular, membrane-spanning, and cytoplasmic domains. Two alternatively spliced mRNA transcripts encode SCF protein products, soluble (S)-SCF and a membrane-associated (MA)–SCF, that differ in sequences amino-terminal of the transmembrane segment.3-7
S-SCF is processed rapidly and efficiently by proteolytic cleavage at a site in exon 6 to produce secreted protein of 164 amino acids. As a consequence, very low levels of S-SCF are detected on the surface of hematopoietic microenvironment (HM)–derived stromal cells.5-9 In contrast, MA-SCF lacks the major proteolytic cleavage site encoded by exon 6 that is responsible for the generation of secreted protein, and thus represents a more stable membrane form of the protein.5-9 Ultimately, in some cells MA-SCF is also processed to produce secreted protein using a secondary cleavage site encoded by exon 7 sequences. This cleavage occurs at a slower rate.5-7 Our laboratory has previously shown using site-directed mutagenesis that loss of proteolytic cleavage sites in both exons 6 and 7 results in the generation of an obligate membrane-restricted (MR) and biologically active form of SCF.7-9
Important insights into the respective role(s) of MA- and S-SCF have come from the characterization of Sl mutants, transgenic and knockout mice that express only the slowly secreted form of SCF (MA-SCF).3,4,8-12 Two alleles at the Sl locus,Steel-dickie (Sld) andSteel17H (Sl17H), are of particular interest with regard to the function of the MA form of SCF.Sld encodes a biologically active but obligately secreted SCF protein as a result of an intragenic deletion of sequences encoding the membrane-spanning domain and cytoplasmic tail of the protein.3,4 Because of the severity of the phenotypes of Sld mice, the membrane form of SCF would appear to be critical for normal function in the affected lineages.1 The Sl17H allele, as a result of a frame shift mutation, encodes a protein in which the cytoplasmic tail is replaced by a novel polypeptide chain of similar length.10 We and others have shown that this frame shift mutation results in a significant reduction in the expression of the MA form of SCF on HM-derived stromal cells.9 12 These animals also demonstrate defective hematopoiesis.
One of the major consequences of the lack of membrane expression (Sld) or defective presentation (Sl17H) of SCF by the stromal cells of the HM is manifested in the cells of erythroid lineage.1,3,4,8,9Both mutants demonstrate lifelong macrocytic anemia in spite of the presence of secreted SCF. Studies performed on transgenic mice that express MR-SCF in Sld orSl17H mutant background have demonstrated a significant correction in both hematocrits and total red cell numbers compared with mutants expressing the S-SCF as a transgene.8,9 Further studies performed in mice expressing only the MA-SCF have also suggested an essential role for membrane presentation of SCF in normal erythroid development.11
In spite of the in vivo studies described above, it remains unclear whether an obligate membrane-restricted form of SCF alone is sufficient for normal erythroid proliferation and survival or whether different isoforms of SCF activate differing signaling pathways in Kit-positive cells. Therefore, to more clearly delineate the role of membrane presentation of SCF and soluble SCF in erythropoiesis, we have compared survival, proliferation, cell cycle progression, and the activation of p38 and ERK MAP kinase pathways in response to stimulation of erythroid cells by HM-derived stromal cells engineered to express either MR-SCF alone, MA-SCF, or S-SCF.
In the present study, the role of ERK and p38 MAP kinases were examined because various hematopoietic cytokines, interleukins, and colony-stimulating factors that regulate hematopoietic cell growth, survival, and differentiation have been found to activate these kinase pathways.13-19 However, the respective role(s) of these distinct MAP kinases in erythropoiesis has not yet been fully elucidated. Our results demonstrate that activation of Kit by membrane isoforms of SCF is associated with significantly lower incidence of apoptosis and cell death of erythroid cells compared with the soluble isoform. Further, activation of Kit by MR-SCF results in proliferation, cell cycle progression, and activation of both p38 and ERK MAP kinase at levels similar to that observed via MA-SCF. In contrast, stimulation by S-SCF results in relatively less proliferation, cell cycle progression, and activation of p38 and ERK MAP kinase. Interestingly, MR- or MA-SCF–induced proliferation was significantly more sensitive to the inhibitory effects of ERK MAP kinase inhibitor compared with S-SCF, whereas S-SCF–induced proliferation was significantly more sensitive to the inhibitory effects of p38 MAP kinase inhibitor compared with MR- or MA-SCF. These data suggest that MR-SCF can substitute for MA form in mediating functional and biochemical responses via Kit in erythroid cells. Further, the extent of use of different signaling biochemical pathways downstream of Kit may depend upon the nature of presentation of the SCF ligand.
Materials and methods
Cell line
SCF-dependent G1E-ER2 cells have been previously described.9 20-22 Unless otherwise specified, G1E-ER2 cells were grown in Iscoves modified Dulbecco medium (IMDM) (Invitrogen, Rockville, MD) with 15% fetal bovine serum (Fisher Scientific, Pittsburg, PA), recombinant erythropoietin (Epo) (2 U/mL), and recombinant rat (rr) SCF (50 ng/mL) (both Amgen, Thousand Oaks, CA). For starvation experiments, cells were washed 3 times in IMDM and then resuspended in the same medium without serum or growth factors for 4 to 6 hours.
Proliferation assays
The effect of SCF isoforms on proliferation of the erythrocytic progenitor cell line G1E-ER2 was assayed using thymidine incorporation. Stromal cells expressing different isoforms of SCF have been previously described.7-9 SCF protein activity in stable stromal cell transfectants was compared with untransfected parental Sl/Sl4 stromal cells by coculture as previously described.9 On the day before assay, parental Sl/Sl4 stromal cells and stromal cells expressing various isoforms of SCF (S-SCF, MA-SCF, and MR-SCF) were treated with 5 μg/mL mitomycin C, washed 3 times in phosphate-buffered saline (PBS), counted, and seeded at 3 × 104 cells/well in 0.1% gelatin-coated 96-well plates. These cultures were incubated in Dulbecco modified Eagle medium (DMEM), 10% calf serum (CS) (Biowhittaker, Walkersville, MD) at 37°C. After 24 hours, 5 × 104 G1E-ER2 cells were added to mitomycin C–treated stromal cells either in the presence or the absence of indicated concentrations of the mitogen/extracellular signal-regulated kinase (MEK) inhibitor PD98059 or p38 inhibitor SB203580 (both Calbiochem, San Diego, CA) and cultured for 48 hours. Subsequently, 1.0 μCi (0.037 MBq) [3H]thymidine (Amersham, Piscataway, NJ) was added to each well for 6 to 8 hours at 37°C. Cells were then harvested using an automated cell harvester (96-well harvester, Brandel, Gaithersburg, MD), and thymidine incorporation was determined in a scintillation counter.
Apoptosis/survival assays
The effect of different SCF isoforms on G1E-ER2 cell survival/death (apoptosis and necrosis) was assayed by staining the cells with annexin V and propidium iodide (PI). On the day before assay, parental Sl/Sl4 stromal cells and stromal cells expressing various isoforms of SCF were prepared as above and seeded at 1 × 105 cells/well in 0.1% gelatin-coated 24-well plates. The cultures were incubated in DMEM, 10% CS at 37°C. After 24 hours, 5 × 105 G1E-ER2 cells were added to each well either in the presence or the absence of indicated concentrations of the MEK inhibitor or p38 inhibitor and cultured for 48 hours. Thereafter, cells were harvested and washed once with PBS and resuspended in 100 uL binding buffer (10 mM HEPES/NaOH, pH 7.4, 140 mM NaCl, 2.5 mM CaCl2). Cells were then stained with 5 uL annexin V (Pharmingen, San Diego, CA) and 500 ng PI (Calbiochem, La Jolla, CA) and incubated at room temperature for 15 minutes in the dark. Then 400 uL binding buffer was added and the cells were analyzed using flow cytometric analysis on a FACScan flow cytometer (Becton Dickinson, Mountain View, CA).
Expression of SCF in stromal cell transfectants and activation of MAP kinase pathways in G1E-ER2 cells
Expression of SCF isoforms in stromal cell transfectants was determined by loading equal amounts of protein (2.4 μg per lane) and performing Western blot analysis using an anti–mouse SCF polyclonal antibody (Peprotech, Rocky Hill, NJ). Activation of p38 and ERKs was determined by using phospho-specific p38 and ERK MAP kinase antibodies (Cell Signaling Technology, Beverly, MA). These antibodies detect p38 and ERK-1 and ERK-2 MAP kinase only when these proteins are catalytically activated by phosphorylation at threonine (Thr) 180/tyrosine (Tyr) 182 for p38 and Thr202 and Tyr204 for ERK-1 and ERK-2. Briefly, Sl/Sl4 cells expressing various SCF isoforms were prepared as described above. The cells were washed and plated on 6-well gelatin-coated plates (1 × 106/well) and cultured for 36 to 48 hours. G1E-ER2 cells were factor-starved for 6 to 8 hours in IMDM, then 5 to 6 × 106 cells were loaded onto stromal cells and further cocultured for various time points at 37°C. Thereafter, cells were harvested and lysed in lysis buffer (10 mM K2HPO4, 1 mL EDTA, 5 mM EGTA, 10 mM MgCl2, 1 mM Na2VO4, 50 mM beta-glycerol-phosphate, 10 ug/mL leupeptin, 1 ug/mL pepstatin, and 10 μg/mL aprotinin) at 4°C for 30 minutes. Cell lysates were clarified by centrifugation for 30 minutes at 10 000g at 4°C. Western blot analysis was performed according to the manufacturer's instructions (New England Biolabs, Beverly, MA). Phosphorylation of ERK and p38 MAP kinase was quantitated by performing densitometry on Western blots using the Eagle Eye II system (Stratagene, La Jolla, CA). Data are presented as relative phosphorylation of ERK and p38, with highest phosphorylation taken as 100.
Results
Activation of Kit by MA-SCF or MR-SCF stimulates enhanced proliferation and cell cycle progression in erythroid cells compared with S-SCF stimulation
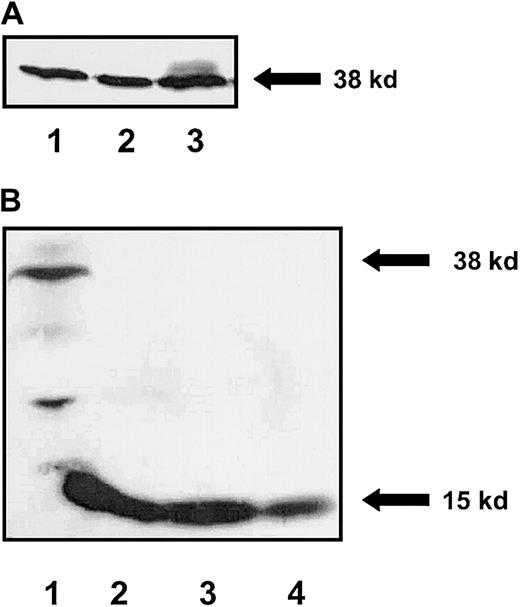
We have previously described stromal cells derived from mice deficient in the expression of endogenous SCF that have been engineered to express different forms of SCF (Sl/Sl4).7-9To compare proliferation, survival, and cell cycle progression of erythroid cells in response to stimulation by soluble, MR-, and MA-SCF, we used an SCF-responsive erythrocytic progenitor cell line, G1E-ER2.9,20-22 To closely mimic the events associated with erythroid cell proliferation, cell cycle progression, and survival within the HM, a coculture assay system was used that allows direct interaction of stromal elements with target erythroid cells. Stromal cell transfectants expressing comparable levels of soluble, MA, or the MR forms of SCF were used in these studies (Figure1A). Concentration of secreted SCF protein in S-SCF stromal cell cultures was estimated by performing Western blot analysis and comparing the amount of secreted SCF protein in conditioned medium with known amounts of Escherichia coli–derived recombinant SCF. As shown in Figure 1B (lane 1), approximately 1 ng/mL SCF protein is secreted in these stromal cell cultures. This level is similar to that noted in our previously published observations.6 Analysis of SCF-induced proliferation of G1E-ER2 cells was performed using a thymidine incorporation assay. G1E-ER2 cells were cocultivated with mitomycin C–treated stromal cell transfectants expressing the soluble, MA, or the MR form of SCF. After 48 hours of coculture, [3H]thymidine was added for 6 hours, and [3H] incorporation was determined. As expected and as previously shown,9 coculturing G1E-ER2 cells with untransfected parental Sl/Sl4 stromal cells (which lack SCF expression) induced only low levels of proliferation (Figure2A). In contrast, a significant increase in proliferation of G1E-ER2 cells was noted in response to cocultivation on stromal cells expressing either soluble, MA-, or MR-SCF over untransfected parental Sl/Sl4 cells. A similar level of proliferation of G1E-ER2 cells was seen in response to stimulation by both MA- and MR-SCF isoforms (Figure 2A). Consistent with the inability of S-SCF to sustain the proliferation of hematopoietic stem and progenitor cells as described previously,6 stimulation of G1E-ER2 cells by S-SCF resulted in significantly less proliferation over a 48-hour coculture period compared to MA- or MR-SCF (Figure 2A). Also, an increase in the entry of cells in the “S” phase of cell cycle was noted when the cells were cocultivated on stroma expressing MA or MR forms of SCF (Figure 2B). These data suggest that the slow secreting and the obligate membrane-restricted form of SCF induce similar levels of proliferation and cell cycle progression. Thus, MR-SCF may substitute for the MA isoform in inducing proliferation of erythroid cells, whereas S-SCF appears to induce quantitatively fewer proliferative signals than isoforms with longer membrane presentation.
Expression of SCF isoforms in stromal cell transfectants.
(A) Stromal cells expressing soluble, MA, and MR forms of SCF were lysed and an equal amount of protein was subjected to Western blot analysis using an anti-SCF antibody. A 38-kilodalton (kD) SCF band can be seen in stromal cells expressing the soluble (lane 1), MR (lane 2), and MA (lane 3) forms of SCF. (B) Quantitation of secreted SCF in conditioned medium from stromal cells expressing S-SCF. Lane 1: concentrated conditioned medium from S-SCF–producing stromal cells. Lanes 2, 3, and 4: 6, 3, and 1 ng recombinant E coli–derived SCF (15 kD), respectively. Shown is a Western blot probed with an anti-SCF antibody. Molecular weight of stromal cell secreted soluble SCF (38 kD) and E coli–derived SCF (15 kD) protein is indicated.
Expression of SCF isoforms in stromal cell transfectants.
(A) Stromal cells expressing soluble, MA, and MR forms of SCF were lysed and an equal amount of protein was subjected to Western blot analysis using an anti-SCF antibody. A 38-kilodalton (kD) SCF band can be seen in stromal cells expressing the soluble (lane 1), MR (lane 2), and MA (lane 3) forms of SCF. (B) Quantitation of secreted SCF in conditioned medium from stromal cells expressing S-SCF. Lane 1: concentrated conditioned medium from S-SCF–producing stromal cells. Lanes 2, 3, and 4: 6, 3, and 1 ng recombinant E coli–derived SCF (15 kD), respectively. Shown is a Western blot probed with an anti-SCF antibody. Molecular weight of stromal cell secreted soluble SCF (38 kD) and E coli–derived SCF (15 kD) protein is indicated.
Proliferation and cell cycle progression of G1E-ER2 cells by stromal cells expressing different isoforms of SCF.
G1E-ER2 cells were cocultured for 48 hours with mitomycin C–treated parental Sl/Sl4 stromal cells or stable stromal cell transfectants expressing S-SCF, MA-SCF, or MR-SCF. (A) Proliferation was measured by thymidine incorporation assay. Bars denote the mean thymidine incorporation (counts per minute [cpm] [± SEM]) of 2 independent experiments performed in replicates of 6. *P < .05 S-SCF, MA-SCF, and MR-SCF versus Sl/Sl.4 **P < .001 S-SCF versus MR-SCF and MA-SCF. MR-SCF versus MA-SCF, P = .2. (B) Cell cycle analysis was performed by propidium iodide staining of G1E-ER2 cells. Percentages of cells in different phases of cell cycle are indicated. Shown is a representative experiment. Similar results were observed in 2 independent experiments performed in replicates of 3.
Proliferation and cell cycle progression of G1E-ER2 cells by stromal cells expressing different isoforms of SCF.
G1E-ER2 cells were cocultured for 48 hours with mitomycin C–treated parental Sl/Sl4 stromal cells or stable stromal cell transfectants expressing S-SCF, MA-SCF, or MR-SCF. (A) Proliferation was measured by thymidine incorporation assay. Bars denote the mean thymidine incorporation (counts per minute [cpm] [± SEM]) of 2 independent experiments performed in replicates of 6. *P < .05 S-SCF, MA-SCF, and MR-SCF versus Sl/Sl.4 **P < .001 S-SCF versus MR-SCF and MA-SCF. MR-SCF versus MA-SCF, P = .2. (B) Cell cycle analysis was performed by propidium iodide staining of G1E-ER2 cells. Percentages of cells in different phases of cell cycle are indicated. Shown is a representative experiment. Similar results were observed in 2 independent experiments performed in replicates of 3.
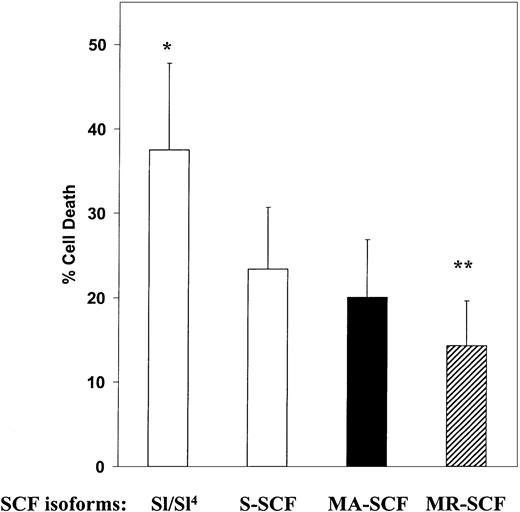
SCF is an important survival factor for Kit-positive cells and has previously been shown to protect some cells from growth factor withdrawal–induced apoptosis.23 To determine the survival-promoting effects of soluble, MA, and MR isoforms of SCF on G1E-ER2 cells, we compared apoptosis in G1E-ER2 cells cocultured on stromal cells expressing SCF isoforms using a combination of propidium iodide (PI) and annexin V staining. G1E-ER2 cells were factor-starved for 4 to 6 hours and then cocultured for 48 hours on mitomycin C–treated parental Sl/Sl4 cells or stromal cells expressing soluble, MA, or MR-SCF. Cells were harvested, stained with annexin V and PI, and scored for the percentage of apoptotic (annexin V+/PI−) and necrotic (annexin V+/PI+) cells using fluorescence-activated cell-sorter (FACS) analysis. G1E-ER2 cells cocultured on untransfected parental Sl/Sl4 stromal cells demonstrated a significantly higher cell death rate compared with cells cocultured on stroma expressing any form of SCF (Figure3). Cocultivation of G1E-ER2 cells on stroma expressing MR-SCF was associated with a significantly lower incidence of apoptosis and cell death compared with stroma expressing either S-SCF or MA-SCF (Figure 3). The reduction in the incidence of apoptosis and cell death noted in cultures stimulated with either S-SCF or MA-SCF was similar (Figure 3). These data suggest that the duration of SCF presentation by HM-derived stromal cells affects cell proliferation and survival to differing degrees.
SCF-induced protection from cell death in G1E-ER2 cells cocultured on stroma expressing different isoforms of SCF.
G1E-ER2 cells were cocultured for 48 hours with parental Sl/Sl4 stromal cells or stable stromal cell transfectants expressing S-SCF, MA-SCF, or MR-SCF. Total cell death was quantitated by performing annexin V and propidium iodide (PI) staining as described in “Materials and methods.” Bars denote the percentage of total cell death (± SEM) of 2 independent experiments performed in replicates of 3. *P < .05 Sl/Sl4 versus S-SCF, MA-SCF, and MR-SCF. **P < .05 MR-SCF versus MA-SCF, S-SCF, and Sl/Sl.4
SCF-induced protection from cell death in G1E-ER2 cells cocultured on stroma expressing different isoforms of SCF.
G1E-ER2 cells were cocultured for 48 hours with parental Sl/Sl4 stromal cells or stable stromal cell transfectants expressing S-SCF, MA-SCF, or MR-SCF. Total cell death was quantitated by performing annexin V and propidium iodide (PI) staining as described in “Materials and methods.” Bars denote the percentage of total cell death (± SEM) of 2 independent experiments performed in replicates of 3. *P < .05 Sl/Sl4 versus S-SCF, MA-SCF, and MR-SCF. **P < .05 MR-SCF versus MA-SCF, S-SCF, and Sl/Sl.4
Sustained and enhanced activation of MAP kinase (ERK) in G1E-ER2 cells is induced by MA and MR forms of SCF
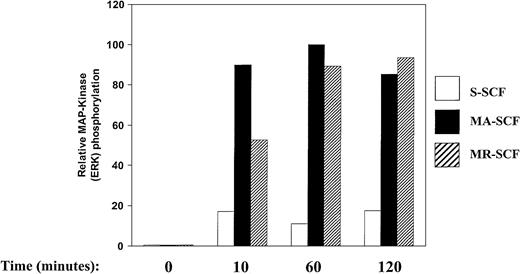
We have previously demonstrated sustained activation of Kit in response to stromal cell presentation of MA- and MR-SCF, and transient activation of Kit in response to soluble SCF.8 24 To examine the consequence(s) of SCF presentation on intracellular kinase signaling pathways associated with MAP kinases in G1E-ER2 cells, we examined the activation of ERK and p38 MAP kinases. Activation of ERK (ERK-1 and ERK-2) was determined by examining the phosphorylation of ERK-1 and ERK-2 using phospho-specific antibodies. G1E-ER2 cells were cocultured on stromal cells expressing S-SCF, MA-SCF, or MR-SCF protein for various time points, and cellular lysates were analyzed. As shown in Figure 4, coculture of G1E-ER2 cells on stroma expressing MA-SCF and MR-SCF resulted in both enhanced and sustained activation of ERK (ERK-1 and ERK-2) over a period of 120 minutes. Coculturing G1E-ER2 cells on stroma expressing S-SCF also resulted in sustained ERK activation, however, at significantly reduced levels.
Sustained and enhanced activation of MAP kinase (ERK) in G1E-ER2 cells by membrane isoforms of SCF.
Factor-starved G1E-ER2 cells were cocultured with mitomycin C–treated stromal cells expressing S-SCF, MA-SCF, or MR-SCF for indicated time points. Subsequently, at various times, cell lysates were collected and subjected to Western blot analysis with a rabbit anti–phospho MAP kinase antibody. Bars show the relative phosphorylation of ERK. Data are presented relative to the phosphorylation of ERK by MA-SCF after 60 minutes (with the level at 60 minutes taken as 100). These experiments were performed at least 3 times with similar results.
Sustained and enhanced activation of MAP kinase (ERK) in G1E-ER2 cells by membrane isoforms of SCF.
Factor-starved G1E-ER2 cells were cocultured with mitomycin C–treated stromal cells expressing S-SCF, MA-SCF, or MR-SCF for indicated time points. Subsequently, at various times, cell lysates were collected and subjected to Western blot analysis with a rabbit anti–phospho MAP kinase antibody. Bars show the relative phosphorylation of ERK. Data are presented relative to the phosphorylation of ERK by MA-SCF after 60 minutes (with the level at 60 minutes taken as 100). These experiments were performed at least 3 times with similar results.
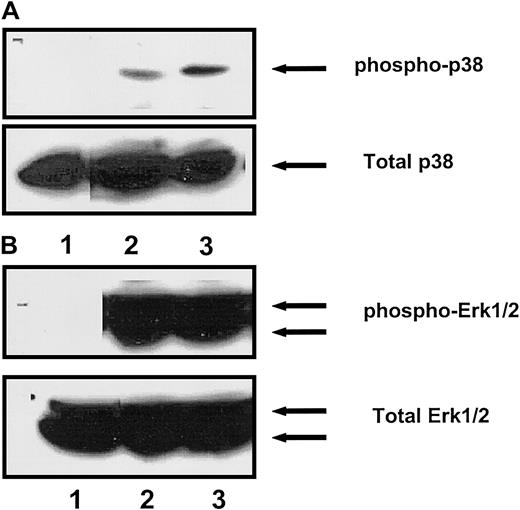
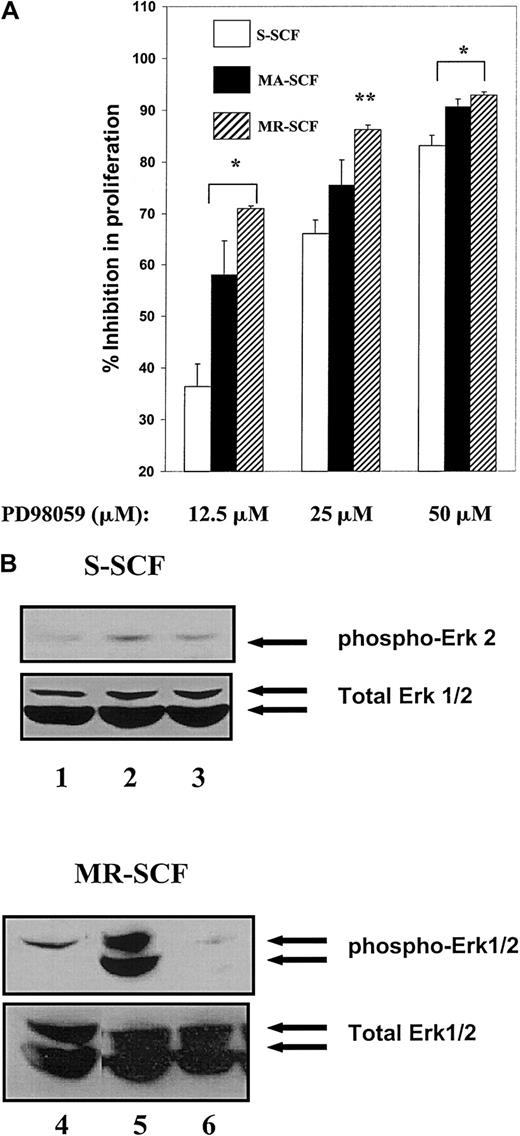
To further characterize the extent of involvement of the ERK MAP kinase pathways in SCF-induced proliferation of G1E-ER2 cells, we used specific pharmacologic inhibitors of the MAP kinase ERK PD98059 and p38 SB203580 pathways. First, we determined the specificity of PD98059 and SB203580. G1E-ER2 cells were treated with the 2 inhibitors, and the effect of each of these inhibitors on the activation of the other MAP kinase pathway was examined in response to membrane SCF stimulation. As shown in Figure 5A (lane 3), treatment of G1E-ER2 cells with the ERK MAP kinase inhibitor PD98059 (12.5 μM) had no effect on the activation of p38 MAP kinase. Conversely, treatment of G1E-ER2 cells with the p38 inhibitor SB203580 (12.5 uM) had no effect on the activation of the ERK MAP kinase (Figure 5B, lane 3). Similar results were obtained in the presence of 25 and 50 μM SB203580 (data not shown). These data demonstrate that the 2 inhibitors used in these studies do not inhibit the activation of each other.
Specificity of inhibition of ERK and p38 MAP kinase by PD98059 and SB203580, respectively.
(A) Starved G1E-ER2 cells were left untreated (lane 1) or stimulated with SCF (lane 2) or pretreated with the ERK inhibitor PD98059 for an hour and then stimulated with SCF (lane 3). Arrow in the top panel indicates phosphorylated p38. Bottom panel shows total p38 in each lane. (B) Starved cells were left untreated (lane 1) or stimulated with SCF (lane 2) or pretreated with the p38 inhibitor SB203580 for an hour and then stimulated with SCF (lane 3). Arrow in the top panel indicates phosphorylated ERK1/2. Bottom panel shows total ERK1/2 protein in each lane.
Specificity of inhibition of ERK and p38 MAP kinase by PD98059 and SB203580, respectively.
(A) Starved G1E-ER2 cells were left untreated (lane 1) or stimulated with SCF (lane 2) or pretreated with the ERK inhibitor PD98059 for an hour and then stimulated with SCF (lane 3). Arrow in the top panel indicates phosphorylated p38. Bottom panel shows total p38 in each lane. (B) Starved cells were left untreated (lane 1) or stimulated with SCF (lane 2) or pretreated with the p38 inhibitor SB203580 for an hour and then stimulated with SCF (lane 3). Arrow in the top panel indicates phosphorylated ERK1/2. Bottom panel shows total ERK1/2 protein in each lane.
Next, we cocultured G1E-ER2 cells on stromal cells expressing various isoforms of SCF in the presence or absence of indicated concentrations of PD98059 (Figures 6A). As an additional positive control, we first performed these experiments with soluble recombinant rat (rr) SCF. After 48 hours of culture, [3H]thymidine was added for 6 hours, and [3H] incorporation was determined. Under these conditions, a dose-dependent inhibition in proliferation of G1E-ER2 cells was observed in the presence of rrSCF and concentrations of PD98059 ranging from 12.5 to 50 μM (data not shown). A dose-dependent inhibition in proliferation of G1E-ER2 cells was also observed when G1E-ER2 cells were stimulated by each isoform of SCF (Figure6A). However, sustained and enhanced activation of ERK in G1E-ER2 cells via MA- or MR-SCF correlated with statistically greater inhibition of proliferation in the presence of PD98059 compared with soluble SCF (Figure 6A). This increase in the inhibition of proliferation induced via PD98059 in G1E-ER2 cells stimulated with MA- or MR-SCF was more apparent at lower concentrations of the inhibitor (Figure 6A). Consistent with these observations, a lower dose of PD98059 (12.5 μM) completely inhibited ERK MAP kinase activation in G1E-ER2 cells cocultivated on MR-SCF, but not the soluble form (Figure 6B, lanes 6 and 3, respectively). These data suggest that enhanced and sustained activation of ERK in G1E-ER2 cells by stromal cells expressing MA- or MR-SCF correlates with greater dependency of these cells on the ERK MAP kinase pathway for proliferation compared to soluble SCF–induced proliferation. Further, these data also demonstrate that MR-SCF resembles MA-SCF with regard to the level and kinetics of ERK activation.
Differential inhibition in proliferation of G1E-ER2 cells by ERK inhibitor and stromal cells expressing different isoforms of SCF.
G1E-ER2 cells were cocultured for 48 hours with mitomycin C–treated stable stromal cell transfectants expressing S-SCF, MA-SCF, or MR-SCF in the absence or presence of indicated concentrations of the ERK inhibitor PD98059. (A) Proliferation was measured by thymidine incorporation assay. Bars denote the mean inhibition in proliferation (± SEM) of 2 independent experiments performed in replicates of 6. *P < .05 MA-SCF and MR-SCF (12.5 μM and 50 μM) versus S-SCF (12.5 μM and 50 μM). **P < .05 MR-SCF versus S-SCF (25 μM). (B) Level of ERK inhibition in G1E-ER2 cells treated with 12.5 μM PD98059 and stimulated with soluble SCF (top panel) or MR-SCF (bottom panel). Lanes 1 and 4: unstimulated G1E-ER2 cells. Lanes 2 and 5: G1E-ER2 cells stimulated with soluble SCF and MR-SCF, respectively. Lanes 3 and 6: PD98059 pretreated G1E-ER2 cells stimulated with soluble and MR-SCF, respectively. Arrows indicate the phosphorylated and total ERK1/2 protein.
Differential inhibition in proliferation of G1E-ER2 cells by ERK inhibitor and stromal cells expressing different isoforms of SCF.
G1E-ER2 cells were cocultured for 48 hours with mitomycin C–treated stable stromal cell transfectants expressing S-SCF, MA-SCF, or MR-SCF in the absence or presence of indicated concentrations of the ERK inhibitor PD98059. (A) Proliferation was measured by thymidine incorporation assay. Bars denote the mean inhibition in proliferation (± SEM) of 2 independent experiments performed in replicates of 6. *P < .05 MA-SCF and MR-SCF (12.5 μM and 50 μM) versus S-SCF (12.5 μM and 50 μM). **P < .05 MR-SCF versus S-SCF (25 μM). (B) Level of ERK inhibition in G1E-ER2 cells treated with 12.5 μM PD98059 and stimulated with soluble SCF (top panel) or MR-SCF (bottom panel). Lanes 1 and 4: unstimulated G1E-ER2 cells. Lanes 2 and 5: G1E-ER2 cells stimulated with soluble SCF and MR-SCF, respectively. Lanes 3 and 6: PD98059 pretreated G1E-ER2 cells stimulated with soluble and MR-SCF, respectively. Arrows indicate the phosphorylated and total ERK1/2 protein.
Sustained and enhanced activation of p38 MAP kinase in G1E-ER2 cells by MA and MR forms of SCF
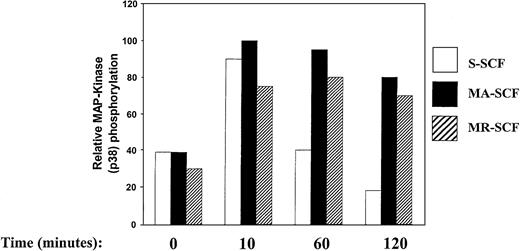
In addition to ERK MAP kinases, p38 MAP kinase also has been implicated in some cells in cytokine-induced proliferation. To investigate the effect of SCF isoforms on activation of p38 in G1E-ER2 cells, we performed coculture experiments as described above and analyzed activation of p38. Activation of p38 was determined by examining the phosphorylation of p38 using an antibody that specifically recognizes the activated form of p38. As shown in Figure7, coculture of G1E-ER2 cells on stroma expressing MA- and MR-SCF resulted in sustained and enhanced activation of p38 over a period of 120 minutes. Coculturing G1E-ER2 cells on stroma expressing S-SCF resulted in an equivalent activation of p38 at 10 minutes, but unlike cultivation with MA- or MR-SCF, this activation was transient, reaching baseline at 60 minutes.
Sustained activation of MAP kinase (p38) in G1E-ER2 cells by membrane isoforms of SCF.
Factor-starved G1E-ER2 cells were cocultured with mitomycin C–treated stromal cells expressing S-SCF, MA-SCF, or MR-SCF for indicated time points. Subsequently, at various times, cell lysates were collected and subjected to Western blot analysis with a rabbit anti–phospho p38 MAP kinase antibody. Bars demonstrate the relative phosphorylation of p38. Data are presented relative to the phosphorylation of p38 by SCF-MA after 10 minutes (with the level at 10 minutes taken as 100). These experiments were performed 2 times with similar results.
Sustained activation of MAP kinase (p38) in G1E-ER2 cells by membrane isoforms of SCF.
Factor-starved G1E-ER2 cells were cocultured with mitomycin C–treated stromal cells expressing S-SCF, MA-SCF, or MR-SCF for indicated time points. Subsequently, at various times, cell lysates were collected and subjected to Western blot analysis with a rabbit anti–phospho p38 MAP kinase antibody. Bars demonstrate the relative phosphorylation of p38. Data are presented relative to the phosphorylation of p38 by SCF-MA after 10 minutes (with the level at 10 minutes taken as 100). These experiments were performed 2 times with similar results.
To evaluate the functional significance of the p38 MAP kinase pathway in SCF isoform-mediated proliferation of erythroid cells and to determine if transient versus sustained activation of p38 via different SCF isoforms results in differential effects on proliferation, G1E-ER2 cells were cocultured on stroma expressing various SCF isoforms in the presence or absence of increasing concentrations of the p38-specific inhibitor SB203580 (Figure 8A). After 48 hours of coculture, [3H]thymidine was added for 6 hours, and [3H] incorporation was determined. As shown in Figure 8A, the extent of SB203580-induced inhibition in proliferation of G1E-ER2 cells depended on the nature of the stimulating SCF isoform. In contrast to reduced sensitivity to the inhibitor of ERK noted previously (Figure 6A), soluble SCF-induced proliferation of G1E-ER2 cells was more sensitive to the p38 inhibitor SB203580 compared with MA or MR forms of SCF, and this sensitivity was similar to that of rrSCF (Figure 8A). No inhibition in proliferation of G1E-ER2 cells was observed via MA- or MR-SCF stimulation in the presence of 12.5 μM SB203580 (Figure 8A). In contrast, at this same concentration of the inhibitor, soluble SCF– or rrSCF-induced stimulation of G1E-ER2 cells resulted in a reduction of 10% in proliferation. As seen in Figure 8A, approximately 40% inhibition in proliferation was observed when GIE-ER2 cells were stimulated with S-SCF or rrSCF protein in the presence of 25 μM SB203580. At this same concentration of SB203580, stimulation of G1E-ER2 cells via MA- or MR-SCF was inhibited only 2% and 5%, respectively (Figure 8A). Consistent with these observations, treatment of G1E-ER2 cells with 12.5 μM and 25 μM SB203580 almost completely inhibited p38 activation in response to soluble SCF treatment (Figure 8B, lanes 3 and 4), but not in response to membrane SCF stimulation (Figure 8B, lanes 7 and 8). In summary, these results suggest that sustained activation of p38 in G1E-ER2 cells in response to the membrane form of SCF is associated with more resistance to the inhibitory effects of SB203580 compared with the soluble form.
Differential inhibition in proliferation of G1E-ER2 cells by p38 inhibitor and stromal cells expressing different isoforms of SCF.
G1E-ER2 cells were cocultured for 48 hours with mitomycin C–treated stable stromal cell transfectants expressing S-SCF, MA-SCF, MR-SCF, or rrSCF in the absence or presence of indicated concentrations of the p38 inhibitor SB203580. (A) Proliferation was measured by thymidine incorporation assay. Bars denote the mean inhibition in proliferation (± SEM) of 2 independent experiments in the presence of an increasing concentration of SB203580 performed in replicates of 6. *P < .05 S-SCF and rrSCF (12.5 and 25 μM) versus MA-SCF (12.5 and 25 μM) and MR-SCF (12.5 and 25 μM). (B) Level of p38 inhibition in G1E-ER2 cells treated with 12.5 or 25 μM SB203580 and stimulated with soluble SCF or MR-SCF. Lanes 1 and 5: unstimulated G1E-ER2 cells. Lanes 2 and 6: G1E-ER2 cells stimulated with soluble SCF and MR-SCF, respectively. Lanes 3 and 7: 12.5 μM SB203580 pretreated G1E-ER2 cells stimulated with soluble and MR-SCF, respectively. Lanes 4 and 8: 25 μM SB203580 pretreated G1E-ER2 cells stimulated with soluble and MR-SCF, respectively. Arrows indicate the phosphorylated and total p38 protein.
Differential inhibition in proliferation of G1E-ER2 cells by p38 inhibitor and stromal cells expressing different isoforms of SCF.
G1E-ER2 cells were cocultured for 48 hours with mitomycin C–treated stable stromal cell transfectants expressing S-SCF, MA-SCF, MR-SCF, or rrSCF in the absence or presence of indicated concentrations of the p38 inhibitor SB203580. (A) Proliferation was measured by thymidine incorporation assay. Bars denote the mean inhibition in proliferation (± SEM) of 2 independent experiments in the presence of an increasing concentration of SB203580 performed in replicates of 6. *P < .05 S-SCF and rrSCF (12.5 and 25 μM) versus MA-SCF (12.5 and 25 μM) and MR-SCF (12.5 and 25 μM). (B) Level of p38 inhibition in G1E-ER2 cells treated with 12.5 or 25 μM SB203580 and stimulated with soluble SCF or MR-SCF. Lanes 1 and 5: unstimulated G1E-ER2 cells. Lanes 2 and 6: G1E-ER2 cells stimulated with soluble SCF and MR-SCF, respectively. Lanes 3 and 7: 12.5 μM SB203580 pretreated G1E-ER2 cells stimulated with soluble and MR-SCF, respectively. Lanes 4 and 8: 25 μM SB203580 pretreated G1E-ER2 cells stimulated with soluble and MR-SCF, respectively. Arrows indicate the phosphorylated and total p38 protein.
Discussion
One of the most overt hematopoietic phenotypes observed in mutants of Sl lacking the expression of MA-SCF (Sld) or possessing a defect in its presentation (Sl17H) is lifelong macrocytic anemia.1-4,9,10 Therefore, erythroid cells provide a unique model to study the biologic and biochemical consequences of Kit stimulation by soluble and membrane forms of SCF in the appropriate cellular context. To more clearly delineate the role of soluble and membrane presentation of SCF in erythropoiesis, we have compared the effect of soluble and membrane presentation of SCF on erythroid cell proliferation and activation of downstream kinase-signaling pathways. The major conclusions of this study are that (1) MR-SCF is as effective as MA isoform in mediating survival, proliferation, and cell cycle progression of erythroid cells; (2) the soluble form of SCF results in significantly less proliferation of G1E-ER2 cells; (3) stimulation of erythroid cells via MA- or MR-SCF results in sustained and enhanced activation of ERK and p38 MAP kinase pathways; and (4) MR- or MA-SCF–induced proliferation of G1E-ER2 cells is significantly more sensitive to the inhibitory effects of ERK MAP kinase inhibitor compared with S-SCF. In contrast, S-SCF–induced proliferation is significantly more sensitive to the inhibitory effects of p38 MAP kinase inhibitor compared with MR- or MA-SCF.
The finding that membrane presentation of SCF is critical for erythroid cell proliferation and survival is consistent with previously published reports.8,9,11 Further, consistent with the inability of soluble SCF to sustain the proliferation of hematopoietic stem and progenitor cells described previously in vivo,6stimulation of G1E-ER2 erythroid cells via soluble SCF results in less proliferation compared with MR- or MA-SCF over a 2-day coculture period in vitro. We have previously shown that soluble and MA-SCF stimulate qualitatively different responses in vitro in MO7e cells.6In combination with other growth factors, MA-SCF but not S-SCF is capable of sustaining the survival of long-term hematopoietic progenitors and erythroblastic leukemia cells.25-28 More recently, using transgenic mice, we have shown that the transgene expression of MR-SCF but not S-SCF results in correction of erythroid cell deficiency associated with the lack or altered expression of MA-SCF in Sld and Sl17Hmice, respectively.8,9 In addition, Tajima et al, using an elegant knockin strategy, also demonstrated that the exclusive expression of MA-SCF in vivo is sufficient for normal erythroid cell development.11 We extend these observations by investigating the response of Kit to an obligate MR form of SCF. Our findings, using stromal cells expressing exclusively the MR form of SCF, provide the biochemical mechanism that is possibly responsible for these in vivo observations. Further, our findings demonstrate no significant differences in erythroid cell proliferation, cell cycle progression, survival, and activation of downstream signaling molecules as a result of stimulation by MR- or MA-SCF. We have exploited the G1E-ER2 cell line in order to better study the biochemical events associated with activation of Kit by different SCF presentation.
Although the biochemical basis for the differences between S-SCF, MA-SCF, and MR-SCF are not completely understood, some signaling differences previously have been reported. After ligand binding to receptor tyrosine kinases such as Kit, signal transduction cascades are initiated by receptor autophosphorylation on key tyrosine residues.29 In the case of S-SCF, tyrosine phosphorylation of Kit is rapid (within minutes), followed by a decline in phosphorylation. This decline in phosphorylation coincides with receptor internalization,30 leading ultimately to receptor degradation. In contrast, phosphorylation of Kit by MA-SCF persists over much longer periods in both MO7e cells as well as in primary erythroid progenitor cells.8,24 This persistence in tyrosine phosphorylation has been attributed to enhanced stability of the Kit receptor on the cell surface after MA-SCF stimulation, likely because of the delayed receptor internalization by this form of ligand presentation. Our results, demonstrating sustained and enhanced phosphorylation of p38 and ERK after stimulation by MA- or MR-SCF, correlate with the persistent phosphorylation of Kit noted previously via these 2 isoforms.8,24 Interestingly, sustained and enhanced activation of p38 by the MA or MR form of SCF did not result in greater dependency of erythroid cells on the p38 pathway for proliferation. In contrast, activation of ERK via these 2 isoforms correlated with significantly greater dependency of erythroid cells on this pathway, as measured by sensitivity to inhibition. The biochemical bases for these differences are currently not known. Differences in the use of other downstream signaling pathways after stimulation with soluble and MA-SCF have been recently described.31 In these studies, significant differences in the use of phospholipase C (PLC)-γ signaling pathway downstream from Kit were observed in the 32D leukemic cell line.
In summary, the results described in the present study, along with the elegant knockin studies by Tajima et al11 demonstrating exclusive expression of MA-SCF in vivo, suggest that, at least under steady-state conditions, S-SCF may play only a minimal role in hematopoiesis in vivo.
Reuben Kapur is a recipient of an American Society of Hematology Junior Faculty Scholar Award.
This work was supported in part by National Institutes of Health grant 2R01 DK48605-06.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Reuben Kapur, Herman B Wells Center for Pediatric Research, Cancer Research Building, 1044 W Walnut St, Room 425, Indianapolis, IN 46202; e-mail: rkapur@iupui.edu.

![Fig. 2. Proliferation and cell cycle progression of G1E-ER2 cells by stromal cells expressing different isoforms of SCF. / G1E-ER2 cells were cocultured for 48 hours with mitomycin C–treated parental Sl/Sl4 stromal cells or stable stromal cell transfectants expressing S-SCF, MA-SCF, or MR-SCF. (A) Proliferation was measured by thymidine incorporation assay. Bars denote the mean thymidine incorporation (counts per minute [cpm] [± SEM]) of 2 independent experiments performed in replicates of 6. *P < .05 S-SCF, MA-SCF, and MR-SCF versus Sl/Sl.4 **P < .001 S-SCF versus MR-SCF and MA-SCF. MR-SCF versus MA-SCF, P = .2. (B) Cell cycle analysis was performed by propidium iodide staining of G1E-ER2 cells. Percentages of cells in different phases of cell cycle are indicated. Shown is a representative experiment. Similar results were observed in 2 independent experiments performed in replicates of 3.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/4/10.1182_blood.v100.4.1287.h81602001287_1287_1293/6/m_h81622980002.jpeg?Expires=1767827956&Signature=P1DoBQNWWKJNo-NLbRFju90jCQkm9tGxupvXGoLTYg1iVssEExoUa~VpuDKtBTjrUzfuP2q9XSMdKoOAAQMdmhTPXxUoa9gJ651Rv0SUq8Ab8KeaCbSBoRHmAUn1dQAIu28PtZSKv-lnVK~ESIcucZmE~UDv-BtxW5yWwkCFF2jH0F536lnZdCiOxUnKaXsvrbCSwktwXv0pIjVR7AzvwovoBw65JCZ9IEN9XtIKOUOGr5qLCeZwcGlMEXOgH4g7oPwroXcl21e8Gfi7APDrgTksOh0MGnLdJ1jzA7Sb5Q~6DVVU4oy-Tk7ZpiKLmGN8OXyj-gq4jRvJCFKCF3gNVg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal