Abstract
We describe data on a 7-year-old girl with congenital dyserythropoietic anemia (CDA), who also had familial Mediterranean fever (FMF). Repeated transfusions required since the age of 6 months to treat her CDA led to iron overload and a persistently high ferritin level. Her relapsing FMF made effective iron chelation therapy very difficult. Consequently, at the age of 4 years, she underwent allogeneic, sibling bone marrow transplantation (BMT). During conditioning for her BMT, symptoms of FMF, including splenomegaly, arthritis, and recurrent abdominal pain, began to resolve and she was gradually weaned off colchicine. Now, 2 years after the transplantation, she remains free from FMF symptomatology and is off all immunosuppressants. This case demonstrates that symptoms of FMF can be alleviated by the therapy used during allogeneic BMT. In this patient it is likely that the missing factor in FMF is now being provided by granulocytes derived from the stem cells within transplanted bone marrow.
Introduction
Familial Mediterranean fever (FMF; Online McKusick Inheritance in Man 249100) is an autosomal recessive disorder characterized by short, episodic bouts of fever, inflammatory serositis causing arthritis, abdominal pain, pleurisy and pericarditis, and an erysipelaslike erythematous rash.1 The erythrocyte sedimentation rate (ESR) is classically increased, with the white blood cell count usually being normal. Amyloidosis can be a long-term complication. Normal serosal fluid contains an inhibitor of neutrophil chemotaxis that acts by antagonizing the complement-derived chemotactic anaphylatoxin C5a. This inhibitory activity is less than 10% of normal in patients with FMF, suggesting that C5a inhibitor deficiency may contribute to the pathogenesis of the inflammatory episodes.2 Colchicine has proved efficacious in minimizing these acute episodes and in preventing the long-term complications of amyloidosis.3
The gene responsible for FMF, MEFV, maps to chromosome 16p,4 and encodes a 791–amino acid protein, variously known as pyrin5 or marenostrin.6 The function of this protein, which is predominantly expressed in granulocytes and synovium, remains to be established, but the most recent evidence suggests it may be an interferon γ–mediated regulator of the inflammatory response.7 A number of mutations have been described,5,6,8-10 with certain mutations associated with particular ethnic groups, suggesting a possible heterozygote advantage.5-10
The congenital dyserythropoietic anemias (CDAs) are rare inherited disorders affecting the normal differentiation-proliferation pathway of the erythroid lineage. The CDAs comprise a group of heterogenous disorders characterized by ineffective erythropoiesis as the predominant mechanism of anemia and distinct morphologic abnormalities of the majority of erythroblasts in the bone marrow.11 The CDAs have been classified into 3 types based on morphologic and serologic findings,12 but there is clearly heterogeneity within the types and many individual cases of CDA have been reported that do not fit into this classification.11
We report data on a patient who had both CDA and FMF, who required allogeneic bone marrow transplantation (BMT) as treatment for the CDA. As a consequence of the BMT she no longer has any symptoms that can be attributed to FMF. We believe this is the first report of treatment of FMF by BMT, which in this patient appears to have been curative.
Patient, materials, and methods
Case report
The patient was the fourth child of consanguineous Coptic Egyptian parents. At the age of 6 weeks she was admitted with a history of irritability and reduced movement of the right arm since soon after birth, with a 1-day history of swelling of the right elbow. Her blood count showed a hemoglobin of 57 g/L, white cell count of 10 × 109/L, and platelets 795 × 109/L. Her blood film showed a leukoerythroblastic picture and a subsequent bone marrow examination showed features typical of congenital dyserythropoietic anemia type II. There were no sideroblasts.
She required further hospital admissions at ages 7 weeks, 10 weeks, 6 months, and 11 months with recurrent episodes of joint swellings associated with fever and marked elevation of C-reactive protein and the ESR. At age 6 months she was admitted with pericarditis, followed by further admissions with episodic vomiting, lethargy, poor feeding, diarrhea, and hepatomegaly. A presumptive diagnosis of FMF was made at age 14 months by one of us (A.M.), and she was started on colchicine, which led to an improvement in her symptoms.
Despite the regular colchicine therapy, she continued to suffer frequent relapses, with abdominal pain, arthritis, fever, and splenomegaly. Her mother would increase her dose of colchicine during these attacks. On 4 occasions these relapses were sufficiently severe to warrant admission, once leading to a laparotomy for suspected small-bowel obstruction.
Her chronic ill health was associated with moderate global developmental delay and failure to thrive. She also had complex feeding difficulties necessitating nasogastric tube feeding from the age of 10 months.
To treat the anemia, she required several blood transfusions from age 6 months, progressing to regular transfusions after 12 months. She developed a rising ferritin level and was started on iron chelation therapy at age 3 years 7 months, with a deferoxamine pump 6 days of 7. Compliance with this regimen became increasingly difficult due to her recurrent episodes of vomiting, diarrhea, and arthritis. Ten months after chelation therapy started her ferritin level was 10 673 μg/L (normal range, 10-150 μg/L). Her mother felt that her symptoms of FMF were frequently exacerbated by the deferoxamine, and so compliance with this remained poor. Her general health was not improved by transfusion. A liver biopsy performed 6 months prior to BMT showed no cholestasis, steatosis, or significant fibrosis, but with a liver iron level of 180.5 μmol/g (normal range < 30 μmol/g).
Tissue typing showed that she had a matched sibling donor. Molecular typing showed identical A, B, and DRB1 markers. The helper T-lymphocyte precursor frequency was 1:1 081 147, suggesting a very low risk of graft- versus-host disease (GVHD).13
Mutation analysis
Genomic DNA was extracted from peripheral blood samples taken from patients and controls using standard techniques.14
Screening for the “common” mutations.
The fragment encompassing the common mutations in exon 10b (Met680Ile, Asp692Ile, Met694Val, Met694Ile, Val726Ala, and Arg761His) was amplified using “hot start” polymerase chain reaction15(PCR) and the oligonucleotide primers described by the International FMF Consortium.5 The cycling conditions used were: initial denaturation step 95°C for 10 minutes, followed by 40 cycles of 95°C for 30 seconds, 63°C for 30 seconds, 72°C for 30 seconds, and a terminal elongation step at 72°C for 4 minutes. The amplification was performed in a total of 25 μL composed of 2.5 μL 10 × PCR buffer II (Perkin Elmer, Scoresby, Victoria, Australia), 15 pmol of each primer, 0.16 mM dNTP solution, 1 U ampliTaq Gold (Perkin Elmer), 2.0 mM MgSO4 solution, and DNA 100 ng.
The presence of the Val726Ala mutation (AluI site created), the Met694Val mutation (HphI site created), and the Met680Ile (NlaIII site destroyed) were analyzed by digesting 5 μL of the PCR product at 37°C overnight with 2 to 2.5 U of appropriate restriction enzyme (New England BioLabs, Beverly, MA) and digestion buffer as per the supplier's specifications to a final volume of 20 μL. The other mutations were screened by automated sequencing of the exon 10b PCR product (SUPAMAC, University of Sydney).
Denaturing high-performance liquid chromatography screening.
The rest of the coding sequence was screened by denaturing high performance liquid chromatography (DHPLC), using the Prostar Helix System (Varian, Walnut Creek, CA). Exons 1, 3, 4, 5, 6, 7, and 10a were amplified using the oligonucleotide sets from the International FMF Consortium.5 Exon 2 was amplified using the oligonucleotide primers described by Aksentijevich et al.9 Exons 8 and 9 were amplified with the following oligonucleotides: exon 8: 8 forward CTCAGGATAGATGGGCTTGG, 8 reverse CAGCACAAGGGAACACTGC; exon 9: 9 forward GACTCATTAGACCACAGTCC, reverse primer for exon 8/9 from the International FMF Consortium.5 Heteroduplexes were formed by denaturing the PCR products at 95°C for 10 minutes then slowly cooling to 55°C over 30 minutes.
The theoretical temperature for each PCR product was determined fromhttp://insertion.stanford.edu/melt.html and then optimized for temperatures ± 2°C the predicted temperature. The optimal temperature was determined by comparing the retention time of the same sample over the 5 different temperatures and on peak morphology. The temperature chosen was either one where a heteroduplex was seen or the temperature at or before the peak started to broaden, in cases where this was difficult to determine 2 temperatures were used. The temperatures used were as follows: exon 1 (63°C), 2a (69°C), 2b (69°C), 3 (63°C), 4 (61°C), 5 (61°C and 62°C), 6 (59°C and 60°C), 7 (61°C), 8 (60°C), 9 (60°C and 61°C), and 10a (59°C and 60°C). Where a heteroduplex was identified, the presence of a sequence variation was confirmed by automated sequencing (Australian Genomic Resource Facility, University of Queensland).
Where no heteroduplex was identified in an amplicon, that amplicon was subjected to automated sequencing. Thus, the whole of the coding region was screened by DHPLC and automated sequencing.
Results
BMT
The young patient was admitted 1 month prior to the planned BMT for 2 weeks of intravenous chelation therapy, bringing her ferritin level down from 5700 μg/L to 3425 μg/L. Her main symptoms of FMF at this stage were swelling of her left elbow, splenomegaly, and diarrhea.
Conditioning therapy, which was commenced at 4 years of age, consisted of 4 doses of busulphan, 150 mg/m2 per day (6.3 mg/kg per day) as a single daily dose on days −9 to −6; 4 doses of cyclophosphamide, 50 mg/kg per day on days −5 to −2; and 3 doses of antithymocyte globulin, 15 mg/kg, on days −8, −6, and −4. GVHD prophylaxis was with cyclosporin, initially at 5 mg/kg per day from day −1. Unmanipulated bone marrow was reinfused on day 0, with a nucleated cell count of 6 × 108/kg recipient body weight. The donor was her healthy brother, who was clinically unaffected by either condition.
The posttransplantation course was uncomplicated. She tolerated nasogastric feedings well throughout the BMT. She received one course of antibiotics for a Klebsiella pneumoniae septicemia. She developed clinical skin GVHD on day +38, which was treated with oral prednisolone from days +38 to +93. She showed full hematologic recovery with neutrophil count of 0.5 and 1.0 × 109/L on day +21 and platelet counts of 25, 50, and 100 × 109/L on days +45, +50, and +87, respectively. Her last transfusion of packed cells was on day +9, and she has been undergoing regular venisection since day +66. Her last serum ferritin level was 207 μg/L at 28 months after BMT and no further venisections are planned.
Donor chimerism was confirmed by analysis of phytohemagglutinin-stimulated lymphocytes from peripheral blood. The first successful analysis was on day +73, when 2 of 30 cells were female. All subsequent analyses, from day +108 to 2 years after BMT revealed male cells only. Cyclosporin therapy was stopped at 8 months after BMT.
Course of symptoms of FMF after BMT
The patient's symptoms of FMF rapidly abated during the conditioning therapy and colchicine treatment was halted the day after BMT. Her persistent left elbow effusion resolved and her splenomegaly rapidly disappeared. She remains free of any symptoms of FMF at 28 months after BMT, 20 months after cessation of cyclosporin therapy. At 14 months following BMT, she had an episode of Enterobactersepticemia. This was associated with fever and transient splenomegaly, but no other FMF symptoms. She is feeding well orally and has started kindergarten. She has shown significant catch-up growth, with an increase in height from a standard deviation score (SDS) score of −3.55 at BMT to −1.83 at 26 months after BMT. Her weight has increased from 19th to 50th percentile (SDS of −0.89 to −0.0).
Mutation analysis
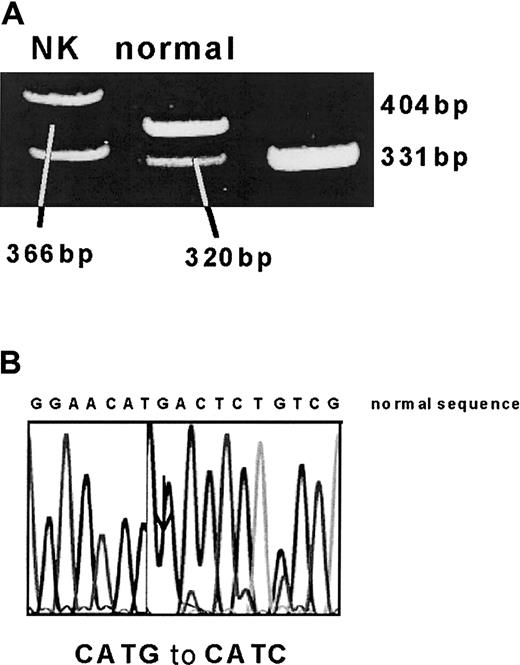
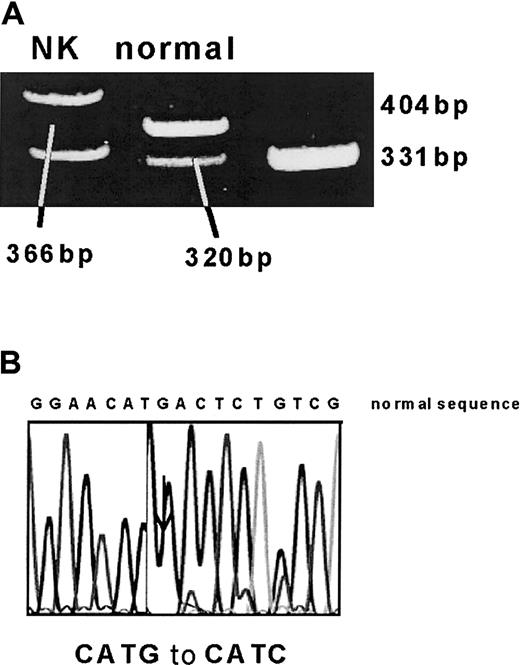
DNA extracted from peripheral blood samples was analyzed before BMT for the patient and at 10 months after BMT for the patient and donor. In the patient's DNA collected before BMT, one allele was found to have the Met680Ile mutation by NlaIII restriction digest and direct sequencing (Figure1). However, despite DHPLC screening and sequencing of the whole coding region, no other disease-causing mutation was identified.
Identification of Met680Ile (2040 G>C) mutation.
(A) A 391-bp PCR fragment was digested with NlaIII. The normal allele is cut to 320-, 46-, and 25-bp fragments, whereas the mutation (which leads to a loss of a NlaIII site) results in 366- and 25-bp fragments. (B) This was confirmed by automated sequencing.
Identification of Met680Ile (2040 G>C) mutation.
(A) A 391-bp PCR fragment was digested with NlaIII. The normal allele is cut to 320-, 46-, and 25-bp fragments, whereas the mutation (which leads to a loss of a NlaIII site) results in 366- and 25-bp fragments. (B) This was confirmed by automated sequencing.
In addition, the patient was found to be heterozygous for the following silent polymorphisms: 414 A>G (Gly138; exon 2), 495 C>A (Ala165; exon 2), 942 C>T (Arg314; exon 3), 1422 A>G (Glu474; exon 5), 1428 G>A (Gln476; exon 5), and 1530 T>C (Asp510; exon 5) [results not shown].
Her clinically healthy donor brother did not have the Met680Ile mutation. He was also found to have the 6 silent polymorphisms, and parental studies revealed that the 6 polymorphisms were inherited from their mother, whereas the Met680Ile mutation was inherited from their father [results not shown].
Discussion
Although the precise function of pyrin/marenostrin remains to be established, available evidence suggests that it plays a role in the regulation of inflammatory processes.16 Recently, it was shown that a pyrin isoform, which has an in-frame deletion of exon 2, shows a markedly different subcellular localization pattern to the full-length protein in transient expression studies, with spliced variant targeted mainly to the nucleus, whereas the full-length form localized to the cytoplasm.7 Based on these results, it has been speculated that the different mutations in the MEFVgene might have different biologic consequences.7 It would be premature at this stage, however, to speculate whether the combination of MEFV mutations in the present patient had a pathogenetic role her development of CDA.
Congenital dyserythropoietic anemia type II (as in this case) is the most frequent form of congenital dyserythropoiesis. It is characterized clinically by an anemia of variable severity, jaundice, and hepatosplenomegaly. Gallbladder disease and secondary hemochromatosis are frequent complications.17 Erythroid hyperplasia with binuclearity or multinuclearity involving late erythroblasts in the bone marrow is a key feature of the diagnosis. In addition, the CDA type II erythrocytes display antibody-mediated sensitivity to lysis in acidified sera of many ABO-compatible donors, which has led to the alternative name of HEMPAS (hereditary erythroblastic multinuclearity with positive acidified serum).18 The erythrocyte membrane proteins show abnormalities of glycosylation, in particular band 3, the anion exchanger.19 Underglycosylated band 3 aggregates in solution and clusters in membranes, resulting in membrane disorganization.20 Evidence has been provided for the localization of the CDA II locus to chromosome 20q11.2 by linkage analysis, although at least 5% to 10% of cases failed to map to 20q stressing genetic heterogeneity.21 In a few patients the underglycosylation of band 3 has been identified as resulting from a deficiency in the activity of either β-4-galactosyltransferase (GalT),N-acetylglucosaminyltransferase (GnT II), or α-mannosidase II (Man II). However, none of the genes for these enzymes localize to chromosome 20,22 suggesting that these deficiencies may be secondary changes, or alternatively result from a deficiency of a tissue-specific transcription factor. Thus, despite progress in the field the primary molecular defect responsible for CDA type II remains unknown.
There has previously been one report of a family with 3 affected members (2 brothers and a cousin) who experienced CDA in association with chronic recurrent multifocal osteomyelitis (CRMO) and Sweet syndrome.23 Interestingly this family was of a consanguineous Palestinian Arab background. The authors proposed that the inherited tendency to the 2 disorders segregated together. Because of this report a diagnosis of CRMO was entertained in our patient early in the course of her disease when she had predominantly bone and joint inflammation. It is interesting to speculate that perhaps the patients in this report in fact had FMF and not CRMO, and perhaps the FMF and CDA segregate together. Alternatively the CDA or the defects of glycosylation may have an impact on the FMF phenotype.
A disease-causing mutation was identified in only one allele of the proband, whereas the other allele harbored 6 apparently silent polymorphisms, which do not coincide with any of the previously reported founder haplotypes.24 It is possible that the pathogenic mutation in the other allele is located in the promoter region of the gene, or in an intronic region not sequenced, which might affect splicing of the messenger RNA (mRNA). Another possibility is that one of the apparent silent exonic polymorphisms affects an exonic splicing enhancer, resulting in perturbations of the normal splicing processes.25,26 It is also possible that in this family the FMF trait has been transmitted with true autosomal dominant inheritance, as has been recently reported,27 28 although this has not been observed to date for the Met680Ile mutation. Finally, it may be that together the combination of silent polymorphisms in cis alters the structure or function of the pyrin polypeptide in a deleterious manner. Confirmation or exclusion of these possibilities may be possible after the development of a functional assay for the pyrin gene product and mRNA studies.
Bone marrow transplantation, once confined to the treatment of hematologic malignancy abnormalities,29,30 has now expanded to treat other stem cell and other monogenic disorders.31-33 Clearly the potential risks of BMT would not routinely be justified in the treatment of FMF, particularly when there is an effective, simple, and relatively safe treatment in the form of colchicine for both acute attacks and long-term complications.
It is unclear whether the patient's hematologic condition exacerbated her FMF, but her parents had the distinct impression that deferoxamine therapy worsened her symptoms, making compliance difficult. Because of severe iron overload, BMT became mandatory to treat the CDA, with the fortuitous result that the BMT appears to have also “cured” her FMF, at least after 28 months of observation off colchicine after BMT, and 20 months off all immunosuppressants.
We believe that this is the first reported case of a patient with FMF receiving a BMT. We suggest that BMT should be considered, albeit as a last resort, in patients who are extremely unresponsive to all therapies including colchicine and interferon α.34
Prepublished online as Blood First Edition Paper, June 14, 2002; DOI 10.1182/blood-2002-02-0651.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Peter J. Shaw, Oncology Department, The Children's Hospital at Westmead, Sydney, NSW 2145, Australia; e-mail:peters@chw.edu.au.